CHAPTER 37 Intracellular Motility
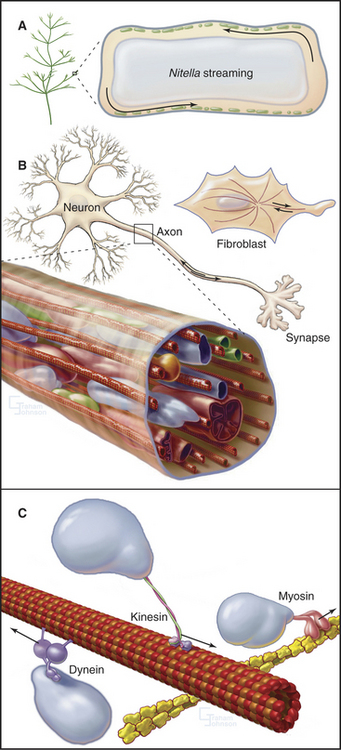
Virtually every component inside living cells moves to some extent, but the magnitude and velocity of these movements vary by orders of magnitude depending on the cell (Table 37-1 and Fig. 37-1). At one extreme, the bulk cytoplasm of algae and giant amoebas streams tens of micrometers per second. At the other extreme, small molecules and macromolecules diffuse through cytoplasm essentially unnoticed. The network of cytoskeletal polymers has a pore size of less than 50 nm, so particles that are larger than the pores must be transported actively. For example, messenger RNA (mRNA) moves from its site of synthesis in the nucleus through nuclear pores into the cytoplasm and then may be carried actively to specific parts of the cell. The nucleus rotates back and forth in most cells. Lysosomes, mitochondria, secretory vesicles, and endosomes all move around actively in cytoplasm, frequently between the centrosome and the cell periphery. Intracellular pathogenic bacteria and viruses subvert the host cell’s actin system to propel themselves randomly through the cytoplasm. Virus particles move along microtubules.
Table 37-1 VELOCITIES OF INTRACELLULAR MOVEMENTS
| System | Velocity (μm s−-1) | Mechanism |
|---|---|---|
| Microtubule Motors | ||
| Anterograde fast axonal transport, squid | 1 | Individual kinesin motors |
| Retrograde fast axonal transport, squid | 2 | Individual dynein motors |
| Chromosome movement in anaphase of mitosis | 0.003–0.2 | Motors plus depolymerization |
| Endoplasmic reticulum sliding, Newt cell | 0.1 | Individual kinesin motors |
| Slow axonal transport, rat nerves | 0.002–0.1 net | Motors on microtubules |
| 1 (intermittent) | ||
| Microtubule Polymerization | ||
| Endoplasmic reticulum tip elongation, Newt cell | 0.1 | Microtubule polymerization |
| Actin-Myosin Motors | ||
| Cytoplasmic streaming, Nitella | 60 | Myosin motors on tracks |
| Cytoplasmic streaming, Physarum | 500 | Actin-myosin contraction |
| Actin Polymerization | ||
| Actin-propelled comet, Listeria | 0.5 | Actin polymerization |
Two ancient mechanisms (Fig. 37-1) account for most intracellular movements in eukaryotes. Transport along microtubules by kinesin or dynein predominates in animal cells. Transport along actin filaments by myosin is more important in plants and fungi. Specialized isoforms of myosin, kinesin, and dynein are dedicated to particular movements. In most cases, transport involves single organelles, but vigorous organelle transport can even result in bulk movement of the cytoplasm. Alternatively, cytoplasmic contractions generated by myosin and actin filaments can produce cytoplasmic stream-ing, like squeezing toothpaste from a tube. Polymerization and depolymerization of microtubules and actin filaments also produce special types of intracellular movements.
Motor-driven movements on microtubules in animal cells generally receive the most attention, but this chapter takes a broad view across biology, highlighting all of the mechanisms for intracellular transport. Chapters on membrane traffic (see Chapters 20 to 21) and mitosis (see Chapter 44) cover more examples of intracellular movements.
Strategies to Identify Tracks and Motors
Historically, experiments with drugs that depolymerize or stabilize actin filaments or microtubules (see Boxes 33-1 and 34-1) provided the first clues about the cytoskeletal polymers that support various biological movements. Identifying the participating motors, if any, has been much more challenging, given a minimum five myosin genes, six kinesin genes, and one dynein gene in budding yeast and about ten times more motor protein genes in humans. A limited number of pharmacologic agents (Box 37-1) can implicate some motors, but the most definitive approach is to alter motor activity or abundance genetically or by RNAi-mediated depletion. Some motors are essential, so deletion mutations are lethal. Conditional mutations have allowed many definitive tests for the functions of these motors in yeast and, in a few cases, in more complex organisms. Other motors are not essential in the sense that cells have alternative strategies that can compensate if the protein is missing or inactive; nevertheless, the protein may have an important function. For example, dynein contributes to mitosis, but Drosophila tissue culture cells that are depleted of dynein can complete mitosis, though only after a long delay during which other motors take over.
Rapid Movements along Microtubules
Organelles in most cells are capable of moving at relatively high velocities, on the order of 1 mms (Table 37-1) along linear microtubule tracks. Thus, the organization of microtubules determines the patterns of these movements (Fig. 37-1; also see Fig. 34-2). Such movements are typically intermittent.
Microtubule-based motor proteins, kinesins and dyneins, power organelle movement along microtubules. Kinesins move organelles toward microtubule plus ends, which are located near the periphery of cells with microtubules radiating from centrosomes. Dyneins are responsible for organelle movements toward the minus ends of microtubules, located at the cell center. Intraflagellar transport of proteins in cilia and flagella (see Fig. 38-20) shares many features with the movements of organelles. Movements of organelles along microtubules have been reconstituted with purified dynein and kinesin. Final proof of the responsible motors usually depends on genetic tests.
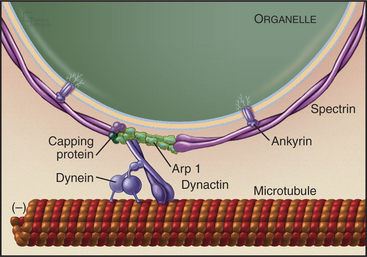
An assembly of proteins called the dynactin complex regulates the ability of dynein to transport membranes along microtubules (Fig. 37-2). This complex consists of a short filament of the actin-related protein Arp1 and seven other subunits, including heterodimeric capping protein. The 150-kD subunit (p150glued) binds to an intermediate chain of dynein. The Arp-1 filament interacts with spectrin associated with the membrane. Mutations in Drosophila p150glued cause developmental defects in the eye and brain. Some patients with inherited forms of motor neuron degeneration also have mutations of p150glued.
Fast Axonal Transport
High-contrast light microscopy of living axons reveals that most membrane-bound organelles move either toward (anterograde) or away from (retrograde) the end of the axon (Fig. 37-3) with some pauses and even occasional changes of direction. Retrograde movements (2.5 mm s−1 or 22 cm/day) are faster than anterograde movements (0.5 mm s−1 or 4 cm/day). At these rates, a round trip from a cell body in the spinal cord of a human to the foot and back takes only three weeks. This might seem slow, but if a 0.1-mm vesicle were the size of a small car, it would be moving anterograde at 50 miles per hour and retrograde at 250 miles per hour. In the axons of vertebrate neurons, mitochondria and autophagic vesicles move back and forth in both directions. Their net movement toward the nerve terminal or cell body depends on physiological conditions.
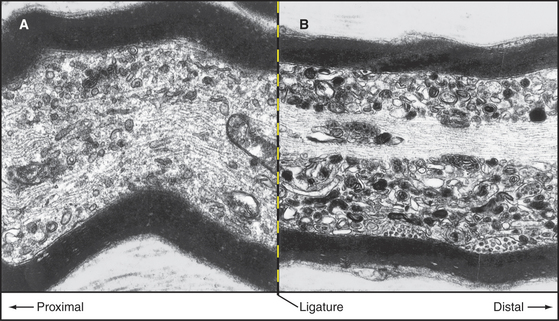
Classic nerve ligation experiments revealed the cargo carried in each direction by fast transport (Fig. 37-4). When mechanical constriction blocks transport, diferent organelles pile up on either side. Small, round, and tubular vesicles, including components of synaptic vesicles (see Fig. 11-8), accumulate on the side near the cell body. Fast anterograde transport carries these cargoes from the cell body toward the end of the axon, where they enter the cycle of synaptic vesicle turnover. Endosomes and multivesicular bodies moving by fast retrograde transport pile up on the distal side of the constriction. Retrograde transport can also move signals from nerve terminals to the cell body. For example, when nerve growth factor activates the TrkA receptor tyrosine kinase (see Fig. 24-4) at nerve terminals, the activated receptor is taken up by endocytosis and transported in endosomes to the perinuclear region, where the MAP kinase pathway regulates cell growth. Some viruses also move by retrograde transport.
Slow Transport of Cytoskeletal Polymers and Associated Proteins in Axons
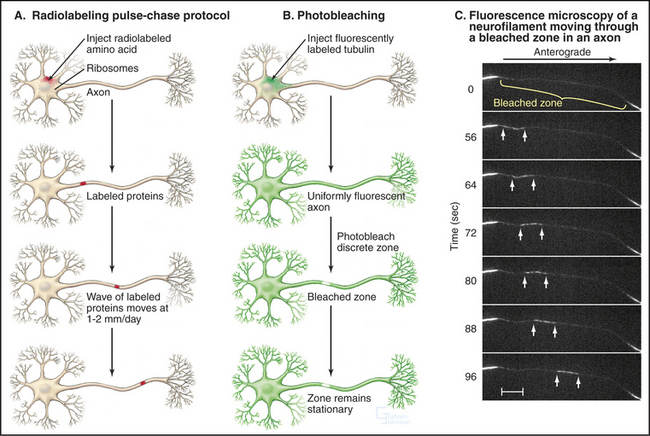
Many neuronal proteins move slowly from their site of synthesis in the cell body toward the ends of axons and dendrites. This transport is essential, as most protein synthesis occurs in the cell body, whereas more than 99% of cell volume can be in axons and dendrites. If nerve cells were smaller or less asymmetrical, we might not even notice such slow movements. These movements along axons can be followed by labeling the proteins with radioactive amino acids during their synthesis in the cell body (Fig. 37-5A). Proteins that are moved by slow axonal transport are classified into two groups based on their velocities. Tubulin, intermediate filament proteins, and spectrin, which compose the “slow component–a,” move exceedingly slowly, about 0.1 to 1.0 mm per day (or 1 to 10 nm s−1). In a human, these molecules take more than 3 months to travel from their site of synthesis in the spinal cord to the foot. “Slow component–b” moves about 10 times faster and includes 10 times more protein than slow component–a. It is a heterogeneous mixture of proteins, including clathrin, glycolytic enzymes, and actin.
Defining the mechanism of slow transport was challenging because various experimental approaches yielded apparently conflicting results. Radioactive labeling established the existence of slow movements and showed that the moving proteins become spread out and diluted as they move away from the cell body (Fig. 37-5A). Photobleaching of fluorescent tubulin and actin in axons of cultured neurons demonstrated that the bulk of these cytoskeletal polymers are stationary (Fig. 37-5B), whereas fluorescent tubulin, photoactivated inside an axon of a cultured Xenopus neuron, moves as a block at a rate that is characteristic of slow transport.
Other Microtubule-Dependent Movements
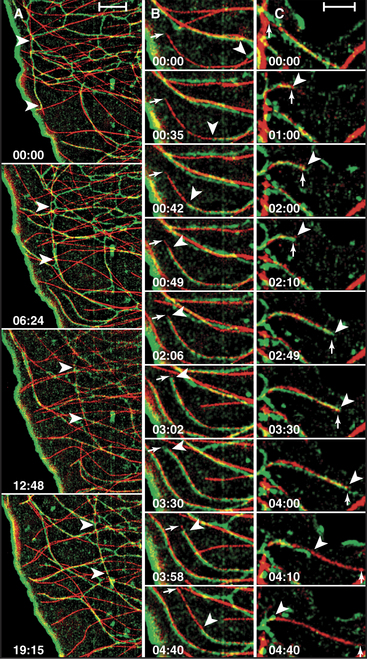
The distribution of the endoplasmic reticulum de-pends on intact microtubules. Strands of the endoplasmic reticulum align with microtubules in cultured cells (Fig. 37-6). This codistribution is achieved in two ways: (1) Motors transport strands of endoplasmic reticulum bidirectionally on microtubules, and (2) other strands of the endoplasmic reticulum attach to the plus end of microtubules and ride the microtubule tip as it grows and shrinks during dynamic instability. This is the best example of movement of an organelle driven by microtubule assembly. The concentration of the Golgi apparatus near the centrosome depends on microtubules, because dynein motors transport Golgi vesicles toward the minus ends of the microtubules. Mitochondria move bidirectionally on microtubules in animal cells but depend on actin filaments in yeast. These examples illustrate how not only the dynamics of the organelles but also the overall organization of a cell depend on the activity of microtubule motors. Thus, cellular architecture is determined actively, not passively.
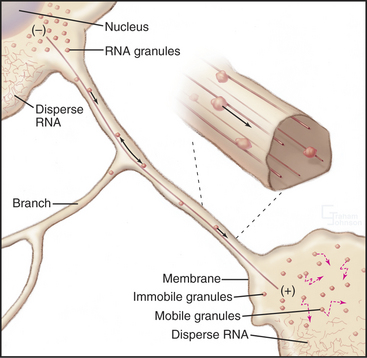
The cellular distribution of nucleoprotein complexes in the cell also depends on active movements. The most obvious example is the movement of chromosomes during mitosis, which depends on microtubule assembly and microtubule motors (see Fig. 44-7). Another example is the asymmetric localization in fly oocytes of certain mRNAs that help to establish the polarity of the embryos. Specific RNA sequences promote the assembly of protein-containing particles that move at steady rates on microtubules over long distances in the cell (Fig. 37-7). Kinesins and dynein are believed to power these movements, but most of the details remain to be determined. Dynein also anchors localized RNAs in oocytes.
Intracellular Movements Driven by Microtubule Polymerization
Microtubule polymerization and depolymerization have long been known to play a central role in the assembly of the mitotic apparatus and the movement of chromosomes (see Fig. 44-7), as well as the establishment of cellular asymmetry (see Fig. 34-2). Polymerizing microtubules can exert substantial forces, but the force will buckle microtubules longer than about 10 mm. Consequently, microtubule pushing mechanisms work best over short distances, such as for positioning the nucleus in fission yeast cells and the mitotic spindle in budding yeast cells.
Remarkably, the depolymerizing end of a micro-tubule can also pull on attached cargo. An in vitro proof-of-principle experiment (Fig. 37-8) showed that chromosomes can ride along on the end of a depolymerizing microtubule, even in the absence of ATP or GTP. Presumably, multiple weak bonds between the chromosome and the side of the microtubule near its end rearrange rapidly enough to maintain attachment, even as tubulin subunits dissociate from the end. In budding yeast, the link between the chromosome and the end of the microtubule is a ring-shaped complex of ten proteins. This ring may slide along the microtubule as protofilaments peel away from the end as it shortens.
Endoplasmic reticulum provides the best example of an intracellular organelle that harnesses microtubule growth for movement as an alternative to motor-driven movements along microtubules (Fig. 37-6). A “tip attachment complex,” yet to be characterized, maintains a connection between the endoplasmic reticulum and the end of a microtubule as its length varies secondary to cycles of polymerization and depolymerization.
Bulk Movement of Cytoplasm Driven by Actin and Myosin
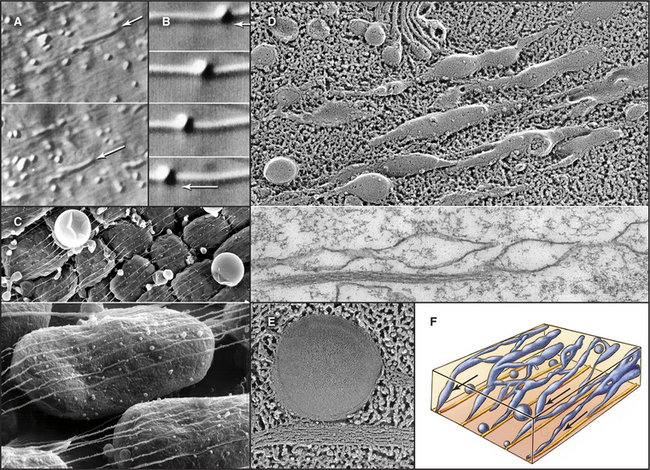
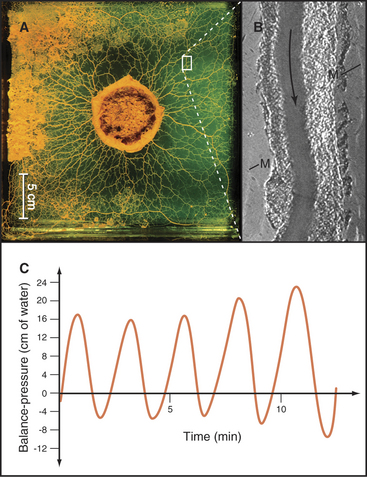
Bulk streaming of cytoplasm is most spectacular in plant cells (Fig. 37-1A). Although confined within rigid walls, plant cell cytoplasm streams vigorously at very high velocities (up to 60 mm s−1). At this rate, cytoplasm moves 5 m/day. Such cytoplasmic streaming is best understood in the giant cells of the green alga Nitella. Streaming occurs continuously in a thin layer of cytoplasm between the large central vacuole and chloroplasts immobilized in the cortex. On each side of the cell, a zone of stationary cytoplasm separates streams moving in opposite directions. The physiological function of this streaming is not clearly understood.
Bulk streaming in Nitella is brought about by movement of endoplasmic reticulum along tracks consisting of bundles of polarized actin filaments associated with chloroplasts (Fig. 37-9C). All of the actin filaments in these bundles have the same polarity, and cytoplasm streams toward their barbed ends. In Nitella extracts, membrane vesicles move along actin filament bundles at the same high velocities that are characteristic of the cytoplasmic streaming. An extraordinary type XI myosin pulls endoplasmic reticulum along cortical actin tracks, dragging along other cytoplasmic components, including organelles and soluble molecules. This myosin moves nearly 10 times faster than the fastest muscle contraction, apparently by taking large steps and by the cooperation of several motors working rapidly on the same membrane.
A completely different actomyosin mechanism produces equally spectacular cytoplasmic streaming in the acellular slime mold Physarum. In these giant, multinucleated cells, cytoplasm flows back and forth rhythmically at high velocities through tubular channels (Fig. 37-10). Cycles of contraction and relaxation of cortical actin filament networks push the relatively fluid endoplasm back and forth in a manner akin to squeezing a toothpaste tube. Myosin-II is thought to generate the cortical contraction, as it is present in high concentration in this cell and can contract actin filament gels in vitro. (This, incidentally, was the first nonmuscle myosin to be purified in the late 1960s.) The cortical contractions that are so prominent in Physarum are also used by giant amoebas for cell locomotion (see Fig. 38-1), cytokinesis (see Fig. 44-23), and movements of some embryonic tissues (see Fig. 38-5).
Actin-Based Movements of Organelles in Other Cells
Like Nitella, budding yeasts transport vesicles along bundles of actin filaments from the mother to the bud (Fig. 37-11), although the movements of these solitary vesicles do not produce cytoplasmic streaming. Myosin-V is the motor, so vesicles fail to move from mother to bud in null mutants of myosin-V genes. A myosin-V also transports certain mRNAs along actin filament cables from the mother to the bud, where they determine cell fate.
Cytoplasmic Movements Driven by Actin Polymerization
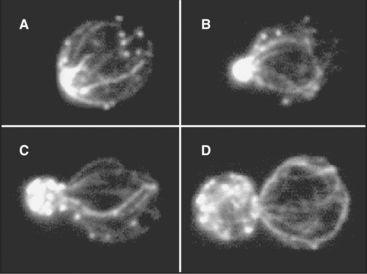
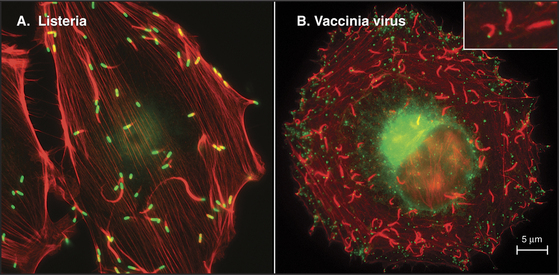
Some intracellular pathogenic bacteria, including Listeria and Shigella, use actin polymerization to move through the cytoplasm of their animal cell hosts at about 0.5 mm s−1 (Fig. 37-12A). These bacteria hijack the machinery that is used to move the leading edge of motile cells to polymerize a comet tail of actin filaments that pushes the bacterium forward. One end of the bacterium has a concentration of proteins that directly (Listeria) or indirectly (Shigella) activate Arp2/3 complex to polymerize a network of branched actin filaments (see Fig. 33-13). Growth of this network pushes the bacterial cell forward. The comet tail of cross-linked actin filaments is stationary and depolymerizes distally at the same rate at which it grows next to the bacterium, so it remains a constant length. Under some conditions, cellular endosomes can induce actin filament comets and move similar to Listeria rather than using microtubules. It is not yet known how widely this phenomenon is used for intracellular motility.
Vaccinia viruses attached to the outer surface of animal cells also use transmembrane proteins to usurp the cytoplasmic actin assembly system to drive their movements at one stage in its life cycle (Fig. 37-12B). Placement of a plastic bead coated with adhesion proteins on the plasma membrane of some animal cells can induce similar propulsive actin comet tails in the cytoplasm.
Chou YH, Helfand BT, Goldman RD. New horizons in cytoskeletal dynamics: Transport of intermediate filaments along microtubule tracks. Curr Opin Cell Biol. 2001;13:106-109.
Cossart P, Pizarro-Cerdá J, Lecuit M. Invasion of mammalian cells by Listeria monocytogenes: Functional mimicry to subvert cellular functions. Trends Cell Biol. 2003;13:23-31.
Engqvist-Goldstein AEY, Drubin DG. Actin assembly and endocytosis: From yeast to mammals. Annu Rev Cell Dev Biol. 2003;19:287-332.
Frank DJ, Noguchi T, Miller KG. Myosin VI: A structural role in actin organization important for protein and organelle localization and trafficking. Curr Opin Cell Biol. 2004;16:189-194.
Guzik BW, Goldstein LSB. Microtubule-dependent transport in neurons: Steps towards an understanding of regulation, function and dysfunction. Curr Opin Cell Biol. 2004;16:443-450.
Hathaway NA, King RW. Dissecting cell biology with chemical scalpels. Curr Opin Cell Biol. 2005;17:12-19.
Kamal A, Goldstein LSB. Principles of cargo attachment to cytoplasmic motor proteins. Curr Opin Cell Biol. 2002;14:63-68.
Kashina A, Rodionov V. Intracellular organelle transport: Few motors, many signals. Trends Cell Biol. 2005;15:396-398.
Lopez de Heredia M, Jansen R-P. mRNA localization and the cytoskeleton. Curr Opin Cell Biol. 2004;16:80-85.
Mandelkow E, Mandelkow E-M. Kinesin motors and disease. Trends Cell Biol. 2002;12:585-591.
Mermall V, Post PL, Mooseker MS. Unconventional myosins in cell movement, membrane traffic and signal transduction. Science. 1998;279:527-533.
Pruyne D, Legesse-Miller A, Gao L, et al. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol. 2004;20:559-591.
Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759-779.
Shah JV, Cleveland DW. Slow axonal transport: Fast motors in the slow lane. Curr Opin Cell Biol. 2004;14:58-62.
Shimmen T, Yokota E. Cytoplasmic streaming in plants. Curr Opin Cell Biol. 2004;16:68-72.
Stokin GB, Goldstein LSB. Axonal trnasport and Alzheimer’s disease. Annu Rev Biochem. 2006;75:607-627.