Interpreting Clinical and Laboratory Data
After reading this chapter you will be able to:
 Describe what a critical value is, and state its importance in clinical practice.
Describe what a critical value is, and state its importance in clinical practice.
 Define the following terms related to clinical laboratory tests: leukocytosis, leukopenia, anemia, polycythemia, and thrombocytopenia.
Define the following terms related to clinical laboratory tests: leukocytosis, leukopenia, anemia, polycythemia, and thrombocytopenia.
 Identify which electrolyte disturbances interfere with normal respiratory function.
Identify which electrolyte disturbances interfere with normal respiratory function.
 Describe clinical tests used to identify cardiac stress and myocardial infarction.
Describe clinical tests used to identify cardiac stress and myocardial infarction.
 Identify the three main tests used to diagnose coagulation disorders.
Identify the three main tests used to diagnose coagulation disorders.
 Describe how the sputum Gram stain and culture are used to diagnose pulmonary infections.
Describe how the sputum Gram stain and culture are used to diagnose pulmonary infections.
Interpreting Clinical Laboratory Tests
Introduction to Laboratory Medicine
Reference Range
Beginning in the 1970s,1 the term normal ranges was slowly replaced with more appropriate terms such as reference ranges, biologic reference intervals, and expected value.2 This change in terminology acknowledged that what we consider normal must take into account variations related to age, gender, race, and ethnicity, which change over time as the demographic composition of society changes. A reference range sets the boundaries for any analyte (e.g., electrolyte, blood cell, protein, enzyme) that would likely be encountered in healthy subjects. This range would encompass the variability reflected in the larger, presumably healthy population.
Reference ranges vary from laboratory to laboratory for various reasons, including differences in measurement techniques, the populations of healthy individuals used to establish the reference intervals, or analytic imprecision when the intervals were constructed. Most differences in reference ranges are relatively small, with reasonably close agreement between most laboratories.2 The reference ranges and critical values given in this chapter are from a single institution, and they serve as representative examples. RTs must become familiar with the reference ranges used at their institutions.
Critical Test Value
In this chapter, critical values are listed along with common pathophysiologic states with which they commonly occur. Not all clinical analytes have an associated critical value. For some tests, there is no general agreement on what a critical value would be. Others have only a one-sided value that exists below or above a critical threshold; this is true particularly for substances that do not normally appear in the blood. For example, certain enzymes and proteins are released only after extensive cellular damage following injury (see later section on enzyme tests). Under normal circumstances, these proteins or enzymes may be virtually undetectable in the serum or plasma.
Complete Blood Count
The complete blood count (CBC) provides a detailed description of the number of circulating white blood cells (WBCs), called leukocytes; red blood cells (RBCs), called erythrocytes; and platelets, called thrombocytes. The WBC count is made up of five different types of cells and is reported under the differential. RBCs are evaluated for size and hemoglobin content. The platelets are evaluated for number present. Table 16-1 lists the normal CBC results for adults.
TABLE 16-1
Reference Range Values for Complete Blood Count in an Adult
| Test | Reference Range |
| Red blood cell count | |
| Men | 4.4-5.9 × 106/mcl |
| Women | 3.8-5.2 × 106/mcl |
| Hemoglobin | |
| Men | 13.3-17.7 g/dl |
| Women | 11.7-15.7 g/dl |
| Hematocrit | |
| Men | 40%-52% |
| Women | 35%-47% |
| White blood cell count | 3.9-11.7 × 103/mcL |
| White blood cell differential | |
| Segmented neutrophils | 40%-75% |
| Bands | 0%-6% |
| Eosinophils | 0%-6% |
| Basophils | 0%-1% |
| Lymphocytes | 20%-45% |
| Monocytes | 2%-10% |
| Platelet count | 150-400 × 103/mcL |
Values for reference ranges and critical test results are from the University of California San Francisco Moffit-Long Hospital and San Francisco General Hospital. http://pathology.ucsf.edu/labmanual/mftlng-mtzn/test/test-index.html and http://pathology.ucsf.edu/sfghlab/test/ReferenceRanges.html. Accessed January 1, 2011.
White Blood Cell Count
White Blood Cell Count Differential
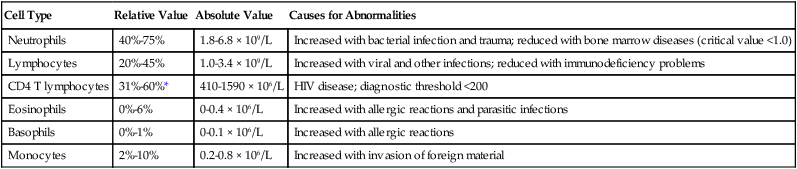
The differential of the WBC count determines the exact number of each type of WBC present in the circulating blood (Table 16-2). Most circulating WBCs are either neutrophils or lymphocytes. Because leukocytosis usually results from only one of the five cell types responding to a problem, significant elevation of the WBC count (>15 × 103/mcl) occurs only when either neutrophils or lymphocytes are responding to an abnormality. Because basophils, eosinophils, and monocytes make up such a small proportion of the circulating WBCs, they are not likely to cause a major increase in the WBC count when responding to disease.
TABLE 16-2
Reference Range Values for White Blood Cell Count Differential and Common Causes for Abnormalities
| Cell Type | Relative Value | Absolute Value | Causes for Abnormalities |
| Neutrophils | 40%-75% | 1.8-6.8 × 109/L | Increased with bacterial infection and trauma; reduced with bone marrow diseases (critical value <1.0) |
| Lymphocytes | 20%-45% | 1.0-3.4 × 109/L | Increased with viral and other infections; reduced with immunodeficiency problems |
| CD4 T lymphocytes | 31%-60%* | 410-1590 × 106/L | HIV disease; diagnostic threshold <200 |
| Eosinophils | 0%-6% | 0-0.4 × 106/L | Increased with allergic reactions and parasitic infections |
| Basophils | 0%-1% | 0-0.1 × 106/L | Increased with allergic reactions |
| Monocytes | 2%-10% | 0.2-0.8 × 106/L | Increased with invasion of foreign material |
Values for reference ranges and critical test results are from the University of California San Francisco Moffit-Long Hospital and San Francisco General Hospital. http://pathology.ucsf.edu/labmanual/mftlng-mtzn/test/test-index.html and http://pathology.ucsf.edu/sfghlab/test/ReferenceRanges.html. Accessed January 1, 2011.
Electrolyte Tests
Basic Concepts for Understanding Electrolyte Balance
Basic Chemistry Panel
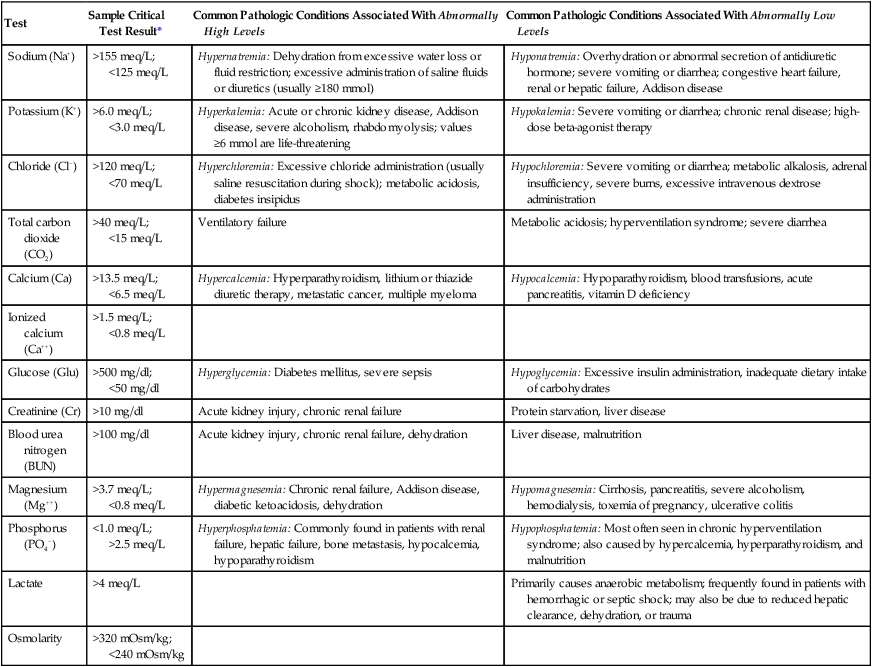
The basic chemistry panel (BCP) or basic metabolic panel includes the predominant electrolytes sodium (Na+), potassium (K+), chloride (Cl−), and total carbon dioxide/bicarbonate (CO2) and glucose. Because the body’s electrolyte balance is controlled by the kidneys, excretion of renal-mediated waste products is included in the panel: creatinine and blood urea nitrogen. A more comprehensive metabolic panel would include other important electrolytes, such as magnesium (Mg++), phosphorus (PO4−), and calcium (Ca++). Each electrolyte plays a crucial role in maintaining normal cellular function. Specific information on the reference range and physiologic significance of each electrolyte and waste product can be found in Table 16-3. Information regarding the terminology used to describe abnormal test values for electrolytes, diseases associated with these disturbances, and sample critical test results are provided in Table 16-4.
TABLE 16-3
| Test | Reference Range* | Physiologic Importance |
| Sodium (Na+) | 136-145 meq/L | Primary extracellular cation; crucial for maintaining fluid balance and nerve impulse conduction |
| Potassium (K+) | 3.5-5.0 meq/L | Primary intracellular cation; crucial for maintaining normal heart and kidney function and acid-base balance |
| Chloride (Cl−) | 98-106 meq/L | Primary extracellular anion; crucial for maintaining serum osmolarity and acid-base balance |
| Total carbon dioxide (CO2) | 22-29 meq/L | Primary metabolic end product of aerobic metabolism; crucial for maintaining acid-base balance |
| Calcium (Ca) | 4.5-5.25 meq/L | Most abundant mineral in the body; essential for bone strength, muscular contraction, nerve impulse conduction, and coagulation |
| Ionized calcium (Ca++) | 2.2-2.7 meq/L | The approximately 50% of calcium not bound to circulating proteins; represents the biologically active portion |
| Glucose (Glu) | 70-139 mg/dl | Primary cellular energy source |
| Creatinine (Cr) | 0.7-1.3 mg/dl | Waste product from muscle catabolism excreted by the kidneys; one of the key markers of kidney function because it provides a gross estimation of glomerular filtration rate |
| Blood urea nitrogen (BUN) | 8-23 mg/dl | Waste product from metabolism of amino acids; a key marker of kidney function |
| Magnesium (Mg++) | 1.7-2.1 meq/L | Essential for regulation of most biochemical processes; important for: normal muscle and neuronal functioning, regulating heart rate and blood pressure, glucose levels, bone strength, and immune function |
| Phosphorus (PO4−) | 1.2-2.3 meq/L | Main intracellular anion (phosphate), exists as phosphorus in serum; combined with calcium in teeth and bones; serum levels inversely related to serum calcium |
| Lactate | 0.7-2.1 meq/L | End product of glucose metabolism under anaerobic conditions; clinically significant levels coincide with regional or systemic tissue hypoxia |
| Osmolarity | 275-295 mOsm/kg | Tonicity or ability to attract water molecules; indicates overall ionic concentration in the serum |
Values for reference ranges and critical test results are from the University of California San Francisco Moffit-Long Hospital and San Francisco General Hospital. http://pathology.ucsf.edu/labmanual/mftlng-mtzn/test/test-index.html and http://pathology.ucsf.edu/sfghlab/test/ReferenceRanges.html. Accessed January 1, 2011.
*Reference ranges vary among clinical laboratories. See text for explanation.
TABLE 16-4
| Test | Sample Critical Test Result* | Common Pathologic Conditions Associated With Abnormally High Levels | Common Pathologic Conditions Associated With Abnormally Low Levels |
| Sodium (Na+) | >155 meq/L; <125 meq/L | Hypernatremia: Dehydration from excessive water loss or fluid restriction; excessive administration of saline fluids or diuretics (usually ≥180 mmol) | Hyponatremia: Overhydration or abnormal secretion of antidiuretic hormone; severe vomiting or diarrhea; congestive heart failure, renal or hepatic failure, Addison disease |
| Potassium (K+) | >6.0 meq/L; <3.0 meq/L | Hyperkalemia: Acute or chronic kidney disease, Addison disease, severe alcoholism, rhabdomyolysis; values ≥6 mmol are life-threatening | Hypokalemia: Severe vomiting or diarrhea; chronic renal disease; high-dose beta-agonist therapy |
| Chloride (Cl−) | >120 meq/L; <70 meq/L | Hyperchloremia: Excessive chloride administration (usually saline resuscitation during shock); metabolic acidosis, diabetes insipidus | Hypochloremia: Severe vomiting or diarrhea; metabolic alkalosis, adrenal insufficiency, severe burns, excessive intravenous dextrose administration |
| Total carbon dioxide (CO2) | >40 meq/L; <15 meq/L | Ventilatory failure | Metabolic acidosis; hyperventilation syndrome; severe diarrhea |
| Calcium (Ca) | >13.5 meq/L; <6.5 meq/L | Hypercalcemia: Hyperparathyroidism, lithium or thiazide diuretic therapy, metastatic cancer, multiple myeloma | Hypocalcemia: Hypoparathyroidism, blood transfusions, acute pancreatitis, vitamin D deficiency |
| Ionized calcium (Ca++) | >1.5 meq/L; <0.8 meq/L | ||
| Glucose (Glu) | >500 mg/dl; <50 mg/dl | Hyperglycemia: Diabetes mellitus, severe sepsis | Hypoglycemia: Excessive insulin administration, inadequate dietary intake of carbohydrates |
| Creatinine (Cr) | >10 mg/dl | Acute kidney injury, chronic renal failure | Protein starvation, liver disease |
| Blood urea nitrogen (BUN) | >100 mg/dl | Acute kidney injury, chronic renal failure, dehydration | Liver disease, malnutrition |
| Magnesium (Mg++) | >3.7 meq/L; <0.8 meq/L | Hypermagnesemia: Chronic renal failure, Addison disease, diabetic ketoacidosis, dehydration | Hypomagnesemia: Cirrhosis, pancreatitis, severe alcoholism, hemodialysis, toxemia of pregnancy, ulcerative colitis |
| Phosphorus (PO4−) | <1.0 meq/L; >2.5 meq/L | Hyperphosphatemia: Commonly found in patients with renal failure, hepatic failure, bone metastasis, hypocalcemia, hypoparathyroidism | Hypophosphatemia: Most often seen in chronic hyperventilation syndrome; also caused by hypercalcemia, hyperparathyroidism, and malnutrition |
| Lactate | >4 meq/L | Primarily causes anaerobic metabolism; frequently found in patients with hemorrhagic or septic shock; may also be due to reduced hepatic clearance, dehydration, or trauma | |
| Osmolarity | >320 mOsm/kg; <240 mOsm/kg |
Values for reference ranges and critical test results are from the University of California San Francisco Moffit-Long Hospital and San Francisco General Hospital. http://pathology.ucsf.edu/labmanual/mftlng-mtzn/test/test-index.html and http://pathology.ucsf.edu/sfghlab/test/ReferenceRanges.html. Accessed January 1, 2011.
*Reference ranges and critical test results vary among clinical laboratories. See text for explanation.
Glucose
In critically ill patients, insulin resistance and hyperglycemia are common. This condition is associated with a higher incidence of multiorgan failure and increased mortality. The condition can be ameliorated with a regimen of intensive insulin therapy to keep blood glucose levels at approximately 110 mg/dl.3 For intensive insulin therapy to be effective, blood glucose levels must be monitored frequently with a bedside (point-of-care) measuring device to facilitate rapid titration of insulin.
Anion Gap
As discussed in Chapter 13, metabolic acidosis is caused by either the addition of nonvolatile acids or a primary loss of HCO3−. The anion gap provides a quick method for determining whether a decrease in HCO3− is caused by a disruption of normal anion balance or the presence of an abnormal acid anion. A balance normally exists between cations and anions in the serum. The normal anion gap occurs because sulfate, phosphate, and organic anions such as lactate are not routinely measured, whereas most cations are measured. The anion gap is calculated by adding the CO2 and Cl− values and then subtracting this total from the serum Na+. The normal anion gap is approximately 8 to 14 meq/L, and gap acidosis usually coincides with an anion gap of 16 mmol/L or greater. However, serum proteins are an important determinant of the anion gap. Hypoalbuminemia (decreased serum albumin) is a common finding in critically ill patients and significantly reduces the anion gap. As a rule, for every 1-g reduction in serum albumin below 4 g/dl, the anion gap is corrected upward by 3 meq/L.
Enzyme Tests
Liver Function Tests
Cardiac Enzyme and Protein Tests
The most common CPK enzyme test is CPK-2 (CPK-MB), which is released from the heart after myocardial infarction. Peak levels occur 12 to 24 hours after injury. Serial CPK-2 measurements are monitored in patients with suspected myocardial infarction and patients with cardiac contusion from chest trauma, open heart surgery, or myocarditis. Troponin is a complex protein that plays an important role in the regulation of skeletal and cardiac muscle contractility. The protein fragment troponin I is associated with cardiac muscle damage. Similar to CPK-2, troponin I levels peak 12 to 16 hours after myocardial infarction. Reference values for these enzyme tests are presented in Table 16-5.
TABLE 16-5
Liver Function and Other Enzymatic Tests
| Test | Reference Range | Sample Critical Test Result* |
| Total bilirubin (T Bil) | 0.1-1.1 mg/dl | ≥15 mg/dl |
| Alanine aminotransferase (ALT) | 7-56 U/L | † |
| Aspartate aminotransferase (AST) | 10-50 U/L | † |
| Alkaline phosphatase (ALK) | 40-125 U/L | † |
| Total protein (TP) | 15-45 mg/dl | † |
| Albumin (ALB) | 3.3-5.2 g/dl | † |
| Ammonia | 18-54 µmol/L | ≥500 mcg/dl |
| Amylase (serum) | 20-110 U/L | >330 U/L |
| Lipase | 10-140 U/L | >420 U/L |
| Creatinine phosphokinase (CPK) | 20-220 U/L | >10,000 U/L |
| Troponin I | 0 ng/ml | >0.05 ng/ml |
| B-type natriuretic peptide | <100 pg/ml | † |
| Lactate dehydrogenase (LDH) | 110-220 U/L | >880 (moderate); >8800 (severe) |
Values for reference ranges and critical test results are from the University of California San Francisco Moffit-Long Hospital and San Francisco General Hospital. http://pathology.ucsf.edu/labmanual/mftlng-mtzn/test/test-index.html and http://pathology.ucsf.edu/sfghlab/test/ReferenceRanges.html. Accessed January 1, 2011.
*Critical test results vary among clinical laboratories based on instrumentation and calibration procedures. Not all tests have an associated critical result that can be reported.
B-type natriuretic peptide (BNP) is a substance secreted by the heart in response to increased stretch in the cardiac muscle. The BNP test primarily is used to evaluate patients for heart failure, in particular, patients who present to the emergency department with dyspnea and pulmonary edema.4 Generally, values greater than 300 pg/ml indicate mild heart failure, whereas values greater than 600 pg/ml are found in patients with moderate heart failure, and values greater than 900 pg/ml are found in patients with severe heart failure.4 Other conditions such as acute respiratory distress syndrome and severe sepsis also cause increased stretch of the cardiac muscle. In these cases, the BNP levels typically are lower (300 to 500 pg/ml) than what is found commonly in critically ill patients with primary heart failure (700 to −1200 pg/ml).5
Coagulation Studies
Because PT test results (Table 16-6) depend on manufactured animal tissue factors, which have unavoidable variability, PT is accompanied by an additional measurement known as the international normalized ratio (INR). The INR expresses PT relative to an established sample value. The reference range for INR is 0.9 to 1.3. INR values of approximately 5.0 indicate a high likelihood for bleeding. Values of 0.5 are associated with a tendency toward increased clotting.
TABLE 16-6
| Test | Reference Range | Critical Test Result |
| Prothrombin time (PT) | 12-15 sec | >30 sec |
| Partial thromboplastin time (PTT) | 25-39 sec | >50 sec |
| International normalized ratio (INR) | 0.8-1.2 | >5 sec |
| Fibrin D-dimer | <200 ng/ml | * |
| Platelet count | 150,000-400,000/mm3 | <25,000/mm3 |
Values for reference ranges and critical test results are from the University of California San Francisco Moffit-Long Hospital and San Francisco General Hospital. http://pathology.ucsf.edu/labmanual/mftlng-mtzn/test/test-index.html and http://pathology.ucsf.edu/sfghlab/test/ReferenceRanges.html. Accessed January 1, 2011.
Protein C has an integral role in the regulation of coagulation. In its activated state (activated protein C), it inhibits coagulation and promotes the degradation of clots. Protein C levels are greatly reduced in patients with severe sepsis and acute respiratory distress syndrome. Low protein C promotes abnormal clot formation and damages blood vessels in the microcirculation throughout the body (disseminated intravascular coagulation). Significant deficiencies in protein C levels (<40% of normal) are associated with increased risk of death in patients with severe sepsis.6 Protein C levels are measured in patients with severe sepsis to assess the appropriateness of therapy with pharmacologic preparations of activated protein C.
Infection Monitoring
Procalcitonin (PCT) is an inactive protein of the hormone calcitonin that is released in response to bacterial infections. PCT levels are directly related with the severity of infection. Because PCT does not increase appreciably in response to viral infections, it is a unique marker for bacterial infections. PCT levels typically increase within 12 hours of infection and promptly decrease once the infection is controlled with appropriate antibiotic therapy. PCT inceasingly is being used to titrate antibiotic therapy. In general, a cutoff valure between 0.25-0.50 mcg/L is used to initiate antibiotic therapy. Measurements of PCT are repeated every 1-2 days to evaluate antibiotic therapy. When PCT decreases by approximately 90% from peak values antibiotic therapy is usually terminated.6
Clinical Application of Laboratory Data
Electrolyte Disorders
Primary electrolyte disorders causing respiratory muscle weakness include low serum levels of calcium, magnesium, and phosphate.7,8 A patient with hypoglycemia often complains of weakness, so that weaning from a ventilator is unlikely to be successful in the patient with hypoglycemia. In addition, abnormally high serum potassium levels, or hyperkalemia (>8.0 mmol/L), and abnormally low serum potassium, or hypokalemia (<2.0 mmol/L), or phosphorus, or hypophosphatemia (<1.0 mg /dl), can lead to respiratory muscle paralysis. In addition, severe hyperkalemia (>6.0 mmol/L) increases the likelihood of cardiac arrhythmias. Severe hypocalcemia (<6.5 mmol/L)sometimes leads to laryngeal stridor and dyspnea.
Electrolyte disorders and other toxins in the bloodstream can depress neurologic function. Pulmonary function is profoundly affected because decreased mental functioning may depress respiratory drive, prevent patients from cooperating with therapy, and suppress the ability of patients to protect their airway and clear secretions by depressing the cough mechanism. In severe cases, hypernatremia is a major cause of central nervous system depression, which can lead to lethargy, coma, and respiratory arrest.9 In patients with severe liver disease, elevated ammonia levels also depress neurologic function.