CHAPTER 355 Intracranial Occlusive Disease
Intracranial Atherosclerosis
Epidemiology
About 750,000 people in the United States suffer ischemic cerebrovascular events each year, and 8% to 10% of these events have been attributed to intracranial atherosclerosis. Stroke secondary to intracranial atherosclerosis has been estimated to occur in 56,000 to 90,000 people each year in the United States.1,2 Moreover, intracranial atherosclerosis accounts for a larger percentage, up to 26%, of patients who develop stroke in the ethnic subgroups of Asian, African American, and Hispanic people.3–5 Analysis of the North American Symptomatic Carotid Endarterectomy Trial (NASCET) population found mild intracranial atherosclerosis (wall irregularities without stenosis) in 26.9% of the patients, moderate disease (<50% stenosis) in 5.8%, and severe stenosis (>50% stenosis) in 0.5%.6 Most studies evaluate symptomatic intracranial arteriosclerosis; therefore, there are few data available to estimate the overall incidence of asymptomatic intracranial disease in the population.
Several large series have provided demographic information on the symptomatic patient population. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial demonstrated that the at-risk population for developing intracranial arteriosclerosis shares common cardiovascular risk factors. The mean age of these patients was 63.5 years, with a male predominance (62%). Associated risk factors included prior cardiac ischemic events, prior cerebrovascular accidents, hypertension, diabetes, hyperlipidemia, and smoking.2 In some studies, about 50% of disease pathology presents in the internal carotid artery (ICA), whereas the remaining cases are distributed throughout the remaining cerebral circulation.7
Intracranial atherosclerosis has been attributed to account for about 22% to 26% of ischemic strokes in the Asian population, 6% to 29% of cerebrovascular events in blacks, and up to 11% of infarcts in Hispanics.3–5,8–10 Although the overall incidence was higher in men, in subgroup analysis women had an 85% greater risk for developing recurrent cerebrovascular events compared with men. This apparent increased risk may be attributed to associated socioeconomic factors.11
Pathophysiology
Intracranial atherosclerosis can lead to an ischemic cerebrovascular event through several differing processes: (1) hypoperfusion, (2) thrombosis at the site of stenosis, (3) thromboembolism, and (4) direct occlusion of small perforating vessels.12–17 Depending on the underlying cause, the clinical presentation may vary from an acute ischemic deficit due to embolic or thrombotic sources to intermittent neurological symptoms from hypoperfusion. This chapter mainly discusses cerebral hypoperfusion secondary to intracranial atherosclerotic lesions; acute ischemic events are discussed in Chapter 344.
Natural History and Medical Management
A meta-analysis by Komotar and colleagues reviewed the natural history of intracranial atherosclerosis based on the identified pathologic vessel and found an annual ipsilateral risk of ischemic events ranging from 3.1% to 8.1% for the ICA.18 In the vertebrobasilar circulation, with a minimum stenosis of 40%, they found the annual risk for stroke in the same vasculature distribution ranged from 0% to 8.7% and annual overall stroke risk of between 3% and 14.3%. For the middle cerebral artery (MCA), the annual ipsilateral stroke risk ranged from 0% to 7.8%, and overall stroke risk ranged from 0% to 5%. The results of this retrospective study, in addition to those of several prospective series, are consistent with the findings from the WASID trial supporting that the risk for ischemic events in the posterior circulation does not exceed that of the anterior circulation.2 However, these investigators did find an increased risk for mortality from posterior circulation and ICA ischemic events compared with the MCA distribution. The discrepancy in mortality rate may simply reflect the relative importance of the neuroanatomic regions supplied by these vessels.
In terms of medical management, several retrospective studies have shown benefit of warfarin sodium (Coumadin) over aspirin to decrease the risk for recurrent ischemic events.19 In one series, patients treated with warfarin after a transient ischemic attack (TIA) or stroke had a 7% risk for stroke or stroke-related death with 14.7 months of follow-up, whereas patients treated with aspirin experienced a 24% risk for stroke or mortality with 19.3 months of follow-up.19 The same retrospective series found that warfarin (3.6% per year) was favorable over aspirin (10.4% per year) in the reduction of cerebrovascular events.
The prospective, randomized WASID study group, however, did not corroborate the prior retrospective conclusions. No statistically significant differences in cerebrovascular event rates between warfarin and high-dose aspirin were found. However, the study did observe a higher risk for noncerebral major hemorrhage from warfarin, not supporting the use of warfarin over high-dose aspirin.2
Recently, a better understanding of the disease evolution has been achieved through additional prospective clinical studies evaluating medical therapy. A prospective, although small, series compared aspirin and warfarin in the treatment of MCA stenosis and found equivocal results looking at the incidence of stroke reduction.20 The Groupe dEtude des Stenoses Intra-Craniennes Atheromateuses Symptomatiques (GESICA) study evaluated 102 nonrandomized patients prospectively treated with varying combinations of antiplatelet and anticoagulant therapies. Even with medical therapy, 38.2% of their sample developed recurrent ischemic events or TIAs within the 23.5 months of follow-up.21 This alarmingly high rate of disease progression was emphasized by a retrospective review that found a medical failure rate of 47.7% for the prevention of TIA or stroke.22 These high rates of recurrent cerebrovascular events even on medical therapy certainly prompt an urgency to identify optimal medical and adjuvant surgical therapies for high-risk patients.
For asymptomatic patients, there is no consensus on optimal management guidelines. The position statement from the American Society of Neuroradiology (ASNR) in 2005 highlighted the uncertainty of treating asymptomatic stenosis.23 The risk for stroke is thought to be lower in asymptomatic patients with MCA stenosis, and the incidence has been estimated at 1.4% to 2.8% per year.24
Risk Factors and Progression
For symptomatic lesions, recent large and prospective studies have helped delineate some of the risk factors contributing to progressive disease. Some authors have found a trend among female gender, extent of intracranial stenosis, and additional presence of asymptomatic stenosis to be associated with a higher risk for future events.25 Progressive MCA stenosis, as evaluated by transcranial Doppler (TCD) studies, appears to be an independent risk factor for increased stroke risk because 32.5% of patients with progressive disease had a stroke by 26 months of follow-up.25 Other series have supported that severe stenosis (>70% stenosis), female gender, National Institutes of Health Stroke Scale score greater than 1, concurrent diabetes, borderline body mass index values, hyperlipidemia, white ethnicity, and presence of hemodynamic stenosis increase the risk for stroke.19,21,22 The GESICA study found that the presence of hemodynamic instability, determined clinically, increased the risk for cerebrovascular events from 31.7% to 60.7%.21 As we identify more high-risk traits in patients, we can better stratify patients into appropriate management protocols.
Radiographic Evaluation
Patency of Vessels
Indirect estimates of flow may be extrapolated from varying imaging modalities. MRA and CTA both provide excellent detail regarding the caliber of vessels, although MRA has been shown to overestimate the degree of stenosis in some cases.26 Correlation between MRI blood volume flow and angiographic flow suggested that MRI may overestimate stenosis in the cavernous ICA, at the distal ICA bifurcation, and at the MCA bifurcation secondary to turbulent flow.26 However, looking at the source images as opposed to the reconstructions can reduce this error. In some series, time-of-flight MRA using 1.5-Tesla MRI had a sensitivity of 90% to 95%, a specificity of 95%, a positive predictive value (PPV) of 84% to 86%, and a negative predictive value (NPV) of 97% to 98%. The diagnostic accuracy ranged from 93% to 95% compared with digital subtraction angiography. However, other authors have postulated that CTA is better than both MRA and TCD to assess intracranial stenosis.27
The WASID trial found that noninvasive techniques carried a 33% false-positive rate in determining more than 50% stenosis.19 The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial enrolled 407 patients to determine the reliability of using TCD or MRA in determining the extent of stenosis. These investigators concluded that TCD had a PPV of 36% and an NPV of 86%, whereas MRA had a PPV of 59% and an NPV of 91%.28 These noninvasive techniques were sufficiently accurate to exclude more than 50% stenosis, but further confirmatory studies were needed to characterize the stenosis. Transcranial color sonography (TCCS) was not ideal to assess MCA stenosis and was less reliable when the ICA had greater than 50% stenosis. Overall, there is still much debate regarding the use of noninvasive techniques to ascertain intracranial stenosis. However, CTA, MRA, and ultrasound techniques have been consistently reliable in the first-line evaluation of stenosis. Currently, their specificity and PPV are not reliable enough to replace invasive evaluations, but with improved technology such as 3-Tesla MRI, the need for invasive procedures may eventually diminish.
Perfusion
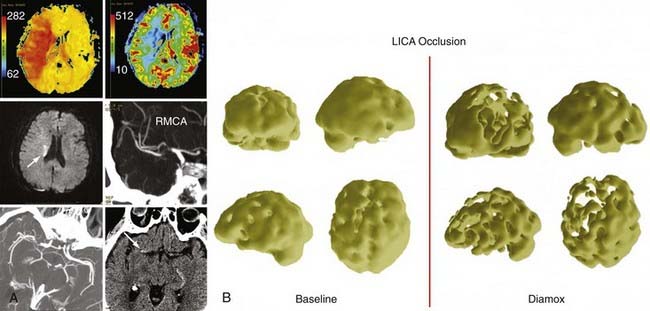
Varying modalities have been used to quantify cerebral perfusion and flow-related changes within the brain parenchyma. Perfusion studies include PET scans, SPECT scans, xenon CT perfusion studies, CT perfusion, and perfusion MRIs (Fig. 355-1).29 These studies permit the extrapolation of CMRO2, OEF, and CBF and provide information on the perfusion of the brain. Varying techniques have been validated through separate studies, but no standard has been established as preferable to others. Individual modalities are discussed in further detail in other chapters.
Mendelowitsch identified hemodynamic instability using SPECT in 65 patients and retrospectively monitored them after they received superficial temporal artery (STA)-MCA bypasses.30 He noted that 88% of patients had neurological function improvement. Other studies have used PET to select patients for revascularization procedures and have correlated clinical improvement with improved radiographic findings as well.31 The Japanese EC-IC Bypass Trial (JET) used SPECT imaging with an acetazolamide challenge to calculate hemodynamic parameters in patients. They used a technique of three-dimensional stereotactic surface projections with stereotactic extraction estimation to objectively quantify hemodynamic instability.32
Currently, the ongoing Carotid Occlusion Surgery Study (COSS) employs measuring OEF by PET.33
Operative Treatment
Surgical Technique
Open surgical revascularization has diminished significantly in popularity since the publication of the JET study.34 However, the procedure and its variations are still beneficial to carefully selected patients. Since the first STA-MCA procedure was described by Yasargil in 1969,35 many variations have been reported. Many of these variations have been developed in dealing with complex cerebral aneurysms and skull base neoplasms in which vessel sacrifice is required. These variations include anastomoses between the bilateral anterior cerebral arteries; occipital artery–to–posterior, inferior cerebral artery (PICA), anterior and inferior cerebral artery (AICA). Others includes PICA to PICA, vertebral artery to PICA, STA to SCA or PCA, subclavian artery to PCA, PCA to SCA, and even a tandem occipital artery to AICA and PICA anastomoses.36 Essentially any two vessels than can be liberated and approximated can be used to create an anastomosis. In terms of the surgical treatment of intracranial atherosclerosis, the main bypass procedure remains the STA-MCA bypass.
Operative Technique
![]() The operative technique (see Video 355-1) proceeds as follows.
The operative technique (see Video 355-1) proceeds as follows.
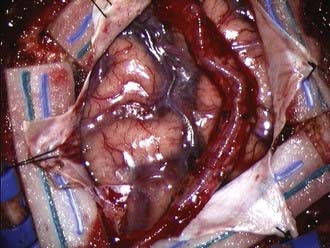
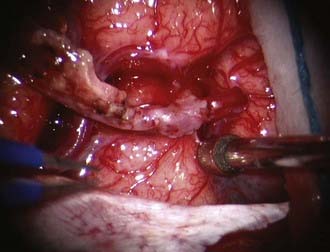
Under loupe magnification or the microscope, the skin is incised over the STA. Using sharp technique, the subcutaneous layer is divided until the layer harboring the STA is encountered. Bleeding points from the skin edges are carefully coagulated using very fine jeweler bipolar forceps. Dissection proceeds along the entire course of the STA until it has been dissected free of the subcutaneous tissues. Using fine bipolar forceps, small branches from the STA are coagulated and divided. A self-retaining retractor is used to retract the skin edges, and the galea surrounding the STA is divided using a needle-tip Bovie, leaving a generous soft tissue cuff. With the STA completely dissected free, it is retracted laterally and protected under a papaverine-soaked cottonoid (Fig. 355-2).
The underlying temporalis muscle and fascia are divided with electrocautery in line with the incision and reflected laterally with the self-retaining retractors. Two bur holes are placed at the inferior and superior limits of the planned craniotomy flap. An elliptical bone flap is then fashioned and removed. The location of the bone flap should be centered about 6 to 7 cm above the external auditory meatus. Alternatively, a mathematical approach to the ideal location of a recipient vessel has been recently published that may permit a more localized craniotomy.37 Dural tenting sutures are placed along the bone edges. At this point, the STA is centered over the craniotomy area; if undue tension is apparent, the STA should be freed further by expanding the dissection proximally and distally. A redundant STA that can be placed over the desired area without tension or stretching is the goal.
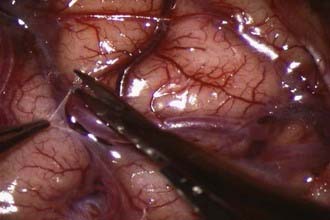
At this point, the dura is opened in a cruciate manner, with care taken not to disturb or injure any naturally developed transdural collaterals. Further, in the event that the middle meningeal artery (MMA) is providing significant dural collaterals to the brain, the incision is modified to preserve the MMA. Under the operating microscope and using microsurgical technique, the brain surface is inspected for a suitable recipient M4 branch. Once identified, the arachnoid over the sulcal area is opened (Fig. 355-3). The recipient vessel is then isolated and any small cortical branches coagulated and divided. Background material is then placed behind this vessel, and a small malleable microsuction device is placed beneath the background material, thus providing continuous suction and a dry field.
At this point, the STA vessel is controlled with a temporary clip and transected distally. The cut flow can be measured at this point if desired using a transonic flow probe.38 One must ensure division of the STA such that the maximal amount of vessel is available. This will ensure the ability to maneuver the vessel back and forth during the anastomosis and facilitate the suturing. The cut end is then trimmed of any remaining adventitial tissue and fish-mouthed to a size 2.5 to 3 times the original orifice size. The STA is flushed with heparinized saline either from the cut end or if possible from a side branch, which can be tied off later when the anastomosis is complete.
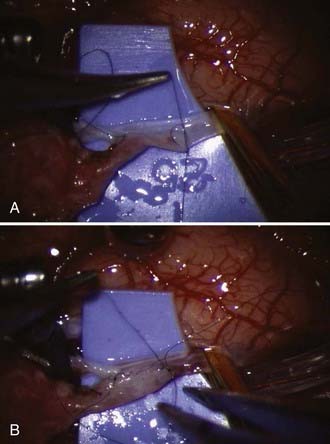
The MCA recipient is then clipped with temporary clips, and an arteriotomy is performed with a sharp, pointed Beaver blade or No. 11 blade. Alternatively, a small portion of vessel wall can be cut away with microscissors, although this may result in a narrower anastomosis. We prefer the former technique and then expand the opening using microscissors. The opening is enlarged to match the STA. At this point, the anastomosis is performed using 10-0 or 11-0 monofilament suture (Fig. 355-4A). The corners are anchored, followed by placing interrupted sutures evenly spaced apart or running the back wall from one corner suture to the other. As the suturing is performed, great care must be taken to ensure that the opposing vessel wall is not incorporated and that too large a bite is not taken, which would result in a narrowed or occluded anastomosis. Throughout the sewing, the assistant flushes the area with heparinized saline. When the back wall is complete, attention is directed to the front wall (Fig. 355-4B). Again, the area must be carefully inspected to ensure appropriate placement of the sutures. After the suturing is completed, the vessels are flushed with heparinized saline before completing the final suture. The proximal temporary clip is removed, allowing flushing of any air into the STA and out the previously mentioned side branch. The distal clip is next removed, followed by the STA temporary clip after ligating the side branch if it was used. Some bleeding from the anastomosis is expected and preferred. This usually stops within a few minutes with the use of normal saline irrigation. If significant bleeding is encountered, an additional stitch may be necessary (Fig. 355-5).
Some authors have applied new technologies to the technique of revascularization. Preoperative MRI and MRA have been used to minimize the craniotomy by identifying an appropriate-sized recipient vessel in conjunction with neuronavigation technology.39 Some authors have also described using laser technology to create the anastomosis for a bypass graft using the saphenous vein. The excimer laser–assisted nonocclusive anastomosis (ELANA) technique attaches a ring to the recipient vessel and then uses high-pressure suction and an excimer laser to create a circular arteriotomy and anastomosis.40
Some authors have also described intracranial endarterectomy to treat significant stenosis. This has mainly been described anecdotally in the MCA territory and extracranial vertebral artery.41,42 This technique for treatment of MCA stenosis has not gained widespread popularity and incurs the risk for endoluminal injury to the vessels as well as having limitations in areas where perforators are affected. Vertebral artery endarterectomies are discussed further in Chapter 354.43,44
Endovascular Technique
Procedural Technique
The decision of whether to perform the procedure under general anesthesia or monitored anesthesia care with local anesthetics is dependent on the surgeon’s preference. With the patient awake, a more accurate and instantaneous neurological assessment can be obtained during the entire procedure. However, patient compliance and breath holding can be more difficult and create a more challenging technical obstacle.45,46
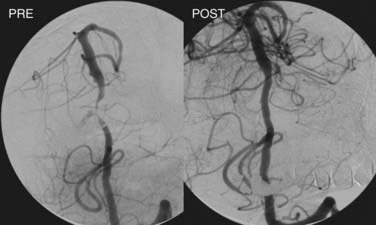
Angioplasty is then performed by gradual inflation of the balloon to 70% to 80% of the vessel diameter. If stenting is planned, the conventional technique involves initial balloon angioplasty followed by stent deployment (Fig. 355-6). In cases of primary stenting with a balloon-expandable stent, the stent is deployed simultaneously as the angioplasty is performed. Stents can be deployed across major branch points, but caution should be used when bridging small perforators because the stent or displaced thrombus can occlude these narrow vessels and cause significant morbidity (“snow-plowing effect”).
Outcomes
Open Surgical Outcomes
The use of extracranial-to-intracranial bypass techniques have diminished since the publication of the ECIC Study. The multicenter study enrolled 1495 patients who were randomized to medical management or surgical intervention. One hundred and eighteen patients were excluded from the study because the referring physicians thought that the patients would benefit more from surgical intervention. Of the remaining 1377 patients, 714 were treated with medical therapy, and 663 underwent surgical revascularization with a mean follow-up of 55.8 months. The study reported an 18% stroke rate in the medical group, compared with 20% in the surgically treated patients. The surgical group had a 1.1% perioperative mortality rate, although 10 of the 30 deaths occurred after patients were randomized but before surgical intervention. Of the patients who had surgery, a graft patency of 96% was obtained.34
The trial was publicly interpreted as not supporting a role for surgical revascularization in patients with intracranial atherosclerosis, but many criticisms were identified. Many patients in the study had very minor symptoms, and no assessment of hemodynamic function was available. Clearly, selection biases affected patient selection because 118 patients were excluded from randomization, and the study did not assess radiographic or clinical perfusion.34 Results from recent randomized studies supporting endovascular management of symptomatic patients with severe stenosis21,47 should prompt reevaluation of the role of bypass revascularization procedures in the management of selected patients with hemodynamic requirements.
Although the randomized prospective ECIC Study questioned the benefit of surgical revascularization, multiple retrospective series have supported a role for extracranial-to-intracranial bypass. A retrospective review of 65 patients who underwent STA-MCA bypass with hemodynamic instability as measured by SPECT reported neurological improvement in 88% of the patients. Postoperative complications, including infection, scalp necrosis, hemorrhages, cerebrospinal fluid leak, seizures, and ischemic events, have been reported in 11% to 26% of cases.30,48,49 The mortality rate ranges from 0% to 4%, and postoperative ischemia has been reported in 2% to 39% of patients. Patency rates of bypass vessels have been reported as high as 90% to 91%, although several ischemic events have still been observed in patients with patent vessels.49 Overall, open bypass revascularization has not been shown to reduce recurrent embolic events but may have a better defined role in the management of patients with increasing hemodynamic demands.
More recently, the JET study was completed, and midterm results were published in the Japanese literature. The JET study prospectively evaluated 206 patients who were randomized into a surgical bypass group or a medical treatment group. Ninety-eight patients were included in each category and had to have an ischemic event within 3 months of enrollment. Patients were selected who had some degree of hemodynamic demand based on acetazolamide challenge using PET, SPECT, and xenon CT. After 2 years of follow-up, they found a benefit in both end points of death and recurrent ischemic events favoring the surgically treated group (P = .046 and P = .045, respectively).50 The ongoing COSS is testing whether STA-MCA anastomosis with adjuvant medical therapy will decrease ipsilateral stroke rates by 40% after 2 years in patients with stage II hemodynamic instability and symptomatic ICA occlusion.33 The results of these studies will help clarify the subset of patients who may gain benefit from surgical revascularization.
Endovascular Outcomes
Sundt and colleagues reported the first endovascular angioplasty for intracranial atherosclerosis in 1980.51 Initial endovascular therapies were fraught with significant morbidity and had technologic limitations. However, more recent technologic advances have enabled the creation of stents tailored for intracranial vessels. Endovascular treatment for intracranial arteriosclerosis has become very popular with the advent of improved neurointerventional tools and techniques. Unfortunately, relatively few randomized, prospective studies exist to validate its widespread use. Recent studies have supported the use of endovascular management for severely stenotic and symptomatic intracranial atherosclerosis, but no comprehensive evaluation has been published comparing open surgical bypass surgery and interventional modalities.
The last position statement by the ASNR in 2005 concluded that symptomatic intracranial atherosclerosis with greater than 50% stenosis after failed medical therapy warranted endovascular treatment. The ASNR also stated that patients with asymptomatic stenosis should be started on antiplatelet agents and possibly “statins” (3-hydroxy-3-methylglutaryl coenzyme A [HMG CoA] inhibitors), but could not recommend prophylactic endovascular treatment.23
Regardless of these uncertainties, an increasing trend toward angioplasty and stenting of intracranial disease exists. Angioplasty alone is associated with high recurrence rates of restenosis. However, with stenting, greater luminal expansion can be achieved with lower rates of restenosis. The Wingspan study had a mean stenosis rate of 74.9%, which was reduced to 50% after angioplasty and again further to 31.9% with stent deployment.47 Fiorella and associates evaluated 82 lesions and reduced a mean stenosis rate of 74.6% to 43.5% with angioplasty alone, but further decreased the stenosis to 27.2% with stenting.52 Multiple studies have suggested that the addition of stenting procedures results in less residual stenosis.
The published technical success rate of endovascular procedures is quite high and will likely continue to improve as balloons and stents continue to be refined and evolve. Although Mori and colleagues were able to achieve successful angioplasty in only 33% of their long segment and tortuous intracranial arterial stenoses, most recent studies have quoted technical success in the range of 92% to 100%.21,47,52–55 The primary endpoints evaluated in most studies are mortality and recurrent stroke. Recurrent stroke is also subdivided into ipsilateral ischemia representing stroke in the vascular distribution of the stenotic vessel, whereas all strokes represent strokes in any distribution. Stroke rates should also be divided into perioperative infarcts within 30 days of the procedure and those at 1 or 2 years of follow-up. The majority of earlier studies do not distinctly separate angioplasty with or without stenting and reported a relatively low peri-operative risk ranging from 4.8% to 14.2% [5.8%,56 4.8%,5514.2%,21 6.6%54].
All prospective studies identified symptomatic patients with greater than 50% intracranial atherosclerosis of the same vessel. The Apollo Stent for Symptomatic Atherosclerotic Intracranial Stenosis (ASSIST) study prospectively enrolled 46 patients who were treated using the Apollo stent. They reported a peri-procedural infarct risk of 8.7% with a cumulative risk of ischemia in the treated vessel at 1 and 2 years of 8.8%. Risk of any strokes after the procedure was 11% at 1 year and 13.3% at 2 years.55
The Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA) study enrolled 61 patients prospectively and reported a 6.6% perioperative stroke risk (3 of 4 were ipsilateral) and a 1-year stroke rate of 13.1%.54 The Wingspan study enrolled 45 patients and had a perioperative risk of 4.5% and a 1-year stroke rate of 9.3%. Most strokes did occur around the perioperative period, but more recent studies have reported slightly lower perioperative ratios.47 Other retrospective series have reported 1-year stroke rates of 4.4% to 7.2%.55,56 Perhaps with improving technology, fewer perioperative complications will be encountered.
Although procedural risks may be decreasing, endovascular therapy is still associated with a serious risk for complications. Nonischemic complications also account for a significant proportion of potential adverse outcomes. Complication rates range from 5.8% to 16.7%.53,55–57 In particular, a higher complication rate has been reported when the stenotic region is near a perforator-rich zone.58 The severity of the stenosis has also been linked to increasing procedural risk. Jiang and colleagues treated 220 stenotic vessels and found a perioperative stroke rate of 4.8% in patients with severe stenosis as opposed to 4.3% in patients with moderate stenosis.59 This increased risk remained with the patients after surgery, culminating in a 1-year risk for stroke of 7.2% compared with 5.3%. As supported by the WASID trial, greater degrees of stenosis may correlate to a higher risk for future ischemic events.2 Using univariate analysis, Jiang and colleagues also found diabetes mellitus, lack of antithrombotic therapy, and stenoses in the posterior circulation to be associated with increasing risk.59
Complication rates may also be minimized by careful patient selection and identification of higher-risk patients. Levy and associates suggested that greater than 90% stenosis, plaques larger than 10 mm, unstable plaques undergoing surgery within 4 weeks of an ischemic event, tortuosity of vessels, thrombus within lesions, calcified plaques, perforator-rich zones, and other significant medical comorbidities contribute to increase the risk.60 Identification of these high-risk patients may also allow us to optimize combinations of medical and therapeutic interventions.
Restenosis Rate
Another important issue with angioplasty and stenting is the occurrence of restenosis. Angioplasty alone resulted in higher restenosis rates, and although the rate is improved with stenting, it is still significant. In the literature, postangioplasty and poststenting restenosis rates were reported between 7.3% and 67% depending on the location and length of follow-up.52–5661 Most restenosis rates range from 7.3% to 32.4% after 3 to 12 months of follow-up.52–5661
Certain lesions, however, have been associated with a higher rate of restenosis. In the SSYLVIA trial, lesions involving the vertebral ostia had a 67% restenosis rate. These investigators found that diabetes mellitus, ostial lesions, degree of posttreatment stenosis, vessel diameter, and age all contributed to increasing restenosis at 6 months of follow-up.54 The recent nationwide Wingspan registry demonstrated a 25% restenosis rate with 6 months of follow-up angiography.47 However, radiographic restenosis may not directly correlate with clinical symptoms. Most patients with restenosis in the ASSIST study remained asymptomatic, and 61% of the patients with restenosis in the SSYLVIA trial were void of any clinical findings.54,55 Recently, additional follow-up of the Wingspan stent was published showing 32.3% in-stent restenosis and 3.9% occlusion with a mean follow-up of 8.5 months. Fifteen of the 41 patients (36.6%) with in-stent restenosis had symptoms.62 Therefore, the clinical relevance and classification of in-stent restenosis remain to be determined.
Drug-Eluting Stents
Just as intracranial management of vessels historically followed coronary treatment, the use of drug-eluting coronary stents prompted investigation of their use intracranially. After stenting, patients are typically treated with long-term, if not permanent, antiplatelet agents to limit thrombosis of the stent. Drug-eluting stents were devised for cardiac disease to decrease the risk for thrombosis and restenosis and have primarily been used with coronary vessels. The stents gradually release either sirolimus, an antifungal medication with antimitotic properties, or paclitaxel, an antineoplastic agent. From the cardiac literature, restenosis rates using drug-eluting stents were decreased from 30% to about 5%.63–65 Intracranial use of drug-eluting stents has been limited to small retrospective series. In small series of 8 to 18 patients, the restenosis rate has ranged from 0% to 14% with 9 to 14 months of follow-up.63,66 However, the use of drug-eluting stents has been associated with delayed thrombosis in coronary vessels up to 4 years later and has raised some concern over safety issues.67 Ongoing coronary studies are underway to compare sirolimus against paclitaxel prospectively.68 Because coronary stents tend to be more rigid and more difficult to navigate in intracranial vessels, drug-eluting stents are unlikely to gain widespread utility intracranially until their role and effect in coronary vessels is better defined and tailored for intracranial anatomy.
Abou-Chebl A, Bashir Q, Yadav J. Drug-eluting stents for the treatment of intracranial atherosclerosis. Stroke. 2005;36:e165-e168.
American Society of Neuroradiology. Intracranial angioplasty and stenting for cerebral atherosclerosis: a position statement of the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, and the American Society of Neuroradiology. Am J Neuroradiol. 2005;26:2323-2327.
Arenillas J, Molina C, Montaner J, et al. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis. Stroke. 2001;32:2898-2904.
Bose A, Hartmann M, Henkes H, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007;38:1531-1537.
Chimowitz M, Kokkinos J, Strong J, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488-1493.
Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis [comment]. N Engl J Med. 2005;352:1305-1316.
EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial. N Engl J Med. 1985;313:1191-1200.
Feldmann E, Wilterdink J, Kosinski A, et al. The stroke outcomes and neuroimaging of intracranial atherosclerosis (SONIA) trial. Neurology. 2007;68:2099-2106.
Freitas J, Zenteno M, Aburto-Murrieta Y, et al. Intracranial arterial stenting for symptomatic stenoses: a Latin American experience. Surg Neurol. 2007;68:378-386.
Grubb RLJr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055-1060.
Grubb RJ, Powers WJ, Derdeyn C, et al. The carotid occlusion surgery study. Neurosurg Focus. 2003;14:1-7.
Jiang W, Xu X, Du B, et al. Comparison of elective stenting severe vs moderate intracranial atherosclerotic stenosis. Neurology. 2007;68:420-426.
Jiang WJ, Xu XT, Jin M, et al. Apollo stent for symptomatic atherosclerotic intracranial stenosis: study results. AJNR Am J Neuroradiol. 2007;28:830-834.
Kappelle LJ, Eliasziw M, Fox AJ, et al. Importance of intracranial atherosclerotic disease in patients with symptomatic stenosis of the internal carotid artery. The North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1999;30:282-286.
Komotar R, Wilson DA, Mocco J, et al. Natural history of intracranial atherosclerosis: a critical review. Neurosurgery. 2006;58:595-601.
Marks M, Wojak J, Al-Ali F, et al. Angioplasty for symptomatic intracranial stenosis: clinical outcomes. Stroke. 2006;37:1016-1020.
Marti-Fabregas J, Cocho D, Mart-Vilalta J, et al. Aspirin or anticoagulants in stenosis of the middle cerebral artery: a randomized trial. Cerebrovasc Dis. 2006;22:162-169.
Mazighi M, Tanasescu R, Ducrocq X, et al. Prospective study of symptomatic atherothrombotic intracranial stenoses. Neurology. 2006;66:1187-1191.
Ogasawara K, Ogawa A. JET study (Japanese EC-IC Bypass trial). Nippon Rinsho. 2006;64(suppl 7):524-527.
SSYLVIA Study Investigators. Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries (SSYLVIA). Stroke. 2004;35:1388-1392.
Thijs V, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology. 2000;55:490-497.
1 Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415-421.
2 Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis [comment]. N Engl J Med. 2005;352:1305-1316.
3 Wityk RJ, Lehman D, Klag M, et al. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974-1980.
4 Feldmann E, Daneaukt N, Kwan E, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990;40:1541-1545.
5 Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: the Northern Manhattan Stroke Study. Stroke. 1995;26:14-20.
6 Kappelle LJ, Eliasziw M, Fox AJ, et al. Importance of intracranial atherosclerotic disease in patients with symptomatic stenosis of the internal carotid artery. The North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1999;30:282-286.
7 Akins PT, Pilgram TK, Cross DT, Moran CJ. Natural history of stenosis from intracranial atherosclerosis by serial angiography. Stroke. 1998;29:433-438.
8 Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986;17:648-655.
9 Moossy J. Pathology of cerebral atherosclerosis. Influence of age, race, and gender. Stroke. 1993;24(suppl 1):I22-I23.
10 Wong KS, Huang YN, Gao S, et al. Intracranial stenosis in Chinese patients with acute stroke. Neurology. 1998;50:812-813.
11 Williams JE, Chimowitz MI, Cotsonis GA, et al. Gender differences in outcomes among patients with symptomatic intracranial arterial stenosis. Stroke. 2007;38:2055-2062.
12 Derdeyn CP, Grubb RLJr, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology. 1999;53:251-259.
13 Naritomi H, Sawada T, Kuriyama Y, et al. Effect of chronic middle cerebral artery stenosis on the local cerebral hemodynamics. Stroke. 1985;16:214-219.
14 Grubb RLJr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055-1060.
15 Constantinides P. Pathogenesis of cerebral artery thrombosis in man. Arch Path. 1967;83:422-428.
16 Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989;39:1246-1250.
17 Bogousslavsky J, Barnett HJ, Fox AJ, et al. Atherosclerotic disease of the middle cerebral artery. Stroke. 1986;17:1112-1120.
18 Komotar R, Wilson DA, Mocco J, et al. Natural history of intracranial atherosclerosis: a critical review. Neurosurgery. 2006;58:595-601.
19 Chimowitz M, Kokkinos J, Strong J, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488-1493.
20 Marti-Fabregas J, Cocho D, Mart-Vilalta J, et al. Aspirin or anticoagulants in stenosis of the middle cerebral artery: a randomized trial. Cerebrovasc Dis. 2006;22:162-169.
21 Mazighi M, Tanasescu R, Ducrocq X, et al. Prospective study of symptomatic atherothrombotic intracranial stenoses. Neurology. 2006;66:1187-1191.
22 Thijs V, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology. 2000;55:490-497.
23 American Society of Neuroradiology. Intracranial angioplasty and stenting for cerebral atherosclerosis: a position statement of the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, and the American Society of Neuroradiology. Am J Neuroradiol. 2005;26:2323-2327.
24 Kern R, Steinke W, Daffertshofer M, et al. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology. 2005;65:859-864.
25 Arenillas J, Molina C, Montaner J, et al. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis. Stroke. 2001;32:2898-2904.
26 Horn P, Vajkoczy P, Schmiedek P, Neff W. Evaluation of extracranial-intracranial arterial bypass function with magnetic resonance angiography. Neuroradiology. 2004;46:723-729.
27 Bash S, Villablanca J, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol. 2005;26:1012-1021.
28 Feldmann E, Wilterdink J, Kosinski A, et al. The stroke outcomes and neuroimaging of intracranial atherosclerosis (SONIA) trial. Neurology. 2007;68:2099-2106.
29 Nagata S, Fujii K, Matsushima T, et al. Evaluation of EC-IC bypass for patients with atherosclerotic occlusive cerebrovascular disease: clinical and positron emission tomographic studies. Neurol Rese. 1991;13:209-216.
30 Mendelowitsch A, Taussky P, Rem J, Gratzl O. Clinical outcome of standard extracranial-intracranial bypass surgery in patients with symptomatic atherosclerotic occlusion of the internal carotid artery. Acta Neurochir. 2004;146:95-101.
31 Sasoh M, Ogasawara K, Kuroda K, et al. Effects of EC-OC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surg Neurol. 2003;59:455-463.
32 Mizumura S, Nakagawara J, Takahashi M, et al. Three-dimensional display in staging hemodynamic brain ischemia for JET study: objective evaluation using SEE analysis and 3D-SSP display. Ann Nucl Med. 2004;18:13-21.
33 Grubb RJ, Powers WJ, Derdeyn C, et al. The carotid occlusion surgery study. Neurosurg Focus. 2003;14:1-7.
34 EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial. N Engl J Med. 1985;313:1191-1200.
35 Yasargil MG. Microsurgery applied to neuro-surgery. Stuttgart: Georg Thieme. 1969:105-115.
36 Ausman JI, Pearce JE, Vacca DF, et al. Tandem bypass: occipital artery to posterior inferior cerebellar artery side-to-side anastomosis and occipital artery to anterior inferior cerebellar artery end-to-side anastomosis—a case report. Neurosurgery. 1988;22:919-922.
37 Kadri PA, Krisht AF, Gandhi GK. An anatomic mathematical measurement to find an adequate recipient M4 branch for superficial temporal artery to middle cerebral artery bypass surgery. Neurosurgery. 2007;61(suppl 3):74-78.
38 Charbel FT, Hoffman WE, Misra M, Ostergren L. Ultrasonic perivascular flow probe: technique and application in neurosurgery. Neurol Res. 1998;20:439-442.
39 Kikuta K, Takagi Y, Fushimi Y, et al. “Target bypass”: a method for preoperative targeting of a recipient artery in superficial temporal artery-to-middle cerebral artery anastomoses. Neurosurgery. 2006;59(suppl 2):ONS320-ONS326.
40 Tulleken C, Streefkerk H, van der Zwan A. Construction of a new posterior communicating artery in a patient with poor posterior fossa circulation: a technical case report. Neurosurgery. 2002;50:415-420.
41 los Reyes RA, Bederson JB, Germano IM. Direct endarterectomy of the middle cerebral artery for treatment of symptomatic stenosis: case report. Neurosurgery. 1993;32:464-467.
42 Jacobson JH, Wallman LJ, Schumacher GA, et al. Microsurgery as an aid to middle cerebral artery endarterectomy [review]. Microsurgery. 1992;13:112-117.
43 Spetzler RF, Hadley MN, Martin NA, et al. Vertebrobasilar insufficiency. Part 1: Microsurgical treatment of extracranial vertebrobasilar disease [review]. J Neurosurg. 1987;66:648-661.
44 Allen GS, Cohen RJ, Preziosi TJ. Microsurgical endarterectomy of the intracranial vertebral artery for vertebrobasilar transient ischemic attacks. Neurosurgery. 1981;8:56-59.
45 Sauvageau E, Ecker R, Levy EI, et al. Recent advances in endoluminal revascularization for intracranial atherosclerotic disease. Neurol Res. 2005;27(suppl 1):S89-S94.
46 Schumacher H, Khaw A, Meyers P, et al. Intracranial angioplasty and stent placement for cerebral atherosclerosis. J Vasc Intervent Radiol. 2004;15:S123-S132.
47 Bose A, Hartmann M, Henkes H, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the wingspan study. Stroke. 2007;38:1531-1537.
48 Diaz F. Extracranial-intracranial bypasses. J Vasc Surg. 1985;2:234-238.
49 Gumerlock M, Ono H, Neuwelt E. Can a patent extracranial-intracranial bypass provoke the conversion of an intracranial arterial stenosis to a symptomatic occlusion? Neurosurgery. 1983;12:391-400.
50 Ogasawara K, Ogawa A. JET study (Japanese EC-IC Bypass trial). Nippon Rinsho. 2006;64(suppl 7):524-527.
51 Sundt TMJr, Smith HC, Campbell JK, et al. Transluminal angioplasty for basilar artery stenosis. Mayo Clin Proc. 1980;55:673-680.
52 Fiorella D, Woo HH. Emerging endovascular therapies for symptomatic intracranial atherosclerotic disease [comment]. Stroke. 2007;38:2391-2396.
53 Freitas J, Zenteno M, Aburto-Murrieta Y, et al. Intracranial arterial stenting for symptomatic stenoses: a Latin American experience. Surg Neurol. 2007;68:378-386.
54 SSYLVIA Study Investigators. Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries (SSYLVIA). Stroke. 2004;35:1388-1392.
55 Jiang WJ, Xu XT, Jin M, et al. Apollo stent for symptomatic atherosclerotic intracranial stenosis: study results. AJNR Am J Neuroradiol. 2007;28:830-834.
56 Marks M, Wojak J, Al-Ali F, et al. Angioplasty for symptomatic intracranial stenosis: clinical outcomes. Stroke. 2006;37:1016-1020.
57 Yu W, Smith W, Singh V, et al. Long-term outcome of endovascular stenting for symptomatic basilar artery stenosis. Neurology. 2005;64:1055-1057.
58 Jiang W, Srivastava T, Gao M, et al. Perforator stroke after elective stenting of symptomatic intracranial stenosis. Neurology. 2006;66:1868-1872.
59 Jiang W, Xu X, Du B, et al. Comparison of elective stenting severe vs moderate intracranial atherosclerotic stenosis. Neurology. 2007;68:420-426.
60 Levy EI, Yonas H. Cerebral revascularization for cerebral ischemia and stroke prevention in patients with atherosclerotic occlusive disease: defining the population that benefits [review]. Neurosurg Clin N Am. 2001;12:489-498.
61 Connors JJ3rd, Wojak J. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. J Neurosurg. 1999;91:415-423.
62 Albuquerque F, Levy EI, Turk A, et al. Angiographic patterns of Wingspan in-stent restenosis. Neurosurgery. 2008;63:23-28.
63 Abou-Chebl A, Bashir Q, Yadav J. Drug-eluting stents for the treatment of intracranial atherosclerosis. Stroke. 2005;36:e165-e168.
64 Holmes DJ, Leon M, Moses J, et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation. 2004;109:634-640.
65 Stone G, Ellis S, Cox D, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004;109:1942-1947.
66 Qureshi A, Kirmani J, Hussein H, et al. Early and intermediate-term outcomes with drug-eluting stents in high-risk patients with symptomatic intracranial stenosis. Neurosurgery. 2006;59:1044-1051.
67 Morice MC, Serruys PW, Barragan P, et al. Long-term clinical outcomes with sirolimus-eluting coronary stents: five-year results of the RAVEL trial. J Am Coll Cardiol. 2007;50:1299-1304.
68 Morice MC, Colombo A, Meier B, et al. REALITY Trial Investigators. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions. The REALITY trial: a randomized controlled trial. JAMA. 2006;295:895-904.