Chapter 10 Inflammation
Inflammation is usually classified according to its time course as:
ACUTE INFLAMMATION
Causes of acute inflammation
Essential macroscopic appearances of acute inflammation
Redness (rubor)
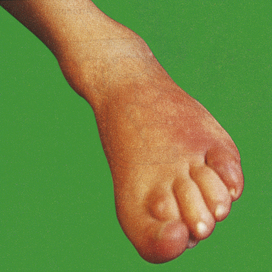
An acutely inflamed tissue appears red, for example skin affected by sunburn, cellulitis due to bacterial infection or acute conjunctivitis. This is due to dilatation of small blood vessels within the damaged area (Fig. 10.1).
Heat (calor)
Increase in temperature is seen only in peripheral parts of the body, such as the skin. It is due to increased blood flow (hyperaemia) through the region, resulting in vascular dilatation and the delivery of warm blood to the area. Systemic fever, which results from some of the chemical mediators of inflammation, also contributes to the local temperature.
Swelling (tumor)
Swelling results from oedema—the accumulation of fluid in the extravascular space as part of the fluid exudate—and, to a much lesser extent, from the physical mass of the inflammatory cells migrating into the area (Fig. 10.2). As the inflammation response progresses, formation of new connective tissue contributes to the swelling.
Early stages of acute inflammation
Changes in vessel calibre
The microcirculation consists of the network of small capillaries lying between arterioles, which have a thick muscular wall, and thin-walled venules. Capillaries have no smooth muscle in their walls to control their calibre, and are so narrow that red blood cells must past through them in single file. The smooth muscle of arteriolar walls forms precapillary sphincters which regulate blood flow through the capillary bed. Flow through the capillaries is intermittent, and some form preferential channels for flow while others are usually shut down (Fig. 10.3).
In blood vessels larger than capillaries, blood cells flow mainly in the centre of the lumen (axial flow), while the area near the vessel wall carries only plasma (plasmatic zone). This feature of normal blood flow keeps blood cells away from the vessel wall.
Increased vascular permeability
Small blood vessels are lined by a single layer of endothelial cells. In some tissues, these form a complete layer of uniform thickness around the vessel wall, while in other tissues there are areas of endothelial cell thinning, known as fenestrations. The walls of small blood vessels act as a microfilter, allowing the passage of water and solutes but blocking that of large molecules and cells. Oxygen, carbon dioxide and some nutrients transfer across the wall by diffusion, but the main transfer of fluid and solutes is by ultrafiltration, as described by Starling. The high colloid osmotic pressure inside the vessel, due to plasma proteins, favours fluid return to the vascular compartment. Under normal circumstances, high hydrostatic pressure at the arteriolar end of capillaries forces fluid out into the extravascular space, but this fluid returns into the capillaries at their venous end, where hydrostatic pressure is low (Fig. 10.4). In acute inflammation, however, not only is capillary hydrostatic pressure increased, but there is also escape of plasma proteins into the extravascular space, increasing the colloid osmotic pressure there. Consequently, much more fluid leaves the vessels than is returned to them. The net escape of protein-rich fluid is called exudation; hence, the fluid is called the fluid exudate.
Ultrastructural basis of increased vascular permeability
The ultrastructural basis of increased vascular permeability was originally determined using an experimental model in which histamine, one of the chemical mediators of increased vascular permeability, was injected under the skin. This caused transient leakage of plasma proteins into the extravascular space. Electron microscopic examination of venules and small veins during this period showed that gaps of 0.1–0.4 μm in diameter had appeared between endothelial cells. These gaps allowed the leakage of injected particles, such as carbon, into the tissues. The endothelial cells are not damaged during this process. They contain contractile proteins such as actin, which, when stimulated by the chemical mediators of acute inflammation, cause contraction of the endothelial cells, pulling open the transient pores. The leakage induced by chemical mediators, such as histamine, is confined to venules and small veins. Although fluid is lost by ultrafiltration from capillaries, there is no evidence that they too become more permeable in acute inflammation.
Other causes of increased vascular permeability
In addition to the transient vascular leakage caused by some inflammatory stimuli, certain other stimuli, e.g. heat, cold, ultraviolet light and X-rays, bacterial toxins and corrosive chemicals, cause delayed prolonged leakage. In these circum-stances, there is direct injury to endothelial cells in several types of vessel within the damaged area (Table 10.1).
| Time course | Mechanisms |
|---|---|
| Immediate transient | Chemical mediators, e.g. histamine, bradykinin, nitric oxide, C5a, leukotriene B4, platelet activating factor |
| Immediate sustained | Severe direct vascular injury, e.g. trauma |
| Delayed prolonged | Endothelial cell injury, e.g. X-rays, bacterial toxins |
Formation of the cellular exudate
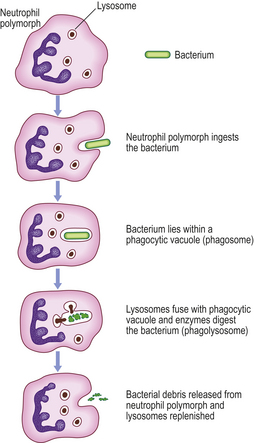
The accumulation of neutrophil polymorphs within the extracellular space is the diagnostic histological feature of acute inflammation. The stages whereby leukocytes reach the tissues are shown in Figure 10.5.
Adhesion of neutrophils
Endothelial cell expression of selectins, such as endothelial– leukocyte adhesion molecule-1 (ELAM-1), which establishes the first loose contact between leukocytes and endothelium (resulting in ‘rolling’), integrins, and intercellular adhesion molecule-1 (ICAM-1), to which the leukocytes’ surface adhesion molecules bond, is increased by:
Later stages of acute inflammation
Chemical mediators of acute inflammation
Chemical mediators released from cells
5-Hydroxytryptamine (serotonin).
This is present in high concentration in platelets. It is a potent vasoconstrictor.
Plasma factors
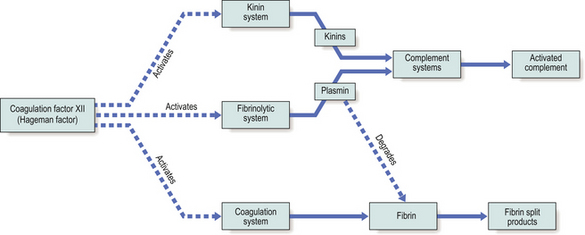
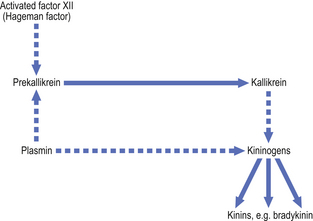
Coagulation factor XII (the Hageman factor), once activated by contact with extracellular materials such as basal lamina, and various proteolytic enzymes of bacterial origin, can activate the coagulation, kinin and fibrinolytic systems. The inter-relationships of these systems are shown in Figure 10.6.
Kinin system.
The kinins are peptides of 9–11 amino acids; the most important is bradykinin. The kinin system is activated by coagulation factor XII (Fig. 10.7). Bradykinin is also a chemical mediator of the pain that is a cardinal feature of acute inflammation.
Complement system.
The products of complement activation most important in acute inflammation include:
Table 10.2 summarises the chemical mediators involved in the three main stages of acute inflammation.
Table 10.2 Endogenous chemical mediators of the acute inflammatory response
| Status of acute inflammatory response | Chemical mediators |
|---|---|
| Vascular dilatation | HistamineProstaglandins PGE2/I2 VIP Nitric oxide PAF |
| Increased vascularpermeability | Transient phase—histamineProlonged phase—mediators such as bradykinin, nitric oxide, C5a, leukotriene B4 and PAF, potentiated by prostaglandins |
| Adhesion of leukocytes to endothelium | Up-regulation of adhesion molecules on endothelium, principally by IL-8, C5a, leukotriene B4, PAF, IL-1 and TNF-alpha |
| Neutrophil polymorph chemotaxis | Leukotriene B4, IL-8 and others |
Role of the neutrophil polymorph
The neutrophil polymorph is the characteristic cell of the acute inflammatory infiltrate (Fig. 10.8). The actions of this cell will now be considered.
Movement
Contraction of cytoplasmic microtubules and gel/sol changes in cytoplasmic fluidity bring about amoeboid movement. These active mechanisms are dependent upon calcium ions and are controlled by intracellular concentrations of cyclic nucleotides. The movement shows a directional response (chemotaxis) to the various chemicals of acute inflammation.
Intracellular killing of micro-organisms
Oxygen-dependent mechanisms.
The neutrophils produce hydrogen peroxide which reacts with myeloperoxidase in the cytoplasmic granules (Fig. 10.8) in the presence of halide, such as C1–, to produce a potent microbicidal agent. Other products of oxygen reduction also contribute to the killing, such as peroxide anions (O2–), hydroxyl radicals (•OH) and singlet oxygen (1O2).
Special macroscopic appearances of acute inflammation
Serous inflammation
In serous inflammation, there is abundant protein-rich fluid exudate with a relatively low cellular content. Examples include inflammation of the serous cavities, such as peritonitis, and inflammation of a synovial joint, acute synovitis. Vascular dilatation may be apparent to the naked eye, the serous surfaces appearing injected (Fig. 10.2), i.e. having dilated, blood-laden vessels on the surface (like the appearance of the conjunctiva in ‘blood-shot’ eyes).
Suppurative (purulent) inflammation
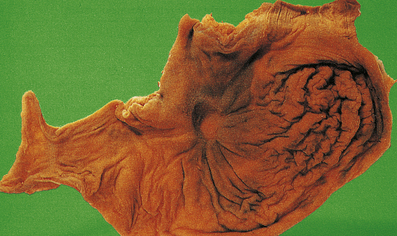
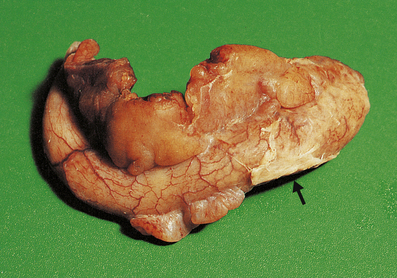
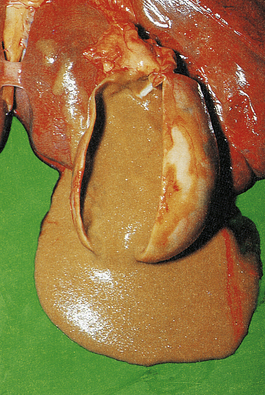
The terms ‘suppurative’ and ‘purulent’ denote the production of pus, which consists of dying and degenerate neutrophils, infecting organisms and liquefied tissues. The pus may become walled-off by granulation tissue or fibrous tissue to produce an abscess (a localised collection of pus in a tissue). If a hollow viscus fills with pus, this is called an empyema, for example empyema of the gallbladder (Fig. 10.9) or of the appendix (Fig. 10.10).
Pseudomembranous inflammation
The term ‘pseudomembranous’ describes superficial mucosal ulceration with an overlying slough of disrupted mucosa, fibrin, mucus and inflammatory cells. This is seen in pseudomembranous colitis due to Clostridium difficile colonisation of the bowel, usually following broad-spectrum antibiotic treatment (Ch. 15).
Effects of acute inflammation
Beneficial effects
Harmful effects
The release of lysosomal enzymes by inflammatory cells may also have harmful effects:
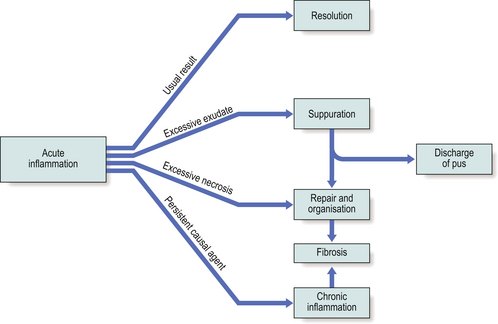
Sequelae of acute inflammation
The sequelae of acute inflammation depend upon the type of tissue involved and the amount of tissue destruction, which depend in turn upon the nature of the injurious agent. The possible outcomes of acute inflammation are shown in Figure 10.12.
Resolution
Following this, the lung parenchyma would appear histologically normal.
Suppuration
Suppuration is the formation of pus, a mixture of living, dying and dead neutrophils and bacteria, cellular debris and sometimes globules of lipid. The causative stimulus must be fairly persistent and is virtually always an infective agent, usually pyogenic bacteria (e.g. Staphylococcus aureus, Streptococcus pyogenes, Neisseria species or coliform organisms). Once pus begins to accumulate in a tissue, it becomes surrounded by a ‘pyogenic membrane’ consisting of sprouting capillaries, neutrophils and occasional fibroblasts; this is a manifestation of healing, eventually resulting in granulation tissue and scarring. Such a collection of pus is called an abscess, and bacteria within the abscess cavity are relatively inaccessible to antibodies and to antibiotic drugs (thus, for example, acute osteomyelitis, an abscess in the bone marrow cavity, is notoriously difficult to treat).
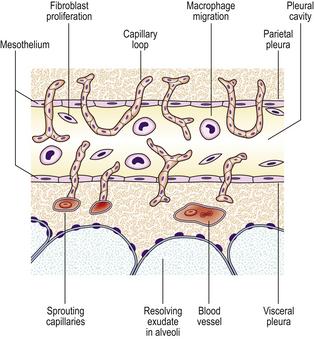
Organisation
During organisation, new capillaries grow into the inert material (inflammatory exudate), macrophages migrate into the zone and fibroblasts proliferate under the influence of TGF-beta, resulting in fibrosis and, possibly, scar formation. A good example of this is seen in the pleural space following acute lobar pneumonia. Resolution usually occurs in the lung parenchyma, but very extensive fibrinous exudate fills the pleural cavity (Fig. 10.11). The fibrin is not easily removed and consequently capillaries grow into the fibrin, accompanied by macrophages and fibroblasts (the exudate becomes ‘organised’). Eventually, fibrous adhesion occurs between the parietal and visceral pleura (Fig. 10.13). Fibrous adhesions also occur commonly in the peritoneal cavity after surgery or an episode of peritonitis; these can hamper further surgery and can also lead to intestinal obstruction.
Progression to chronic inflammation
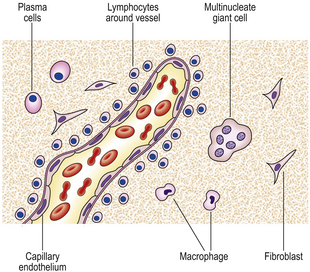
If the agent causing acute inflammation is not removed, the acute inflammation may progress to the chronic stage. In addition to organisation of the tissue just described, the character of the cellular exudate changes, with lymphocytes, plasma cells and macrophages (sometimes including multinucleate giant cells) replacing the neutrophil polymorphs (Fig. 10.14). Often, however, chronic inflammation occurs as a primary event, there being no preceding period of acute inflammation.
CHRONIC INFLAMMATION
Causes of chronic inflammation
Primary chronic inflammation
In most cases of chronic inflammation, the inflammatory response has all the histological features of chronic inflammation from the onset, and there is no initial phase of acute inflammation. Some examples of primary chronic inflammation are listed in Table 10.3.
| Cause of inflammation | Examples |
|---|---|
| Resistance of infective agent to phagocytosis and intracellular killing | Tuberculosis, leprosy, brucellosis, viral infections |
| Endogenous materials | Necrotic adipose tissue, bone, uric acid crystals |
| Exogenous materials | Silica, asbestos fibres, suture materials, implanted prostheses |
| Some autoimmune diseases |
Macroscopic appearances of chronic inflammation
The commonest appearances of chronic inflammation are:
Microscopic features of chronic inflammation
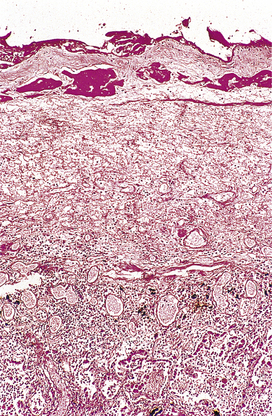
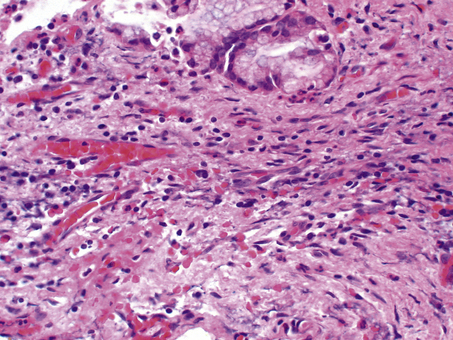
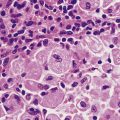
The cellular infiltrate consists characteristically of lymphocytes, plasma cells and macrophages. A few eosinophil polymorphs may be present, but neutrophil polymorphs are scarce. Some of the macrophages may form multinucleate giant cells. Exudation of fluid is not a prominent feature, but there may be production of new fibrous tissue from granulation tissue (Figs 10.15–10.17). There may be evidence of continuing destruction of tissue at the same time as tissue regeneration and repair. Tissue necrosis may be a prominent feature, especially in granulomatous conditions such as tuberculosis. It is not usually possible to predict the causative factor from the histological appearances in chronic inflammation.
Paracrine stimulation of connective tissue proliferation
Healing involves regeneration and migration of specialised cells, while the predominant features in repair are angiogenesis followed by fibroblast proliferation and collagen synthesis resulting in granulation tissue. These processes are regulated by low molecular weight proteins called growth factors which bind to specific receptors on cell membranes and trigger a series of events culminating in cell proliferation (Table 10.4).
Table 10.4 Growth factors involved in healing and repair associated with inflammation
| Growth factor | Abbreviation | Main function |
|---|---|---|
| Epidermal growth factor | EGF | Regeneration of epithelial cells |
| Transforming growth factor-alpha | TGF-alpha | Regeneration of epithelial cells |
| Transforming growth factor-beta | TGF-beta |
Cellular co-operation in chronic inflammation
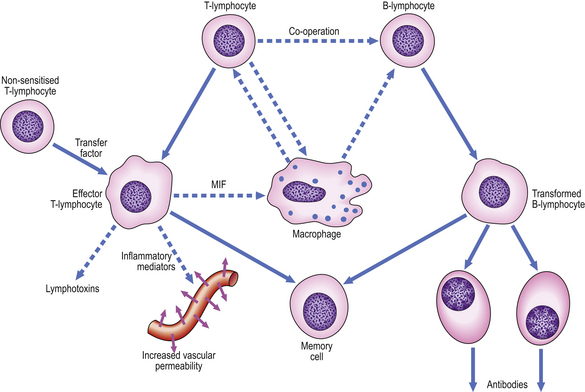
The lymphocytic tissue infiltrate contains two main types of lymphocyte (described more fully in Ch. 9). B-lymphocytes, on contact with antigen, become progressively transformed into plasma cells, which are cells specially adapted for the production of antibodies. The other main type of lymphocyte, the T-lymphocyte, is responsible for cell-mediated immunity. On contact with antigen, T-lymphocytes produce a range of soluble factors called cytokines, which have a number of important activities.
These pathways of cellular co-operation are summarised in Figure 10.18.
Macrophages in chronic inflammation
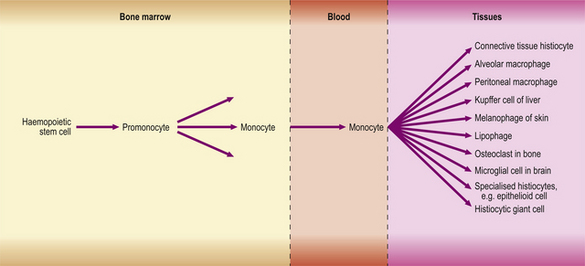
Macrophages in inflamed tissues are derived from blood monocytes that have migrated out of vessels and have become transformed in the tissues. They are thus part of the mononuclear phagocyte system (Fig. 10.19), also known as the reticulo-endothelial system.
The mononuclear phagocyte system, shown in Figure 10.19, is now known to include macrophages, fixed tissue histiocytes in many organs and, probably, the osteoclasts of bone. All are derived from monocytes, which in turn are derived from a haemopoietic stem cell in the bone marrow.
Specialised forms of macrophages and granulomatous inflammation
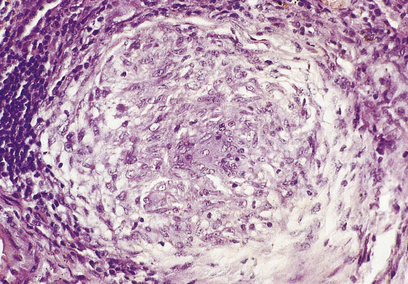
A granuloma is an aggregate of epithelioid histiocytes (Fig. 10.20). It may also contain other cell types such as lymphocytes and histiocytic giant cells. Granulomatous diseases comprise some of the most widespread and serious diseases in the world, such as tuberculosis and leprosy.
Epithelioid histiocytes
The appearance of granulomas may be augmented by the presence of caseous necrosis (as in tuberculosis) or by the conversion of some of the histiocytes into multinucleate giant cells. The association of granulomas with eosinophils often indicates a parasitic infection (e.g. worms). A common feature of many of the stimuli that induce granulomatous inflammation is indigestibility of particulate matter by macrophages. In other conditions, such as the systemic granulomatous disease sarcoidosis, there appear to be far-reaching derangements in immune responsiveness favouring granulomatous inflammation. In other instances, small traces of elements such as beryllium induce granuloma formation, but the way in which they induce the inflammation is unknown. Some of the commoner granulomatous conditions are shown in Table 10.5.
| Cause | Example |
|---|---|
| Specific infections |
Role of inflammation in systemic and organ-specific diseases
Acute inflammation is involved in the cardiovascular system in the response to acute myocardial infarction (Ch. 13) and the generation of some complications of myocardial infarction such as cardiac rupture. It is also involved in infective endocarditis, pericarditis and myocarditis, and in some vasculitic syndromes. One mechanism of vasculitis is that immune complexes deposit in the vessel wall, activate complement, and thus excite an inflammatory response.
Commonly confused conditions and entities relating to inflammation
| Commonly confused | Distinction and explanation |
|---|---|
| Acute and chronic | In inflammation, acute and chronic denote both the dynamics and character of the process. Acute inflammation has a relatively rapid onset and, usually, resolution, and neutrophil polymorphs are the most abundant cells. Chronic inflammation has a relatively insidious onset, prolonged course and slow resolution, and lymphocytes, plasma cells and macrophages (sometimes with granuloma formation) are the most abundant cells. |
| Exudate and transudate | Exudates have a high protein content because they result from increased vascular permeability. Transudates have a low protein content because the vessels have normal permeability characteristics. |
| Granuloma and granulation tissue | A granuloma is an aggregate of epithelioid histiocytes and a feature of some specific chronic inflammatory disorders. Granulation tissue is an important component of healing and comprises small blood vessels in a connective tissue matrix with myofibroblasts. |
| Monocytes, macrophages and histiocytes | Monocytes are the newly formed cells of the mononuclear phagocyte system. After a few hours in the blood, they enter tissues and undergo further differentiation into macrophages. Some macrophages in tissues have specific features and names (e.g. Kupffer cells); others are referred to as histiocytes. |
| Fibrin and fibrous | Fibrin is deposited in blood vessels and tissues or on surfaces (e.g. in acute inflammation) as a result of the action of thrombin on fibrinogen. Fibrous describes the texture of a non-mineralised tissue of which the principal component is collagen (e.g. scar tissue). |
Chronic inflammation is involved in myocardial fibrosis after myocardial infarction.
Inflammation also features in the tissue injury associated with neurodegenerative disorders of the central nervous system. Multiple sclerosis is a relatively common chronic demyelinating neurodegenerative disorder in which chronic inflammation plays an important role. Perivascular cuffing by plasma cells and T lymphocytes is seen in zones of white matter where macrophages break down myelin.
Badolato R., Oppenheim J.J.. Role of cytokines, acute-phase proteins, and chemokines in the progression of rheumatoid arthritis. Seminars in Arthritis and Rheumatism. 1996;26:526-538.
Balkwill F.. Cytokine amplification and inhibition of immune and inflammatory responses. Journal of Viral Hepatitis. 1997;4(Suppl 2):6-15.
Ballou S.P., Kushner I.. Chronic inflammation in older people: recognition, consequences, and potential intervention. Clinics in Geriatric Medicine. 1997;13:653-669.
Baumert P.W.Jr. Acute inflammation after injury. Quick control speeds rehabilitation. Postgraduate Medicine. 1995;97:35-36.
Borregard N., Sørensen O.E., Theilgaard-Mönch K.. Neutrophil granules: a library of innate immunity proteins. Trends in Immunology. 2007;28:340-345.
Brewer D.P.. Activities of the neutrophil polymorph. British Medical Journal. 1972;5810:396-400.
Burger D., Dayer J.M.. Inhibitory cytokines and cytokine inhibitors. Neurology. 1995;45(Suppl 6):S39-S43.
Cohen M.S.. Molecular events in the activation of human neutrophils for microbial killing. Clinical Infectious Diseases. 1994;18(Suppl 2):170-179.
Collins T.. Leukocyte recruitment, endothelial cell adhesion molecules, and transcriptional control. Insights for drug discovery. London: Kluwer; 2001.
Di Perry G., Bonora S., Allegranzi B., Concia E.. Granulomatous inflammation and transmission of infectious disease. Immunology Today. 20, 1999. 337–338
Edwards S.W.. Biochemistry and physiology of the neutrophil. Cambridge: Cambridge University Press; 1994.
Epstein W.L., Fukuyama K.. Mechanisms of granulomatous inflammation. Immunology Series. 1989;46:687-721.
Faurschou M., Borregaard N.. Neutrophil granules and secretory vesicles in inflammation. Microbes and Infection. 2003;5:1317-1327.
Formela L.J., Galloway S.W., Kingsnorth A.N.. Inflammatory mediators in acute pancreatitis. British Journal of Surgery. 1995;82:6-13.
Furie M.B., Randolph G.J.. Chemokines and tissue injury. American Journal of Pathology. 1995;146:1287-1301.
Galli S.J.. The Paul Kallos Memorial Lecture. The mast cell: a versatile effector cell for a challenging world. International Archives of Allergy and Immunology. 1997;113:14-22.
Goldblatt D., Thrasher A.J.. Chronic granulomatous disease. Clinical and Experimental Immunology. 1998;122:1-9.
Gordon S.. The role of the macrophage in immune regulation. Research in Immunology. 1998;149:685-688.
Kasama T., Miwa Y., Isozaki T., Odai T., Adachi M., Kunkel S.L.. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Current Drug Targets. 2005;4:273-279.
Kobayashi S.D., Voyich J.M., DeLeo T.. Regulation of neutrophil-mediated inflammatory response to infection. Microbes and Infection. 2003;5:1337-1344.
Kornfeld H., Mancino G., Colizzi V.. The role of macrophage in cell death and tuberculosis. Cell Death and Differentiation. 1999;6:71-78.
Lakhani S.R., Dilly S.A., Finlayson C.J., Dogan A.. Basic pathology, 3rd edn. London: Hodder Arnold; 2003.
Lawrence T., Gilroy D.W.. Chronic inflammation: a failure of resolution. International Journal of Experimental Pathology. 2007;88:85-94.
Lee W.Y., Chin A.C., Voss S., Parkos C.A.. In vitro neutrophil transepithelial migration. Methods in Molecular Biology. 341, 2006. 205–215
Leung D.Y.. Immunologic basis of chronic allergic diseases: clinical messages from the laboratory bench. Pediatric Research. 1997;42:559-568.
Levin N.W., Ronco C.. Chronic inflammation: an overview. Contributions to Nephrology. 137, 2002. 364–370
Levy J.H.. The human inflammatory response. Journal of Cardiovascular Pharmacology. 1996;27(Suppl 1):S31-S37.
Ley K.. Physiology of inflammation. Oxford: Oxford University Press; 2001.
Lue H., Kleemann R., Calandra T., Roger T., Bernhagen J.. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes and Infection. 2002;4:449-460.
Mahmoudi M., Curzen N., Gallagher P.J.. Atherogenesis: the role of inflammation and infection. Histopathology. 2007;50:535-546.
Majno G.. Chronic inflammation: links with angiogenesis and wound healing. American Journal of Surgical Pathology. 153, 1998. 1045–1049
Mannaioni P.F., Di Bello M.G., Masini E.. Platelets and inflammation: role of platelet-derived growth factor, adhesion molecules and histamine. Inflammation Research. 1997;46:4-18.
Sartor R.B.. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. American Journal of Gastroenterology. 1997;92(Suppl):5S-11S.
Savill J.. Apoptosis in resolution of inflammation. Journal of Leukocyte Biology. 1997;61:375-380.
Schultz D.R., Tozman E.C.. Antineutrophil cytoplasmic antibodies: major autoantigens, pathophysiology, and disease associations. Seminars in Arthritis and Rheumatism. 1995;25:143-159.
Serhan C.N., Brain S.D., Buckley C.D., et al. Resolution of inflammation: state of the art, definitions and terms. FASEB Journal. 2007;21:325-332.
Thomson A.W., Lotze M.T.. The cytokine handbook, 4th edn. London: Academic Press; 2003.
Tremblay G.M., Janelle M.F., Bourbonnais Y.. Anti-inflammatory activity of neutrophil elastase inhibitors. Current Opinion in Investigational Drugs. 4, 2000. 556–565
Vallance P., Collier J., Bhagat K.. Infection, inflammation, and infarction: does acute endothelial dysfunction provide a link? Lancet. 1997;349:1391-1392.
Walker A., Ward C., Taylor E.L., et al. Regulation of neutrophil apoptosis and removal of apoptotic cells. Current Drug Targets. 2005;4:447-454.






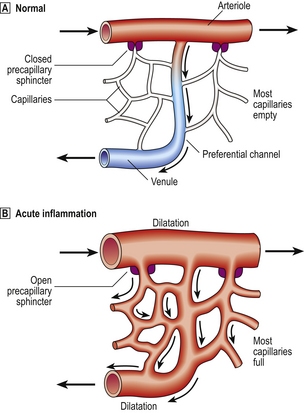
 Normally, most of the capillary bed is closed down by precapillary sphincters.
Normally, most of the capillary bed is closed down by precapillary sphincters.  In acute inflammation, the sphincters open, causing blood to flow through all capillaries.
In acute inflammation, the sphincters open, causing blood to flow through all capillaries.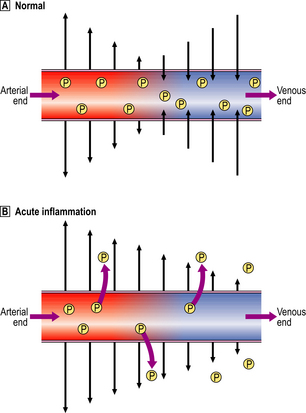
 Normally, fluid leaving and entering the vessel is in equilibrium.
Normally, fluid leaving and entering the vessel is in equilibrium.  In acute inflammation, there is a net loss of fluid together with plasma protein molecules (P) into the extracellular space, resulting in oedema.
In acute inflammation, there is a net loss of fluid together with plasma protein molecules (P) into the extracellular space, resulting in oedema.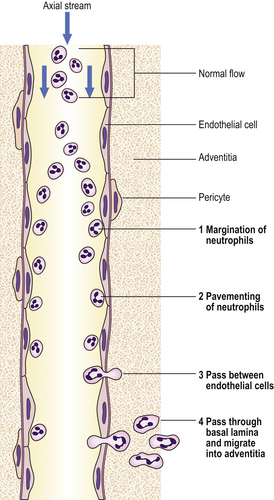
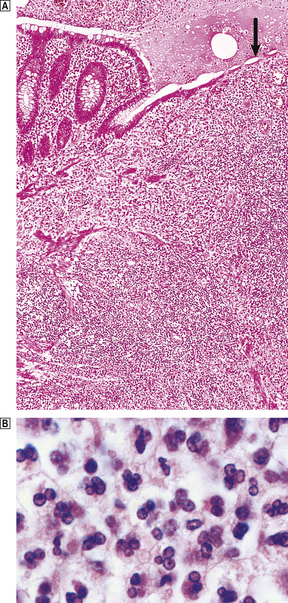
 The appendix lumen is filled with pus, there is focal mucosal ulceration (arrow) and the appendicular wall and mesoappendix (bottom) are thickened due to an acute inflammatory exudate.
The appendix lumen is filled with pus, there is focal mucosal ulceration (arrow) and the appendicular wall and mesoappendix (bottom) are thickened due to an acute inflammatory exudate.  Pus in the lumen of the appendix. Pus consists of living and degenerate neutrophil polymorphs together with liquefied tissue debris.
Pus in the lumen of the appendix. Pus consists of living and degenerate neutrophil polymorphs together with liquefied tissue debris.