Chapter 23 Intervertebral Disc
Anatomy, Physiology, and Aging
Anatomy
Intervertebral Disc
The spinal column has 23 IVDs, starting from the C2-3 interspace to the L5-S1 interspace. These discs constitute approximately 25% of the total height of the spinal column. The discs vary in thickness from the thinnest disc in the thoracic region to the thickest in the lumbar region.1 The IVD is a vital component of the joint system present at each spinal level. This system allows for the range of motion permitted at each segment.2 Each IVD works in conjunction with the paired dorsal zygapophyseal joints to form a “three-joint complex.” The function of the individual components of this complex are intimately related, as are their effects on one another during the degenerative process.
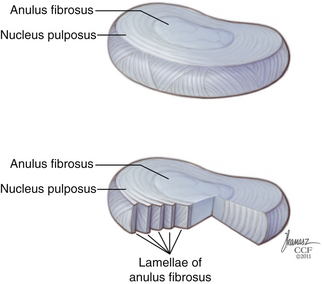
The IVD is constructed of three distinct components. The bulk of the disc is made up of a central nucleus pulposus (NP) and the surrounding anulus fibrosus (AF) (Fig. 23-1). The disc is flanked rostrally and caudally by cartilaginous end plates that serve as a transitional zone between the disc and the adjacent vertebral bodies.3
Nucleus Pulposus
The central core of the IVD is composed of the NP. It is formed from remnants of the notochord, a derivation of the endoderm—unlike the remaining components of the IVD, which are derived from the mesoderm.4 The NP is composed of a soft gelatinous material consisting of proteoglycans, surrounded circumferentially by the AF. The NP is 80% water, with proteoglycans contributing to 50% of its dry weight. Aggrecans, a type of proteoglycan known as leucine-rich repeat proteins, are the predominant type of proteoglycans in the NP.5 The high-proteoglycan content allows for the NP’s ability to maintain an increased hydration state, which creates the viscoelastic properties of the NP, which are essential to its load-bearing properties in the spine. The NP is rich in type II collagens, which constitute 80% of the collagen content in the NP.6 There is a clear distinction between the NP and the surrounding AF; however, this distinction becomes less obvious during the course of aging.1 Elastin fibers can also be found in the NP. These fibers are observed to be situated in both a radial distribution from the center to the periphery in the NP, as well as in a vertical orientation, anchoring the NP to the end plates.7 This orientation likely contributes to maintaining the structure of the NP within the AF, restoring the NP to its original form following load bearing, as well as playing a role in load transmission to surrounding AF.
Anulus Fibrosus
The AF is a concentrically organized structure that surrounds the NP, occupying the majority of the disc space. It is composed of bundles of collagen fibers that are arranged in concentric lamellae. Approximately 15 to 25 lamellae compose each anulus.8 The collagen fiber bundles within the lamellae are generally oriented at 30 degrees from the horizontal axis, although closer examination shows that throughout the course of the lamellae, the fiber angle can vary from 20 to 55 degrees.9 Collagen fibrils in adjacent lamellae run in the opposite direction, allowing for greater tensile strength during stretching.
The AF can be further separated into an outer and inner anulus. The outer AF is a tougher, less flexible component, composed primarily of densely packed type I collagen fibers. The cells in the outer AF are more ellipsoidally shaped cells and are fibroblast-like in nature. At the periphery of the outer anulus, fibrillar bundles known as Sharpey fibers extend superiorly and inferiorly to anchor the disc into the periosteal fibrils of the adjacent vertebral bodies.10 The inner AF is a softer, less dense, and more fibrocartilagenous component, composed primarily of type II collagen fibers. It possesses a less structured cellular morphology with more widely spaced cells than that of type I collagen. Type II collagen has been shown to be able to maintain 50% to 100% greater water content than type I collagen.11 The outer lamellae are composed of up to approximately 70% type I collagen and only 20% type II collagen, whereas in the inner AF the percentage of type II collagen increases to 70%. There is also a progressive increase in the concentration of proteoglycans from 10% to 30% when moving from the outer to inner AF.12 Other types of collagen are also present in the anulus, with type VI collagen making up as much as 10% of the collagen in the AF.5
Cartilage End Plates
On the superior and inferior surfaces of the IVD lies the cartilage end plates. They are composed of a thin layer of hyaline cartilage, which serves as the interface between the bony vertebral body and the disc itself. Their composition is similar to the disc; that is, made up of proteoglycans, type II collagen, and water.13 The end plates range from 0.5 to 1.5 mm in thickness. They are thinnest at the center where they interface with the NP.14 Each end plate is attached to the adjacent vertebral body by a thin layer of calcium. The calcium is absent in areas where numerous perforations exist throughout the end plates. These perforations in the end plates allow for the passage of vascular channels that traverse from the adjacent vertebral body into the disc.1 These channels become progressively rare and eventually nonexistent, as the disc ages past the second decade of life.15.The end plate serve as a stiff but porous barrier between the vertebral body and the IVD. They allow for the diffusion of nutrients and fluid movement into and out of the disc and prevent protrusion of the soft malleable disc material into the vertebral body (Schmorl nodes). Weaknesses in the end plates resulting in Schmorl nodes may be due to a dysregulation of the end-plate composition since those areas have been found to have significantly less proteoglycan concentration.16
Vascular Supply
The IVD is generally considered to be an avascular tissue structure, but this is only partially accurate. In infancy, the native disc has a direct vascular supply. This, however, nearly completely regresses by the third decade of life. Vascular “buds” extend from the vertebral bodies through the porous channels in the cartilage end plates and traverse into the disc to supply nutrients.16 These channels are overtaken by scarring or collapse, likely as a result of weight bearing, past the second decade of life and become occluded and eventually nonexistent.15,17 Without a direct vascular supply, nutrient exchange occurs through diffusion from blood vessels that surround the periphery of the anulus. The surrounding blood supply comes from segmental branches of the spinal artery.18
Innervation
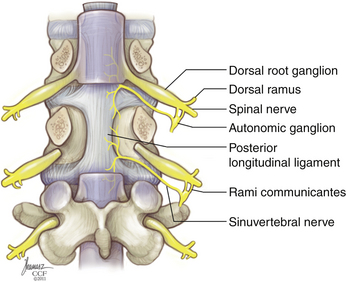
The IVD receives innervations primarily from two sources: the sinuvertebral nerve and the sympathetic trunk via the multiple gray rami communicantes (Fig. 23-2). The sinuvertebral nerve is formed from a branch of the ventral primary ramus and the gray ramus communicans, which branches from the sympathetic trunk. The sinuvertebral nerve enters the spinal canal caudal to the pedicle through the intervertebral foramen and branches into a larger superior division and a lesser inferior division. The superior division travels lateral to the posterior longitudinal ligament in the ventral aspect of the spinal canal, supplying sensation to the dorsal and dorsolateral anulus, as well as the posterior longitudinal ligament. The inferior trunk passes medially and caudally. The lateral and ventral portions of the AF are supplied by multiple gray rami communicantes from the nearby sympathetic trunk.19,20 The sympathetic trunk runs in parallel, ventral and lateral to the vertebral column, composed of nerve root contributions from the thoracic and upper two segments of the lumbar spinal cord. The sympathetic trunk provides a number of gray rami comminicantes that help supply the anulus. At least one, but often more, rami communicantes travel around the vertebral body deep to the psoas muscle to join with each ventral primary ramus. Rami communicantes also traverse the psoas to join with the ventral primary ramus. In addition, “paradiscal” rami run along the surface of the IVD in the perianular connective tissue.19 Innervation of the end plate extends from intraosseous nerves that branch from the ventral primary rami and the basivertebral nerve, a branch of the sinuvertebral nerve, which enters the end plate dorsally.6
Innervation of the disc interspace is restricted to the outer anulus. A quantitative analysis of nerve density in multiple IVDs showed innervation to extend to a depth of four to seven lamellae ventrally and no more than three lamellae dorsally. This is indeed very shallow, given that a usual AF contains an average of 15 to 25 lamellae.8 Unlike the anulus, innervation of the end plate is densest near the center, adjacent to the NP. Innervation of the end plates originates from the intraosseous nerves that penetrate the caudal portion of the vertebral body ventrally and dorsally and travel to the center of the end plate.21
Physiology
Extracellular Matrix
The main purpose of the IVD is to serve as a load-bearing structure. The disc performs this task by absorbing axial loads and redistributing them across the entire disc. The capacity for load redistribution is determined, in part, by the molecular structure of the disc. The extracellular matrix is primarily composed of collagen and proteoglycan. These molecules allow the different components of the disc to complete the aforementioned tasks. As previously noted, the AF is a mix of type I and type II collagen, which gives it its necessary tensile strength. In addition to type I and II collagen, multiple other collagen types, including types V and XI, are present in smaller amounts. These collagens play a role in interlinking the collagen fibrils, thus contributing to the overall strong fibrillar collagen network.5,22 The differences in the composition in different areas of the disc create a dynamic structure that is able to bear and distribute loads across the entire structure. When axial loading takes place, the NP and inner AF absorb the weight and conform to generate hydrostatic pressure that is distributed evenly to the adjacent outer anulus. The alternating lamellar structure of the type I collagen network in the outer AF creates the tensile strength to absorb the redistributed loading force.
Proteoglycans are the other main molecular component of the disc. They constitute 50% of the cells in the NP. Numerous types of proteoglycans are present in the extracellular matrix, including aggregan, versican, decorin, biglycan, fibromodulin, lumican, and perlecan. Aggregan is the most important proteoglycan found in the NP. Proteoglycans comprise a central core protein with attached side chains of keratin sulfate and chondroiton sulfate. Early in life, aggrecans are rich in chondroitin sulfate chains, but these side chains are gradually replaced by keratin sulfate as the disc matures. At their N-terminus, aggrecans attach to hyaluronic acid,12 and at their C-terminus, they can attach to various molecules in the extracellular matrix, including collagen.23 The proteogylycans are attached to hyaluronic acid molecules through link proteins, which allow them to form aggregates.5 Proteoglycans have negative charges on their surface and are therefore hydrophilic molecules able to bind water—thus allowing the NP to retain its hydrated state. The percentages of proteoglycan aggregates are highest in infancy and decrease with age. Breakdown of aggregans that occurs with aging causes them to be replaced by nonaggregated proteoglycans, which are less able to absorb water than their aggregated counterparts.
Metabolic Balance
The extracellular matrix of a healthy intervertebral disc is a dynamic environment, which is maintained at equilibrium due to a fine balance between anabolism and catabolism. The mature NP is essentially void of any vascular supply, and therefore diffusion and glycolysis are the primary sources of nutrient metabolism. Several growth factors, including insulin-like growth factor (IGF), bone morphogenetic protein (BMP), and transforming growth factor-β (TGF-β), contribute to the anabolism within the IVD. IGF and TGF-β have been shown to play a role in stimulating prostaglandin synthesis. BMP-2 also has been shown to stimulate prostaglandin synthesis, as well increase cell proliferation and expression of type II collagen and aggregans.24 These growth factors work against catabolic factors that lead to breakdown of matrix products. Multiple forms of a zinc-dependent family of enzymes called matrix metalloproteinases (MMPs) are responsible for the degradation of several types of collagen and noncollagenous matrix proteins, including proteoglycans and glycoproteins.25 MMPs are a vital component of the disc’s natural biologic balance between synthesis and breakdown of the extracellular matrix, but have been shown to be up-regulated in diseased discs.26 The activity of MMPs is tempered by the expression of countering enzymes known as tissue inhibitors of metalloproteinases (TIMPs). TIMPs act to inhibit the activity of MMPs so a proper balance between tissue construction and degradation is achieved.27 Dysregulation of this system can be seen in degenerative disc disease.
Disc Nutrition
Several factors must be considered to fully understand the nature of the nutrient supply to the IVD. To make things more complex, this landscape changes with age. Only a direct vascular supply exists in the immature disc, which travels from the adjacent vertebral bodies and passes through porous channels in the cartilage end plate. These channels decrease with age and are nearly nonexistent past the second decade as a result of calcification. In addition to the reduction in direct vascular supply, in the early stages of disc maturation, the disc height increases, thus making it more difficult for a direct blood supply to exist in the deeper areas of the disc. Therefore, the nutritional supply and removal of metabolic waste products for the majority of the life of the disc is restricted to diffusion or convection transport. Smaller molecules such as glucose and oxygen are able to effectively diffuse through the disc aided by brownian movements as a result of concentration gradients.28 This occurs either through the AF circumferentially or through the end plates from the vertebral bodies. The end plates act as a selectively permeable barrier to certain solutes. Calcification of the end plates that occurs with aging can hinder nutrient supply through this pathway.29 Convection transport, as a result of bulk fluid flow, is the alternative method of solute transport and is likely the preferential method for the transport of larger molecules that are not effectively transported via diffusion. Cycling of mechanical loading between activity and rest can mobilize almost 22% of the total disc volume.30 Therefore, the diurnal loading cycle can account for a substantial amount of metabolite transfer and is a necessary element of IVD metabolism.31
Despite these mechanisms of nutrient supply, the disc lives in a relatively hypoxic environment. The periphery of the disc receives the greatest nutrient supply. This is evident in the increased cell density at the periphery of the AF.32 In the center of the disc, the levels of glucose and oxygen are lower, due to restrictions in solute diffusion, resulting in increased lactic acid concentration and a more acidic environment, which can hinder proper maintenance of the cell matrix.28,33 As a result of the natural course of aging and degeneration, further inhibition of nutrient supply pathways can occur, which results in an even more accelerated degeneration within the disc matrix. External factors such as smoking, which can hinder vascular supply, or accelerated calcification of the end plates, as seen in scoliosis, can compound this effect.34
Biomechanics
Load sharing is also performed by the dorsal zygapophyseal joints of the three-joint complex.6 Aging of the IVD, leading to loss of disc height, can transfer additional load-bearing responsibilities to the joints, and vice versa. The interplay between the IVD and the zygapophyseal joints constitutes the interaction of the three-joint complex, which is a key part of the degenerative process. Despite the interaction between the individual components, studies have shown that degeneration of the disc occurs before that of the joints, indicating the importance of the role of the disc in this sytem.35
Aging
Extracellular Matrix Changes
Maturation of the IVD is characterized by a general shift from a thick, well-hydrated, elastic material capable of absorption of compressive loads to a thin, stiff, fibrous material that has lost its ability to properly distribute loads. This is related to the fact that several changes take place at the microscopic level. One of the main changes is the degradation of type II collagen and an increased percentage of type I collagen. This is evident in the elderly in whom less than 30% of the collagen is type II. This is due to selective collagen degradation by various types of MMPs. The collagen fibers also change in composition, as they develop cross-links to adjacent collagen fibers. In addition to enzymatic cross-linking, uncontrolled nonenzymatic cross-linking also occurs, which results in an excessively cross-linked collagen network that increases the stiffness of the entire matrix.36 Nonenzymatic glycation of this type is a result of a lack of turnover of the collagen supplies in the matrix, which is a consequence of the avascular nature of the disc precluding optimal material turnover. This type of cross-linking results in a yellow-brown pigmentation that is commonly seen in aging discs.
In the NP, significant changes occur to proteoglycans during the course of aging. Early in the disc’s life, proteoglycans are rich in chondroiton sulfate side chains, with few surrounding collagen fibers. The proteoglycans interact with nearby hyaluronic acid to form proteoglycan aggregates.37 As the disc ages, the percentage of proteoglycans in aggregate form significantly decreases, probably secondary to proteolytic degradation. Also, as the number of chondroiton sulfate chains decreases, the number of keratin sulfate chains increases. Further aging is associated with the degradation of the proteoglycan aggregates as well as the link proteins. These effects reduce the NP’s ability to create a hydrophilic environment, which in turn leads to decreased water retention in the disc.
Although the exact mechanism is unclear, there appears to be an imbalance between the anabolic and catabolic mechanisms within the disc. An up-regulation of MMPs in herniated and diseased discs has been observed.38 Proinflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α), are stimulated from collagen degradation products and lead to further activation of MMPs. TNF-α has been shown to decrease aggregan and type II collagen synthesis and induce the expression of multiple MMPs and aggrecanases.39 Interestingly, an up-regulation of tissue inhibitors of metalloproteinases (TIMPs),26 is also noted, suggesting that a repair mechanism is attempting to compensate for the increase in catabolic activity. Nitric oxide has also been shown to play a role in the evolution of the extracellular matrix during aging and degeneration. Although the mechanism by which this is performed is unclear, studies have shown the nitric oxide can inhibit proteoglycan synthesis in response to hydrostatic pressure.40 This course of events indicates that during the course of disc aging and into degeneration, an imbalance toward catabolism develops, which disrupts the normal equilibrium of the extracellular matrix. The disc’s natural ability to compensate for these mechanisms is overtaken, leading to continued activation of these mechanisms and eventually resulting in disc degeneration.
Structural and Functional Changes
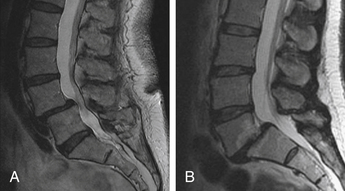
The molecular changes that occur during the course of aging result in significant structural and functional changes in the IVD. The NP, which was once a thick viscoelastic substance, becomes thinner and more fibrous, and consequently unable to conform to the compressive loads placed upon it (Fig. 23-3). The distinction between the AF and the NP becomes less clear as the collagen composition becomes stiffer and more fibrous. Anular degeneration results in a reduced number of layers of lamellae, although the thickness of the AF remains the same due to thickening of the remaining lamellae.8 The proteoglycan concentration decreases, resulting in decreased water content in the NP and a loss of its hydrostatically related ability to dissipate the compressive loads. This, along with thinning of the NP, forces the AF to become directly exposed to the axial-loading pressures of the spine, which in turn results in further injury to the anulus. During the course of aging, reduction in the functional diameter of the NP results in an increase of stress forces applied to the outer AF of up to 160%.41 In essence, the central NP load-bearing characteristics of youth are transformed directly into a peripheral or perimeter AF load bearing as we age.
Degenerative Cascade
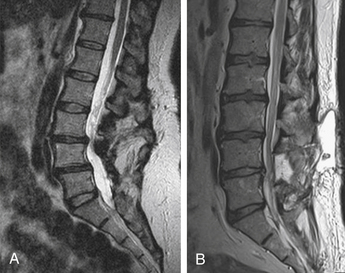
Yong-Hing and Kirkaldy-Willis categorized the aging and degeneration of the spine into three stages to further define how the natural history of the IVD and zygapophyseal joint relate to each other.2 The first state is the dysfunction stage, which is characterized by synovial reaction in the dorsal joints and small tears in the IVDs. These injuries occur as a result of natural load bearing and the inevitable aging process of the disc. This progresses to the destabilization stage, which may be better described as the stage of instability. This stage is defined as a progression of the disc past the normal aging process into a degenerative process that involves all components of the three-joint complex. Disc degeneration in this stage results in a loss of disc height and load-bearing capacity of the disc, thus increasing the loading pressure delivered to the zygapophyseal joints. Excess load bearing on the joints in turn can lead to joint capsule laxity and subluxation of the joints. Instability in the joints can lead to anterior translation of the rostral vertebral body with respect to the caudal vertebral body. Such translation of the vertebral segments, in combination with loss of disc interspace height, can result in further narrowing of the neural foramina (Fig. 23-4). Weakness of the joints and movement of the vertebral bodies result in an even greater load applied to the already damaged discs, continuing a cycling cascade of injury within the joint complex.
The final stage is the restabilization stage. As a result of the joint instability and bony movement, osteophyte formation occurs between the vertebral segments. Further disc degeneration and calcification combined with extensive osteophyte formation results in fusion of the intervertebral level and loss of joint motion, thus restabilizing the given motion segment. At this stage, the IVD essentially becomes obsolete since its original role in maintaining joint mobility and load bearing has been eliminated. Once this fusion has occurred, the degenerative process is complete.
Adams M.A., McNally D.S., Dolan P. “Stress” distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg [Br]. 1996;78:965.
Antoniou J., Steffen T., Nelson F., et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996.
Buckwalter J.A., Einhorn T.A., Simon S.R. Orthopaedic basic science: biology and biomechanics of the musculoskeletal system. Rosemont, IL, 2000, American Academy of Orthopaedic Surgeons. 2000.
Philips F.M. Lauryssen: the lumbar intervertebral disc. NewYork: Thieme Medical; 2010.
Roberts S., Caterson B., Menage J. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976). 2000;25:3005.
Yong-Hing K., Kirkaldy-Willis W.H. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am. 1983;14:491.
1. Coventry M.B., Ghormley R.K., Kernohan J.W. The intervertebral disc: its microscopic anatomy and pathology Part I. Anatomy, development and physiology. J Bone Joint Surg [Am]. 1945;27:105-112.
2. Yong-Hing K., Kirkaldy-Willis W.H. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am. 1983;14:491-504.
3. Taylor J.R. Growth of human intervertebral discs and vertebral bodies. J Anat. 1975;120:49-68.
4. Roberts S., Evans H., Trivedi J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg [Am]. 2006;88:10-14.
5. Buckwalter J.A., Einhorn T.A., Simon S.R. Orthopaedic basic science: biology and biomechanics of the musculoskeletal system. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2000.
6. Philips F.M. Lauryssen: the lumbar intervertebral disc. NewYork: Thieme Medical; 2010.
7. Yu J., Winlove P.C., Roberts S., Urban J.P. Elastic fibre organization in the intervertebral discs of the bovine tail. J Anat. 2002;201:465-475.
8. Marchand F., Ahmed A.M. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine (Phila Pa 1976). 1990;15:402-410.
9. Berthet C., Hulmes D.J., Miller A., et al. Structure of collagen in cartilage of intervertebral disk. Science. 1978;199:547-549.
10. Inoue H. Three-dimensional architecture of lumbar intervertebral discs. Spine (Phila Pa 1976). 1981;6:139-146.
11. Grynpas M.D., Eyre D.R., Kirschner D.A. Collagen type II differs from type I in native molecular packing. Biochim Biophys Acta. 1980;626:346-355.
12. Antoniou J., Steffen T., Nelson F., et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996-1003.
13. Raj P.P. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18-44.
14. Grignon B., Grignon Y., Mainard D., et al. The structure of the cartilaginous end-plates in elder people. Surg Radiol Anat. 2000;22:13-19.
15. Coventry M.B., Ghormley R.K., Kernohan J.W. The intervertebral disc: its microscopic anatomy and pathology. Part II. Changes in the intervertebral disc concomitant with age. J Bone Joint Surg [Am]. 1945;27:233-247.
16. Roberts S., Menage J., Urban J.P. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine (Phila Pa 1976). 1989;14:166-174.
17. Nerlich A.G., Schaaf R., Wälchli B., et al. Temporo-spatial distribution of blood vessels in human lumbar intervertebral discs. Eur Spine J. 2007;16:547-555.
18. Moore K.L., Dalley A.F., Agur A.M.R. Clinically oriented anatomy. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
19. Bogduk N., Tynan W., Wilson A.S. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132:39-56.
20. Bogduk N. The innervation of the lumbar spine. Spine (Phila Pa 1976). 1983;8:286-293.
21. Fagan A., Moore R. Vernon Roberts B: ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine (Phila Pa 1976). 2003;28:2570-2576.
22. Setton L.A., Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg [Am]. 2006;88:52-57.
23. Feng H., Danfelter M., Strömqvist B. Extracellular matrix in disc degeneration. J Bone Joint Surg [Am]. 2006;88:25-29.
24. Tim Yoon S., Su Kim K., Li J. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine (Phila Pa 1976). 2003;28:1773-1780.
25. Goupille P., Jayson M.I., Valat J.P. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine (Phila Pa 1976). 1998;23:1612-1626.
26. Roberts S., Caterson B., Menage J. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976). 2000;25:3005-3013.
27. Bachmeier B.E., Nerlich A., Mittermaier N. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur Spine J. 2009;18:1573-1586.
28. Urban J.P., Smith S., Fairbank J.C. Nutrition of the intervertebral disc. Spine (Phila Pa 1976). 2004;29:2700-2709.
29. Roberts S., Menage J., Eisenstein S.M. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11:747-757.
30. Botsford D.J., Esses S.I., Ogilvie-Harris D.J. In vivo diurnal variation in intervertebral disc volume and morphology. Spine (Phila Pa 1976). 1994;19:935-940.
31. Ferguson S.J., Ito K., Nolte L.P. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech. 2004;37:213-221.
32. Maroudas A., Stockwell R.A., Nachemson A., et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113-130.
33. Ishihara H., Urban J.P. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829-835.
34. Battié M.C., Videman T., Gill K., et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine (Phila Pa 1976). 1991;16:1015-1021.
35. Butler D., Trafimow J.H., Andersson G.B.J., et al. Discs degenerate before facets. Spine (Phila Pa 1976). 1990;15:111-113.
36. Duance V.C., Crean J.K., Sims T.J., et al. Changes in collagen cross-linking in degenerative disc disease and scoliosis. Spine (Phila Pa 1976). 1998;23:2545-2551.
37. Oegema T.R.Jr., Bradford D.S., Cooper K.M. Aggregated proteoglycan synthesis in organ cultures of human NP. J Biol Chem. 1979;254:10579-10581.
38. Le Maitre C.L., Freemont A.J., Hoyland J.A. Human disc degeneration is associated with increased MMP 7 expression. Biotech Histochem. 2006;81:125-131.
39. Séguin C.A., Bojarski M., Pilliar R.M., et al. Differential regulation of matrix degrading enzymes in a TNFalpha-induced model of NP tissue degeneration. Matrix Biol. 2006;25:409-418.
40. Liu G., Ishihara H., Osada R., et al. Nitric oxide mediates the change of proteoglycan synthesis in the human lumbar intervertebral disc in response to hydrostatic pressure. Spine (Phila Pa 1976). 2001;26:134-141.
41. Adams M.A., McNally D.S., Dolan P. “Stress” distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg [Br]. 1996;78:965-972.