General principles77
9.2 Vascular events in inflammation79
9.3 Cellular processes of inflammation79
Inflammatory processes are part of the body’s natural defence and repair mechanisms. However, their beneficial effects can be accompanied by significant tissue damage. Inflammation involves the coordination of many different functions by a wide variety of cells. Soluble molecules called inflammatory mediators (e.g. cytokines) are important in this process, as are cell–cell and cell–matrix interactions. To help conceptualise this complex web of activity, it is helpful to think about these processes as vascular events (changes in blood vessels) and cellular events (the activities of inflammatory cells).
9.1. General principles
Inflammation is the local response to injury in living, vascularised tissues. Its purpose is to localise and eliminate the injurious agent, such as infecting organisms, and then, as far as possible, to restore the tissue to normal structure and function. However, inflammation may be seen as a two-edged sword. On the one hand, it has beneficial effects by localising and eliminating an injurious agent; on the other, the consequent tissue damage may be detrimental to the host. Inflamed tissues are named with the suffix ‘-itis’; thus appendicitis is inflammation of the appendix and hepatitis is inflammation of the liver.
Chemical inflammatory mediators
Chemical inflammatory mediators have an important role in orchestrating the inflammatory response. They are widely distributed throughout the body (Table 15). Some circulate as plasma proteins (the clotting factors, fibrinolytic factors, kinin system and complement proteins); the others are released, synthesised or activated locally. Inactivation occurs rapidly after release, which is important for the control and localisation of inflammation.
| Mediator | Common sources | Important functions |
|---|---|---|
| Histamine and serotonin | Mast cells and basophils, platelets | Vasodilatation and early increase in vascular permeability |
| Cytokines | Lymphocytes, macrophages, many other cells | Activation and modulation of inflammatory cell activity, chemotaxis of inflammatory cells (chemotactic cytokines are called chemokines) |
| Platelet activating factor (PAF) | Most white cells, vascular endothelium | Increases vascular permeability, increases adhesion of white cells to endothelium and induces platelet aggregation |
| Prostaglandins (PG) and PG-like substances | Mast cells, endothelium, platelets | PGE2 causes pain, PGE2 and PGI2 cause vasodilatation, thromboxane A2 (produced by platelets) causes vasoconstriction |
| Leukotrienes | Neutrophils | Neutrophil chemotaxis, increased vascular permeability, vasoconstriction |
| Nitric oxide | Vascular endothelium, macrophages | Vasodilatation, toxic to bacteria |
| Lysosomal compounds (proteases, acid hydrolases) | Neutrophils, macrophages | Increased vascular permeability, activation of complement, increased tissue damage |
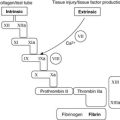
| Coagulation factors | Plasma proteins | Thrombin activates many cells, including platelets, endothelial cells and smooth muscle cells; fibrin forms a clot-like mesh in the inflammatory exudate |
| Fibrinolytic system | Plasma proteins | Plasmin dissolves fibrin but also activates C3 |
| Kinin system | Plasma proteins | Pain, vasodilatation, increased vascular permeability |
| Complement cascade | Plasma proteins | See Chapter 5 |
Clinical features of inflammation
There are four clinical features that have been known to be associated with inflammation since antiquity. They are known as the ‘cardinal signs’:
• redness (from dilatation of blood vessels)
• pain (from oedema and inflammatory mediators)
• heat (from vasodilatation)
• swelling (from oedema).
In addition, patients with inflammation frequently experience systemic effects due to circulating cytokines such as tumour necrosis factor alpha (TNF-α) and interleukin 1 (IL-1) released from the inflamed tissue. Malaise and loss of appetite are common, and fever is due to re-setting of the set-point of the thermostat in the hypothalamus to a higher temperature. The liver releases a variety of proteins called acute-phase reactants that can have a role in the inflammatory response, e.g. C-reactive protein and serum amyloid-A protein (SAA), which have antibacterial effects, and clotting factors such as fibrinogen. The numbers of circulating leucocytes increase as leucocytes are released from the bone marrow. When neutrophils are released from the bone marrow in this way, the proportion of less mature forms in the blood increases; haematologists describe this as a ‘shift to the left’ in neutrophil morphology. Weight loss is common in long-standing inflammation due to a negative nitrogen balance caused by a combination of increased metabolic activity and loss of appetite.
Acute inflammation
Acute inflammation is defined as the early inflammatory response to an injurious agent, and is characterised by the presence of neutrophil polymorphs and later macrophages. Acute inflammation usually lasts for a few hours or days.
Causes of acute inflammation
• Hypersensitivity reactions: types I–IV
• Physical agents: physical trauma, burns, UV light, radiation
• Chemicals: acids, alkalis, oxidising agents
• Tissue necrosis: e.g. infarction
The acute inflammatory response is characterised by increased blood flow, exudation of protein-rich fluid, and the accumulation of neutrophils (Figure 22). The mechanisms responsible are discussed later in this chapter. Within tissues, the exudate accumulates as oedema fluid, but exudate from inflamed serous membranes can accumulate in body cavities such as the pleural, pericardial and peritoneal cavities. The exudate contains large amounts of fibrin that can cause clots to form. Histologically, the presence of neutrophils is an important feature indicating the presence of acute inflammation to the pathologist.
 |
| Figure 22 |
Outcomes of acute inflammation
Healing by resolution
Acute inflammation may disappear after a few days and the tissue returns to normal. Fluid and degraded proteins are drained by the lymphatic channels and the exudate, cell debris and fibrin are removed by neutrophils and monocytes. To prevent further acute inflammation, the inflammatory cells are finally removed by apoptosis. The end result is that the tissue returns to normal and normal anatomy is restored. Resolution is only possible if there is minimal destruction of the extracellular matrix and the tissue has cells capable of dividing by mitosis to replace cells lost in the inflammatory process.
Healing by fibrosis
This is the process of organisation by granulation tissue (see Ch. 10).
Progression to chronic inflammation
Chronic inflammation
Chronic inflammation is an inflammatory response lasting more than a few days and is characterised by the presence of lymphocytes, plasma cells and macrophages. It is generally associated with significant tissue damage and so healing occurs by granulation tissue formation and fibrosis (see Ch. 10).
Clinically, chronic inflammation may occur as a result of:
• progression from acute inflammation due to persistence of the inflammatory stimulus, such as a foreign body or persistent microorganisms
• chronic inflammation ab initio (i.e. without preceding acute inflammation): typically seen as the response to intracellular organisms (e.g. mycobacteria, viruses), in many autoimmune diseases (e.g. rheumatoid arthritis, contact dermatitis), and in cases of chronic rejection of transplants.
9.2. Vascular events in inflammation
Vasodilatation
This occurs rapidly in the early acute inflammatory response and leads to increased blood flow into the damaged tissue. It is principally due to relaxation of arterioles.
Increased vascular permeability
Venules and capillaries are lined by a continuous layer of endothelium which keeps blood cells and large proteins within the vessel; water, oxygen and carbon dioxide pass freely through the vessel wall. In inflamed tissue, there is an increase in hydrostatic pressure due to the relaxation of arterioles. There is also an increase in the permeability (‘leakiness’) of the blood vessel to proteins, which can occur in a number of ways:
• gaps between endothelial cells induced by histamine, leukotrienes, etc.
• direct injury to the endothelial cells by the injurious agent (toxins, ischaemia, burns, etc.)
• increased transport of proteins across cell membranes stimulated by cytokines.
The increased intravascular hydrostatic pressure and increased permeability allow fluid and proteins to leak into the extracellular tissues. This protein-rich fluid is called exudate and is responsible for the oedema of inflammation. Within the dilated vascular beds, blood flow will slow dramatically as exudate is lost from the vascular space into tissues. Many of the proteins in the exudate are important in the inflammatory reaction, e.g. antibodies and acute-phase reactants.
9.3. Cellular processes of inflammation
Cells of the inflammatory response
Neutrophil polymorphs
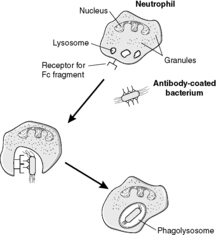
Neutrophils are the first cells to appear at the site of acute inflammation. They are phagocytes, and their function is to degrade cell debris and to ingest and kill microbes. Neutrophils originate in the bone marrow and have a short tissue lifespan of only 3–4days.
They kill microorganisms by two main mechanisms: digestive enzymes in lysosomes and oxygen-dependent production of free radicals (see Box 12 and Figure 23). Neutrophils are an important component of pus (Box 13).
Box 12
Neutrophils and macrophages both kill microorganisms by two main methods:
• Their lysosomes contain bactericidal intracellular enzymes:
• myeloperoxidase
• lysozyme
• acid hydrolase.
The lysosomes fuse with the vacuole containing the ingested material, releasing the enzymes which can destroy the contents. The resulting structure is called a phagolysosome.
• Highly reactive oxygen species and other free radicals that are toxic to microorganisms are produced in the wall of the phagolysosome. This process requires a burst of oxidative phosphorylation, and so it is called the oxygen-dependent mechanism.
Neutrophils, macrophages and eosinophils can all release their antimicrobial products into the extracellular space. This process occurs if the antigen cannot be ingested. Although of potential benefit, for example by attacking parasites that are too big for phagocytosis, there is also the potential for considerable damage to adjacent tissues.
Box 13
Pus is thick, sticky fluid, white, yellow or greenish, formed from neutrophils (both alive and dead), cell debris from necrotic tissue, bacteria (both alive and dead) and inflammatory fluid exudate. Although it occurs under a wide variety of different circumstances, it is particularly characteristic of inflammation caused by pyogenic bacteria (see Glossary). Inflammation characterised by pus is called suppurative inflammation.
A collection of pus within the tissues is called an abscess. Unless drained, abscesses become surrounded by granulation tissue and fibrosis. The pressure inside abscesses tends to increase due to osmotic influx of water into the abscess cavity from the surrounding tissues. Abscesses may discharge their contents via a sinus tract, which is defined as an abnormal connection, lined by granulation tissue or epithelium, connecting an abscess with a mucosal surface or the skin. Sometimes, this process leads to an abnormal passage between two mucosal surfaces or a mucosal surface and the skin, called a fistula. If pus accumulates in a pre-existing body cavity, e.g. the gall bladder or pleural cavity, the term empyema is used.
Eosinophils
These phagocytic cells originate in the bone marrow and have striking red/pink intracytoplasmic granules when visualised using a haematoxylin and eosin stain. They are associated with type I hypersensitivity responses and parasitic infestations. Their granules contain substances such as major basic protein which are toxic to parasites. Large numbers of eosinophils may alert the pathologist to the possibility of an allergic reaction or the presence of a parasite.
Basophils and mast cells
Mast cells have cytoplasmic granules which contain heparin and histamine and enzymes such as tryptase and acid hydrolase. They release these substances rapidly by degranulation in response to a variety of stimuli. An important cause of mast cell degranulation is the type I hypersensitivity reaction mediated by IgE. They have IgE Fc receptors on their cell membranes, and binding of IgE to the Fc receptors leads to release of the granule contents into the tissues. Other causes of degranulation include physical agents (heat, cold, trauma), various cytokines, and complement components C3a and C5a.
Basophils resemble mast cells but are found circulating in the blood rather than in tissues.
Macrophages
Macrophages are the major scavenger cells of the body. They are derived from blood monocytes and are attracted to sites of inflammation by chemotactic factors, appearing 12–24 hours later than neutrophils. A term sometimes used for macrophages by pathologists is ‘histiocytes’. Macrophages are long-lived phagocytic cells and contain powerful intracellular enzymes which degrade particulate matter including dead neutrophils and microorganisms (see Box 12 and Figure 23). In addition to their phagocytic function they orchestrate many of the cellular, vascular and reparative responses of inflammation by releasing cytokines (e.g. interleukins, interferons, TNF-α, transforming growth factor beta (TGF-β) and neutrophil chemotactic factor). They also produce coagulation factors such as factor VIII. The role of macrophages in antigen presentation is discussed in Chapters 7 and 8.
Lymphocytes and plasma cells
These cells are seen in a wide variety of different inflammatory reactions. They are discussed in Chapters 7 and 8.
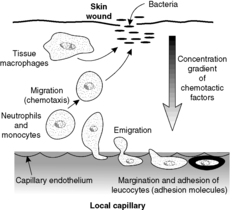
Recruitment of inflammatory cells to sites of inflammation
The recruitment of inflammatory cells involves a number of steps, all of which are controlled by a variety of mechanisms. The overall process is called extravasation or emigration.
Margination
In inflammation, blood flow slows as vessels dilate and fluid enters the interstitium, as described previously. Consequently, leucocytes move out of the central (axial) column of blood, where they usually flow, and accumulate at the periphery of the vessels, where they can interact with endothelial cells. This process, the first step in extravasation, is called margination and occurs particularly in the post-capillary venules.
Rolling and adhesion
Both endothelial cells and leucocytes can display a number of cell adhesion molecules on their surface membranes. In inflammation, under the influence of cytokines, cell adhesion molecules are up-regulated. For example, vascular endothelial cells can display adhesion molecules which will bind to neutrophils and monocytes; some are already present within the endothelial cell and are redistributed to the surface, others are newly produced. The neutrophils and monocytes also have surface adhesion molecules (integrins) that can bind to their ligands on the endothelial surface.
The interactions between leucocytes and endothelial cells result in the former rolling along the endothelial surface. Finally, the leucocytes become firmly stuck (adhesion; see Box 14).
Box 14
Interactions between different cells and between cells and connective tissue are mediated by cell adhesion molecules, which are transmembrane molecules with extracellular and intracellular domains. The latter may attach to the cytoskeleton. The interactions are essentially those of receptor and ligand, i.e. they link to one another in a similar manner to a lock and key. Cell adhesion molecules are involved in a wide variety of cellular processes. In normal tissues, they are the mechanism whereby cells adhere to each other and the interstitial matrix. Furthermore, this binding is not simply a passive process – the intracellular domain can act a the first step in a signal transduction sequence. Thus, the differentiation of a cell depends in part on the nature of the underlying matrix. When cells move through tissues (whether during tissue remodelling or inflammation), cell adhesion molecules are involved. Malignant cells show abnormalities of cell adhesion molecule expression, allowing them to detach from adjacent cells and then move abnormally through the tissues (i.e. invade). In addition, cell adhesion molecules can be a component of immune reactions.
Emigration
The leucocytes are able to move into the tissues through the endothelium, mainly by passing between endothelial cells and then degrading the basement membrane with collagenases. This process is called emigration. The cells move in an amoeboid fashion.
Chemotaxis
After extravasation, the leucocytes are attracted by substances produced in the inflamed tissue. To be precise, the leucocytes move through the tissues along a concentration gradient (chemotaxis), serving to bring them to the site of inflammation. Substances that may be chemotactic for leucocytes include bacterial products, complement components (especially C5a) and cytokines.
9.4. Granulomatous inflammation
Under some circumstances, macrophages may transform into epithelioid macrophages or fuse to form giant cells. Epithelioid macrophages have developed into cells that specialise in secreting cytokines and other products; they have a secretory morphology, i.e. abundant ribosomes and endoplasmic reticulum, and only limited phagocytic capabilities. Giant cells are large multinucleated cells, sometimes with dozens of nuclei. They form when multiple macrophages attempt to engulf indigestible material such as foreign substances or certain microorganisms, and the macrophages fuse together producing a multinucleated giant cell. Giant cells in which the nuclei are arranged in a peripheral rim are known as Langhans’ giant cells; they are often (though not always) associated with tuberculosis.
A granuloma is an aggregate of epithelioid macrophages, often with giant cells as well. Thus, granulomatous inflammation is a special type of chronic inflammation in which granulomas are present (Figure 24). The identification of granulomas in a specimen may suggest the diagnosis of one of the specific causes of granulomatous inflammation, for example:
• indigestible organisms, e.g. mycobacteria, some fungi, parasites
• exogenous foreign materials, e.g. silica, talc, suture material, beryllium
• endogenous foreign materials, e.g. cholesterol crystals, uric acid crystals, free keratin
• unknown mechanisms, as seen in conditions such as rheumatoid arthritis, Crohn’s disease and sarcoidosis.
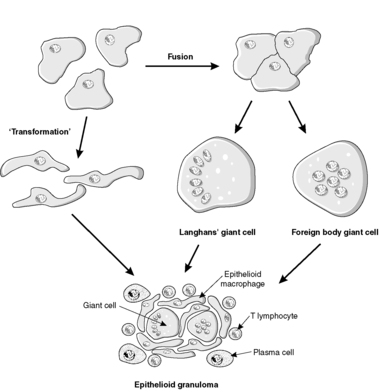 |
| Figure 24 |
The presence of caseating necrosis in the centre of a granuloma is particularly associated with mycobacterial infection (see Ch. 4). Note that granulation tissue is different from a granuloma – the two are sometimes confused by students.
Self-assessment: questions
True-false questions
1. During the acute inflammatory response:
a. histamine causes vasodilatation
b. the exuded fibrin is formed by local fibroblasts
c. there is upregulation of cell adhesion molecules on endothelial cells
d. red cell extravasation is a passive phenomenon
e. complement components may act as chemoattractants
2. The following are correctly paired:
a. granulomatous inflammation – Mycobacterium tuberculosis
b. plasma cells – phagocytosis
c. pus – collection of neutrophils
d. eosinophils – parasitic infection
e. Langerhans’ cells – Mycobacterium tuberculosis
Extended matching items questions (EMIs)
EMI 1
Theme: Inflammatory mediators
A. C-reactive protein
B. histamine
C. immunoglobulin
D. IL-1
E. neutrophil acid hydrolase
F. nitric oxide
For each of the following, select the most appropriate response from the list:
1. An acute-phase reactant.
2. A cytokine.
3. A principal product of mast cells.
Case history questions
Case history 1
A 10-year-old girl is admitted to hospital with a 3-day history of right iliac fossa pain and fever. On examination there is severe abdominal tenderness. Appendicectomy is performed. At operation, the tip of the appendix is swollen and the serosal surface covered in a purulent exudate.
1. State the most likely diagnosis.
2. The appendix is submitted to the histopathology laboratory for examination. What are the likely histological features of the tip of the appendix?
3. Explain the likely pathogenesis of the raised temperature.
4. What are the possible outcomes of acute appendicitis?
5. What is the difference between an exudate and a transudate?
Case history 2
A 63-year-old man has part of his large bowel removed through an abdominal incision. Five days after operation, the wound is red, hot and painful and pus is seen coming out from one end.
1. Briefly describe the likely pathogenesis.
2. The patient’s doctor wishes to identify the organism responsible. What sample should be taken and where should it be sent?
3. What is the nature of the giant cells in this case?
4. Name three other types of pathological giant cell and briefly state their main characteristics.
Self-assessment: answers
One best answer
1. b. The inflammatory exudate is rich in fibrinogen (an acute-phase reactant), which becomes converted to a fibrin meshwork by thrombin. This inhibits the movement of organisms and gives phagocytes a supporting structure.
2. a. It is worth knowing the cardinal signs, if only because they are a favoured question for medical students!
3. e. The clinical description is of a skin abscess called a boil; the fluid that was drained was pus. Such lesions are typically due to pyogenic organisms such as Staph. aureus. These organisms cause suppurative inflammation. Pus may form diffusely in tissue planes, or collect in discrete foci that may become walled-off by fibrin, granulation tissue and eventually fibrous scarring (i.e. an abscess). An abscess wall effectively isolates the infected locus, but this can prevent the body’s inflammatory cells reaching it and also prevent antibiotics from being effective. This is why surgical intervention to drain the abscess is the most effective treatment. In this case, incision and drainage (sometimes called lancing the boil) was performed. Clostridium perfringens causes gas gangrene (see Ch. 4) and not discrete abscesses. Guinea worm is a parasite, so eosinophil-rich inflammation would be expected rather than an abscess (unless there is secondary infection with a skin organism such as Staph. aureus). Lice cause an itchy rash, although they may also be a vector for various systemic infections such as typhus. Mycobacteria cause caseous inflammation, not suppuration.
4. c. Neutrophils characterise acute inflammation. Plasma cells, lymphocytes and eosinophils are found in chronic inflammatory reactions (although some neutrophils may be present also).
True-false answers
1.
a. True. Histamine is a vasoactive amine secreted by mast cells. It acts via receptors on post-capillary venules during the early phase of acute inflammation to increase permeability of the vessel.
b. False. This is a common examiner’s trick. Fibroblasts secrete procollagen, which is converted to collagen outside the cell. In fact no cells produce fibrin; fibrin is derived from fibrinogen, a hepatocyte-derived clotting factor.
c. True. Upregulation of adhesion molecules promotes adherence by circulating neutrophils.
d. True. Whereas the process of inflammatory cell emigration is an active, ‘purposeful’ phenomenon, red cells leave the vessel as a result of the combined effects of raised intravascular hydrostatic pressure and vascular leakiness. This passive emigration of red cells is called diapedesis.
e. True. Chemotaxis is the unidirectional, purposeful flow of neutrophils or macrophages towards a chemoattractant. The cells typically have receptors for the molecule on their surfaces. Components of the complement pathway such as C5a can attract inflammatory cells such as neutrophils into the inflamed area.
2.
a. True. Tuberculosis is a classic example of a chronic granulomatous inflammatory disease.
b. False. Phagocytosis is the process by which neutrophils and macrophages engulf and then dispose of particulate material. Plasma cells have no such ability; their function is to produce antibody.
c. True. Pus is a collection of viable and dead or dying neutrophils, admixed with exudate and the non-viable tissue in which the acute inflammatory reaction has occurred.
d. True. Eosinophils are often the dominant inflammatory cell involved in parasitic infections. Eosinophil products make the microenvironment around the parasites unsuitable for their continuing survival.
e. False. This is a common misconception. The giant cells in a tuberculosis granuloma are known as Langhans’ giant cells not Langerhans’ cells, which are dendritic antigen-presenting cells in the epidermis.
EMI answers
EMI 1
Theme: Inflammatory mediators
1. A. C-reactive protein is one of the acute-phase proteins released from the liver as part of the systemic inflammatory response. Its function seems to be to act as an opsonin, promoting phagocytosis of bacteria.
2. D. IL-1 is an important cytokine with local and systemic effects. A useful way of thinking about cytokines is to divide them into pro-inflammatory cytokines that upregulate inflammatory responses, and anti-inflammatory cytokines that inhibit inflammation and damp down the process. IL-1 is a pro-inflammatory cytokine (others are TNF-α, IL-2 and IL-6). Anti-inflammatory cytokines include IL-4, IL-10 and TGF-β.
3. B. Histamine is stored in mast cell granules, ready for instant action when released on degranulation.
Case history answers
Case history 2
1. The patient’s wound site exhibits features of inflammation with pus formation. The probable cause is acute suppurative inflammation due to infection of the wound. The causative bacteria could derive from the skin or bowel.
2. Swabs of the pus and wound edges should be taken and submitted to the microbiology laboratory for microscopy and culture.
3. Foreign body giant cells are derived from macrophages. They have multiple nuclei and contain indigestible foreign material in their cytoplasm.
4.
• Langhans’ giant cells: often found in granulomas; have nuclei disposed in a rim (Figure 24).
• Touton giant cells: found in lipid-containing lesions such as xanthelasma and areas of fat necrosis; they have a central, ring-like arrangement of nuclei and peripheral fat droplets in their cytoplasm.
• Multinucleated tumour giant cell: seen in some neoplasms.
Comment: You might have thought of other types of giant cell, but note that not all large, multinucleated cells are pathological: examples include bone-resorbing osteoclasts and platelet-producing megakaryocytes.