Chapter 79 Intermediate Uveitis
Introduction
Intermediate uveitis is defined anatomically, according to the Standardized Uveitis Nomenclature working group, as intraocular inflammation in which the primary site is the vitreous, but it commonly involves the peripheral retina as well.1 The origin of the inflammatory cells includes the ciliary body pars plana, the peripheral retinal vessels, and the peripheral choroid. Previously used terminology for this entity includes pars planitis, peripheral uveitis, peripheral cyclitis, hyalitis, and vitritis. The term “pars planitis” now specifically refers to a subcategory of idiopathic intermediate uveitis characterized by the presence of snowbanks and snowballs, which will be described in more detail below.
Epidemiology and demographics
Because the currently used nomenclature for uveitis was agreed upon in 2005, current prevalence and incidence figures for this anatomic category of uveitis are sparse. Although various groups have reported the frequency of intermediate uveitis in their uveitis practices, ranging from 4 to 15.4%, these figures are subject to referral bias since the reports originate from tertiary care referral clinics.2–6 In pediatric uveitis, it accounts for up to 25% of cases.7,8 The overall population prevalence of different types of uveitis based on anatomic location including intermediate uveitis has been investigated in several population studies since 2005. Gritz and Wong found a prevalence ratio for intermediate uveitis of 4.0 per 100 000 persons in a cross-sectional retrospective study using a database of individuals in northern California receiving care through a single large health maintenance organization.9 The incidence was 1.5 per 100 000 person-years. These figures were reported with the stipulation that the prevalence may have been underestimated because a significant proportion of included patients did not have dilated fundus examinations, and the anatomic uveitis location was, therefore, unknown. Another study utilizing the Veterans Affairs database in the Pacific Northwest region found that the prevalence of intermediate uveitis was approximately 3.3 per 100 000 persons.10 The annual incidence of pars planitis in Olmstead County, Minnesota, was 2.08 per 100 000 persons.11
Although intermediate uveitis can occur at any age, it tends to occur in a younger age range. Average age of onset was 31 years (range 8–64 years) in one study,12 and 30 years (range 6–76 years) in another.13 There appears to be no gender predilection for this anatomic subtype of uveitis, although certain entities that can cause intermediate uveitis, such as sarcoidosis, have a strong female bias.14 In certain uveitis practices, such as that reported by Thorne and colleagues, intermediate uveitis appears to be slightly more common in women (66.4%).13 Racial predilection likely depends on the etiology, but there appears to be no race preference in pars planitis.
Presentation and clinical findings
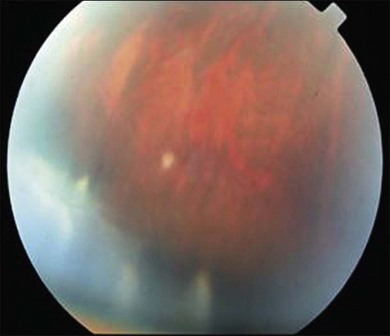
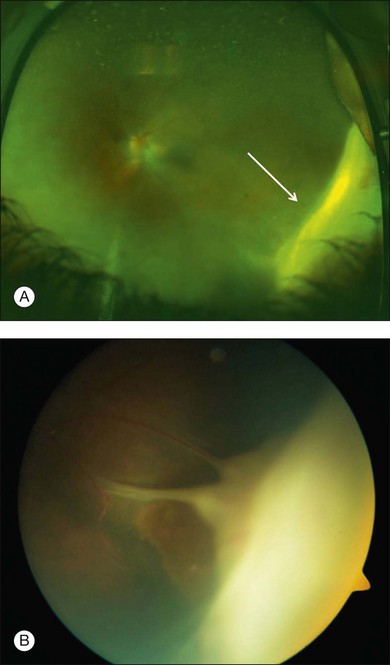
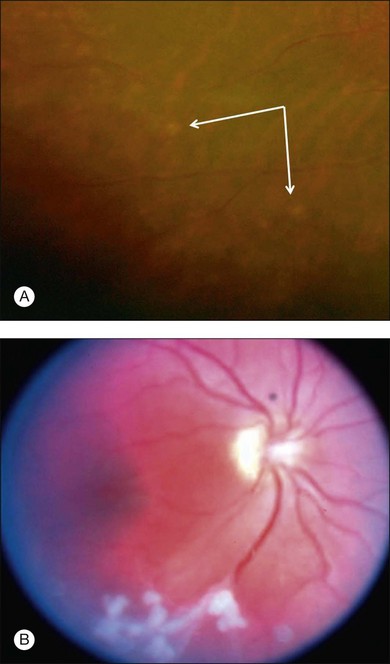
The most common symptoms of intermediate uveitis include blurry vision and floaters, whereas pain, redness, and photophobia are less common than in other types of uveitis. Although typically bilateral over time (74.5–80% bilateral),3,12,13,15 it is frequently asymmetric and may begin unilaterally. Findings on clinical examination at onset include anterior vitreous cells or diffuse vitreous haze, and, to a lesser extent, anterior chamber involvement. Vitreous haze is graded on a scale of 0–4+ based on the level of optic nerve and retinal vessel obscuration.16 Snowballs (see below for discussion on histopathology) are whitish inflammatory vitreous opacities in clusters frequently found in the inferior vitreous cavity (Fig. 79.1). Snowbanks are identified as whitish gray confluent preretinal membranes most commonly seen along the inferior pars plana, and peripheral retina (Fig. 79.2A). Over time, these may develop into organized fibrovascular membranes that are prone to vitreous hemorrhage and retinal detachment. The vascular component of this snowbank is continuous with the retinal vessels (Fig. 79.2B). Commonly, a peripheral vasculitis manifested by perivascular sheathing is seen in intermediate uveitis, and may indicate an increased probability of associated systemic disease.12 According to one study, periphlebitis in the setting of intermediate uveitis was associated with an increased rate of multiple sclerosis (MS) or optic neuritis.17 It is important to note that sarcoidosis can also present as an isolated periphlebitis or as granulomatous inflammation in the form of snowballs. Periphlebitis in sarcoidosis can present with a wide spectrum of appearances, from scant focal periphlebitis to exudative candle-wax drippings (taches de bougie) (Fig. 79.3). Yellowish peripheral punched-out chorioretinal lesions that might represent active or old choroidal granulomata are highly suspicious for sarcoidosis, although once these are present, the anatomic designation is panuveitis rather than intermediate uveitis. Obliterative peripheral vasculitis may result in peripheral neovascularization and resultant vitreous hemorrhage (Fig. 79.4). Cystoid macular edema (CME) is a common finding, occurring in over 40% of individuals at diagnosis of intermediate uveitis (Fig. 79.5).13 Clinically apparent optic nerve edema also occurs in a small percentage of patients with intermediate uveitis.
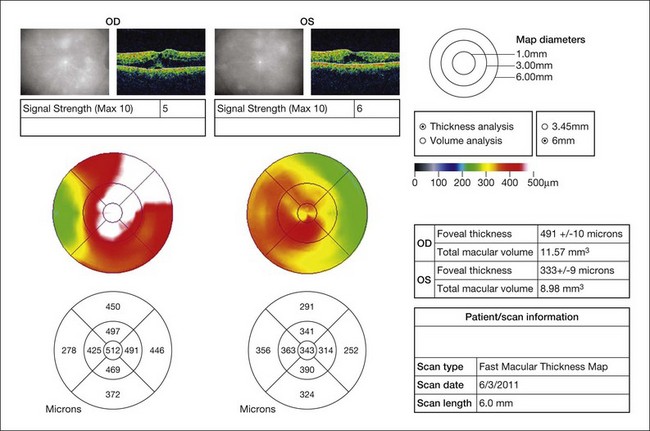
Fig. 79.5 Bilateral cystoid macular edema in a patient with otherwise quiescent intermediate uveitis.
Imaging
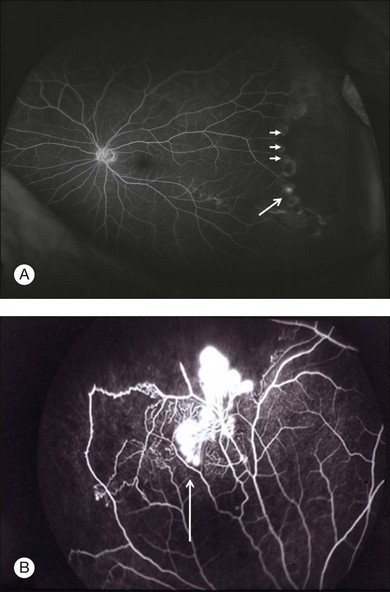
Although the majority of cases of intermediate uveitis are diagnosed by clinical examination, various imaging modalities give useful adjunctive information to guide diagnosis and management. For instance, in the setting of media opacity from cataract, ultrasound biomicroscopy (UBM) can be used to detect pars plana exudates. Identification of cyclitic membranes by UBM can be of great value in preoperative planning. B-scan ultrasonography can be used to diagnose and measure macular thickening with a great degree of accuracy, sensitivity, and specificity in the setting of media opacity or intolerance to fluorescein angiography and optical coherence tomography (OCT).18 Fluorescein angiography is useful to detect CME, vasculitis, peripheral nonperfusion, and peripheral neovascularization. OCT is important to identify and follow CME as a gauge of disease activity in intermediate uveitis.19 In some cases, concentric macular retinal thickening without frank CME, as determined by OCT, can be used to monitor response to treatment.20
Differential diagnosis and workup
The cause of intermediate uveitis can be determined by investigating the presence of systemic findings or historical clues. Although the vast majority of cases are idiopathic (nearly 70%),21 the most common known inflammatory etiologies include sarcoidosis and MS, whereas infectious causes can include syphilis, Lyme disease, and tuberculosis (Table 79.1). Pars planitis is, by definition, idiopathic intermediate uveitis characterized by the presence of snowbanks and snowballs. Thorne et al. found that 36% of their intermediate uveitis patients had pars planitis.13 In one study by Rodriguez et al., 22.2% of cases of intermediate uveitis at a referral center were due to sarcoidosis, whereas 8.0% were due to MS.21 One out of 112 cases at this center was due to Lyme disease. In two other studies, 14.8 and 16.2% had MS, respectively.17,22
HTLV-1, human T-lymphotropic virus-1; NHL, non-Hodgkin’s lymphoma; CME, cystoid macular edema.
Tests that warrant investigation on all patients with intermediate uveitis include rapid plasma reagin and fluorescent treponemal antibody absorption test to test for syphilis, chest X-ray, angiotensin-converting enzyme (ACE), and tuberculin skin test with anergy panel, to evaluate sarcoidosis and tuberculosis. If clinical suspicion is high for sarcoidosis in the setting of a negative ACE and chest X-ray, one should consider a gallium scan, pulmonary function tests, or a chest computed tomography scan. A careful review of systems should be performed to determine if there are risks associated with MS, including urinary retention, neurologic weakness or symptoms, or evidence of previous optic neuritis (Table 79.2).23 In a significant number of patients, as is the case in our experience, pars planitis can be the first manifestation of a central nervous system demyelinating process, so it is important to elicit the appropriate history and keep a low threshold for the diagnosis of MS. Because there is growing evidence that early treatment of MS with systemic interferon therapy may reduce long-term disability, it is important to consider this systemic association seriously upon initial presentation.24–26 Evaluation by a neurologist, magnetic resonance imaging (MRI) of the brain, and cerebrospinal fluid studies should be considered in new-onset intermediate uveitis, even without the above symptoms, given that early disease can by asymptomatic.
Table 79.2 Systemic symptoms and signs of multiple sclerosis by site
| Anatomic location | Symptoms | Signs |
|---|---|---|
| Cerebrum | Cognitive impairment | Deficits in attention, reasoning, and executive function; dementia (late) |
| Depression | Flat affect | |
| Hemisensory and motor | Upper motor neuron signs | |
| Optic nerve | Unilateral vision loss | rAPD, Uthoff phenomenon (exacerbation in hot temperatures), Pulfrich effect (difficulty judging path of oncoming cars) |
| Cerebellum | Tremor | Action tremor |
| Clumsiness and poor balance | Gait ataxia | |
| Brainstem | Diplopia, oscillopsia | INO, nystagmus |
| Vertigo | ||
| Impaired swallowing | ||
| Impaired speech | ||
| Spinal cord | Weakness | |
| Stiffness and painful spasms | Spasticity, Lhermitte’s sign (painful electric-shock sensation down spine upon neck flexion) | |
| Bladder dysfunction | ||
| Retention | ||
| Frequency, urgency | ||
| Other | Pain | |
| Fatigue | ||
| Temperature sensitivity and exercise intolerance |
rAPD, relative afferent papillary defect; INO, internuclear ophthalmoplegia.
(Modified from Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–17.)
If there is a history of exposure to deer or ticks, especially if the patient lives or travels in an endemic area, and there is an associated history of a target lesion, or systemic flu-like symptoms, then Borrelia burgdorferi antibody should be tested. Exposure to cats might prompt serum antibody testing for Bartonella.27 In the elderly population, intraocular lymphoma should be suspected and there should be a lower threshold to perform diagnostic vitrectomy, especially if there is limited response to corticosteroids.28,29 If intraocular lymphoma is highly suspected, a lumbar puncture for cytological evaluation of the cerebrospinal fluid and neuroimaging are adjunctive diagnostic tests to consider. Bilateral pars planitis has been found in patients who have human T-cell lymphotropic virus type 1 (HTLV-1) infection, which is endemic in Japan, the Caribbean islands, parts of Central Africa, and South America.30 The latter can be diagnosed with serological testing for antibodies against HTLV-1. Gastrointestinal disturbances associated with a pars planitis-like clinical presentation should prompt referral to a gastroenterologist since it has been reported in association with inflammatory bowel disease and Whipple’s disease.31,32 Other masquerade syndromes, such as intermediate uveitis secondary to tumor necrosis from retinoblastoma or uveal melanoma or even metastasis from other types of systemic cancer, must be evaluated in the appropriate clinical settings.33 Finally, mild vitritis and CME in the setting of recent cataract surgery should prompt consideration of pseudophakic CME. One should consider indolent endogenous or exogenous endophthalmitis as a possibility if there is a poor response or worsening with corticosteroids in the setting of the appropriate risk factors, such as immunocompromised state or recent intraocular surgery.
Histopathology and pathophysiology
On histopathologic examination, snowballs are isolated vitreous granulomas consisting of lymphocytes, macrophages, epithelioid cells, and multinucleated giant cells.34 Snowbanks consist of collapsed vitreous collagen, membranous fibroglial cells, blood vessels, and lymphocytes as well as hyperplastic pars plana nonpigmented epithelium.35 Periphlebitis is denoted by lymphocytic infiltration and cuffing of the peripheral retinal venules.34 Because the term “intermediate uveitis” is an anatomic designation, one should avoid assigning a single etiologic or pathophysiologic process to it given that various systemic diseases and infectious processes can result in inflammation in this anatomic location. The pathogenesis of the autoimmune causes of intermediate uveitis involves overlapping principles as well as some distinguishing characteristics, and will be discussed here for pars planitis and sarcoidosis.
Pars planitis
Although it is thought that noninfectious intermediate uveitis arises from an autoimmune response, the antigenic stimulus has not yet been clearly identified. Bora et al. described a novel 36 kDa nucleopore complex protein that was found to be six to eight times higher in the sera of 81% of active pars planitis patients compared with controls (P < 0.05).36 The exact function of this protein and its role in the pathogenesis of pars planitis are unknown, although there are indications that other nucleopore complex proteins may be involved in myeloid leukemogenesis and autoantibody formation.37,38 Wetzig et al. found that endstage idiopathic intermediate uveitis in a familial case of bilateral intermediate uveitis was characterized immunopathologically by a predominance of CD4+ T lymphocytes and overwhelming predominance of glial cells in the pars plana snowbank; the latter is a characteristic unique to this type of uveitis.39 It has been proposed that pars planitis represents a similar pathoetiological entity as MS, although with expression either isolated to the eye or starting in the eye.40 Indeed, patients with MS have increased circulating CD54+ lymphocytes and antibodies that recognize glial proteins.41 Raja et al.22 discovered that a human leukocyte antigen (HLA)-DR15 allele, which is known to be associated with MS, was significantly associated with pars planitis compared with controls (odds ratio, 2.86; P = 0.004). Although no specific autoantigen has been clearly determined, components of vitreous, in addition to the glial elements mentioned above, have been implicated. Circulating lymphocytes isolated from pars planitis patients appear to proliferate in response to type II collagen.35 Furthermore, Hultsch produced a clinical entity similar to pars planitis by giving owl monkeys multiple intravitreal injections of hyaluronic acid.42
Interleukin-8 (IL-8), a cytokine that is involved in neutrophil and T-lymphocyte recruitment, and soluble intercellular adhesion molecule 1 (sICAM-1), which is expressed at sites of inflammation to aid in leukocyte adhesion and migration, were elevated in the blood of patients with intermediate uveitis compared to controls. Furthermore, elevated sICAM-1 and IL-8 were associated with development of systemic disease. Elevated IL-8 was associated with signs of inflammatory activity such as periphlebitis and the presence of vitreous exudates. Rather than explaining the pathogenesis of this ocular condition, these markers may be indicators of systemic disease or activity, even in the absence of systemic symptoms.43,44
Sarcoidosis
Sarcoidosis is also thought to be a CD4 T-lymphocyte-mediated process in the eye and in other actively affected tissues. Efforts to elucidate an infectious cause for sarcoidosis have met with little success. Patients with active sarcoidosis have a predominantly Th1 cytokine profile with elevated levels of IL-2 and interferon-gamma, and diminished levels of IL-4. Despite the predominance of CD4+ T cells in ocular tissues affected by the disease, elevated peripheral levels of immunoglobulin, B-cell hyperactivity in the peripheral blood, and a loss of delayed-type hypersensitivity to the tuberculin skin test (anergy) argues for an unusual partitioning of the immune response.41,45 We have found that approximately 50% of patients with sarcoid-associated uveitis are anergic to delayed-type hypersensitivity testing.46 The latter observations underline the importance of an anergy panel to accompany a tuberculin skin test in the initial workup of intermediate uveitis. HLA associations in sarcoidosis also differ from pars planitis and have not been clearly elucidated. In one study by Rossman et al., ocular sarcoidosis was associated with the HLA-DRB1*0401 allele in both blacks and whites.47
Treatment
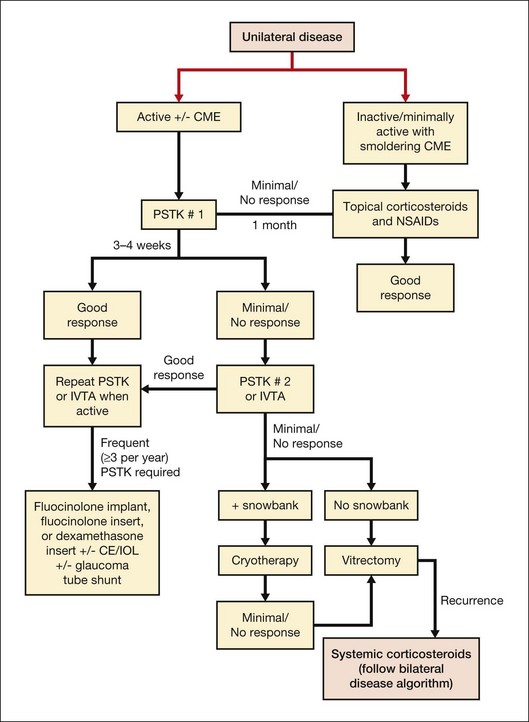
Unilateral disease
Once an infectious cause has been ruled out, the primary treatment of choice is corticosteroids. If an underlying systemic condition is suspected, then systemic treatment with appropriate referral to a rheumatologist or infectious disease specialist is warranted. For unilateral noninfectious intermediate uveitis in the absence of systemic disease, periocular corticosteroids are initiated (Fig. 79.6), although some clinicians utilize systemic steroids. In our practice, posterior subtenon Kenalog is given at 40 mg/mL, 1 mL total. If there is little to no response in 3–4 weeks, a second injection can be given, or alternatively, if the patient is pseudophakic and does not have glaucoma, an intravitreal injection of preservative-free triamcinolone acetonide can be offered. If smoldering uveitic CME is present without other evidence of active inflammatory disease, a trial of topical corticosteroids in combination with nonsteroidal anti-inflammatory drops can be applied prior to considering either periocular or intravitreal corticosteroids.48 Alternatively, bevacizumab has been investigated for its efficacy in treating uveitic CME. In a study comparing intravitreal bevacizumab with intravitreal triamcinolone acetonide for uveitic CME, after removing the effect on cataract formation the triamcinolone group appeared to have significantly better visual acuity outcomes. Triamcinolone was also more effective in reducing macular thickness than bevacizumab.49
If the patient has benefited from periocular or intraocular corticosteroid, but has documented recurrence when the corticosteroids have waned, then a corticosteroid intravitreal implant can be considered, especially if the patient is already pseudophakic (Fig. 79.7). If the patient is phakic, combined lens extraction, intraocular lens implantation, and insertion of a fluocinolone acetonide sustained drug delivery system (Retisert) can be performed.50 If the patient has known glaucoma, then combined glaucoma tube shunt and fluocinolone implant surgery should be considered and has been found to be effective.51 In young patients, combined surgery should proceed only after achieving relative quiescence given the robust inflammatory response after incisional surgery and careful consideration of the loss of accommodation that will occur after removing the crystalline lens. Alternatively, a dexamethasone intravitreal insert (Ozurdex)52 can be applied to circumvent the need for incisional surgery. If there is little to no response after two corticosteroid injections (either posterior subtenon or intravitreal), then therapeutic/diagnostic vitrectomy can be considered. If a snowbank is present, cryotherapy can be applied.53 Peripheral laser photocoagulation has also been used to treat pars planitis, resulting in a decrease in the amount of corticosteroid needed and in decreased vitritis, although the rate of epiretinal membrane increased.54 If peripheral neovascularization occurs, then peripheral laser is indicated. Finally, oral prednisone can be used, applying the algorithm for bilateral disease below.
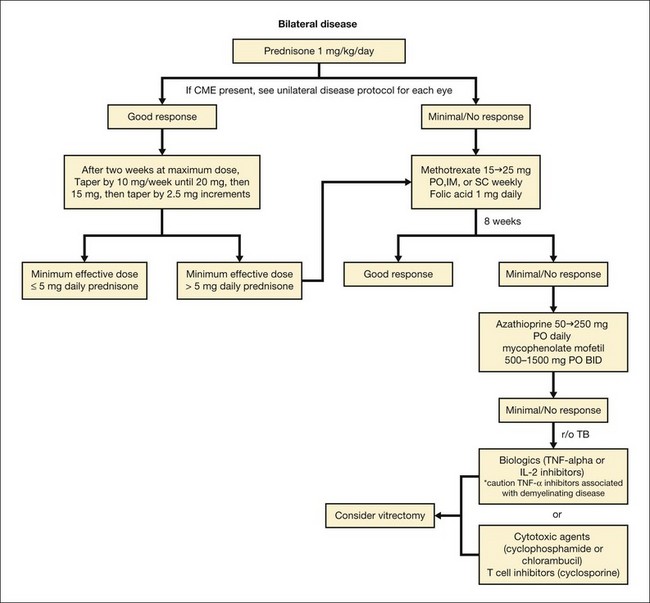
Bilateral disease
Bilateral disease usually warrants treatment with systemic corticosteroids, starting at 1 mg/kg/day tapered to the lowest levels required to achieve quiescence over a 2-month period. If greater than 10 mg of oral prednisone is required for longer than 4 months, or when treating children, in whom long-term corticosteroids can cause growth retardation, steroid-sparing immunosuppressives should be initiated (Fig. 79.8). If one is not aware of the systemic risks or does not choose to monitor appropriately for systemic risks referral to or co-management with an internist or rheumatologist is highly recommended.
We have had good success treating sarcoidosis-associated intermediate and panuveitis with methotrexate starting at 12.5–15 mg PO weekly and escalating to 25 mg PO weekly, along with folic acid, 1 mg/day. Dosage is based on treatment response and tolerability. Because of its relative safety in children, methotrexate is often used as a first-line agent with a short course of bridging corticosteroids. It should be noted that methotrexate should not be used in pregnancy given its teratogenicity and fetal abortive effects. Common side-effects of methotrexate include nausea, vomiting, and oral stomatitis. If gastrointestinal side-effects prevent oral dosing, intramuscular or subcutaneous injections can be administered instead. Hepatotoxicity, cytopenia, and interstitial pneumonitis can also occur with methotrexate use. Liver function tests and complete blood counts are evaluated before initiation of therapy and should be monitored every 8–12 weeks thereafter. The dose of methotrexate should be reduced if the aspartate aminotransferase or alanine aminotransferase level is more than twice normal on two separate occasions, but any elevation may warrant at least dose reduction. In the first study on the use of methotrexate in ocular sarcoidosis, 100% of 11 patients decreased their dosage of corticosteroids, 86% successfully discontinued prednisone, and 90% had preserved or improved visual acuity, although all patients in this study had panuveitis rather than isolated intermediate uveitis.55In a multicentered retrospective study in which methotrexate was used to treat noninfectious uveitis patients of all anatomic subtypes, methotrexate was found to be moderately effective in controlling inflammation, although the side-effect profile was beneficial.56 Information regarding the effectiveness of methotrexate on the intermediate uveitis subgroup in the latter study was difficult to interpret given that 76.2% were inactive at the time of starting methotrexate.
In patients unable to tolerate methotrexate or who fail to achieve treatment success on this agent, other antimetabolites, including azathioprine or mycophenolate mofetil, can be initiated. Galor et al. compared the three antimetabolites in the treatment of noninfectious ocular inflammation in a retrospective cohort study. They suggested that mycophenolate mofetil controlled inflammation more rapidly than methotrexate, when “control of inflammation” was defined by quiescent inflammation on ≤10 mg oral prednisone.57 There were several caveats in the latter study, however, including significantly younger age in the methotrexate group, and the use of prior immunosuppressive drug therapy in the azathioprine and mycophenolate groups. If antimetabolites are minimally effective, a course of cytotoxic agents (cyclophosphamide or chlorambucil) or T-cell inhibitors (cyclosporine) can be given with close attention to toxic side-effects. Alternatively, the tumor necrosis alpha (TNF-α) inhibitors adalimumab or infliximab can be initiated in combination with methotrexate,58 although it is important to note that there are a number of reports of these biologics causing demyelinating disease such as MS and progressive multifocal leukoencephalopathy.59 Because they have also been implicated in the reactivation of latent tuberculosis,60 an updated tuberculin skin test should be performed prior to initiating these drugs. If the patient is anergic to the tuberculin skin test due to sarcoidosis, a quantiferon tuberculosis test can be performed.61
The biologics daclizumab and infliximab have both been used to treat ocular sarcoidosis with success.62,63 Etanercept is a TNF-α inhibitor that is no longer used as often in the treatment of uveitis since it has been associated with increased inflammation or lack of ocular inflammation control. In one study of 22 uveitis patients who received anti-TNF-α therapy, infliximab was more effective than etanercept in controlling ocular inflammation.64 Referral to a rheumatologist is typically sought once the patient requires immunosuppressive medication stronger than the antimetabolites.
Recently, the results of the Multicenter Uveitis Steroid Treatment trial were published, comparing systemic anti-inflammatory therapy with the fluocinolone acetonide implant for intermediate, posterior, and panuveitis.65 The study demonstrated no significant difference in visual acuity outcome at 24 months, but improved control of uveitis activity, vitreous haze, and a trend towards better control of CME in the implant group by 24 months. The rate of ocular complications, such as elevated intraocular pressure requiring glaucoma surgery or cataracts, was significantly higher in the implant group, as expected, whereas adverse systemic events, while infrequent in both groups, were slightly lower in the implant group, although the risk of hospitalization did not differ between the two groups.65
Diagnostic and therapeutic vitrectomy
Diagnostic and therapeutic vitrectomy is considered in patients who are not responsive to the above treatment regimens or if intraocular lymphoma is highly suspected. In a prospective randomized study, Tranos et al. demonstrated better visual acuity and improved fluorescein angiographic CME characteristics after vitrectomy, compared with standard medical therapy consisting of systemic corticosteroids and immunosuppressives. However, this study was underpowered to achieve statistical significance.66 A number of other studies also appear to show a moderate level of success in treating CME with pars plana vitrectomy, which is thought to be effective by debulking soluble and cellular vitreous inflammatory mediators, and by allowing easier access to the vitreous cavity of aqueous inhibitory molecules such as transforming growth factor-ß and alpha-melanocyte stimulating hormone.67–69 We perform a diagnostic vitrectomy if an infectious source such as herpetic viral infection or toxoplasmosis is suspected but cannot be confirmed using other methods, or if intraocular lymphoma is suspected, in which case the specimen is sent for cytopathological evaluation and/or flow cytometry. If intraocular lymphoma is diagnosed, a neurologic workup and MRI may be necessary to determine if there is central nervous system disease. If isolated to the eye or in the setting of central nervous system lymphoma with a recurrence restricted to the eye, orbital irradiation or intravitreal rituximab alone or in combination with methotrexate can be administered with success.70–72
Clinical course and complications
Clinical course
Patients with inflammatory intermediate uveitis often have a favorable visual acuity outcome. In one study of patients with pars planitis, mean visual acuity after 10 years of follow-up was 20/30, 75% maintained a visual acuity of 20/40 or better, and one-third maintained normal visual acuity without treatment.11 In a study by Kalinina Ayuso and colleagues, younger age at onset decreased the visual prognosis and increased the rates of complications;73 however, another study showed higher rates of remission in patients who presented at a younger age. Mean time to remission in the latter study, which occurred at a rate of 34%, was 8.6 years.12
Complications
Causes of vision loss in intermediate uveitis include CME, uveitic glaucoma, retinal detachment, vitreous hemorrhage, cataracts, and epiretinal membranes. Band keratopathy, especially prominent in children with chronic intermediate uveitis, can also cause vision loss. Chronic CME accounts for most cases of permanent visual loss, and occurred at a rate of 41.2% over 15 years according to one large retrospective cohort study, and at a rate of 45.7% in a separate study.11,13 Concentric retinal macular thickening can also be seen in patients with intermediate uveitis, although it does not necessarily need to be treated.20 The 15-year rates of cataract and epiretinal membranes were 34.2% and 44.4%, respectively, in the report by Donaldson et al.11 An increased rate of CME appears to be associated with cigarette smoking. In one large case-control study, the odds ratio of a smoker (versus a nonsmoker) having intermediate uveitis with CME was 8.4 compared with an odds ratio of 1.5 in intermediate uveitis without CME.4 In the report from Thorne et al., the odds ratio of having CME at presentation was 3.9 compared with a reference group of patients who had never smoked.13
In the late stages of intermediate uveitis peripheral neovascular and fibrous membranes can extend on to the ciliary body to form cyclitic and/or retrolenticular membranes, resulting in ciliary body detachment and hypotony. Prevention of progression to this stage is the mainstay of management. Once this develops, surgical intervention can be attempted to remove the cyclitic membrane and reverse hypotony to prevent phthisis, but success in improving visual outcome is poor.74 Retinal detachment in the setting of active inflammatory disease should be repaired after relative quiescence is achieved, if possible. Combined surgery with fluocinolone implant and placement of silicone oil can be considered given the lipophilic nature of fluocinolone and its ability to be dispersed throughout the silicone oil.75
1 Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516.
2 Bonfioli AA, Damico FM, Curi AL, et al. Intermediate uveitis. Semin Ophthalmol. 2005;20:147–154.
3 Lai WW, Pulido JS. Intermediate uveitis. Ophthalmol Clin North Am. 2002;15:309–317.
4 Lin P, Loh AR, Margolis TP, et al. Cigarette smoking as a risk factor for uveitis. Ophthalmology. 2010;117:585–590.
5 Lin P, Tessler HH, Goldstein DA. Family history of inflammatory bowel disease in patients with idiopathic ocular inflammation. Am J Ophthalmol. 2006;141:1097–1104.
6 Henderly DE, Genstler AJ, Smith RE, et al. Changing patterns of uveitis. Am J Ophthalmol. 1987;103:131–136.
7 Kump LI, Cervantes-Castaneda RA, Androudi SN, et al. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005;112:1287–1292.
8 Tugal-Tutkun I, Havrlikova K, Power WJ, et al. Changing patterns in uveitis of childhood. Ophthalmology. 1996;103:375–383.
9 Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. discussion
10 Suhler EB, Lloyd MJ, Choi D, et al. Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am J Ophthalmol. 2008;146:890–896. e8
11 Donaldson MJ, Pulido JS, Herman DC, et al. Pars planitis: a 20-year study of incidence, clinical features, and outcomes. Am J Ophthalmol. 2007;144:812–817.
12 Vidovic-Valentincic N, Kraut A, Hawlina M, et al. Intermediate uveitis: long-term course and visual outcome. Br J Ophthalmol. 2009;93:477–480.
13 Thorne JE, Daniel E, Jabs DA, et al. Smoking as a risk factor for cystoid macular edema complicating intermediate uveitis. Am J Ophthalmol. 2008;145:841–846.
14 Tugal-Tutkun I, Aydin-Akova Y, Guney-Tefekli E, et al. Referral patterns, demographic and clinical features, and visual prognosis of Turkish patients with sarcoid uveitis. Ocul Immunol Inflamm. 2007;15:337–343.
15 Althaus C, Sundmacher R. Intermediate uveitis: epidemiology, age and sex distribution. Dev Ophthalmol. 1992;23:9–14.
16 Madow B, Galor A, Feuer WJ, et al. Validation of a photographic vitreous haze grading technique for clinical trials in uveitis. Am J Ophthalmol. 2011;152:170–176.
17 Malinowski SM, Pulido JS, Folk JC. Long-term visual outcome and complications associated with pars planitis. Ophthalmology. 1993;100:818–824. discussion 25
18 Lai JC, Stinnett SS, Jaffe GJ. B-scan ultrasonography for the detection of macular thickening. Am J Ophthalmol. 2003;136:55–61.
19 de Smet MD, Okada AA. Cystoid macular edema in uveitis. Dev Ophthalmol. 2010;47:136–147.
20 Castellano CG, Stinnett SS, Mettu PS, et al. Retinal thickening in iridocyclitis. Am J Ophthalmol. 2009;148:341–349.
21 Rodriguez A, Calonge M, Pedroza-Seres M, et al. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol. 1996;114:593–599.
22 Raja SC, Jabs DA, Dunn JP, et al. Pars planitis: clinical features and class II HLA associations. Ophthalmology. 1999;106:594–599.
23 Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517.
24 Curkendall SM, Wang C, Johnson BH, et al. Potential health care cost savings associated with early treatment of multiple sclerosis using disease-modifying therapy. Clin Ther. 2011;33:914–925.
25 Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000;343:898–904.
26 Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 2001;357:1576–1582.
27 Ormerod LD, Dailey JP. Ocular manifestations of cat-scratch disease. Curr Opin Ophthalmol. 1999;10:209–216.
28 Sabet-Peyman EJ, Eberhart CG, Janjua K, et al. Persistent intermediate uveitis associated with latent manifestation of facial large B-cell non-Hodgkin lymphoma. Ocul Immunol Inflamm. 2009;17:322–324.
29 Mruthyunjaya P, Jumper JM, McCallum R, et al. Diagnostic yield of vitrectomy in eyes with suspected posterior segment infection or malignancy. Ophthalmology. 2002;109:1123–1129.
30 Buggage RR. Ocular manifestations of human T-cell lymphotropic virus type 1 infection. Curr Opin Ophthalmol. 2003;14:420–425.
31 Boskovich SA, Lowder CY, Meisler DM, et al. Systemic diseases associated with intermediate uveitis. Cleve Clin J Med. 1993;60:460–465.
32 Thaler S, Grisanti S, Klingel K, et al. Intermediate uveitis and arthralgia as early symptoms in Whipple’s disease. Int J Infect Dis. 2010;14(Suppl 3):e388–e389.
33 Soheilian M, Mirbabai F, Shahsavari M, et al. Metastatic cutaneous melanoma to the vitreous cavity masquerading as intermediate uveitis. Eur J Ophthalmol. 2002;12:324–327.
34 Eichenbaum JW, Friedman AH, Mamelok AE. A clinical and histopathological review of intermediate uveitis (“pars planitis”). Bull N Y Acad Med. 1988;64:164–174.
35 Pederson JE, Kenyon KR, Green WR, et al. Pathology of pars planitis. Am J Ophthalmol. 1978;86:762–774.
36 Bora NS, Bora PS, Tandhasetti MT, et al. Molecular cloning, sequencing, and expression of the 36 kDa protein present in pars planitis. Sequence homology with yeast nucleopore complex protein. Invest Ophthalmol Vis Sci. 1996;37:1877–1883.
37 Courvalin JC, Lassoued K, Bartnik E, et al. The 210-kD nuclear envelope polypeptide recognized by human autoantibodies in primary biliary cirrhosis is the major glycoprotein of the nuclear pore. J Clin Invest. 1990;86:279–285.
38 Kraemer D, Wozniak RW, Blobel G, et al. The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm. Proc Natl Acad Sci U S A. 1994;91:1519–1523.
39 Wetzig RP, Chan CC, Nussenblatt RB, et al. Clinical and immunopathological studies of pars planitis in a family. Br J Ophthalmol. 1988;72:5–10.
40 Nissenblatt MJ, Masciulli L, Yarian DL, et al. Pars planitis – a demyelinating disease? Arch Ophthalmol. 1981;99:697.
41 Boyd SR, Young S, Lightman S. Immunopathology of the noninfectious posterior and intermediate uveitides. Surv Ophthalmol. 2001;46:209–233.
42 Hultsch E. Peripheral uveitis in the owl monkey. Experimental model. Mod Probl Ophthalmol. 1977;18:247–251.
43 Klok AM, Luyendijk L, Zaal MJ, et al. Elevated serum IL-8 levels are associated with disease activity in idiopathic intermediate uveitis. Br J Ophthalmol. 1998;82:871–874.
44 Klok AM, Luyendijk L, Zaal MJ, et al. Soluble ICAM-1 serum levels in patients with intermediate uveitis. Br J Ophthalmol. 1999;83:847–851.
45 Bianco A, Spiteri MA. Peripheral anergy and local immune hyperactivation in sarcoidosis: a paradox or birds of a feather. Clin Exp Immunol. 1997;110:1–3.
46 Chow JH, Mettu PS, Srivastava SK, et al. PPD and positive skin test controls in the evaluation of anergy in uveitis patients. ARVO Meeting Abstracts. April 11, 2008.
47 Rossman MD, Thompson B, Frederick M, et al. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet. 2003;73:720–735.
48 Hogewind BF, Zijlstra C, Klevering BJ, et al. Intravitreal triamcinolone for the treatment of refractory macular edema in idiopathic intermediate or posterior uveitis. Eur J Ophthalmol. 2008;18:429–434.
49 Soheilian M, Rabbanikhah Z, Ramezani A, et al. Intravitreal bevacizumab versus triamcinolone acetonide for refractory uveitic cystoid macular edema: a randomized pilot study. J Ocul Pharmacol Ther. 2010;26:199–206.
50 Chieh JJ, Carlson AN, Jaffe GJ. Combined fluocinolone acetonide intraocular delivery system insertion, phacoemulsification, and intraocular lens implantation for severe uveitis. Am J Ophthalmol. 2008;146:589–594.
51 Malone PE, Herndon LW, Muir KW, et al. Combined fluocinolone acetonide intravitreal insertion and glaucoma drainage device placement for chronic uveitis and glaucoma. Am J Ophthalmol. 2010;149:800–806. e1
52 Ghosn CR, Li Y, Orilla WC, et al. Treatment of experimental anterior and intermediate uveitis by a dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52:2917–2923.
53 Okinami S, Sunakawa M, Arai I, et al. Treatment of pars planitis with cryotherapy. Ophthalmologica. 1991;202:180–186.
54 Pulido JS, Mieler WF, Walton D, et al. Results of peripheral laser photocoagulation in pars planitis. Trans Am Ophthalmol Soc. 1998;96:127–137. discussion 37–41
55 Dev S, McCallum RM, Jaffe GJ. Methotrexate treatment for sarcoid-associated panuveitis. Ophthalmology. 1999;106:111–118.
56 Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116:2188–2198. e1
57 Galor A, Jabs DA, Leder HA, et al. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology. 2008;115:1826–1832.
58 Neri P, Zucchi M, Allegri P, et al. Adalimumab (Humira): a promising monoclonal anti-tumor necrosis factor alpha in ophthalmology. Int Ophthalmol. 2011;31:165–173.
59 Li SY, Birnbaum AD, Goldstein DA. Optic neuritis associated with adalimumab in the treatment of uveitis. Ocul Immunol Inflamm. 2010;18:475–481.
60 Salgado E, Gomez-Reino JJ. The risk of tuberculosis in patients treated with TNF antagonists. Expert Rev Clin Immunol. 2011;7:329–340.
61 Milman N, Soborg B, Bo Svendsen C, et al. Quantiferon test for tuberculosis screening in sarcoidosis patients. Scand J Infect Dis. 2011.
62 Gallagher M, Quinones K, Cervantes-Castaneda RA, et al. Biological response modifier therapy for refractory childhood uveitis. Br J Ophthalmol. 2007;91:1341–1344.
63 Lindstedt EW, Baarsma GS, Kuijpers RW, et al. Anti-TNF-alpha therapy for sight threatening uveitis. Br J Ophthalmol. 2005;89:533–536.
64 Galor A, Perez VL, Hammel JP, et al. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology. 2006;113:2317–2323.
65 Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the Multicenter Uveitis Steroid Treatment Trial. Ophthalmology. 2011;118:1916–1926.
66 Tranos P, Scott R, Zambarakji H, et al. The effect of pars plana vitrectomy on cystoid macular oedema associated with chronic uveitis: a randomised, controlled pilot study. Br J Ophthalmol. 2006;90:1107–1110.
67 Becker M, Davis J. Vitrectomy in the treatment of uveitis. Am J Ophthalmol. 2005;140:1096–1105.
68 Dugel PU, Rao NA, Ozler S, et al. Pars plana vitrectomy for intraocular inflammation-related cystoid macular edema unresponsive to corticosteroids. A preliminary study. Ophthalmology. 1992;99:1535–1541.
69 Verbraeken H. Therapeutic pars plana vitrectomy for chronic uveitis: a retrospective study of the long-term results. Graefes Arch Clin Exp Ophthalmol. 1996;234:288–293.
70 Levy-Clarke GA, Chan CC, Nussenblatt RB. Diagnosis and management of primary intraocular lymphoma. Hematol Oncol Clin North Am. 2005;19:739–749. viii
71 Pe’er J, Hochberg FH, Foster CS. Clinical review: treatment of vitreoretinal lymphoma. Ocul Immunol Inflamm. 2009;17:299–306.
72 Smith JR, Rosenbaum JT, Wilson DJ, et al. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology. 2002;109:1709–1716.
73 Kalinina Ayuso V, ten Cate HA, van den Does P, et al. Young age as a risk factor for complicated course and visual outcome in intermediate uveitis in children. Br J Ophthalmol. 2011;95:646–651.
74 Yu EN, Paredes I, Foster CS. Surgery for hypotony in patients with juvenile idiopathic arthritis-associated uveitis. Ocul Immunol Inflamm. 2007;15:11–17.
75 Dayani PN, Chow J, Stinnett SS, et al. Pars plana vitrectomy, fluocinolone acetonide implantation, and silicone oil infusion for the treatment of chronic, refractory uveitic hypotony. Am J Ophthalmol. 2011;152:849–856. e1