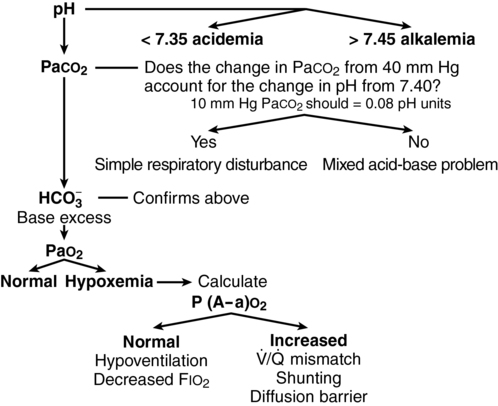
Interpretation of arterial blood gases
Ventilation
Normal PaCO2 values range between 36 and 44 mm Hg. Production of CO2 is relatively constant in most clinical settings, so the elimination of CO2 is proportional to alveolar ventilation. As diagrammed in Figure 23-1, a PaCO2 below 36 mm Hg implies hyperventilation and a PaCO2 above 44 mm Hg implies hypoventilation, unless these situations occur as respiratory compensation for metabolic acid-base abnormalities.
Oxygenation
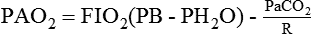
Simplified for clinical use, this equation reads as follows:
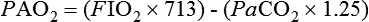
If the P(A−a)O2 gradient is normal, (i.e., ≤ 20), then the hypoxemia must be the result of hypoventilation or decreased FIO2 concentration. If the P(A−a)O2 gradient is increased, then the hypoxemia is the results of a ![]() /
/![]() mismatch, shunting, or, rarely, a diffusion barrier (see Figure 23-1).
mismatch, shunting, or, rarely, a diffusion barrier (see Figure 23-1).

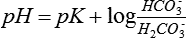
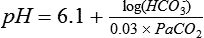
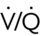 , ventilation/perfusion.
, ventilation/perfusion.