CHAPTER 15 Intraocular lenses
Introduction
The first intraocular lens was inserted on the 29th November 1949 and made out of polymethyl methacrylate (PMMA). The lenses were manufactured by Rayner in the United Kingdom and the name of the surgeon Harold Ridley became synonymous with innovation in ophthalmic surgery1. The lens was a disc inserted into the posterior chamber after extra-capsular cataract extraction (ECCE).
Materials for lens implant manufacture
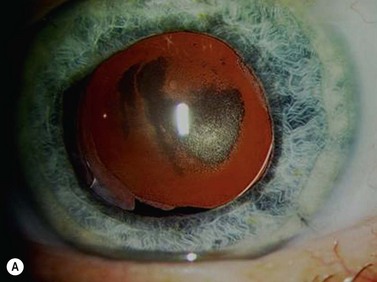
There have also been problems with lens implants with the hydrogel lenses causing opacification/calcification (Fig. 15.1) and glistenings/trapped fluid in vacuoles occurring in the hydrophobic acrylic lenses although these do not seem to cause visual symptoms2,3.
Optical design
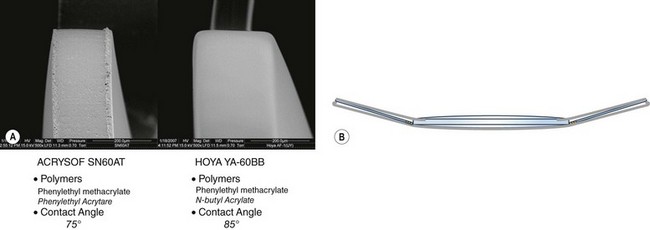
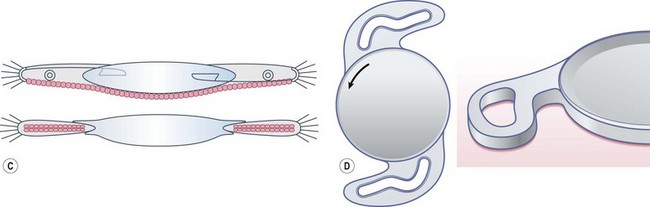
Intraocular lens design has to minimize posterior capsule opacification, by the incorporation of a square edge at the junction of the posterior surface and lateral surface, which provides a mechanical barrier to lens epithelial cell migration onto the posterior capsule (Fig. 15.2). It should be noted that not all ‘square edges’ are square and the radius of curvature of the junction of these two surfaces should be as small as possible. This, combined with material and surgical technique, is an important factor in the prevention of posterior capsular opacification. Another concept is the bag in the lens (M-J Tassignon) where the lens is supported by anterior and posterior capsulorrhexis.
Aspheric intraocular lenses
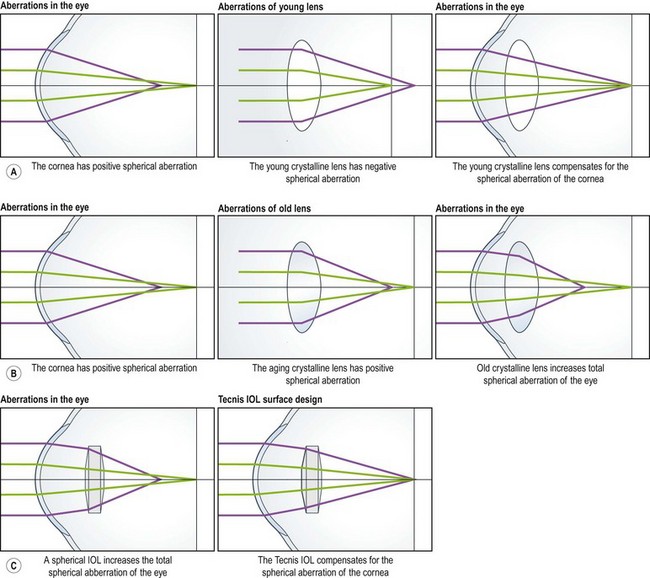
Optical aberrations occur when light from a point object does not form a perfect point after passing through the optical system. The refraction of peripheral rays of light is greater than those passing near the lens center. This is pupil-dependent and may account for the symptoms of dysphotopsia when the Snellen visual acuity is good, but the patient has a poor subjective outcome. The cornea has a positive spherical aberration and the young crystalline lens has negative spherical aberration, resulting in neutralization (Fig. 15.3). However, later in life the aging crystalline lens develops positive spherical aberration and therefore both combine to increase the spherical aberration of the eye. Therefore if one inserts an intraocular lens with a negative spherical aberration to neutralize that of the cornea, visual function will theoretically improve.
Blue blocking intraocular lenses
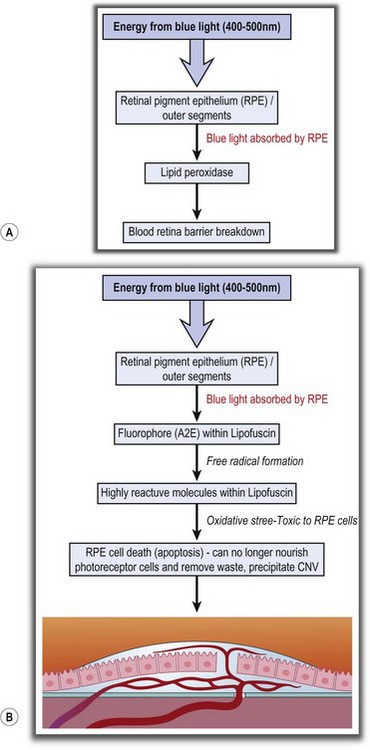
The risk factors for progression of age-related macular degeneration are hypertension, hyperopia, smoking, and confluent drusen. Age-related macular degeneration can progress following cataract surgery due to acute (class II photochemical reaction) blue light damage and perioperative inflammation, the latter being associated with compliment activation (Fig. 15.4A). It might also progress in the long term due to increased exposure to blue light and is proportional to the level and duration of exposure and the wavelength. The process is said to occur because energy from blue light is absorbed by the retinal pigment epithelium which interacts with fluorophore (A2E) within lipofuscin to produce free radicals, which subsequently cause oxidative stress to the RPE cells and apoptosis (Fig. 15.4B).
Therefore, there is a clear rationale for blue light filters, experimental evidence of efficacy, but little clinical proof. It is likely that their total light exposure is a risk factor for progression of macular degeneration, which is dependent on geographical location and lifestyle4.
Multifocal intraocular lenses
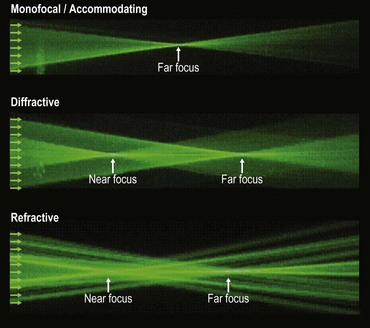
There are three types of multifocal/accommodating intraocular lenses. The path of monochromatic light (Fig. 15.5) illustrates the optical effect of the different types of lens.
The concept of monovision is important and should always be borne in mind when assessing patients.
1 Apple DJ. Sir Harold Ridley and His Fight For Sight: He Changed the World So That We May Better See It. Thorofare, NJ: Slack Publications. ISBN 13 978-1-55642-786-2.
2 Oshika T, Shiokawa Y, Amano S, et al. Influence of glistenings on the optical quality of acrylic foldable intraocular lens. Br J Ophthalmol. 2001;85(9):1034-1037.
3 Werner L. Biocompatibility of intraocular lens materials. Curr Opin Ophthalmol. 2008;19(1):41-49.
4 Cuthbertson FM, Peirson SN, Wulff K, et al. Blue light-filtering intraocular lenses: review of potential benefits and side effects. J Cataract Refract Surg. 2009;35(7):1281-1297.
5 Buznego C, Trattler WB. Presbyopia-correcting intraocular lenses. Curr Opin Ophthalmol. 2009;20(1):13-18.