Chapter 4 Intraoperative Neurophysiology
A Tool to Prevent and/or Document Intraoperative Injury to the Nervous System
Supratentorial Surgery
Surgery for brain gliomas has become more and more aggressive. This is based on clinical data that support better patient survival and quality of life after gross total removal of both low- and high-grade lesions.1,2
However, the resection of tumors located in eloquent brain areas, such as the rolandic region and frontotemporal speech areas, requires the identification of functional cortical and subcortical areas that must be respected during surgery. Moreover, the dogmatic assumption that tumoral tissue could not retain function has been repeatedly questioned by neurophysiologic and functional magnetic resonance imaging studies.3–5 In response to the need for a safe surgery in eloquent brain areas, the past decade has seen the development of a number of techniques to map brain functions, including, but not limited to, functional magnetic resonance imaging, magnetoencephalography, and positron emission tomography.6–11
The neurophysiologic contribution to brain mapping has been evident since the late 19th century with the pioneering work of Fritsch and Hitzig12 and Bartholow.13 In the 20th century, Penfield and colleagues14,15 made invaluable contributions through intraoperative mapping of the sensorimotor cortex, whose findings have been substantiated by a number of recent studies.16–18
Somatosensory-Evoked Potential Phase-Reversal Technique
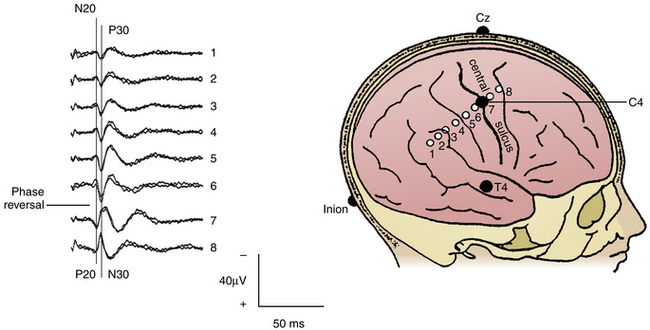
To indirectly identify the central sulcus, SEPs can be recorded from the exposed cerebral cortex by using the phase-reversal technique. SEPs are elicited by stimulation of the median nerve at the wrist and the posterior tibial nerve at the ankle (40-mA intensity, 0.2-msec duration, 4.3-Hz repetition rate). Recordings are performed from the scalp at CZ′-FZ (for legs) and C3′/C4′-CZ′ (for arms) according to the 10–20 International Electroencephalography System. After craniotomy, a strip electrode is placed across the exposed motor cortex and primary somatosensory cortex, transversing the central sulcus. This technique is based on the principle that an SEP, elicited by median nerve stimulation at the wrist, can be recorded from the primary sensory cortex.19 Its mirror-image waveform can be identified if some of the contacts of the strip electrode are placed on the opposite side of the central sulcus, over the motor cortex20–22 (Fig. 4-1). For phase reversal, a strip electrode with four to eight stainless steel contacts with an intercontact distance of 1 cm is used. In the literature, the success rate of the phase reversal technique to indirectly localize the primary motor cortex ranges between 91%20,21 and 97%.18 Interestingly, identification of the central sulcus by magnetic resonance imaging provided contradictory results when compared with intraoperative phase reversal.20 Although it is expected that ongoing progress in the field of functional magnetic resonance imaging will eventually replace the need for neurophysiologic tests, ION still retains the highest reliability in mapping of the motor cortex and language areas when compared with functional neuroimaging.23–26
Direct Cortical Stimulation (60-Hz Penfield Technique)
Once the motor strip has indirectly been identified by the phase reversal technique, direct cortical stimulation is needed to confirm the localization of the motor cortex. Most current methods are based on the original Penfield technique. This calls for continuous direct cortical stimulation over a period of a few seconds with a frequency of stimulation of 50 to 60 Hz and observation of muscle movements.14,16,27 An initial current intensity of 4 mA is used and, if no movements are elicited in contralateral muscles of the limbs and face, stimulation is increased in steps of 2 mA to the point at which movements are elicited.16 Muscle responses can either be observed visually or documented by multichannel electromyography, which appears to be more sensitive.28 If no response is elicited with an intensity as high as approximately 16 mA, that area of cortex is considered not functional and can therefore be removed.29 It should be emphasized that a negative mapping does not always ensure safety. To increase the chances of obtaining a positive mapping result, technical and anesthesiologic drawbacks have to be carefully ruled out and cortical exposure should be generous.
More in general, a limitation to the reliability of cortical mapping is the large variability of threshold for a positive mapping response across and within individuals.30 A motor response from the same muscular group can be elicited from more than one cortical site, using different stimulation intensities.
1. Some authors apply the concept that thresholds (the minimum stimulation current required to induce functional changes) vary across the exposed cortex depending on the task being assessed and the location being maped. This is in keeping with the observation that even afterdischarge (AD) thresholds can vary significantly, not only across the population but in the same subject at different cortical sites.30,31 Accordingly, they attempt to maximize stimulation currents at each cortical site to ensure the absence of eloquent function. Doing so, it is more common to exceed AD thresholds in adjacent cortices, and there is a higher risk of distal activation due to current spreading to adjacent sites.
2. Other authors29,32,33 keep stimulation intensity constant while mapping the entire cortex and set threshold just below the lowest current observed to induce AD. This strategy is aimed to minimize the risk of inducing ADs (which may invalidate the results) and clinical seizures, but may miss the identification of eloquent cortical sites.
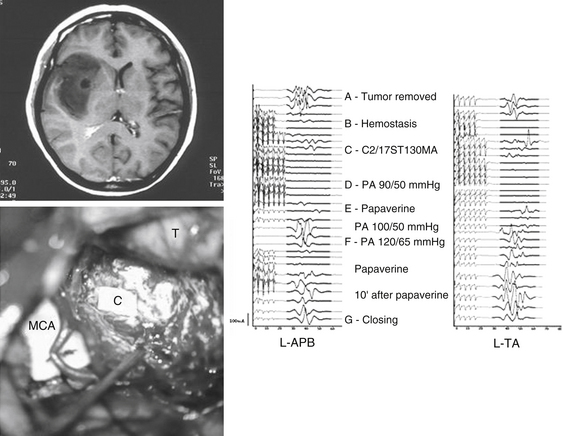
Spreading of the current using the 60-Hz stimulation technique is limited to 2 to 3 mm as detected by optical imaging in monkeys.34 Accordingly, one can assume that using this technique is safe for removal of tumors very close to the motor and sensory pathways as long as stimulation is repeated whenever a 2- to 3-mm section of tumoral tissue is removed.29 Similarly, this technique allows us to map motor pathways subcortically while removing tumors that arise or extend to the insular, subinsular, or thalamic areas.27,35 At the subcortical level, the stimulation intensity required to elicit a motor response is usually lower than that required for cortical mapping. When performing subcortical mapping, however, we have to keep in mind that a distal muscle response after stimulation of subcortical motor pathways can be misleading. Although this stimulation activates axons distal to the stimulation point, the possibility of damage to the pathways proximal to that point cannot be ruled out. This is a concern, especially when dealing with an insular tumor where there is a risk of cortical or subcortical ischemia induction secondary to manipulation of perforating vessels (Fig. 4-2).
Despite its popularity in the past, this 60-Hz Penfield technique has some disadvantages. With the exception of speech mapping, it is our opinion that these disadvantages should prevent its use as a motor cortex/pathways mapping technique. First, this technique can induce seizures in as many as 20% of patients, despite therapeutic levels of anticonvulsants and regardless of whether there is a preoperative history of intractable epilepsy.36,37 Second, in children younger than 5 years old, direct stimulation of the motor cortex for mapping purposes may not yield localizing information because of the relative unexcitability of the motor cortex.19,38 Third, because this is a mapping and not a monitoring technique, no matter how often cortical or subcortical stimulation is repeated, the functional integrity of the motor pathways cannot be assessed continuously during surgery.
Direct Cortical Stimulation and Motor-Evoked Potential Monitoring (Short Train of Stimuli Technique)
Recently, mapping techniques have integrated monitoring techniques to continuously assess the functional integrity of the motor pathways and therefore increase the safety of these procedures.20,39–41 The following is a description of the technique that we use at our institutions and have found suitable for both mapping and monitoring.
After exposure of the cortex and once phase reversal has been performed, direct cortical stimulation of the motor cortex can be achieved by using a monopolar-stimulating probe to identify the cortical representation of contralateral facial and limb muscles. The same parameters of stimulation used for TES, except for a much lower intensity (≤20 mA), can be used.39 Sometimes the short train of stimuli technique requires slightly higher current intensities than those required by the Penfield technique. However, by using a very short train, the charge applied to the brain is significantly reduced42 and, consequently, the risk of inducing seizures. The number of pulses in the short-train technique is five to seven pulses per second, whereas in the Penfield technique, there are 60 pulses per second. The effect of stimulation on the cerebral cortex, from a neurophysiologic point of view, differs between the Penfield technique and the short train of stimuli technique. The Penfield technique delivers one stimulus every 15 to 20 msec continuously for a couple of seconds. The short train of stimuli technique delivers five to seven stimuli in a period of approximately 30 msec with a long pause between trains (470 to 970 msec, which depends on train repetition rate—1 or 2 Hz). Therefore, the Penfield technique is more prone to produce seizures, activating the cortical circuitry more easily than short-train stimuli do. Furthermore, compared with the Penfield technique, the short-train technique does not induce strong muscle twitches that may interfere with the surgical procedure. Responses are usually recorded from needle electrodes used to record muscle MEPs elicited by TES. However, any combination of recording muscles can be used, according to the tumor location. The larger the number of monitored muscles, the lower the chance of a false-negative mapping result. We suggest that stimulation of the tumoral area should always be performed to rule out the presence of some functional cortex. As already described, this is especially true in the case of low-grade gliomas.3–5
In the illustrative case presented in Fig. 4-2,39 an impairment of muscle MEPs occurred at the end of tumor removal when opening and closing mapping procedures had already been done and confirmed the integrity of motor pathways distal to the stimulation point at the level of the internal capsule. However, ischemia of the pyramidal tracts secondary to severe vasospasm of the main perforating branches of the middle cerebral artery occurred during hemostasis and was detected by muscle MEP monitoring. If not detected in time, this event would have likely resulted in an irreversible loss of muscle MEPs and, consequently, a permanent motor deficit. Mapping techniques are unlikely to detect these events because they do not allow a continuous “online” assessment of the functional integrity of neural pathways.
In our experience with using the short-train technique, a threshold lower than 5 mA for eliciting muscle MEPs usually indicated proximity to the motor cortex. When muscle responses are elicited through higher stimulation intensities, activation of the CT is of less localizing value because of the possibility of spreading of the current to adjacent areas.39
When removing a tumor that extends subcortically, preservation of muscle MEPs during monitoring from the strip electrode will guarantee the functional integrity of motor pathways and avoid the need for periodic remapping of the cortex at known functional sites.39
For insular tumors where the motor cortex is not exposed by the craniotomy, a strip electrode can still be gently inserted into the subdural space to overlap the motor cortex. Phase reversal and/or direct cortical stimulation can be used to identify the electrode with the lowest threshold to elicit muscle MEPs. The use of MEPs during surgery for insular tumors has proved very useful to identify impending vascular derangements to subcortical motor pathways in time for corrective measures to be taken. In spite of the observation that intraoperative MEP changes occurred in nearly half of the procedures, these were reversible in two thirds of the cases.43
Warning Criteria and Correlation with Postoperative Outcome
Still debated are the warning criteria for changes in muscle MEPs that are used to inform the surgeon about an impending injury to the motor system. It should be stressed that although for spinal cord surgery, a “presence/absence” of muscle MEPs criterion has proved to be reliable and strictly correlates with postoperative results,44,45 there are not definite MEP parameters indicative of significant impairment during supratentorial surgery.46 We believe that the predictive value of muscle MEPs is different for supratentorial and spinal cord surgeries. As such, different warning criteria must be employed. This judgment is based on the difference in types of CT fibers in supratentorial portion of the CT as compared with the spinal cord. Different groups with established experience in this field have proposed similar criteria,40,46 suggesting that a shift in latency between 10% and 15% and a decrease in amplitude of more than 50% to 80% correlate with some degree of postoperative motor deficit. However, a permanent new motor deficit has consistently correlated only with irreversible complete loss of muscle MEPs.46
A persistent increase in the threshold to elicit muscle MEPs or a persistent drop in muscle MEP amplitude, despite stable systemic blood pressure, anesthesia, and body temperature, represents a warning sign. However, it should be noted that muscle MEPs are easily affected by muscle relaxants, bolus of intravenous anesthetics and high concentrations of volatile (and other) anesthetics such that wide variation in muscle MEP amplitude and latency can be observed.47 Due to this variability, the multisynaptic nature of the pathways involved in the generation of muscle MEPs, and the nonlinear relationship between stimulus intensity and the amplitude of muscle MEPs, the correlation between intraoperative changes in muscle MEPs (amplitude and/or latency) and the motor outcome are not linear. Further clinical investigation is needed to clarify sensitive and specific neurophysiologic warning criteria for brain surgery.
Brain Stem Surgery
The neurosurgeon faces two major problems when attempting to remove brain stem tumors. First, if the tumor is intrinsic and does not protrude on the brain stem surface, approaching the tumor implies a violation of the anatomic integrity of the brain stem. Knowledge of the location of critical neural pathways and nuclei is mandatory when considering a safe entry into the brain stem,48,49 but may not suffice when anatomy is distorted. Morota and colleagues50 reported that visual identification of the facial colliculus based on anatomic landmarks was possible in only three of seven medullary tumors and was not possible in five pontine tumors. The striae medullares were visible in four of five patients with pontine tumors and in five of nine patients with medullary tumors.
Mapping Techniques
Neurophysiologic mapping techniques have been increasingly used to localize CT and cranial nerve motor nuclei on the lateral aspect of the midbrain and on the floor of the fourth ventricle.50–54
Mapping of the Corticospinal Tract at the Level of the Cerebral Peduncle
This is a recently described technique used to map the CT tract within the brain stem at the level of the cerebral peduncle.54,55 To identify the CT, we use a hand-held monopolar-stimulating probe (0.75-mm tip diameter) as a cathode, with a needle electrode inserted in a nearby muscle as an anode. If the response (D wave) is recorded from an epidural electrode, a single stimulus is used. Conversely, if the response is recorded as a compound muscle action potential from one or more muscles of contralateral limbs, a short train of stimuli should be used.
This technique is particularly useful for midbrain tumors that have displaced the CT tract from its original position. Usually, the so-called midbrain lateral vein described by Rhoton56 represents a useful anatomic landmark because it allows an indirect identification of the CT tract, located anterior to the vein. However, when an expansive lesion distorts anatomy, only neurophysiologic mapping allows the identification of the CT and, consequently, a safe entry zone to the lateral midbrain.
In the case of a cystic midbrain lesion, sometimes mapping of the CT is negative at the beginning of the procedure, but a positive response can be recorded when mapping from within the cystic cavity toward the anterolateral cystic wall.57
Mapping of Motor Nuclei of Cranial Nerves on the Floor of the Fourth Ventricle
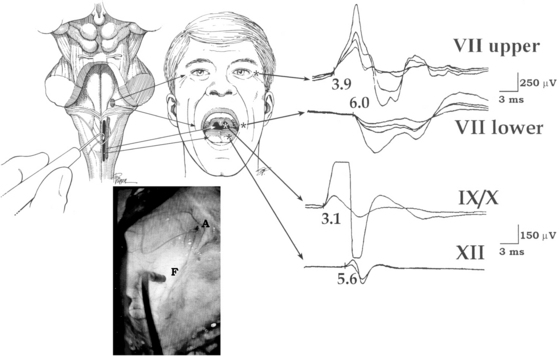
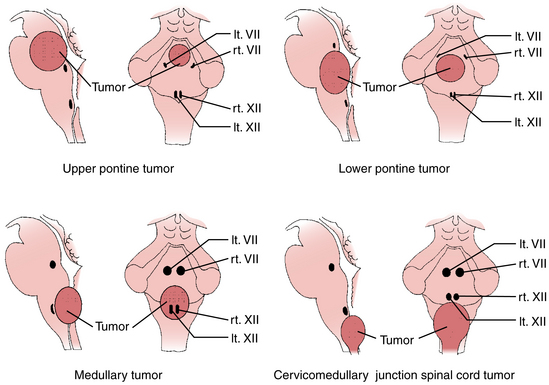
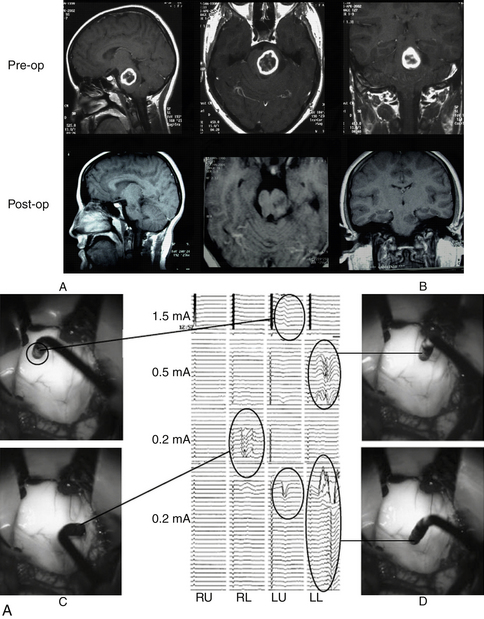
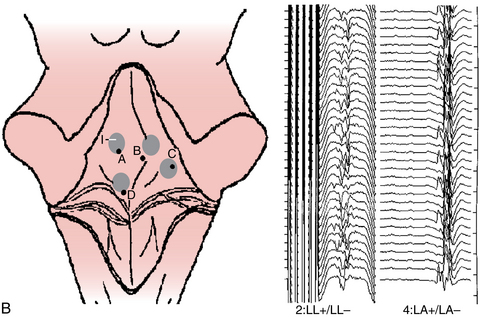
This technique is based on intraoperative electrical stimulation of the motor nuclei of the cranial nerves on the floor of the fourth ventricle, using a hand-held monopolar stimulating probe. Compound muscle action potentials are then elicited in the muscles innervated by the cranial motor nerves. A single stimulus of 0.2-msec duration is delivered at a repetitive rate of 2.0 Hz. Stimulation intensity starts at approximately 1 mA and is then reduced to determine the point with the lowest threshold that elicits muscle responses corresponding with the mapped nucleus (Fig. 4-3). No stimulation intensity higher than 2 mA should be used.50,51 To record the responses from cranial motor nerves VII, IX/X, and XII, wire electrodes are inserted into the orbicularis oculi and orbicularis oris muscles, the posterior wall of the pharynx, and the lateral aspect of the tongue muscles, respectively. Based on mapping studies, characteristic patterns of motor cranial nerve displacement, secondary to tumor growth, have been described (Fig. 4-4).58 The case described in Fig. 4-5 is consistent with this observation.
A similar methodology can be applied to identify the motor nuclei of cranial nerves innervating ocular muscles (nerves III, IV, and VI) during a dorsal approach to midbrain lesions as well as quadrigeminal plate, tectal, and pineal region tumors.59,60
Despite the relative straightforwardness of the fourth ventricle mapping technique and its indisputable usefulness in planning the most appropriate surgical strategy to enter the brain stem, postoperative functional outcome is not always predicted by postresection responses.50 In the case of mapping of the motor nuclei of the seventh cranial nerve, brain stem mapping cannot detect injury to the supranuclear tracts originating in the motor cortex and ending on the cranial nerve motor nuclei. Consequently, a supranuclear paralysis would not be detected, although lower motoneuron integrity has been preserved. Similarly, the possibility of stimulating the intramedullary root more than the nuclei itself exists. This could result in a false-negative peripheral response still being recorded despite an injury to the motor nuclei.50
Mapping of the glossopharyngeal nuclei is also of limited benefit. Recording activity from the muscles of the posterior pharyngeal wall after stimulation of the ninth cranial nerve motor nuclei on the floor of the fourth ventricle assesses the functional integrity of the efferent arc of the swallowing reflex. This technique, however, does not provide information on the integrity of afferent pathways and afferent/efferent connections within brain stem, which are indeed necessary to provide functions involving reflexive swallowing, coughing, and the complex act of articulation. Recently, however, Sakuma and colleagues61 succeeded in monitoring glossopharyngeal nerve compound action potentials after stimulation of the tongue in dogs, opening the possibility of a new field of investigation in humans. Functional magnetic resonance imaging of the brain stem has also provided a new, yet experimental, tool to localize cranial nerve nuclei in humans.62
An intrinsic limitation of all mapping techniques, however, is that these do not allow the continuous evaluation of the functional integrity of a neural pathway. The identification of the safe entry zone for approaching a pontine astrocytoma, as in Fig. 4-5, does not provide any information on the well-being of the adjacent corticospinal, corticobulbar, sensory, and auditory pathways during the surgical manipulation aimed to remove the tumor. Therefore, it is essential to combine mapping with monitoring techniques.
Monitoring Techniques
SEPs and brain stem auditory-evoked potentials have been extensively used to assess the functional integrity of the brain stem, and we refer the reader to the related literature for a review of these classic techniques. Unfortunately, SEPs and brain stem auditory-evoked potentials can evaluate only approximately 20% of brain stem pathways.63 As a result, their use is of limited valued when the major concern is related to corticospinal and cranial nerve motor function. Still, brain stem auditory-evoked potentials can provide useful information on the general well-being of the brain stem, especially during those procedures in which a significant surgical manipulation of the brain stem and/or of the cerebellum is expected. When interpreting brain stem auditory-evoked potential recordings, a thoughtful analysis of the waveform and of their correlation with neural generators provides useful information about the localization of the changes.
When an initial myelotomy is performed at the region of dorsal column nuclei of the medulla, further monitoring with SEPs is compromised due to limitations similar to those related to intramedullary spinal cord tumor surgery after myelotomy.54 For pontine and midbrain tumors, SEPs have little localizing value but can still be used to provide nonspecific information about the general functional integrity of the brain stem (because it is expected that a major impending brain stem failure will be detected by changes in SEP parameters).
With regard to motor function within the brain stem, standard techniques for continuously assessing the functional integrity of motor cranial nerves relies on the recording of spontaneous electromyographic activity in the muscles innervated by motor cranial nerves.60,64,65 Several criteria have been proposed to identify electromyographic activity patterns that may anticipate transitory or permanent nerve injury, but these patterns are not always easily recognizable and criteria remain vague or at least subjective. Overall, convincing data regarding a clinical correlation between electromyographic activity and clinical outcomes is still lacking.60,64
Seeking more reliable techniques in the neurophysiologic monitoring of motor cranial nerve integrity, the possibility of extending the principles of CT monitoring to the corticobulbar tracts is currently under investigation.57,66
Monitoring of Corticobulbar (Corticonuclear) Pathways
For this purpose, TES with a train of four stimuli, with an interstimulus interval of 4 msec, and a train-stimulating rate of 1 to 2 Hz, intensity between 60 and 100 mA can be used. The stimulating electrode montage is usually C3/Cz for right-side muscles and C4/Cz for left-side muscles. For recording muscle MEPs, electromyographic wire electrodes are inserted in the orbicularis oris and orbicularis oculi muscles for nerve VII, in the posterior pharyngeal wall for the cranial motor nerves IX and X, and in the tongue muscles for the hypoglossal nerve (i.e., in the same manner as described for mapping of motor nuclei of the cranial nerves). Reproducible muscle MEPs can be continuously recorded from the facial, pharyngeal, and tongue muscles while the brain stem is surgically manipulated (see Fig. 4-5). This technique allows one to monitor the entire pathway, from the motor cortex down to the neuromuscular junction so that a supranuclear injury can be detected. However, the corticobulbar tract monitoring technique is still far from becoming standardized due to some theoretical and practical drawbacks. First, from a neurophysiologic perspective, use of the lateral montage as an anodal stimulating electrode (C3 or C4) increases the risk that strong TES may not activate the corticobulbar pathways but the cranial nerve directly. Accordingly, an injury to the corticobulbar pathway rostral to the point of activation may be masked by a misleading preservation of the muscle MEP. To minimize this risk, stimulation intensity should be kept as low as possible. One of the ways to recognize structure generating this response is as follows; 90 msec after delivering train of stimuli a single stimulus was delivered through the same stimulating montage. The rationale for this kind of stimulation is the fact that in most patients under general anesthesia, only a short train of stimuli can elicit “central” responses generated by the motor cortex or subcortical part of corticobulbar tract (CBT). If a single stimulus elicits a response, this should be considered a “peripheral” response which activates the cranial nerve directly by spreading current more distally.67
Furthermore, given the continuous fluctuations in the threshold required to elicit muscle MEPs intraoperatively (i.e., due to variability in room temperature, anesthesiologic regimen, and physiologic variability in muscle MEP threshold, and so on), the appropriate threshold for monitoring corticobulbar pathways should be rechecked throughout the surgical procedure. Another limitation of this technique is that spontaneous electromyographic activity can sometimes hinder the recording of reliable muscle MEPs from the same muscles. In our experience, this spontaneous activity appears to be more common in the pharyngeal muscles as compared with the facial and tongue muscles. Further experience with this technique will indicate the extent to which monitoring of the corticobulbar tract predicts postoperative function and allows an impending injury to the motor cranial nerves to be recognized in time to be corrected (see Fig. 4-5).
Due to the complexity of the brain stem’s functional anatomy, the more neurophysiologic techniques that can be rationally integrated, the better the chances for successful monitoring.68 The battery of techniques to be used should be tailored to each individual patient according to tumor location and clinical status. Keeping this in mind, SEPs and brain stem auditory-evoked potentials should always be considered. However, unlike in the past decade when these classic monitoring methods allowed only a very limited assessment of the brain stem functional integrity, current techniques of MEP monitoring and motor nuclei mapping are receiving increasing credit in assisting the neurosurgeon during brain stem surgery.
Spinal and Spinal Cord Surgery
These surgeries are potentially burdened with serious neurologic deficits such as para- or quadri-plegia (paresis). As a rule, the closer to the spinal cord that the neurosurgeon operates, the higher is the risk of injury. Of course, there is always the possibility that surgeries on the bony structures of the spinal cord can result in paraplegia.69,70 Furthermore, long-lasting intraoperative hypotension can be disastrous for the spinal cord if neurophysiologic monitoring has not been used because no other routine methods are available to evaluate the functional integrity of the spinal cord during hypotension.
It has been shown that the use of SEPs to monitor the functional integrity of the spinal cord is inadequate and can result in false-negative results (i.e., no changes in SEP parameters intraoperatively, but the patient wakes up paraplegic after surgery69,70). Therefore, it is mandatory that during spinal and spinal cord surgeries, monitoring of both sensory and motor modalities of evoked potentials is conducted. Each of these methods evaluates different long tracts; SEPs evaluate the dorsal columns, whereas MEPs evaluate CT If a lesion to the spinal cord is diffuse in nature, affecting both long tracts, monitoring one of them may suffice. Unfortunately, this is not always the case. A typical example is anterior spinal cord artery syndrome with preservation of SEPs and disappearance of MEPs.70
Surgery for intramedullary spinal cord tumors requires a special approach concerning monitoring with MEPs. During this type of surgery, very precise surgical instruments are used, such as the Contact Laser System (SLT, Montgomeryville, PA),71 and a very selective lesion within the spinal cord can occur. Therefore, monitoring this type of surgery using only MEPs recorded from limb muscles can be insufficient. Monitoring the D wave (i.e., recording descending activity of the CT using catheter electrodes placed over the exposed spinal cord) should be combined with MEP recording from limb muscles. Combining both of these techniques proved highly effective in preventing paraplegia/quadriplegia. This gives the neurosurgeon the opportunity to be more radical in tumor resection. This combined type of monitoring can precisely predict transient postoperative motor deficits45,72 and clearly distinguish them from permanent ones.
Neurophysiologic Monitoring of the Spinal Cord and Spinal Surgeries with Motor-Evoked Potentials
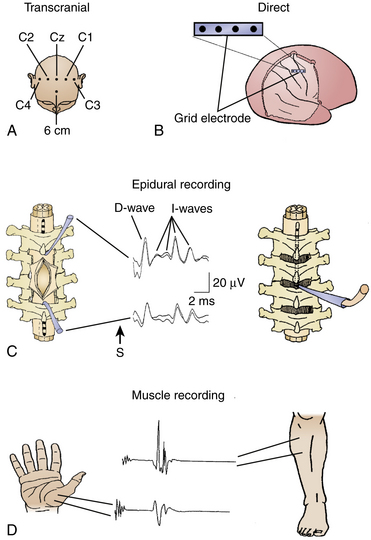
A schematic drawing of techniques for eliciting MEPs by TES or direct electrical stimulation of the exposed motor cortex while recording them from the spinal cord (D wave) or limb muscles (muscle MEPs) is presented inFig. 4-6.
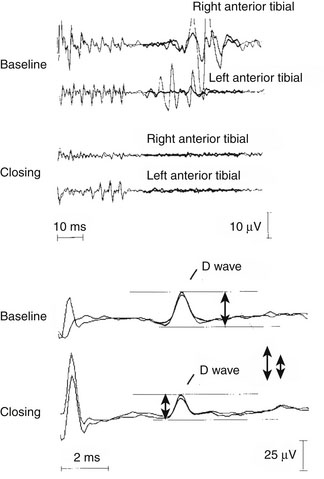
Table 4-1 summarizes the results from the combined use of D-wave and muscle MEP recordings during surgery for intramedullary spinal cord tumors. The neurosurgeon can proceed with the surgery aggressively, without jeopardizing the patient’s motor status despite the complete disappearance of the muscle MEPs during surgery. This is only allowed when the D-wave amplitude does not decrease more than 50% from the baseline amplitude. After disappearance of muscle MEPs, patients will have only transient postoperative motor deficits, with a full recovery of muscle strength later on. Therefore, to achieve a good postoperative motor outcome, it is imperative that the critical decrement in D-wave amplitude not be permitted. The neurophysiologic explanation for transient paraplegia is that the D wave is generated exclusively by the descending activity of the transcranially activated fast neurons of the CT, whereas muscle MEPs are generated by the combined action of the fast neurons of the CT and propriospinal and other descending tracts within the spinal cord (of course, with consecutive activation of alpha motoneurons, peripheral nerves, and muscles). The selective lesion to the propriospinal and other descending tracts can occur with the use of precise neurosurgical instruments (e.g., contact laser with a tip of 200 μm, producing minimal collateral damage).71 Lesioning of the propriospinal system and other descending tracts can be functionally compensated postoperatively, whereas a lesion to the fast neurons of the CT cannot. Empirically, we have discovered that decrements in the amplitude of the D wave occur in a stepwise fashion (except in the case of anterior spinal artery lesion). Therefore, the neurosurgeon has enough time to make a decision and can immediately stop the surgery when a critical decrement in D-wave amplitude occurs. The previous statement has been tested in 100 surgeries for intramedullary spinal cord tumors performed by one neurosurgeon and using a combined monitoring method. This approach showed a sensitivity of 100% and a specificity of 91%.45 Based on these results, Table 4-1 was produced to explain the meaning and predictive features of combined monitoring of the spinal cord surgery using muscle MEPs and D wave.
TABLE 4-1 Principles of Motor-Evoked Potential Interpretation
| D Wave | Muscle MEP∗ | Motor Status |
|---|---|---|
| Unchanged or 30%–50% decrease | Preserved | Unchanged |
| Unchanged or 30%–50% decrease | Lost uni- or bilaterally | Transient motor deficit |
| >50% decrease | Lost bilaterally | Long-term motor deficit |
∗ In the tibial anterior muscle(s).
Source: Deletis V. Intraoperative neurophysiological monitoring. In: McLone D. Pediatric Neurosurgery: Surgery of the Developing Nervous System, 4th ed. Philadelphia: WB Saunders; 1999:1204-1213.
Combined monitoring of MEPs in one typical patient with an intramedullary spinal cord tumor showed a disappearance of muscle MEPs and a sustained D wave. This resulted in a transient postoperative paraplegia, with a complete recovery from paraplegia, as presented in Fig. 4-7.
Mapping of the Corticospinal Tract within the Surgically Exposed Spinal Cord
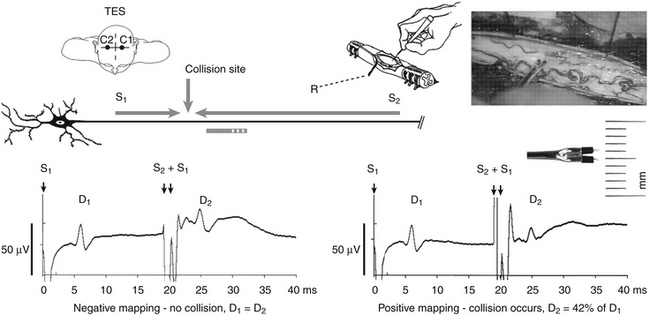
Further improvement in the prevention of lesioning of the CT during spinal cord surgery has been achieved by introducing a neurophysiologic method of mapping the CT by using a D-wave collision technique. This technique has recently been developed by our group and has allowed us to precisely map the anatomic location of the CT when the anatomy of the spinal cord has been distorted.73,74 The anatomic position of the CT is difficult to determine by visual inspection alone. D-wave collision is accomplished by simultaneously stimulating the exposed spinal cord (with a small handheld probe delivering a 2-mA intensity stimulus), with TES to elicit a D wave. Because the resulting signals are transmitted along the same axons, the descending D wave collides with the ascending signal carried antidromically along the CT (Fig. 4-8). This results in a decrease in the D-wave amplitude recorded cranially to the collision site. This phenomenon indicates that the spinal cord-stimulating probe is in close proximity to the CT. This could potentially guide surgeons to stay away from the “hot spot.” Mapping of the CT is now in the process of a technical refinement. Its initial use indicates an impressive ability to selectively map the spinal cord for the CT’s anatomic location. Using this method, the CT can be localized within 1 mm. This is in concordance with the other CT mapping techniques used within the brain stem that show the same degree of selectivity.50
Mapping of the Dorsal Columns of the Spinal Cord
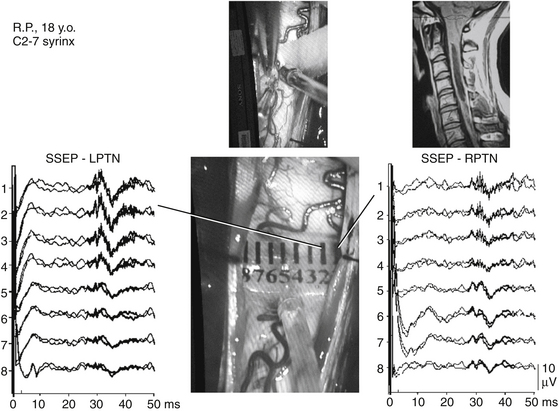
To protect the dorsal columns from lesioning during myelotomy, a novel neurophysiologic technique has been developed. To approach an intramedullary tumor, accepted neurosurgical techniques require a midline dorsal myelotomy. Distorted anatomy, however, often does not allow a precise determination of the anatomic midline by visual inspection and anatomic landmarks. Therefore, to facilitate the precise determination of the medial border between the left and right dorsal columns of the spinal cord, a technique for dorsal column mapping has been developed.75 Dorsal column mapping is based on two basic principles. First, after stimulation of the peripheral nerves, evoked potentials traveling through the dorsal columns can be recorded. Second, the area over the dorsal columns of the spinal cord where the maximal amplitude of the SEPs is recorded represents the point on the recording electrode in closest proximity to the dorsal columns. For recording these traveling waves, a miniature multielectrode is placed over the surgically exposed dorsal columns of the spinal cord. This electrode consists of eight parallel wires, 76 μm in diameter and 2 mm in length, placed 1 mm apart and embedded in a 1-cm2 (approximately) silicone plate (Fig. 4-9). An extremely precise amplitude gradient of the SEPs is recorded as the conducted potentials pass beneath the electrodes after alternating stimulation of the tibial nerves.
The amplitude gradient of the conducted potentials indicates the precise location of the functional midline corresponding to the dorsal fissure of the spinal cord (i.e., the optimal site for myelotomy). These data can be used by the neurosurgeon to prevent injury to the dorsal columns that could occur through an imprecise midline myelotomy. This is especially useful during surgery for intramedullary spinal cord tumors or during the placement of a shunt to drain syringomyelic cysts (see Fig. 4-9).74
Neurophysiologic Monitoring during Spinal Endovascular Procedures
Endovascular procedures for the embolization of spinal and spinal cord vascular lesions carry the risk of spinal cord ischemia.76 Whenever these procedures are performed under general anesthesia, only neurophysiologic monitoring can provide an “online” assessment of the functional integrity of sensory and motor pathways. Monitoring of SEPs and muscle MEPs is performed in the same fashion as described for monitoring of intramedullary spinal cord tumor surgery.77,78 The D wave, in contrast, is usually not monitored during these procedures because these patients receive a considerable amount of heparin in the perioperative period and the percutaneous placement of the recording epidural electrode would expose the patient to the risk of an epidural bleed. Besides safety issues, there is also no evidence that monitoring the D wave is an essential adjunct to muscle MEPs during these endovascular procedures. Both peripheral and myogenic MEPs have, in fact, been proven to be more sensitive than the D wave in detecting spinal cord ischemia. Similar results have been consistently reported both in experimental and clinical studies, supporting the hypothesis that whenever the mechanism of spinal cord injury is purely ischemic, muscle MEPs may suffice.79–82 Given the complexity of spinal cord hemodynamics, which is even more unpredictable in the presence of a spinal cord hypervascularized lesion, it is mandatory to perform both SEP and MEP monitoring to enhance the safety of these risky procedures.78
A critical step regarding neurophysiologic monitoring during these procedures consists of the provocative tests.78,83,84 These tests rely on the properties of two drugs, lidocaine and amobarbital, to selectively block axonal and neuronal conduction (respectively) when injected intra-arterially in the spinal cord.85 Provocative tests are usually performed once the endovascular catheter has reached the embolizing position, before any embolizing material is injected. If that specific vessel not only feeds the target of the embolization (e.g., spinal cord arteriovenous malformation, hemangioblastoma, arteriovenous fistula) but also perfuses normal spinal cord, it is expected that the provocative drug will block the white and/or gray matter conduction, and this will be reflected in neurophysiologic tests. Criteria for positive provocative tests are the disappearance of the MEPs and/or a 50% decrease in SEP amplitude. If the test is positive, embolization from that specific catheter position is not performed and embolization from a different feeder or from a more selectively advanced catheter position is attempted. Provocative tests mimic the effect of the embolization and select those patients amenable to a safe embolization. Although the specificity of provocative tests has not been tested (because the procedure is abandoned whenever provocative tests are consistently positive), their sensitivity has proven to be very high and no false-negative results (i.e., new postoperative neurologic deficit despite embolization performed after a negative provocative test) have so far been reported.78
Surgery of the Lumbosacral Nervous System
Intraoperative monitoring during surgery of the lumbosacral nervous system is a very demanding task and is still not developed in comparison with monitoring of the surgeries for other parts of the central and peripheral nervous systems. The neurophysiologic techniques used to monitor the lumbosacral nervous system are dependent on the pathology and structures involved. Generally, monitoring of the lumbosacral system involves the epiconus, conus, and cauda equina. These structures are essential in both voluntary and reflexive control of micturition, defecation, and sexual function as well as somatosensory and motor innervation of the pelvis and lower extremities. So far, only methodologies for monitoring and mapping of the somatomotor and somatosensory components of the lumbosacral nervous system have been developed. Intraoperative monitoring of the vegetative component of the lumbosacral nervous system is still in the embryonic stage.86
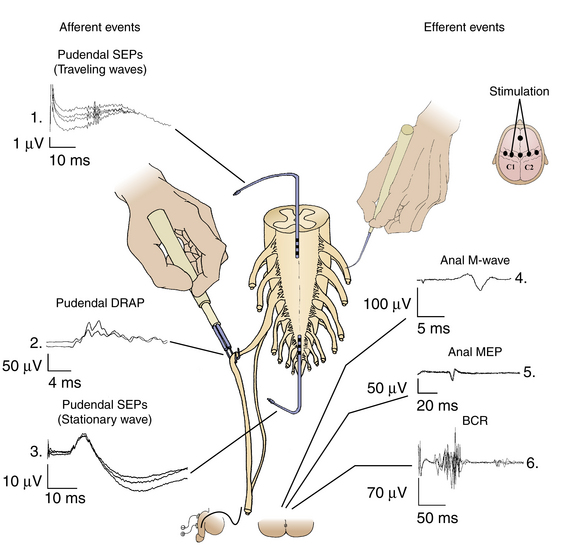
One of the most widely used applications of intraoperative monitoring of the lumbosacral nervous system, at least in the pediatric population, is for patients undergoing surgery for a tethered spinal cord. During these procedures, the surgeon cuts the filum terminale or removes the tethering tissue that envelopes the conus and/or the cauda equina roots. In a large series of patients operated on for tethered spinal cords, permanent neurologic complications have been described in as many as 4.5%.87,88 The rate increased to 10.9% when transient complications were considered. Due to the tethering, the lumbosacral nerve roots leave the spinal cord in different directions than in a healthy spinal cord. Furthermore, the cord may be skewed and sometimes a nerve root may pass through a lipoma. Nerve roots may also be involved in the thickened filum terminale that is cut during untethering. Direct electric stimulation of these structures in the surgical field or direct recording from them after peripheral nerve stimulation has proven helpful. Using mapping techniques, functional neural structures of the lumbosacral region can be correctly identified and thus possibly preserved. In Fig. 4-10, schematic drawings of the most important neurophysiologic techniques for monitoring afferent and efferent events (i.e., recording and monitoring neurophysiologic signals from sensory or motor parts of the lumbosacral system) are presented. During intraoperative testing with these techniques, it has been found that some of them are more important than others, from pragmatic point of view. Only these are described.
Pudendal Dorsal Root Action Potentials
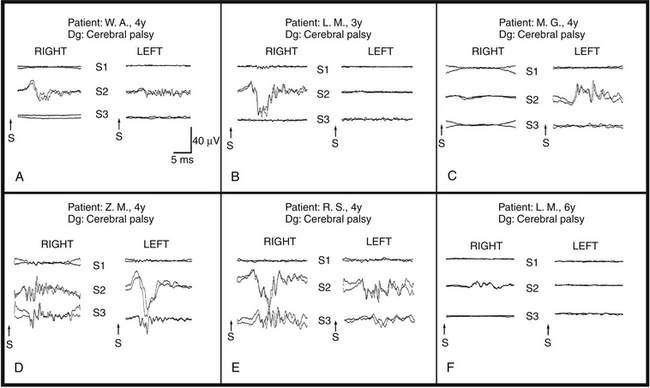
In the treatment of spasticity (e.g., in cerebral palsy), sacral roots are increasingly being included during rhizotomy procedures.89 Children who underwent L2–S2 rhizotomies had an 81% greater reduction in plantar/flexor spasticity compared with children who underwent only L2–S1 rhizotomies. However, as more sacral dorsal roots have been included in rhizotomies, neurosurgeons have experienced an increased rate of postoperative complications, especially with regards to bowel and bladder functions.
To spare sacral function, we have attempted to identify those sacral dorsal roots carrying afferents from pudendal nerves using recordings of dorsal root action potentials after stimulation of dorsal penile or clitoral nerves. Patients were anesthetized with isoflurane, nitrous oxide, fentanyl, and a short-acting muscle relaxant introduced only at the time of intubation. The cauda equina was exposed through a T12–S2 laminotomy/laminectomy, and the sacral roots were identified using bony anatomy. The dorsal roots were separated from the ventral ones, and dorsal root action potentials were recorded by a hand-held sterile bipolar hooked electrode (the root being lifted outside the spinal canal) (Fig. 4-11). The dorsal root action potentials were evoked by electrical stimulation of the penile or clitoral nerves. One hundred responses were averaged together and filtered between 1.5 and 2100 Hz. Each average response was repeated to assess its reliability. Afferent activity from the right and left dorsal roots of S1, S2, and S3 were always recorded, along with occasional recordings from the S4–S5 dorsal roots. Of special relevance was the finding that in 7.6% of these children, all afferent activity was carried by only one S2 root (see Fig. 4-11C and F). These findings were confirmed by a later analysis of results of mapping in 114 children (72 male, 42 female; mean age, 3.8 years).90 Mapping was successful in 105 of 114 patients. S1 roots contributed 4%, S2 roots 60.5%, and S3 roots 35.5% of the overall pudendal afferent activity. The distribution of responses was asymmetrical in 56% of the patients (see Fig. 4-11B, C, and F). Pudendal afferent distribution was confined to a single level in 18% (see Fig. 4-11A) and even to a single root in 7.6% of patients (see Fig. 4-11C and F). Fifty-six percent of the pathologically responding S2 roots during rhizotomy testing (using electrical stimulation of dorsal roots with spreading activity in adjacent myotomes) were preserved because of the significant afferent activity (as demonstrated during pudendal mapping). None of the 105 patients developed long-term bowel or bladder complications.
All our results in the early series of dorsal root mapping with 19 patients have been confirmed by analysis of the larger series of 105 patients.90 With this series, we showed that selective S2 rhizotomy can be performed safely without an associated increase in residual spasticity while preserving bowel and bladder function by performing pudendal afferent mapping.89 Therefore, we suggest that the mapping of pudendal afferents in the dorsal roots should be employed whenever these roots are considered for rhizotomy in children with cerebral palsy without urinary retention. In children with cerebral palsy with hyper-reflexive detrusor dysfunction, in whom sacral rhizotomy may be considered to alleviate the problem, preoperative neurologic investigation of the child should help in making appropriate decisions. In any case, intraoperative mapping of sacral afferents should make selective surgical approaches possible and provide the maximal benefit for children with cerebral palsy. Mapping of pudendal afferents has been further expanded by introducing methodology that maps afferents from the anal mucosa by stimulating them using anal plug electrodes and recording them in the same way as penile/clitoral afferents.91
Mapping and Monitoring of Motor Responses from the Anal Sphincter
These responses can be elicited by direct stimulation of the S2 to S5 motor roots and recording from the anal sphincters, after surgical exposure of the cauda equina, or by TES of the motor cortex. The first method of cauda equina stimulation, using a small hand-held monopolar probe, is easy to perform with recording of responses from each anal hemisphincter with intramuscular wire electrodes identical to the ones used to record the bulbocavernosus reflex.92 This mapping method is very useful when it becomes necessary to identify roots within the cauda equina during tethered cord or tumor surgeries in which the normal anatomy is distorted. To perform mapping, the surgeon must stop surgery and map the roots with a monopolar probe. To continuously monitor the functional integrity of parapyramidal motor fibers (for volitional control of the anal sphincters) and the motor aspects of the pudendal nerves from the anterior horns to the anal sphincters, the method of TES and recording of anal responses was introduced. Because the response recorded from the anal sphincter after TES has to pass multiple synapses at the level of the spinal cord, this method is moderately sensitive to anesthetics and rather light anesthesia should be maintained. So far no clinical correlation using this method has been published.
Monitoring of the Bulbocavernosus Reflex
The bulbocavernosus reflex is an oligosynaptic reflex mediated through the S2–S4 spinal cord segments that is elicited by electrical stimulation of the dorsal penis/clitoris nerves with the reflex response recorded from any pelvic floor muscles. The afferent paths of the bulbocavernosus reflex are the sensory fibers of the pudendal nerves and the reflex center in the S2–S4 spinal segment. The efferent paths are the motor fibers of the pudendal nerves and anal sphincter muscles. In neurophysiologic laboratories, the bulbocavernosus reflex is usually recorded from the bulbocavernosus muscles, and this is where it gets its name. We have described an intraoperative method for recording the bulbocavernosus reflex from the anal sphincter muscle, with improvement in methodology reported by others.92–94 The advantage of bulbocavernosus reflex monitoring is that it tests the functional integrity of the three different anatomic structures: the sensory and motor fibers of the pudendal nerves and the gray matter of the S2–S4 sacral segments (see Fig. 4-10). Because of a lack of published statistical data collected for large groups of patients, the reliability of monitoring for conus and cauda equina surgeries remains unclear.
Special Consideration for ION in Children
With regard to D-wave monitoring, Szelenyi et al. reviewed D-wave data from 19 children aged 8 to 36 months operated on for intramedullary spinal cord tumors.95 The D-wave was present in 50% of children aged 21 months or older but was never recorded in children younger than 21 months. Although the preoperative neurologic status of these children was not specified, a 50% D-wave monitor ability rate compares unfavorably to the data reported in adults, where mean monitor ability rate is around 66% and reaches 80% in patients in McCormick grade I.45 This is likely due to the immaturity of the CT in younger children where incompletely myelinated fibers have variable conduction velocities resulting in desynchronization.96 A few studies have recently looked at the feasibility of muscle MEP monitoring in children after TES, and consistently reported higher threshold in younger children.95,97,98 Our data are in agreement with those reported by Journee et al., who suggested preconditioning TES to overcome some of the limitations in eliciting MEPs in this subgroup of patients. 96,99
According to Nezu, electrophysiologic maturation of the CT innervating hand muscles is complete by the age of 13 and the CT appears to be the only spinal cord pathway with incomplete myelination at birth.100,101 There is likely a discrepancy between the anatomic and neurophysiologic development of the CT. Cortico-motoneuronal connections reach sacral levels between 18 and 28 weeks PCA, and are completed at birth.102 Myelination of the lumbar spinal cord occurs between 1 and 2 years of age, with a slower development for lower extremities than for upper extremities.103 However, the neurophysiologic maturation of the CT progresses throughout childhood and adolescence with myelogenesis and synaptogenesis that continues in to the second decade of life. Overall, a transition from development to motor control function exists for the CT.104 In conclusion, in Pediatric Neurosurgery essentially the same techniques used in adults can be applied to map and monitor the motor system. Yet, younger children have less excitable motor cortex and pathways due to their neurophysiologic—rather than anatomic—immaturity. Different stimulating parameters may be required to overcome these limitations.
Bricolo A., Turazzi S.. Surgery for gliomas and other mass lesions of the brainstem, Symon L., editor, Advances and Technical Standards in Neurosurgery, Vienna, Springer-Verlag, 1995;vol 22:261-341.
Cedzich C., Taniguchi M., Schafer S., et al. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996;38:962-970.
Deletis V. Intraoperative neurophysiology and methodology used to monitor the functional integrity of the motor system. In: Deletis V., Shils J.L. Neurophysiology in Neurosurgery: A Modern Intraoperative Approach. San Diego: Academic Press; 2002:25-51.
Deletis V., Vodusek D.B. Intraoperative recording of the bulbocavernosus reflex. Neurosurgery. 1997;40:88-92.
Dong C.C., Macdonald D.B., Akagami R., et al. Intraoperative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base surgery. Clin Neurophysiol. 2005;116(3):588-596.
Duffau H., Capelle L., Sichez J.P., et al. Intra-operative direct electrical stimulations of the central nervous system: the Salpetrière experience with 60 patients. Acta Neurochir (Wien). 1999;141:1157-1167.
Eyre J.A. Development and plasticity of the corticospinal system in man. Neural Plast. 2003;10:93-106.
Huang J.C., Deletis V., Vodusek D.B., et al. Preservation of pudendal afferents in sacral rhizotomies. Neurosurgery. 1997;41:411-415.
Jones S.J., Buonamassa S., Crockard H.A. Two cases of quadriparesis following anterior cervical discectomy, with normal perioperative somatosensory evoked potentials. J Neurol Neurosurg Psychiatry. 2003;44:273-276.
Kothbauer K., Deletis V., Epstein F.J. Motor evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998;4:1-9.
Minahan R.E., Sepkuty J.P., Lesser R.P., et al. Anterior spinal cord injury with preserved neurogenic “motor” evoked potentials. Clin Neurophysiol. 2001;112:1442-1450.
Morota N., Deletis V., Epstein F.J., et al. Brain stem mapping: neurophysiological localization of motor nuclei on the floor of the fourth ventricle. Neurosurgery. 1995;37:922-930.
Morota N., Deletis V., Lee M., et al. Functional anatomic relationship between brain stem tumors and cranial motor nuclei. Neurosurgery. 1996;39:787-794.
Neuloh G., Pechstein U., Schramm J. Motor tract monitoring during insular glioma surgery. J Neurosurg. 2007;106:582-592.
Neuloh G., Schramm J. Mapping and monitoring of supratentorial procedures. In: Deletis V., Shils J.L. Neurophysiology in Neurosurgery: A Modern Intraoperative Approach. San Diego: Academic Press; 2002:339-401.
Ojemann G.A. Functional mapping of cortical language areas in adults. Intraoperative approaches. Adv Neurol. 1993;63:155-163.
Penfield W., Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389-443.
Pouratian N., Cannestra A.F., Bookheimer S.Y., et al. Variability of intraoperative electrocortical stimulation mapping parameters across and within individuals. J Neurosurg. 2004;101:458-466.
Rodi Z., Vodusek D.B. Intraoperative monitoring of bulbocavernous reflex—the method and its problems. Clin Neurophysiol. 2001;112:879-883.
Sala F., Krzan M.J., Deletis V. Intraoperative neurophysiological monitoring in pediatric neurosurgery: why, when, how? Childs Nerv Syst. 2002;18:264-287.
Sala F., Manganotti P., Grossauer S., et al. Intraoperative neurophysiology of the motor system in children: a tailored approach. Child’s Nerv Syst. 2010;26:473-490.
Strauss C., Romstock J., Fahlbusch R. Pericollicular approaches to the rhomboid fossa. Part II: Neurophysiological basis. J Neurosurg. 1999;91:768-775.
Vodušek D.B., Deletis V. Sacral roots and nerves, and monitoring for neuro-urologic procedure. In: Nuwer M., editor. Monitoring neural function during surgery. Elsevier; 2007:423-433.
Yingling C.D., Ojemann S., Dodson B., et al. Identification of motor pathways during tumor surgery facilitated by multichannel electromyographic recording. J Neurosurg. 1999;91:922-927.
1. Nitta T., Sato K. Prognostic implications of the extent of surgical resection in patients with intracranial malignant gliomas. Cancer. 1995;75:2727-2731.
2. Hentschel S.J., Sawaya R. Optimizing outcomes with maximal surgical resection of malignant gliomas. Cancer Control. 2003;10:109-114.
3. Skirboll S.S., Ojemann G.A., Berger M.S., et al. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38:678-685.
4. Ojemann J.G., Miller J.W., Silbergeld D.L. Preserved function in brain invaded by tumor. Neurosurgery. 1996;39:253-259.
5. Schiffbauer H., Ferrari P., Rowley H.A., et al. Functional activity within brain tumors: a magnetic source imaging study. Neurosurgery. 2001;49:1313-1321.
6. George J.S., Aine C.J., Mosher J.C., et al. Mapping function in the human brain with magnetoencephalography, anatomical magnetic resonance imaging, and functional magnetic resonance imaging. J Clin Neurophysiol. 1995;12:406-431.
7. Kober H., Nimsky C., Moller M., et al. Correlation of sensorimotor activation with functional magnetic resonance imaging and magnetoencephalography in presurgical functional imaging: a spatial analysis. Neuroimage. 2001;14:1214-1228.
8. Kaplan A.M., Bandy D.J., Manwaring K.H., et al. Functional brain mapping using positron emission tomography scanning in preoperative neurosurgical planning for pediatric brain tumors. J Neurosurg. 1999;91:797-803.
9. Meyer P.T., Sturz L., Sabri O., et al. Preoperative motor system brain mapping using positron emission tomography and statistical parametric mapping: hints on cortical reorganisation. J Neurol Neurosurg Psychiatry. 2003;74:471-478.
10. Sobottka S.B., Bredow J., Beuthien-Baumann B., et al. Comparison of functional brain PET images and intraoperative brain-mapping data using image-guided surgery. Comput Aided Surg. 2002;7:317-325.
11. Schulder M., Maldjian J.A., Liu W.C., et al. Functional image-guided surgery of intracranial tumors located in or near the sensorimotor cortex. J Neurosurg. 1998;89:412-418.
12. Fritsch G., Hitzig E. Uber die elektrische Errgebarkeit des Grosshirns. Arch Anat Physiol Wiss Med. 1870;37:300-332.
13. Bartholow R. Experimental investigations into the functions of the human brain. Am J Med Sci. 1874;67:305-313.
14. Penfield W., Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389-443.
15. Penfield W., Rasmussen T. The Cerebral Cortex of Man: A Clinical Study of Localization and Function. New York: Macmillan; 1950.
16. Berger M.S., Ojemann G.A., et al. Techniques for functional brain mapping during glioma surgery. In: Berger M.S., Wilson C.B. The Gliomas. Philadelphia: WB Saunders; 1999:421-435.
17. Duffau H.L., Capelle J., Sichez P., et al. Intéret des stimulations électriques corticales et sous-corticales directes peropératoires dans la chirurgie cérébrale en zone fonctionnelle. Rev Neurol. 1999;155:553-568.
18. Kombos T., Suess O., Funk T., et al. Intra-operative mapping of the motor cortex during surgery in and around the motor cortex. Acta Neurochir. 2000;142:263-268.
19. Goldring S., Gregorie E.M. Surgical management of epilepsy using epidural recordings to localize the seizure focus: review of 100 cases. J Neurosurg. 1984;60:457-466.
20. Cedzich C., Taniguchi M., Schafer S., et al. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996;38:962-970.
21. Wood C.C., Spencer D.D., Allison T., et al. Localization of human sensorimotor cortex during surgery by cortical surface recording of somatosensory evoked potentials. J Neurosurg. 1988;68:99-111.
22. King R.B., Schell G.R. Cortical localization and monitoring during cerebral operations. J Neurosurg. 1987;67:210-219.
23. Lehericy S., Duffau H., Cornu P., et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg. 2000;92:589-598.
24. Puce A.R., Constable T., Luby M.L., et al. Functional magnetic resonance imaging of sensory and motor cortex: comparison with electrophysiological localization. J Neurosurg. 1995;83:262-270.
25. Fandino J., Kollias S.S., Weiser H.G., et al. Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J Neurosurg. 1999;91:238-250.
26. Roux F.E., Boulanouar K., Lotterie J.A., et al. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52:1335-1347.
27. Duffau H., Capelle L., Sichez J.P., et al. Intra-operative direct electrical stimulations of the central nervous system: the Salpetrière experience with 60 patients. Acta Neurochir (Wien). 1999;141:1157-1167.
28. Yingling C.D., Ojemann S., Dodson B., et al. Identification of motor pathways during tumor surgery facilitated by multichannel electromyographic recording. J Neurosurg. 1999;91:922-927.
29. Berger M.S. Functional mapping-guided resection of low-grade gliomas. Clin Neurosurg. 1995;42:437-452.
30. Pouratian N., Cannestra A.F., Bookheimer S.Y., et al. Variability of intraoperative electrocortical stimulation mapping parameters across and within individuals. J Neurosurg. 2004;101:458-466.
31. Lesser R.P., Luders H., Klem G. Cortical afterdischarge and functional response thresholds: results of extraoperative testing. Epilepsia. 1984;25:615-621.
32. Ojemann G.A. Functional mapping of cortical language areas in adults. Intraoperative approaches. Adv Neurol. 1993;63:155-163.
33. Van Buren J.M., Fedio P., Frederick G.C. Mechanisms and localization of speech in the parietotemporal cortex. Neurosurgery. 1978;2:233-239.
34. Haglund M.M., Ojemann G.A., Blasdel G.G. Optical imaging of bipolar cortical stimulation. J Neurosurg. 1993;78:785-793.
35. Berger M.S. Lesions in functional (“eloquent”) cortex and subcortical white matter. Clin Neurosurg. 1994;41:444-463.
36. Sartorius C.J., Wright G. Intraoperative brain mapping in a community setting: technical considerations. Surg Neurol. 1997;47:380-388.
37. Sartorius C.J., Berger M.S. Rapid termination of intraoperative stimulation-evoked seizures with application of cold Ringer’s lactate to the cortex: technical note. J Neurosurg. 1998;88:349-351.
38. Hines M., Boynton E.P. The maturation of “excitability” in the precentral gyrus of the young monkey (Macaca mulatta). Contrib Embryol. 1940;178:313-451.
39. Sala F., Lanteri P. Brain surgery in motor areas: the invaluable assistance of intraoperative neurophysiological monitoring. J Neurosurg Sci. 2003;47:79-88.
40. Kombos T., Suess O., Ciklatekerlio O., et al. Monitoring of intraoperative motor evoked potentials to increase the safety of surgery in and around the motor cortex. J Neurosurg. 2001;95:608-614.
41. Zhou H.H., Kelly P.J. Transcranial electrical motor evoked potential monitoring for brain tumor resection. Neurosurgery. 2001;48:1075-1081.
42. Riviello J.J., Kull L., Troup C., et al. Cortical stimulation in children: techniques and precautions. Tech Neurosurg. 2001;7:12-18.
43. Neuloh G., Pechstein U., Schramm J. Motor tract monitoring during insular glioma surgery. J Neurosurg. 2007;106:582-592.
44. Kothbauer K., Deletis V., Epstein F.J. Intraoperative spinal cord monitoring for intramedullary surgery: an essential adjunct. Pediatr Neurosurg. 1997;26:247-254.
45. Kothbauer K., Deletis V., Epstein F.J. Motor evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998;4:1-9.
46. Neuloh G., Schramm J. Mapping and monitoring of supratentorial procedures. In: Deletis V., Shils J.L. Neurophysiology in Neurosurgery: A Modern Intraoperative Approach. San Diego: Academic Press; 2002:339-401.
47. Deletis V., Camargo A.B. Transcranial electrical motor evoked potential monitoring for brain tumor resection. Neurosurgery. 2001;49:1488-1489.
48. Bricolo A., Turazzi S.. Surgery for gliomas and other mass lesions of the brainstem, Symon L., editor, Advances and Technical Standards in Neurosurgery, Vienna, Springer-Verlag, 1995;vol 22:261-341.
49. Kyoshima K., Kobayashi S., Gibo H., et al. A study of safe entry zones via the floor of the fourth ventricle for brain-stem lesions. J Neurosurg. 1993;78:987-993.
50. Morota N., Deletis V., Epstein F.J., et al. Brain stem mapping: neurophysiological localization of motor nuclei on the floor of the fourth ventricle. Neurosurgery. 1995;37:922-930.
51. Suzuki K., Matsumoto M., Ohta M., et al. Experimental study for identification of the facial colliculus using electromyography and antidromic evoked potentials. Neurosurgery. 1997;41:1130-1136.
52. Strauss C., Romstock J., Fahlbusch R. Intraoperative mapping of the floor of the fourth ventricle. In: Loftus C.M., Traynelis V.C. Intraoperative Monitoring Techniques in Neurosurgery. New York: McGraw-Hill; 1994:213-218.
53. Strauss C., Romstock J., Fahlbusch R. Pericollicular approaches to the rhomboid fossa. Part II: Neurophysiological basis. J Neurosurg. 1999;91:768-775.
54. Deletis V., Sala F., Morota N. Intraoperative neurophysiological monitoring and mapping during brain stem surgery: a modern approach. Oper Tech Neurosurg. 2000;3:109-113.
55. Quiñones-Hinojosa A., Russ L., Rose D., Lawton M. Intraoperative motor mapping of the cerebral peduncle during resection of a midbrain cavernous malformation: technical case report. J Neurosurg. 56, 2005. E439
56. Rhoton A.L. The posterior fossa veins. Neurosurgery. 2000;47:S69-S92.
57. Sala F., Lanteri P., Bricolo A. Intraoperative neurophysiological monitoring of motor evoked potentials during brain stem and spinal cord surgery. Adv Tech Stand Neurosurg. 2004;29:133-169.
58. Morota N., Deletis V., Lee M., et al. Functional anatomic relationship between brain stem tumors and cranial motor nuclei. Neurosurgery. 1996;39:787-794.
59. Sekiya T., Hatayama T., Shimamura N., et al. Intraoperative electrophysiological monitoring of oculomotor nuclei and their intramedullary tracts during midbrain tumor surgery. Neurosurgery. 2000;47:1170-1177.
60. Schlake H.P., Goldbrunner R., Siebert M., et al. Intra-operative electromyographic monitoring of extra-ocular motor nerves (Nn. III, VI) in skull base surgery. Acta Neurochir (Wien). 2001;143:251-261.
61. Sakuma J., Matsumoto M., Ohta M., et al. Glossopharyngeal nerve evoked potentials after stimulation of the posterior part of the tongue in dogs. Neurosurgery. 2002;51:1026-1033.
62. Komisaruk B.R., Mosier K.M., Liu W.C., et al. Functional localization of brainstem and cervical spinal cord nuclei in humans with fMRI. AJNR Am J Neuroradiol. 2002;23:609-617.
63. Fahlbusch R., Strauss C. The surgical significance of brain stem cavernous hemangiomas. Zentralbl Neurochir. 1991;52:25-32.
64. Grabb P.A., Albright L., Sclabassi R.J., et al. Continuous intraoperative electromyographic monitoring of cranial nerves during resection of fourth ventricular tumors in children. J Neurosurg. 1997;86:1-4.
65. Romstock J., Strauss C., Fahlbusch R. Continuous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J Neurosurg. 2000;93:586-593.
66. Dong C.C., Macdonald D.B., Akagami R., et al. Intraoperative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base surgery. Clin Neurophysiol. 2005;116(3):588-596.
67. Ulkatan S., Deletis V., Fernandez-Conejero I. Central or peripheral activations of the facial nerve? J Neurosur. 2007;106(3):519-520.
68. Sala F., Krzan M.J., Deletis V. Intraoperative neurophysiological monitoring in pediatric neurosurgery: why, when, how? Childs Nerv Syst. 2002;18:264-287.
69. Jones S.J., Buonamassa S., Crockard H.A. Two cases of quadriparesis following anterior cervical discectomy, with normal perioperative somatosensory evoked potentials. J Neurol Neurosurg Psychiatry. 2003;44:273-276.
70. Minahan R.E., Sepkuty J.P., Lesser R.P., et al. Anterior spinal cord injury with preserved neurogenic “motor” evoked potentials. Clin Neurophysiol. 2001;112:1442-1450.
71. Jallo G., Kothbauer K., Epstein F. Contact laser microsurgery. Childs Nerv Syst. 2002;18:333-336.
72. Deletis V. Intraoperative neurophysiology and methodology used to monitor the functional integrity of the motor system. In: Deletis V., Shils J.L. Neurophysiology in Neurosurgery: A Modern Intraoperative Approach. San Diego: Academic Press; 2002:25-51.
73. Deletis V., Camargo A.B. Interventional neurophysiological mapping during spinal cord procedures. Stereotact Funct Neurosurg. 2001;77:25-28.
74. Yanni D.S., Ulkatan S., Deletis V., et al. Utility of neurophysiological monitoring using dorsal column mapping in intramedullary spinal cord surgery. J Neurosurg (Spine). 2010;12:623-628.
75. Krzan M.J. Neurophysiology in Neurosurgery: A Modern Intraoperative Approach. San Diego: Academic Press; 2002. 153–164
76. Berenstein A., Lasjaunias P. Spine and spinal cord vascular lesions. In: Berenstein A., Lasjaunias P. Surgical Neuroangiography: Endovascular Treatment of Spine and Spinal Cord Lesions. Berlin, Heidelberg: Springer; 1992:1-109.
77. Berenstein A., Young W., Benjamin V., Merkin H. Somatosensory evoked potentials during spinal angiography and therapeutic transvascular embolization. J Neurosurg. 1984;60:777-785.
78. Sala F., Niimi Y. Neurophysiological monitoring during endovascular procedures on the spine and the spinal cord. In: Deletis V., Shils J.L. Neurophysiology in Neurosurgery: A Modern Intraoperative Approach. San Diego: Academic Press; 2002:119-151.
79. de Haan P., Kalkman C.J., de Mol B.A. Efficacy of transcranial motorevoked myogenic potentials to detect spinal cord ischemia during operations for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg. 1997;113:87-101.
80. de Haan P., Kalkman C.J., Jacobs M.J. Spinal cord monitoring with myogenic motor evoked potentials: early detection of spinal cord ischemia as an integral part of spinal cord protective strategies during thoracoabdominal aneurysm surgery. Semin Thorac Cardiovasc Surg. 1998;10:19-24.
81. Konrad P.E., Tacker W.A.Jr., Levy W.J., et al. Motor evoked potentials in the dog: effects of global ischemia on spinal cord and peripheral nerve signals. Neurosurgery. 1987;20:117-124.
82. Kai Y., Owen J.H., Allen B.T., et al. Relationship between evoked potentials and clinical status in spinal cord ischemia. Spine. 1994;19:1162-1168.
83. Sadato A., Taki W., Nakamura I., et al. Improved provocative tests for the embolization of arteriovenous malformations: technical note. Neurol Med Chir (Tokyo). 1994;34:187-190.
84. Touho H., Karasawa J., Ohnishi H., et al. Intravascular treatment of spinal arteriovenous malformations using a microcatheter: with special reference to serial Xylocaine tests and intravascular pressure monitoring. Surg Neurol. 1994;42:148-156.
85. Tanaka K., Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidocaine. Nature. 1966;209:207-208.
86. Vodušek D.B., Deletis V. Sacral roots and nerves, and monitoring for neuro-urologic procedure. In: Nuwer M., editor. Monitoring neural function during surgery. New York: Elsevier; 2007:423-433.
87. Choux M., Lena G., Genitori L., et al. The surgery of occult spinal dysraphism. Adv Tech Stand Neurosurg. 1994;21:183-238.
88. Piere-Kahn A., Zerah M., Renier D., et al. Congenital lumbosacral lipomas. Childs Nerv Syst. 1997;13:298-335.
89. Lang F.F., Deletis V., Valasquez L., et al. Inclusion the S2 dorsal rootlets in functional posterior rhizotomy for spasticity in children with cerebral palsy. Neurosurgery. 1994;34:847-853.
90. Huang J.C., Deletis V., Vodusek D.B., et al. Preservation of pudendal afferents in sacral rhizotomies. Neurosurgery. 1997;41:411-415.
91. Deletis V., Krzan M.J., Bueno de Camargo A., Abbott R. Functional anatomical asymmetry of pudendal nerve sensory fibers. In: Bruch H.P., Kocherling F., Bouchard R., Schug-Pass C. New Aspects of High Technology in Medicine. Bologna, Italy: Monduzzi; 2001:153-158.
92. Deletis V., Vodusek D.B. Intraoperative recording of the bulbocavernosus reflex. Neurosurgery. 1997;40:88-92.
93. Deletis V. Intraoperative neurophysiological monitoring. In: McLone D., editor. Pediatric Neurosurgery: Surgery of the Developing Nervous System. Philadelphia: WB Saunders; 1999:1204-1213.
94. Rodi Z., Vodusek D.B. Intraoperative monitoring of bulbocavernous reflex—the method and its problems. Clin Neurophysiol. 2001;112:879-883.
95. Szelenyi A., Bueno de Camargo A., Deletis V. Neurophysiological evaluation of the corticospinal tract by D-wave recordings in young children. Childs Nerv Syst. 2003;19:30-34.
96. Sala F., Manganotti P., Grossauer S., et al. Intraoperative neurophysiology of the motor system in children: a tailored approach. Childs Nerv Syst. 2010;26:473-490.
97. Frei F.J., Ryhult S.E., Duitmann E., et al. Intraoperative monitoring of motor-evoked potentials in children undergoing spinal surgery. Spine. 2007;32:911-917.
98. Liebermann A., Jeremy Lyon R., Diab M., Gregory A.G. The effect of age on motor evoked potentials in children under propofol/isoflurane anesthesia. Anesth Analg. 2006;103:316-321.
99. Journee H.L., Hoving E., Mooij J. Stimulation threshold-age relationship and improvement of muscle potentials by preconditioning transcranial stimulation in young children. Clin Neurophysiol. 2006;117:115.
100. Nezu A., Kimura S., Uehara S., et al. Magnetic stimulation of motor cortex in children: maturity of corticospinal pathway and problem of clinical application. Brain Dev. 1997;19:176-180.
101. Kubis N., Catala M. [Development and maturation of the pyramidal tract]. Neurochirurgie. 2003;49:145-153.
102. Eyre J.A. Development and plasticity of the corticospinal system in man. Neural Plast. 2003;10:93-106.
103. Brody B.A., Kinney C.H., Kloman A.S., Gilles F.H. Sequence of central nervous system myelination in human infancy. I. An autopsy study on myelination. J Neuropathol Exp Neurol. 1997;46:283-301.
104. Meng Z., Li Q., Martin J.H. The transition from development to motor control function in the corticospinal system. J Neurosci. 2004;24:605-614.