CHAPTER 83 Injection Procedures
PATHOPHYSIOLOGY OF RADICULAR LUMBAR PAIN
Lumbar radicular pain, i.e. sciatica, in this context is defined as pain referred from the back into the dermatome of the affected nerve root along the femoral or sciatic nerve trunk. This has to be differentiated from nonradicular pain, which refers symptoms into the leg in a nondermatomal pattern.1 Radicular pain is shooting and bandlike, whereas somatic referred pain is usually constant in position but poorly localized and diffuse, and is aching in quality. True radiculopathy is defined as radicular pain in the presence of a neurological deficit.2 The prevalence of lumbar disc syndrome (herniated disc or typical sciatica) was studied as part of the Mini-Finland Health Survey.3 A diagnosis of lumbar disc syndrome was made for 5.1% of men and 3.7% of women aged 30 years or over. In a Finnish longitudinal birth cohort study, symptomatic lumbar disc disease (herniated nucleus pulposus or sciatica) appeared around the age of 15 years, and the incidence rose more sharply from the age of 19 years.4
Tissue origin of lumbar radicular pain
The tissue origin of sciatic pain has been studied during decompression operations performed with local anesthesia. In these studies, sciatic pain could be produced only by pressure on the compressed, swollen nerve root, or on the dorsal root ganglion (DRG). Pressure on normal nerve roots or on other tissue did not produce sciatica.5,6
Intervertebral disc
Disc herniation is the single most common cause of radicular pain.2 Mixter and Barr7 discovered that soft tissue ‘tumours’ were actually derived from the intervertebral discs, and that their surgical removal relieved sciatica symptoms. The causal link between herniated nucleus pulposus (HNP) and radicular pain is, however, not so straightforward since (1) HNP can be found in 20–36%, depending on the age, of asymptomatic subjects,8–11 and (2) internal disc ruptures (without HNP) may also induce disabling radicular pain,12,13 indicating the existence of an alternative mechanism to neural compression. Even though this chapter does not cover the clinical diagnosis of lumbar radicular pain, the authors stress that nerve root tension signs, assessed by the straight leg-raising test, can be positive in sciatica patients without HNP in MRI.14
Central and lateral stenosis
Spinal stenosis is a condition associated with degenerative changes of the disc and zygapophyseal joints at multiple levels, which may include degenerative spondylolisthesis.15 Spinal stenosis has both structural and dynamic components. When the spinal canal is structurally narrowed, slight extension can cause compression of the nerves.16,17 Extension can also cause an increase in epidural pressure.18 Flexion has the reverse effect, widening the spinal canal and foramina and reducing the epidural pressure. These typical features can be used in the practical clinical diagnosis of spinal stenosis and also in the algorithm of radicular pain.
Lateral lumbar spinal stenosis due to osteoarthritis can be divided into entrance zone, midzone and exit zone stenosis.19 When a nerve root is laterally entrapped, it gives unilateral pain that is worse on walking. When central canal is narrow, pain radiates to one or both legs while walking and is relieved with flexion postures.20,21
Midzone stenosis is clinically the most relevant entity, because the DRG occupies a large part of the midzone.19 Recent experimental data also support the critical role played by the DRG in the pathophysiology of painful stenosis.22 The authors found that neither demyelinization nor axonal degeneration in the cauda equina induced mechanical allodynia, i.e. neuropathic pain, whereas lesions distal or immediately proximal to it are painful. They concluded that DRG apoptosis may be important for the production and maintenance of mechanical allodynia.22
Pathophysiological mechanisms of radicular pain
Evidence of other mechanisms that can elicit lumbar radicular pain other than nerve root compression comes from many directions. We have already cited the findings of experimental surgery in anesthesia, existence of HNP in asymptomatics, and on the other hand, sciatica syndromes in those without an HNP. Additional evidence comes from animal experiments. McCarron et al.23 demonstrated that nuclear material of the intervertebral disc is chemically inflammatory and neurotoxic. Olmarker et al. showed that nuclear material – without any compression – can induce structural and functional changes in porcine nerve roots.24 The functional changes included focal degeneration of myelinated fibers and focal Schwann cell damage in nondegenerated axons. The damage to the Schwann cells resulted in a disintegration of Schmidt-Lantermann incisures, which represent connections of Schwann cell cytoplasm inside and outside the myelin sheath.25 Additional evidence supporting inflammation comes from the finding that nucleus pulposus is chemotactic, attracting leukocytes, and it may also induce macromolecular leakage and spontaneous firing of axons in vitro.26 Inflammation-induced capillary leakage increases endoneural pressure and reduces blood flow, thereby causing a ‘compartment syndrome’ in the DRG.27 A similar decrease in blood flow has been observed also in the canine nerve root. This reduction correlated with decrease in nerve conduction velocity, and was maximal within 1 week and recovered within 1 month. The pattern of nucleus-exposed DRG was, however, different, showing no clear recovery.28 These findings suggest that DRG irritation may lead into a different – perhaps more conservative treatment-resistant – radicular pain entity than nerve root involvement only. An additional, important landmark study is that of Kawakami et al.29 They nicely showed that leukocytes are essential in experimental radicular pain. In a rat model of mechanical hyperalgesia induced by application of nucleus pulposus to nerve roots, depletion of leukocytes with nitrogen mustard inhibited the generation of hyperalgesia. This indicated that the leukocytes are important in the production of pain-related behavior. The cells first appearing in and around the HNP on nerve–nuclear interface were polymorphonuclear leukocytes. Macrophages, originating from monocytes, did predominate a few days later and then remain in the affected region until the inflammation subsided.29 The implication of the observations is that lumbar radicular pain is a systemic disease, at least in the early stages of the disease.
What is the leukotactic signal(s) of extruded nuclear material? Many substances, including hydrogen ions and glycoproteins, have been suspected of causing chemical radiculitis.30–32 A crucial finding was the one reported by Olmarker et al.33 They noted that the neurotoxicity of the nucleus seems to be associated with disc cells, as freezing prevented the neuronal damage. This observation limited the number of possible inflammatory candidates, but several were still ‘without alibi.’
Phospholipase A2 (PLA2) was a promising suspect, as it is the rate-limiting enzyme in the synthesis of proinflammatory lipid mediators (prostaglandins, leukotrienes, lipoxenies, and platelet-activating factor). It is calcium-dependent, adsorbing tightly to plasma membranes and intact cells. PLA2 liberates arachidonic acid from the membrane phospholipids, and is secreted extracellularly by activated phagocytes in response to cytokines.34 Additionally, it is released from rabbit chondrocytes in response to interleukin (IL)-1.35 It was found in extraordinarily high concentrations in herniated and painful discs,36 although this finding has since been questioned.37 It is also itself inflammatory38 and neurotoxic.39 When PLA2 was injected epidurally, motor weakness, demyelinization, and increased sensitivity of dorsal roots to mechanical stimulation were observed 3 days after the injection, but not beyond 3 weeks.40
Tumor necrosis factor alpha (TNF-α) is another potential candidate in HNP-induced nerve root irritation. TNF-α is a cytokine produced mainly by activated macrophages and T cells in response to inflammation, and by mast cells and Schwann cells in response to peripheral nerve injury.41,42 It activates the transcription factors NF-κB and AP-1 by binding to its p55 TNF-receptor (TNFR1), thereby inducing the production of proinflammatory and immunomodulatory genes.43 Endoneurial TNF-α causes demyelinization, axonal degeneration, and hyperalgesic pain states.44 In thermal hyperalgesia, two peaks have been associated with Wallerian degeneration, and can be reproduced in chronic injury to peripheral nerves.45 These peaks are also related to changes in TNF-α expression. It seems that the first peak, 6 hours after the nerve injury, is due to the local expression of the cytotoxic transmembrane 26 kDa TNF-α protein released by the resident Schwann cells. The second peak occurs 5 days after the injury, and may represent TNF-α protein released by hematogenously recruited macrophages.45 It has been shown immunohistochemically that TNF-α is expressed in the porcine nucleus pulposus.46 In a rat model, the concentration of TNF-α was found to be approximately 0.5 ng per herniated rat disc.47 Moreover, exogenous TNF-α produced neuropathological and behavioral changes (Wallerian degeneration of nerve fibers, macrophage recruitment to phagocytoze the debris, splitting of the myelin sheath) that mimicked those of the nucleus pulposus.47 Application of TNF-α on porcine nerve roots induced a reduction of the nerve conduction velocity that was even more pronounced than for nucleus pulposus, whereas application of IL-1β and IFNδ induced slight reductions of conduction velocity compared with fat, but they were not statistically significant.48 Additional evidence for a crucial role of TNF-α comes from an animal study in which soluble TNF-α receptor (etanercept, Enbrel™) reversed nucleus pulposus-induced nerve conduction block and nerve root edema.49 However, TNF-α is not just a ‘bad guy’ as it also has an important role in the resorption of disc herniations. Macrophages secrete matrix metalloproteinase (MMP)-7 (=matrilysin) enzyme, which liberates soluble TNF-α from macrophage cell membranes. Soluble TNF-α induces disc chondrocytes to secrete MMP-3 (stromelysin), required for the release of a macrophage chemoattractant and subsequent macrophage infiltration of the disc.50,51
In addition to TNF-α, other inflammatory mediators may take part in the inflammatory component of radicular pain. These mediators could be either proximal to TNF-α, i.e. increase the expression of TNF-α, or distal to TNF-α, i.e. they are upregulated by TNF-α. Kang et al.52 observed increased matrix metalloproteinase activity, and increased levels of nitric oxide, prostaglandin E2, and IL-6 in HNP culture media compared with the control discs. Similarly, Burke et al.53 also detected increased levels of IL-6 in disc extracts from patients undergoing fusion for discogenic pain. They found additionally increased levels of a chemokine, IL-8. Interleukin-6 is an interesting interleukin, as it regulates to a large extent the hepatic acute phase and cachectic responses to an acute inflammatory stimulus.54 Recently, it was found that sciatica patients have an elevated acute phase response.55 Mean sensitized C-reactive protein (CRP) levels were significantly higher in sciatica patients compared to age- and sex-matched controls (1.68 versus 0.74 mg/L; p=0.002). We have genotyped sciatica patients with regard to some inflammatory genes and compared these patients to asymptomatic subjects. A genotype leading to increased production of IL-6 was overexpressed in sciatica patients.56
Additionally, in the HNP homogenates IL-1α, IL-1β and granulocyte-macrophage colony stimulating factor are detectable.57 The exact role of IL-1 in HNP-induced radicular pain is not known but it may have separate activity as it has in experimental arthritis.58
Natural course of lumbar radicular pain
The long-term prognosis of lumbar radicular pain is considered to be good59 although in one study only one-third of sciatica patients recovered fully within 1 year, whereas one-third underwent surgery and one-third had residual symptoms.60 This study by Balague60 is in concordance with a systematic review on the long-term course of low back pain (LBP).61 Sixty-two percent of LBP patients still experienced pain at 12 months, 16% were sick-listed 6 months after inclusion into the study, and 60% experienced relapses of pain. A cohort of primary care patients with sciatica was followed in the Netherlands.62 An unfavorable outcome was predicted by a disease duration of more than 30 days, increased pain on sitting, pain upon coughing, and straight leg raising restriction.
Magnetic resonance imaging (MRI) follow-up examinations have shown that HNP tends to regress over time, with partial to complete resolution after 6 months in two-thirds of people.63 We have recently rescanned 21 patients with HNP-induced severe sciatica at 2 weeks, 3 months, and 6 months in an intervention trial. Significant resorption seemed to occur already as early as 3 months in most patients.64 There is a predilection for large extrusions to resorb well.65,66 The resorption process seems to associate with HNP-encircling rim enhancement,67 which is thought to represent a neovascularized zone with macrophage infiltration.68 Neovascularization probably remains high in extrusions, as these have ruptured the posterior longitudinal ligament and entered the epidural space, allowing small vessels to penetrate the disc tissue more easily, whereas subligamentous herniations are more or less immunoprivileged.69 This is supported by the higher resorption rate for extrusion-type disc herniations.70 We have recently analyzed determinants of HNP resorption.71 In the final model, the only significant determinants for resorption were thickness of rim enhancement and Komor classification, i.e. herniation extending above or below 67% of the adjacent vertebra.72
INTERVENTIONAL TREATMENT OPTIONS FOR LUMBAR RADICULAR PAIN
Evidence on substantiating the best method to achieve successful treatment of lumbar radicular pain is still sparse. A recent systematic review found only 19 randomized, controlled trials (RCTs), of which eight met the three major requirements (comparability of groups, observer blinding, and intention-to-treat analysis).73 From the perspective of this review, no significant effect was demonstrated for nonsteroidal antiinflammatory agents (NSAIDs), traction, or intramuscular steroids. Considering the, at least partial, inflammatory nature of lumbar radicular pain, blocking of the cytokine cascade by local or systematic corticosteroids might, however, be effective. It is known from animal experiments that methylprednisolone injected within 2 days after the application of the nucleus pulposus inhibits the nucleus-induced vascular permeability and functional impairment, i.e. decrease of nerve conduction velocity.74 Any clinically useful intervention for radicular pain should be (1) effective in pain alleviation, (2) safe (i.e. no harmful complications), and (3) the technical details of the procedure or the equipment used should not be too complicated so that the intervention can be used widely in clinical practice. Moreover, if two different interventions are found equally effective and safe on radicular pain, the more cost-effective procedure should be chosen. When designing and using interventions for radicular pain, one should not tamper with the benign natural course of sciatica. In the ensuing, epidural injections, selective nerve root blocks, and anticytokine therapy are discussed in more extensive detail.
Epidural injections
Epidural injections in the cervical, thoracic, and lumbosacral spine have been used for both diagnostic and therapeutic purposes in modern interventional spine practice. Epidural injections should preferably be combined with other therapeutic modalities, e.g. physical training and musculoskeletal rehabilitation. Epidural injection of medication allows a concentrated amount of the treatment agents (i.e. mostly corticosteroids) to be deposited and retained, thereby exposing the nerve roots to the medication for a prolonged period of time. The ability of steroids injected through an epidural route to reach their target in the anterior or anterolateral epidural compartment has been questioned. Indeed, even in experienced hands 25–45% of blind interlaminar or caudal epidural needle placements may be incorrect.75–77
Technical procedure
There are two different routes to perform epidural injections: a caudal route through the sacral hiatus, and a lumbar interlaminar route. Epidural injection can be done with fluoroscopy or without it. Many specialists recommend fluoroscopy, because without fluoroscopy the needle is not always placed in the epidural space. Additionally, fluoroscopy prevents accidental intravascular injection. The incidence of intravascular uptake during lumbar spinal injection procedures was found to be approximately 8.5%. Absence of flashback of blood upon preinjection aspiration did not predict extravascular needle placement.78 Recently, fluoroscopic guidance has evolved into the standard approach in the US, although some clinicians stubbornly perform blind injections (Curtis Slipman, personal communication). Typically, blind injections are reserved for pregnant women and heavy patients who exceed the weight limits of the fluoroscopy table. It is easiest and safest to insert the needle at L2–3 or L3–4, close to the superior spinous process. The standard technique used in epidural injections is the loss of resistance technique, where a controlled and well-defined loss of resistance occurs upon entering the epidural space through the ligamentum flavum. One study found that in the non-obese patient, lumbar interlaminar injections can be accurately placed without X-ray screening, in contrast to caudal injections, which require X-ray screening independent of the weight of the patient.79 In the caudal epidural injection, the quantity of corticosteroid that is possible to apply near to the inflamed nerve root is usually small. As well, the precise application is always uncertain, because anatomical structures such as septas may interfere with the flow of the injectate. However, caudal epidural route is useful in cases when the lesion is at L5–S1, but the interspinous route is preferable if the lesion is located at L4–5 or above.
Efficacy
Epidural steroid injections are found to have a high success rate when evaluated in terms of long-term alleviation of radicular symptoms due to lumbar HNP.80,81 One published meta-analysis concluded that epidural corticosteroids are effective in both the short and long term in low back pain and sciatica.82 In contrast, the systematic review by Koes et al. that included higher-quality trials, found at most a short-term effect on sciatica.83 To further complicate the matter, the systematic review by Vroomen et al. on treatment of sciatica concluded that epidural steroids may produce a short-term benefit for lumbar radicular pain.73 Following these aforementioned studies, two RCTs on epidural steroids for sciatica have been published. In the trial of Buchner et al.,84 patients with lumbar radicular pain consequent to a confirmed HNP were randomized into the epidural group (3 injections of 100 mg methylprednisolone in 0.25% bupivacaine; n=17) and control group (n=19). At 2 weeks, patients receiving methylprednisolone injection showed a significant improvement in straight leg raising test results and a tendency for a greater pain relief. At 6 weeks and 6 months, no significant differences were observed in any of the outcomes. In the trial of Valat and colleagues,85 three epidural injections of 50 mg prednisolone acetate (n = 43) were compared to epidural saline injections (n = 42) in HNP-induced sciatica. A significant improvement was observed in both groups, but epidural steroid injections provided no additional benefit over saline. Our conclusion, which stems from the reviews and subsequent RCTs, is that epidural steroids may have, at best, a short-term beneficial effect on lumbar radicular pain. Additionally, cost minimization analysis suggests that epidural injections under fluoroscopy may not be justified on the basis of the current literature.86 The authors’ personal preference is to use selective nerve root blocks (SNRB) in lieu of interlaminar epidurals for lesions at L4–5 or above. At L5–S1, our present view is to prefer computed tomography (CT)-guided SNRBs over caudal epidurals, and caudal epidurals over fluoroscopy-guided SNRBs. We do acknowledge that there is no uniform consensus regarding the approach at L5–S1.
Safety
A major concern when administrating anesthetics into the epidural space is systemic toxicity. There is the theoretical risk of cardiovascular toxicity and central nervous system (CNS) effects. These complications can be avoided by adhering to careful technique and using lower doses of less concentrated anesthetics as discussed in the spinal injections technique chapter (Ch. 23). The maximum epidural dose recommended for a single injection is 500 mg for lidocaine and 225 mg for bupivacaine. The amount of local anesthetic agent used for SNRBs is much less.
Epidural injections are usually considered to be extremely safe when performed with the proper technique.87 Nevertheless, the interlaminar route may be prone to complications, which include dural puncture-caused spinal headache, transient hypotension, Cushing’s syndrome, bacterial meningitis, chemical meningitis, epidural abscess, sinus arrhythmia, respiratory distress from spinal anesthesia, transiently increasing back or leg pain, numbness, transient dizziness, and cardiopulmonary arrest.88 In the meta-analysis of Watts and Silagy, which was based on seven trials with 431 patients, 2.5% suffered from dural taps, 2.3% transient headache, 1.9% transient increase in pain, and 0.2% irregular menstrual cycle.82 Long-term complications were not covered in the original reports, but, according to data from the American Society of Anesthesiologists Closed Claims Project database (1970–1999), epidural steroid injections accounted for 40% of all chronic pain management claims. Serious injuries, involving brain damage or death, occurred, especially with local anesthetics and/or opioids.89
Selective nerve root blocks
Derby et al.90 have postulated that the transforaminal approach may get corticosteroid more reliably in the anterior epidural space, where most of the pain-sensitive structures are located. In the procedure, the pharmaceutical agents are injected between the nerve root and the epidural sheath, depicting the nerve root in tubular fashion.91 Hereafter, we use the term selective nerve root block (SNRB), but synonymous terms include selective nerve root injection, periradicular infiltration, transforaminal injection, and perineural injection. The mechanism of therapeutic effect is postulated to rely on mainly on the antiinflammatory effect of corticosteroid, which blocks the afferent impulses from the periphery.91 However, the anesthetic component may have an effect on its own, as lidocaine has been shown to increase intraradicular blood flow identical to the responses of a sympathetic ganglion block.92
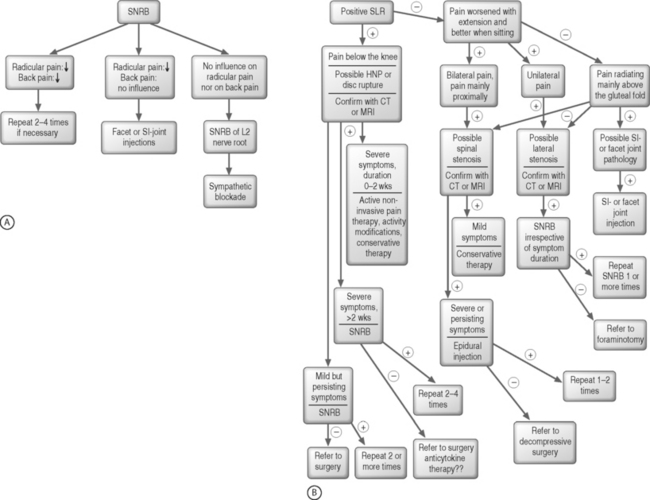
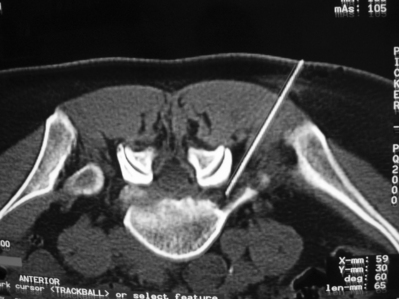
SNRBs are useful in the diagnosis of radicular pain in atypical presentations. They have an accuracy of 85–94% in identifying a single symptomatic root, sensitivity of 100%, and a positive predictive value of 93–95% has been presented for root blocks.1,91,93,94 Indications are: (1) atypical extremity pain; (2) when imaging studies and clinical presentation do not correlate; (3) when electromyography and MRI do not correlate; (4) anomalous innervations, such as conjoint nerve roots or furcal nerves; (5) failed back surgery syndrome with atypical extremity pain; and (6) transitional vertebrae.88 A diagnostic SNRB is usually done without any antiinflammatory drug such as steroid in order to confirm the identity of the affected nerve root, whereas in therapeutic injections the ultimate goal is a therapeutic effect (typically achieved with a corticosteroid with or without local anesthetic). See the algorithm on diagnostic SNRBs and treatment of lumbar radicular pain, Figures 83.1A and 83.1B, respectively.
Technical procedure
Fluoroscopy-guided SNRB is typically the simplest, most rapid, and cost-effective technique. The details of this procedure have been thoroughly explained in Chapter 23. There are, however, two other techniques to confirm the proper needle insertion and placement: CT and MRI guidance.
CT guidance
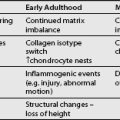
During CT-guided SNRBs, patients are in prone position on the scanner table. Axial slides are taken and analyzed before the procedure. The safe triangle described within the numerous technique chapters is the targeted area. The trajectory of the injection can be preplanned according to the information obtained from the CT scans. The entry point is marked on the skin with ink and can be controlled with a new CT scan (Fig. 83.2). Injection angle and the distance between skin and target area can be measured accurately. The interventionalist can check the correct angle of the injection needle with an angle measurement device and thus guide the clinician throughout the procedure. The correct injection site can also be controlled with contrast-enhanced CT scans when needed. For postoperative radicular pain, CT-guided injection seems to be superior to fluoroscope-assisted injection for both its visualization and a longer-lasting effect.95
MRI guidance
MRI guidance is another method, though it has not gained wide popularity because of the expensive MRI equipment required. The technical details of the MRI imaging system include an open-configuration c-arm magnet, an MR-compatible in-room console, large screen display unit, and optical navigator.96 An MRI-guided procedure lacks the disadvantages of the other two methods. There is no ionizing radiation risk, and because of its ability to provide superior soft tissue contrast detail, contrast agent is not required. MRI guidance offers three-dimensional information during the nerve root injection, which is particularly advantageous for S1 infiltrations,96 which tend to produce unsatisfactory results by fluoroscopy.97
Efficacy
It was observed in diagnostic studies that patients with lumbar spinal stenosis due to spondylosis or degenerative spondylolisthesis had experienced a better therapeutic benefit from SNRBs than those with radicular symptoms referable to a disc herniation or to spondylolytic spondylolisthesis.91,98 Several uncontrolled follow-up studies confirmed these observations of a therapeutic effect.99,100 In HNP-induced radiculopathy, there was a 75% long-term recovery after an average of 1.8 transforaminal injections per patient of betamethasone acetate combined with Xylocaine. The outcome was better for symptom duration of less than 36 weeks.100 Weiner and Fraser used fluoroscopy-guided SNRB for patients with severe lumbar radiculopathy secondary to foraminal and extraforaminal disc herniation, which had not resolved with rest and nonsteroidal antiinflammatory agents. They observed a considerable and sustained pain relief in 22 out of 28 (79%) patients.99 Derby and colleagues observed that a successful SNRB is a good prognostic sign for a positive surgical result for those with symptomatology of at least 1-year duration.101
Fluoroscopically guided transforaminal steroid injections seem to be beneficial in radicular pain due to lumbar spinal stenosis in terms of both pain reduction and improved walking tolerance.102 However, a prospective cohort study involving radicular patients with a disc herniation or spinal stenosis indicated that the response was significantly better in patients with a HNP.103
So far, only 6 RCTs comparing perineural corticosteroid injection to a nonsteroid regimen have been published. Kraemer et al.104 used an interlaminar injection of triamcinolone and lidocaine (n=47), which was compared to paravertebral local anesthetic (n=46). Actually, they did not use SNRB but an oblique interlaminar method. This injection method, however, is somewhat similar to the SNRB precision technique and therefore it is reviewed in this context. Three injections were given with 1-week intervals. They used a composite score and prevention of back surgery in the assessments. Their results indicated that epidural perineural injections were more effective than conventional posterior epidural injections. Devulder et al.105 used transforaminal injections for failed back surgery. The combination of methylprednisolone, bupivacaine, and hyaluronidase (n=20) was compared to a combination of saline, bupivacaine, and hyaluronidase (n=20). Their main outcome measure was at least 50% reduction in leg pain. No statistical differences were found between the treatment groups, although it is unlikely that SNRBs are effective in postoperative radicular syndromes with a high probability of neuropathic pain component. Riew et al.106 used transforaminal injections for degenerative lumbar radicular pain in patients indicated for surgery. Patients had either a disc herniation or central or lateral stenosis confirmed in MRI and/or CT. Patients were randomized to a selective nerve root injection with either bupivacaine alone or bupivacaine with betamethasone. Nineteen patients received multiple injections. Eighteen out of 27 patients in the control group underwent back surgery, as compared to 8 out of 28 patients in the active group. The difference in the operative rates between the two groups was statistically and clinically significant (p<0.004). In the Finnish RCT,107 transforaminal injection was given to nonoperated patients with radicular pain extending below the knee. Eighty-two percent of all randomized patients had an MRI-confirmed HNP. In the study, a single injection of methylprednisolone (80–120 mg) with bupivacaine (n=80) was compared to isotonic transforaminal saline (n=80). A short-term effect was observed in favor of the steroid injection, but at later follow-up assessments the treatments were of equal efficacy. In a later subgroup analysis, it was found that the effect of steroid was greater at L3–4 or L4–5 level and in case of contained, i.e. subligamentous, herniations.108 Vad et al.109 used transforaminal injection of betamethasone with Xylocaine (n=25), which was compared to lumbar paraspinal trigger-point injections with saline (n=25) for nonoperated sciatica patients. The active group had a success rate of 84% in the long term, as compared to with 48% in the control group. The effect estimate may be, however, biased because patients were not blinded to treatment allocation. An additional factor, which may increase treatment effect is the fact that patients did not undergo a trial of conservative care before the SNRB. Ng et al.110 compared 0.25% bupivacaine and 40 mg methylprednisolone to 0.25% bupivacaine only for chronic leg pain. Patients had MRI-verified root compromise due to herniation or lateral stenosis with a mean symptom duration of more than 12 months. The final study population included 43 patients in both groups. At 3 months, there was no statistically significant difference in the outcome measures between the groups (Oswestry Disability Index, visual analogue scores for back and leg pain, change in walking distance).
A recent systematic review found moderate evidence in support of SNRBs in treating painful radicular symptoms.111 The authors of the review concluded that current studies support use of SNRBs as a safe and minimally invasive adjunct treatment for lumbar radicular symptoms. However, conclusive evidence is still lacking.111 The current authors agree with the conclusion of the review – SNRB is a safe intervention for lumbar radicular pain in experienced hands. In clinical practice most patients with HNP- or lateral stenosis-induced lumbar radicular pain seem to improve permanently (from the current episode) with a single SNRB. In the subanalysis of our RCT on SNRB, injection with steroid was cost-effective in case of subligamentous, i.e. contained, herniations, whereas saline was cost-effective in case of large herniations, i.e. extrusions and sequestrations.108 The difference in the cost-effectiveness was due to different surgery rates in these subgroups; the steroid injection patients with large HNP tended to undergo surgery more commonly in comparison to the saline injection and vice versa in the subgroup of contained herniations. This led us to suspect the existence of a negative effect of steroids on the resorption process as described by Minamide et al. in rabbits.112 Yet, in our analyses we did not observe such an effect exerted by SNRBs when using steroids.113 Readers are invited to view the algorithm constructed by the authors for the treatment of lumbar radicular pain (see Fig. 83.1B).
Safety
In a retrospective evaluation of 322 SNRB injections, complications included transient nonpositional headache (3.1%), increased back pain (2.4%), increased leg pain (0.6%), facial flushing (1.2%), vasovagal reactions (0.3%), increased blood sugar in a insulin-dependent diabetic (0.3%), and intraoperative hypertension (0.3%). No dural punctures occurred and all reactions resolved without morbidity.114 Intravascular injections have been encountered in 11.2% of SNRBs, the rate being higher at S1.115 Serious complications of SNRBs have also been encountered, and include epidural abscess, arachnoiditis, epidural hematoma, cerebrospinal fluid fistula, hypersensitivity reaction to injectate, and even persisting paraplegia.116 However, in a recent prospective 3-month follow-up study of cervical and lumbosacral SNRBs (with complications as the primary outcome) no major complications were encountered.117 Minor complications of lumbosacral procedures included increased pain at the injection site (17.1% of 306 injections performed), increased radicular pain (8.8%), lightheadedness (6.5%), increased spine pain (5.1%), non-specific headache (1.4%), and vomiting (0.5%).117
Practical considerations
The authors use SNRBs only for lumbar radicular pain regardless of the presence of motor and/or sensory defects. Those who experience a partial or total muscle paresis without concurrent pain represent a unique challenge. We have observed that moderate motor weakness improves with a SNRB, whereas sensory defect and abnormal tendon reflexes mainly do not. The differential recovery rate of sensory and motor fibers has been verified also in animal studies.117 We try to encourage patients that the prognosis of motor paresis is benign, and although sensory disturbances are likely to remain they do not result in any functional limitation (of course, there must be an awareness about the possibility of a burn or other tissue injury because of the anesthetic skin). Basically, we use SNBRs only for patients with radicular pain but some painless patients might benefit from the procedure. However, we try an SNRB only once in these cases and refer them to surgery in case motor weakness does not improve following this intervention. Recently published long-term results of lumbar discectomy showed that preoperative foot drops recovered almost completely irrespective the cause (HNP or lumbar spinal stenosis).118
Our patients with lumbar radicular pain typically demonstrate an HNP that clinically corroborates with the distribution of the pain. We do not consider surgery the initial management step. Patients must be apprised of the benign natural course of the symptom manifestation of disc herniations and the variety of treatment options for the radicular component. Invariably, we supplement the performance of SNRBs with physical therapy that emphasizes the McKenzie approach.119 Centralization of pain is a good prognostic sign.120,121 For more chronic patients with intermittent radicular symptoms we use additional therapies that involve physical conditioning and a cognitive behavioral approach.122 One of these approaches is the DBC method, which is an active outpatient therapy using specific devices to enhance lumbar spine function in combination with a behavioral approach.123,124 A combination of an SNRB and exercise is usually effective for patients with HNP to avoid surgery, as indicated in the trial of Riew et al.106
Anticytokine therapy
Animal studies indicate that TNF-α assumes a crucial role in the pathophysiology of radicular pain. Either intervertebral disc-contained TNF-α or another molecule, acting through the upregulation of TNF-α expression, causes hematogenously recruited leukocytes and macrophages to accumulate in the environment of the nerve root and/or DRG. This leads to increased capillary permeability and endoneural pressure, and ultimately to endoneural ischemia. Thus, a strong theoretical rationale exists for the use of anti-TNF-α therapy in lumbar radicular pain. Other cytokines may also be involved,53 but so far anticytokine therapy other than anti-TNF-α or anti-IL-1 has not been exploited for radicular pain. Several new anti-TNF-α preparations, including an orally administered one, will be commercially available shortly. The different preparations may differ in the safety profile and the efficacy for lumbar radicular pain. Therefore, before a wide-scale clinical implementation of the various anticytokine preparations both proof of concept trials and phase IV studies are needed to elucidate the clinical effectiveness and safety issues.
Technical procedure
Prior to the infusion, patients must be screened for serious infections, hepatitis B or hepatitis C, human immunodeficiency virus (HIV), opportunistic infections, malignancies, latent or active tuberculosis, lymphoproliferative disease, pregnancy, and severe progressive or uncontrolled renal, hepatic, hematologic, gastrointestinal, endocrine, pulmonary, cardiac (e.g. congestive heart failure), neurologic or cerebral diseases including demyelinating diseases such as multiple sclerosis.
Efficacy
An open-label study indicated that a single infusion of infliximab 3 mg/kg is efficacious for the treatment of HNP-induced acute sciatica. The curative effect occurred within 3 hours after infusion initiation and was sustained throughout the 3-month follow-up period.125 In the 1-year follow-up of the open-label study, the effect was sustained in all but one patient over the follow-up period as evidenced by the outcomes reported for both leg pain and disability measured with the Oswestry scale. The nonresponder was operated on shortly before the 1-year assessment.126 The dose of 1 mg/kg was given to two patients. One of them had a rapid and complete relief of radicular pain, but the other had no benefit from the infusion. Furthermore, a single infusion of infliximab did not interfere with the spontaneous resorption of disc herniations. The observation of a nondeleterious effect on herniation resorption was later confirmed in a randomized setting.64 Recently, we finished a 3-month follow-up of an RCT where intravenous infliximab 5 mg/kg was compared to intravenous saline.127 Unfortunately, the positive results of the open-label study125,126 could not be replicated. Patients in both groups had a similarly good response, with approximately two-thirds responding well (i.e., at least 75% reduction in leg pain). Seven patients in both groups had discectomy by 3 months. The pronounced placebo effect could be best observed in the straight leg raising test as 3 hours after the i.v. saline infusion this test had improved by 15 degrees. Our conclusion is therefore that routine clinical use of infliximab is not recommended until efficacy is established.
In addition to infliximab, etanercept has also been used for lumbar radicular pain. In a Swiss study,128 five consecutive patients with acute severe sciatica received three injections of etanercept (25 mg every 3 days). They were compared to seven consecutive patients who received 3 intravenous injections of methylprednisolone (250 mg every 3 days). At 10 days, patients treated with etanercept exhibited a significant improvement in both leg pain and disability. Further improvement was observed at 6 week. In the steroid group, significant improvement was noted at 10 days, but at 6 weeks symptoms reoccurred. Tobinick and Britschgi-Davoodifar129 have documented their experiences on etanercept for discogenic pain. Most of their patients had a rapid and sustained benefit after a single subcutaneous injection of 25 mg etanercept. They had 14 patients with clear-cut lumbar or cervical radiculopathy, and five patients with suspected lumbar radicular pain. In a case of lumbar radicular pain, the author administered etanercept by a perispinal subcutaneous injection to the lower lumbar region
Wehling et al.130 compared steroid injections to IL-1 receptor antagonist injections in experimental allergic radiculitis. Prednisolone appeared to be somewhat more effective than IL-1 receptor antagonist but a causative role for IL-1 in sciatica was speculated.
Safety
All of the anti-TNF-α drugs are prone to potentially serious adverse effects. At the moment, clinical safety data are available for infliximab and etanercept, as both of these drugs have been used in clinical practice for several years. Both are contraindicated in patients with severe infection, anemia, neutropenia, lymphoma, demyelinating disease, or who have active or latent tuberculosis.131 Infliximab and etanercept should not be used in patients with moderate or severe heart failure. A potential problem with all biological medicines is immunogenicity. Antibodies against all of the anti-TNF-α drugs may develop and may cause serious allergic reactions. A possible acute infusion reaction is defined as any adverse event that occurs during or within 1 hour after the administration of the infusion. A possible delayed hypersensitivity reaction consists of myalgia and/or arthralgia with fever and/or rash (that does not represent signs and symptoms of other recognized clinical syndromes) occurring 3–12 days after an infusion of study drug. These may be accompanied by other events including pruritus, facial, hand, or lip edema, dysphagia, urticaria, sore throat and/or headache. Delayed hypersensitivity reactions may occur in patients retreated with these agents following a period of several years without any exposure to the drug in question.
In the open-label study, we have treated 12 patients with a single infusion of infliximab (10 with a 3 mg/kg dose and 2 with 1 mg/kg dose), but no immediate or delayed adverse effects have been encountered.126 In the RCT,127 three mild adverse effects (rhinitis, diarrhea, otitis media with sinusitis maxillaris) were encountered.
ALGORITHM FOR DIAGNOSIS AND TREATMENT OF RADICULAR PAIN
To establish the diagnosis of radicular pain, we always prefer SNRBs (see Fig. 83.1A). Our preference is to use CT guidance, especially in case of S1 injections, and with a minimal injectate volume in order to minimize or prevent caudal spreading. If there is a positive response in both radicular and low back pain, diagnosis of the affected level is confirmed. SNRB can be repeated if necessary (see also Fig. 83.1B on treatment of radicular pain). If the SNRB relieves radicular pain but LBP remains, a facet joint or SI joint injection may need to be performed. If the SNRB does not have effect on radicular pain nor on LBP, SNRB can be performed at the L2 level, as it is hypothesized that discogenic pain is transmitted mainly by sympathetic afferent fibers in the L2 nerve root.132 It may be that L2 SNRB is not very effective for the radicular component but it seems to alleviate the local pain component effectively.132
There is, so far, no hard scientific evidence to justify any intervention for treatment of lumbar radicular pain. This does not mean that such interventions should not be useful in clinical practice because uncontrolled studies suggest that a variety of interventions are useful in radicular pain. One specific intervention is, however, unlikely to benefit all patients with radicular pain. Therefore, we need more information on prognostic factors, which may increase treatment effect of different therapeutic interventions. Lumbar radicular pain may not be as homogeneous an entity as we have thought. Time since onset of symptoms is one prognostic factor that ought to be considered. In clinical practice, patients who have more acute radicular pain (less than 3 months) respond better to epidural and transforaminal injections than those with long-lasting radicular symptoms (longer than 1 year). In animal experiments, methylprednisolone reversed the nucleus pulposus-induced nerve root injury but steroid was injected within 2 days of the experimental lesion.74 Acute symptoms could thus be more susceptible to treatment and, on the contrary, it is unlikely that local steroids would be beneficial in case of neuropathic radicular pain. Other prognostic factors to be considered are DRG involvement,28 the level of the lesion,108 type of symptomatic herniation (subligamentous versus transligamentous),108 having workers’ compensation, and work requiring lifting.133
In Figure 83.1B, an algorithm for treatment of lumbar radicular pain is presented. If SLR is positive and pain extends below the knee, an HNP or disc rupture should be suspected. MRI can also visualize disc ruptures, whereas CT is useful only in the case of HNP. When symptoms have only been present for a short interval, regardless of their severity, noninvasive active pain therapy including NSAIDs and opiates, activity modifications and conservative therapy (see Practical considerations, above) is preferred. Unfortunately, NSAIDs may often not be sufficient to alleviate radicular pain. Patients with indications for emergency surgery (cauda equine, progressive muscle paresis, or rapidly increasing pain) are, of course, referred for surgery, as these are considered the sole indications for immediate/emergency surgery. Patients should avoid bed rest134 and stay active. Transforaminal steroids is the option to be chosen for continuing disabling radicular pain but usually patients should have demonstrated a failure to improve with less invasive treatment options (rehabilitation techniques). Transforaminal steroids are preferred and repeated as necessary as they may prevent surgery.106 SNRBs are usually repeated 2–4 times. In case of severe short-term pain not responding to SNRBs, consideration of a more aggressive approach should be entertained, such as open surgery. The exact indications for different invasive techniques are reviewed elsewhere in this book. It has been our observation that patients with an L4–5 HNP and major trunk list do not respond well with SNRBs and typically require surgery. Anticytokine therapy may be indicated for the more severe cases but so far we have no evidence based on randomized, controlled trials to justify its use. Therefore, we strongly discourage off-label use, despite some optimistic commentaries,135 because anticytokine therapies may potentially produce serious adverse effects. At present, evidence has been demonstrated only for surgery in herniation-induced sciatica.136 However, the conclusion drawn from this classic by Weber has been questioned, as a recent Finnish trial could not replicate his findings.137 Interestingly, a subanalysis of this intention-to-treat trial found evidence that surgery may be effective at L4–5.
When the SLR test is negative but radicular pain is worsened with extension and walking and better with sitting, a stenotic process should be suspected. When there are bilateral symptoms, central spinal stenosis is the probable cause. In case of central stenosis, we prefer epidural injections, which can be repeated 1–2 times. In case of mild symptoms, conservative rehabilitation methods are always the initial treatment intervention, which may be followed by more invasive procedures. If symptoms persist or the intensity of pain or bothersomeness increases, epidural injections are indicated. Surgery should be reserved only for patients with severe symptoms.138 In case of lateral stenosis, we prefer SNRBs always, regardless of symptom duration. If pain is relieved, but only temporarily, patients may be referred for other more aggressive treatments ranging from percutaneous disc decompression to decompressive foraminotomy (see Fig. 83.1B). Indications and techniques for SI joint and facet joint injections are discussed elsewhere in this book.
1 van Akkerveeken PF. Pain patterns and diagnostic blocks. In: Wiesel SW, Weinstein JN, Herkowitz H, et al, editors. The lumbar spine. Philadelphia: WB Saunders; 1996:105-122.
2 Bogduk N. Clinical anatomy of the lumbar spine and sacrum. New York: Churchill Livingstone, 1997.
3 Heliövaara M. Body height, obesity, and risk of herniated lumbar intervertebral disc. Spine. 1987;12:469-472.
4 Zitting P, Rantakallio P, Vanharanta H. Cumulative incidence of lumbar disc diseases leading to hospitalization up to the age of 28 years. Spine. 1998;23:2337-2343.
5 Smyth MJ, Wright J. Sciatica and the intervertebral disk. An experimental study. J Bone Joint Surg. 1958:1401-1418.
6 Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin N Am. 1991;22:181-187.
7 Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-215.
8 Hitselberger WE, Witten RM. Abnormal myelograms in asymptomatic patients. J Neurosurg. 1968;28:204-206.
9 Wiesel SW, Tsourmas N, Feffer HL, et al. A study of computer-assisted tomography. I. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9:549-551.
10 Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg [Am]. 1990;72:403-408.
11 Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
12 Ohnmeiss DD, Vanharanta H, Ekholm J. Degree of disc disruption and lower extremity pain. Spine. 1997;22:1600-1605.
13 Ohnmeiss DD, Vanharanta H, Ekholm J. Relation between pain location and disc pathology: a study of pain drawings and CT/discography [In Process Citation]. Clin J Pain. 1999;15:210-217.
14 Karppinen J, Malmivaara A, Tervonen O, et al. Severity of symptoms and signs in relation to magnetic resonance imaging findings among sciatic patients. Spine. 2001;26:E149-E154.
15 Amundsen T, Weber H, Lilleas F, et al. Lumbar spinal stenosis. Clinical and radiologic features. Spine. 1995;20:1178-1186.
16 Penning L, Wilmink JT. Posture-dependent bilateral compression of L4 or L5 nerve roots in facet hypertrophy. A dynamic CT-myelographic study. Spine. 1987;12:488-500.
17 Willen J, Danielson B, Gaulitz A, et al. Dynamic effects on the lumbar spinal canal: axially loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine. 1997;22:2968-2976.
18 Takahashi K, Kagechika K, Takino T, et al. Changes in epidural pressure during walking in patients with lumbar spinal stenosis. Spine. 1995;20:2746-2749.
19 Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine. 1988;13:313-320.
20 Porter RW, Hibbert CS. Symptoms associated with lysis of the pars interarticularis. Spine. 1984;9:755-758.
21 Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21:2046-2052.
22 Sekiguchi M, Kikuchi S, Myers RR. Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain. Spine. 2004;29:1105-1111.
23 McCarron RF, Wimpee MW, Hudkins PG, et al. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine. 1987;12:760-764.
24 Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425-1432.
25 Olmarker K, Nordborg C, Larsson K, et al. Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus. Spine. 1996;21:411-414.
26 Olmarker K, Blomquist J, Stromberg J, et al. Inflammatogenic properties of nucleus pulposus. Spine. 1995;20:665-669.
27 Yabuki S, Kikuchi S, Olmarker K, et al. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglia. Spine. 1998;23:2517-2523.
28 Otani K, Arai I, Mao GP, et al. Nucleus pulposus-induced nerve root injury: relationship between blood flow and motor nerve conduction velocity. Neurosurgery. 1999;45:614-619.
29 Kawakami M, Tamaki T, Matsumoto T, et al. Role of leukocytes in radicular pain secondary to herniated nucleus pulposus. Clin Orthop. 2000;376:268-277.
30 Nachemson A. Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop Scand. 1969;40:23-42.
31 Marshall LL, Trethewie ER. Chemical irritation of nerve-root in disc prolapse. Lancet. 1973;2:320.
32 Marshall LL, Trethewie ER, Curtain CC. Chemical radiculitis. A clinical, physiological and immunological study. Clin Orthop. 1977;129:61-67.
33 Olmarker K, Brisby H, Yabuki S, et al. The effects of normal, frozen, and hyaluronidase-digested nucleus pulposus on nerve root structure and function. Spine. 1997;22:471-475.
34 Vadas P, Pruzanski W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab Invest. 1986;55:391-404.
35 Chang J, Gilman SC, Lewis AJ. Interleukin 1 activates phospholipase A2 in rabbit chondrocytes: a possible signal for IL-1 action. J Immunol. 1986;136:1283-1287.
36 Saal JS, Franson RC, Dobrow R, et al. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine. 1990;15:674-678.
37 Gronblad M, Virri J, Ronkko S, et al. A controlled biochemical and immunohistochemical study of human synovial-type (group II) phospholipase A2 and inflammatory cells in macroscopically normal, degenerated, and herniated human lumbar disc tissues. Spine. 1996;21:2531-2538.
38 Franson RC, Saal JS, Saal JA. Human disc phospholipase A2 is inflammatory. Spine. 1992;17:S129-S132.
39 Ozaktay AC, Kallakuri S, Cavanaugh JM. Phospholipase A2 sensitivity of the dorsal root and dorsal root ganglion. Spine. 1998;23:1297-1306.
40 Chen C, Cavanaugh JM, Ozaktay AC, et al. Effects of phospholipase A2 on lumbar nerve root structure and function. Spine. 1997;22:1057-1064.
41 Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996;73:625-629.
42 Bemelmans MH, van Tits LJ, Buurman WA. Tumor necrosis factor: function, release and clearance. Crit Rev Immunol. 1996;16:1-11.
43 Darnay BG, Aggarwal BB. Early events in TNF signaling: a story of associations and dissociations. J Leukoc Biol. 1997;61:559-566.
44 Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport. 1996;7:2897-2901.
45 Shubayev VI, Myers RR. Upregulation and interaction of TNF alpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855:83-89.
46 Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus pulposus-induced nerve root injury. Spine. 1998;23:2538-2544.
47 Igarashi T, Kikuchi S, Shubayev V, et al. Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975-2980.
48 Aoki Y, Rydevik B, Kikuchi S, et al. Local application of disc-related cytokines on spinal nerve roots. Spine. 2002;27:1614-1617.
49 Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine. 2001;26:863-869.
50 Haro H, Crawford HC, Fingleton B, et al. Matrix metalloproteinase-3-dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J Clin Invest. 2000;105:133-141.
51 Haro H, Crawford HC, Fingleton B, et al. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000;105:143-150.
52 Kang JD, Georgescu HI, McIntyre-Larkin L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271-277.
53 Burke JG, Watson RW, McCormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg [Br]. 2002;84:196-201.
54 Oldenburg HS, Rogy MA, Lazarus DD, et al. Cachexia and the acute-phase protein response in inflammation are regulated by interleukin-6. Eur J Immunol. 1993;23:1889-1894.
55 Le Gars L, Borderie D, Kaplan G, et al. Systemic inflammatory response with plasma C-reactive protein elevation in disk-related lumbosciatic syndrome. Joint Bone Spine. 2000;67:452-455.
56 Noponen-Hietala N, Virtanen I, Karttunen R, et al. Genetic variations in IL-6 associate with intervertebral disc disease characterized by sciatica. Pain. 2005;114:186-194.
57 Takahashi H, Suguro T, Okazima Y, et al. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218-224.
58 van den Berg WB, Joosten LA, Kollias G, et al. Role of tumour necrosis factor alpha in experimental arthritis: separate activity of interleukin-1beta in chronicity and cartilage destruction. Ann Rheum Dis. 1999;58(Suppl 1):I40-I48.
59 Hakelius A. Prognosis in sciatica. A clinical follow-up of surgical and non-surgical treatment. Acta Orthop Scand. 1970;Suppl 129:1-76.
60 Balague F, Nordin M, Sheikhzadeh A, et al. Recovery of severe sciatica. Spine. 1999;24:2516-2524.
61 Hestbaek L, Leboeuf-Yde C, Manniche C. Low back pain: what is the long-term course? A review of studies of general patient populations. Eur Spine J. 2003;12:149-165.
62 Vroomen PC, Wilmink JT. Prognostic value of MRI findings in sciatica. Neuroradiology. 2002;44:59-63.
63 Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363-370.
64 Autio RA, Karppinen J, Niinimäki J, et al. The effect of infliximab, a monoclonal antibody against TNFα, on disc herniation resorption: a randomized controlled study. Spine. 2006;31:2641-2645.
65 Saal JA, Saal JS, Herzog RJ. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine. 1990;15:683-686.
66 Maigne JY, Rime B, Deligne B. Computed tomographic follow-up study of forty-eight cases of nonoperatively treated lumbar intervertebral disc herniation. Spine. 1992;17:1071-1074.
67 Komori H, Okawa A, Haro H, et al. Contrast-enhanced magnetic resonance imaging in conservative management of lumbar disc herniation. Spine. 1998;23:67-73.
68 Rothoerl RD, Woertgen C, Holzschuh M, et al. Is there a clinical correlate to the histologic evidence of inflammation in herniated lumbar disc tissue? Spine. 1998;23:1197-1200.
69 Ozaki S, Muro T, Ito S, et al. Neovascularization of the outermost area of herniated lumbar intervertebral discs. J Orthop Sci. 1999;4:286-292.
70 Ahn SH, Ahn MW, Byun WM. Effect of the transligamentous extension of lumbar disc herniations on their regression and the clinical outcome of sciatica. Spine. 2000;25:475-480.
71 Autio R, Karppinen J, Niinimäki J, et al. Determinants of spontaneous resorption of intervertebral disc herniations. Spine. 2006;31:1247-1252.
72 Komori H, Shinomiya K, Nakai O, et al. The natural history of herniated nucleus pulposus with radiculopathy. Spine. 1996;21:225-229.
73 Vroomen PC, de Krom MC, Slofstra PD, et al. Conservative treatment of sciatica: a systematic review. J Spinal Disord. 2000;13:463-469.
74 Olmarker K, Byrod G, Cornefjord M, et al. Effects of methylprednisolone on nucleus pulposus-induced nerve root injury. Spine. 1994;19:1803-1808.
75 White AH. Injection techniques for the diagnosis and treatment of low back pain. Orthop Clin N Am. 1983;14:553-567.
76 Weinstein SM, Herring SA, Derby R. Contemporary concepts in spine care. Epidural steroid injections. Spine. 1995;20:1842-1846.
77 Stitz MY, Sommer HM. Accuracy of blind versus fluoroscopically guided caudal epidural injection. Spine. 1999;24:1371-1376.
78 Sullivan WJ, Willick SE, Chira-Adisai W, et al. Incidence of intravascular uptake in lumbar spinal injection procedures. Spine. 2000;25:481-486.
79 Price CM, Rogers PD, Prosser AS, et al. Comparison of the caudal and lumbar approaches to the epidural space. Ann Rheum Dis. 2000;59:879-882.
80 Cannon DT, Aprill CN. Lumbosacral epidural steroid injections. Arch Phys Med Rehabil. 2000;81:S87-S98.
81 Wang JC, Lin E, Brodke DS, et al. Epidural injections for the treatment of symptomatic lumbar herniated discs. J Spinal Disord Tech. 2002;15:269-272.
82 Watts RW, Silagy CA. A meta-analysis on the efficacy of epidural corticosteroids in the treatment of sciatica. Anaesth Intensive Care. 1995;23:564-569.
83 Koes BW, Scholten RJ, Mens JM, et al. Efficacy of epidural steroid injections for low-back pain and sciatica: a systematic review of randomized clinical trials. Pain. 1995;63:279-288.
84 Buchner M, Zeifang F, Brocai DR, et al. Epidural corticosteroid injection in the conservative management of sciatica. Clin Orthop. 2000;375:149-156.
85 Valat JP, Giraudeau B, Rozenberg S, et al. Epidural corticosteroid injections for sciatica: a randomised, double blind, controlled clinical trial. Ann Rheum Dis. 2003;62:639-643.
86 Straus BN. Chronic pain of spinal origin: the costs of intervention. Spine. 2002;27:2614-2619.
87 Botwin KP, Gruber RD. Lumbar epidural steroid injections in the patient with lumbar spinal stenosis. Phys Med Rehabil Clin N Am. 2003;14:121-141.
88 Huston CW, Slipman CW. Diagnostic selective nerve root blocks: indications and usefulness. Phys Med Rehabil Clin N Am. 2002;13:545-565.
89 Fitzgibbon DR, Posner KL, Domino KB, et al. Chronic pain management: American Society of Anesthesiologists Closed Claims Project. Anesthesiology. 2004;100:98-105.
90 Derby R, Bogduk N, Kine G. Precision percutaneous blocking procedures for localizing spinal pain. Part 2. The lumbar neuraxial compartment. Pain Digest. 1993;3:175-188.
91 Hasue M, Kikuchi S. Nerve root injections. In: Frymoyer J, editor. The adult spine: principles and practice. Philadelphia: Lippincott-Raven; 1997:647-653.
92 Yabuki S, Kikuchi S. Nerve root infiltration and sympathetic block. An experimental study of intraradicular blood flow. Spine. 1995;20:901-906.
93 Haueisen DC, Smith BS, Myers SR, et al. The diagnostic accuracy of spinal nerve injection studies. Their role in the evaluation of recurrent sciatica. Clin Orthop. 1985;198:179-183.
94 Dooley JF, McBroom RJ, Taguchi T, et al. Nerve root infiltration in the diagnosis of radicular pain. Spine. 1988;13:79-83.
95 Lutze M, Stendel R, Vesper J, et al. Periradicular therapy in lumbar radicular syndromes: methodology and results. Acta Neurochirurgica. 1997;139:719-724.
96 Ojala R, Vähälä E, Karppinen J, et al. Nerve root infiltration of the first sacral root with MRI guidance. J Magn Reson Imaging. 2000;12:556-561.
97 Viton JM, Rubino T, Peretti-Viton P, et al. Short-term evaluation of periradicular corticosteroid injections in the treatment of lumbar radiculopathy associated with disc disease. Revue Du Rhumatisme, English Edition. 1998;65:195-200.
98 Kikuchi S, Hasue M, Nishiyama K, et al. Anatomic and clinical studies of radicular symptoms. Spine. 1984;9:23-30.
99 Weiner BK, Fraser RD. Foraminal injection for lateral lumbar disc herniation. J Bone Joint Surg [Br]. 1997;79:804-807.
100 Lutz GE, Vad VB, Wisneski RJ. Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Arch Phys Med Rehabil. 1998;79:1362-1366.
101 Derby R, Kine G, Saal JA, et al. Response to steroid and duration of radicular pain as predictors of surgical outcome. Spine. 1992;17:S176-S183.
102 Botwin KP, Gruber RD, Bouchlas CG, et al. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: an outcome study. Am J Phys Med Rehabil. 2002;81:898-905.
103 Ng LC, Sell P. Outcomes of a prospective cohort study on peri-radicular infiltration for radicular pain in patients with lumbar disc herniation and spinal stenosis. Eur Spine J. 2004;13:325-329.
104 Kraemer J, Ludwig J, Bickert U, et al. Lumbar epidural perineural injection: a new technique. Eur Spine J. 1997;6:357-361.
105 Devulder J, Deene P, De Laat M, et al. Nerve root sleeve injections in patients with failed back surgery syndrome: a comparison of three solutions. Clin J Pain. 1999;15:132-135.
106 Riew KD, Yin Y, Gilula L, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg [Am]. 2000;82:1589-1593.
107 Karppinen J, Malmivaara A, Kurunlahti M, et al. Periradicular infiltration for sciatica. A randomized controlled trial. Spine. 2001;26:1059-1067.
108 Karppinen J, Ohinmaa A, Malmivaara A, et al. Cost effectiveness of periradicular infiltration for sciatica. Subgroup analysis of a randomized controlled trial. Spine. 2001;26:2587-2595.
109 Vad VB, Bhat AL, Lutz GE, et al. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine. 2002;27:11-16.
110 Ng L, Chaudhary N, Sell P. The efficacy of corticosteroids in periradicular infiltration for chronic radicular pain: a randomized, double-blind, controlled trial. Spine. 2005;30:857-862.
111 DePalma MJ, Bhargava A, Slipman CW. A critical appraisal of the evidence for selective nerve root injection in the treatment of lumbosacral radiculopathy. Arch Phys Med Rehabil. 2005;86:1477-1483.
112 Minamide A, Tamaki T, Hashizume H, et al. Effects of steroid and lipopolysaccharide on spontaneous resorption of herniated intervertebral discs. An experimental study in the rabbit. Spine. 1998;23:870-876.
113 Autio RA, Karppinen J, Kurunlahti M, et al. Effect of periradicular methylprednisolone on spontaneous resorption of intervertebral disc herniations. Spine. 2004;29:1601-1607.
114 Botwin KP, Gruber RD, Bouchlas CG, et al. Complications of fluoroscopically guided transforaminal lumbar epidural injections. Arch Phys Med Rehabil. 2000;81:1045-1050.
115 Furman MB, O’Brien EM, Zgleszewski TM. Incidence of intravascular penetration in transforaminal lumbosacral epidural steroid injections. Spine. 2000;25:2628-2632.
116 Houten JK, Errico TJ. Paraplegia after lumbosacral nerve root block: report of three cases. Spine J. 2002;2:70-75.
117 Huston CW, Slipman CW, Garvin C. Complications and side effects of cervical and lumbosacral selective nerve root injections. Arch Phys Med Rehabil. 2005;86:277-283.
118 Girardi FP, Cammisa FPJ, Huang RC, et al. Improvement of preoperative foot drop after lumbar surgery. J Spinal Disord Tech. 2002;15:490-494.
119 Taylor MD. The McKenzie method: a general practice interpretation: the lumbar spine. Aust Fam Physician. 1996;25:189-197. 200
120 Sufka A, Hauger B, Trenary M, et al. Centralization of low back pain and perceived functional outcome. J Orthop Sports Phys Ther. 1998;27:205-212.
121 Long A, Donelson R, Fung T. Does it matter which exercise? A randomized control trial of exercise for low back pain. Spine. 2004;29:2593-2602.
122 Schonstein E, Kenny D, Keating J, et al. Physical conditioning programs for workers with back and neck pain: a Cochrane Systematic Review. Spine. 2003;28:E391-E395.
123 Kankaanpää M, Taimela S, Airaksinen O, et al. The efficacy of active rehabilitation in chronic low back pain. Effect on pain intensity, self-experienced disability, and lumbar fatigability. Spine. 1999;24:1034-1042.
124 Taimela S, Diederich C, Hubsch M, et al. The role of physical exercise and inactivity in pain recurrence and absenteeism from work after active outpatient rehabilitation for recurrent or chronic low back pain: a follow-up study. Spine. 2000;25:1809-1816.
125 Karppinen J, Korhonen T, Malmivaara A, et al. Tumor necrosis factor-alpha monoclonal antibody, infliximab, used to manage severe sciatica. Spine. 2003;28:750-753.
126 Korhonen T, Karppinen J, Malmivaara A, et al. Efficacy of infliximab for disc herniation-induced sciatica. One-year follow-up. Spine. 2004;29:2115-2119.
127 Korhonen T, Karppinen J, Paimela L, et al. The treatment of disc herniation-induced sciatica with infliximab. Results of a randomized, controlled, 3-month follow-up study. Spine. 2005;30:2724-2728.
128 Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute and severe sciatica. A pilot study. Ann Rheum Dis. 2004;63:1120-1123.
129 Tobinick EL, Britschgi-Davoodifar S. Perispinal TNF-alpha inhibition for discogenic pain. Swiss Med Wkly. 2003;133:170-177.
130 Wehling P, Cleveland SJ, Heininger K, et al. Neurophysiologic changes in lumbar nerve root inflammation in the rat after treatment with cytokine inhibitors. Evidence for a role of interleukin-1. Spine. 1996;21:931-935.
131 Bencsath M, Blaskovits A, Borvendeg J. Biomolecular cytokine therapy. Pathol Oncol Res. 2003;9:24-29.
132 Nakamura SI, Takahashi K, Takahashi Y, et al. The afferent pathways of discogenic low-back pain. Evaluation of L2 spinal nerve infiltration. J Bone Joint Surg [Br]. 1996;78:606-612.
133 Tong HC, Williams JC, Haig AJ, et al. Predicting outcomes of transforaminal epidural injections for sciatica. Spine J. 2003;3:430-434.
134 Vroomen PC, de Krom MC, Wilmink JT, et al. Lack of effectiveness of bed rest for sciatica. N Engl J Med. 1999;340:418-423.
135 Cooper RG, Freemont AJ. TNF-alpha blockade for herniated intervertebral disc-induced sciatica: a way forward at last? Rheumatology (Oxford). 2004;43:119-121.
136 Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine. 1983;8:131-140.
137 Österman H, Seitsalo S, Karppinen J, et al. Effectiveness of microdiscectomy for lumbar disc herniation. A randomised controlled trial with two years of follow-up. Spine. 2006;31:2409-2414.
138 Herno A, Airaksinen O, Saari T, et al. Lumbar spinal stenosis: a matched-pair study of operated and non-operated patients. Br J Neurosurg. 1996;10:461-465.