Infections of the Lower Respiratory System
1. Define the trachea, bronchi, bronchioles, and alveoli, and explain the anatomic structure of the lower respiratory system.
2. List the most common etiologic agents responsible for lower respiratory disease and pneumonia in patients of various ages and categories: children <5 years of age, school-age children, young adults, older adults, and immunocompromised patients.
3. Describe the virulence factors found in bacteria and viruses associated with infection of the lower respiratory tract.
4. List the four possible routes of transmission or dissemination within the body that allow organisms to cause an infection in the lungs.
5. Name the most important decision for physicians regarding the treatment of pneumonia in older individuals, and list the three-step process used to guide them in this decision.
6. List the most prevalent cause of community-acquired pneumonia in adults.
7. Differentiate between community-acquired and hospital-acquired pneumonia.
8. State the factors anaerobic bacteria possess that enhance their ability to produce disease; explain how these anaerobes gain entrance to the lungs.
9. Define Lukens trap, and explain the type of patient or specimen associated with the method.
10. Describe the difference between early-onset or late-onset hospital- or ventilator-associated pneumonia.
11. List the etiologic agent of lung infections identified in cystic fibrosis patients.
12. Name the organisms most often associated with pneumo-opportunistic infection in HIV-positive individuals.
13. Explain the mechanisms that, because of the bacterial production of toxins, enable microorganisms to produce respiratory-associated disease.
14. Explain how the host immune system can contribute to microorganism growth in the respiratory disease process.
15. Explain why Mycobacterium tuberculosis is a classic representative of an intracellular pathogen.
16. Describe specimens collected for respiratory infections including determination of specimen quality and rejection criteria for the following: sputum, induced sputum, endotracheal suction, pleural fluid, bronchoalveolar lavage, bronchial washing, and bronchial brush sample.
17. Explain how the microbiologist would test for the less common causes of respiratory infection, including Pneumocystis jiroveci, Legionella spp., Chlamydophila pneumonia, Bordetella pertussis, Mycoplasma pneumonia, and Norcardia.
General Considerations
Anatomy
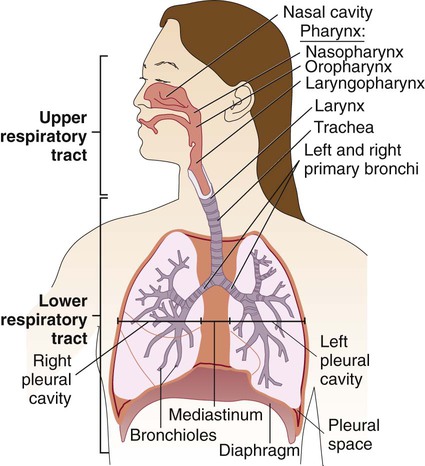
The respiratory tract can be divided into two major areas: the upper respiratory tract consists of all structures above the larynx, whereas the lower respiratory tract follows airflow below the larynx through the trachea to the bronchi and bronchioles and then into the alveolar spaces where gas exchange occurs (Figure 69-1). The respiratory and gastrointestinal tracts are the two major connections between the interior of the body and the outside environment. The respiratory tract is the pathway through which the body acquires fresh oxygen and removes unneeded carbon dioxide. It begins with the nasal and oral passages, which humidify inspired air, and extends past the nasopharynx and oropharynx to the trachea and then into the lungs. The trachea divides into bronchi, which subdivide into bronchioles, the smallest branches that terminate in the alveoli. Some 300 million alveoli are estimated to be present in the lungs; these are the primary microscopic gas exchange structures of the respiratory tract.
Familiarization with the anatomic structure of the thoracic cavity ensures proper specimen collection from various sites in the lower respiratory tract for processing by the laboratory. The thoracic cavity, which contains the heart and lungs, has three partitions separated from one another by pleura (see Figure 69-1). The lungs occupy the right and left pleural cavities, whereas the mediastinum (space between the lungs) is occupied mainly by the esophagus, trachea, large blood vessels, and heart.
Pathogenesis of the Respiratory Tract: Basic Concepts
Microorganisms primarily cause disease by a limited number of pathogenic mechanisms (see Chapter 3). Because these mechanisms relate to respiratory tract infections, they are discussed briefly. Encounters between the human body and microorganisms occur many times each day. However, establishment of infection after such contact tends to be the exception rather than the rule. Whether an organism is successful in establishing an infection depends not only on the organism’s ability to cause disease (pathogenicity) but also on the human host’s ability to prevent the infection.
Host Factors
In addition to these nonspecific host defenses, normal flora of the nasopharynx and oropharynx help prevent colonization by pathogenic organisms of the upper respiratory tract. Normal bacterial flora prevent the colonization by pathogens by competing for the same space and nutrients as well as production of bacteriocins and metabolic products that are toxic to invading organisms. Some of the bacteria that can be isolated as part of the indigenous flora of healthy hosts, as well as many species that may cause disease under certain circumstances and are often isolated from the respiratory tracts of healthy persons, are listed in Box 69-1. Under certain circumstances and for unknown reasons, these colonizing organisms can cause disease—perhaps because of previous damage by a viral infection, loss of some host immunity, or physical damage to the respiratory epithelium (e.g., from smoking). Differentiation of normal flora of the respiratory tract is important for determining the importance of an isolate in the clinical laboratory. Colonization does not always represent an infection. It is important to differentiate colonization from infection based on the specimen source, number of organisms present, and presence or quantity of white blood cells. (Organisms isolated from normally sterile sites in the respiratory tract by sterile methods that avoid contamination with normal flora should be definitively identified and reported to the clinician.)
Microorganism Factors
Toxins.
Certain microorganisms are almost always considered to be etiologic agents of disease if they are present in any numbers in the respiratory tract because they possess virulence factors that are expressed in every host. These organisms are listed in Box 69-2. The production of extracellular toxin was one of the first pathogenic mechanisms discovered among bacteria. Corynebacterium diphtheriae is a classic example of a bacterium that produces disease through the action of an extracellular toxin. Once the organism colonizes the upper respiratory epithelium, it produces a toxin that is disseminated systemically, adhering preferentially to central nervous system cells and muscle cells of the heart. Systemic disease is characterized by myocarditis, peripheral neuritis, and local disease that can lead to respiratory distress. Growth of C. diphtheriae causes necrosis and sloughing of the epithelial mucosa, producing a “diphtheritic (pseudo) membrane,” which may extend from the anterior nasal mucosa to the bronchi or may be limited to any area between—most often the tonsillar and peritonsillar areas. The membrane may cause sore throat and interfere with respiration and swallowing. Although nontoxic strains of C. diphtheriae can cause local disease, it is much milder than disease associated with toxigenic strains.
Diseases of the Lower Respiratory Tract
Bronchitis
Acute
The value of microbiologic studies to determine the cause of acute bronchitis in otherwise healthy individuals has not been established. Acute bronchitis is caused by viral agents, such as influenza and respiratory syncytial virus (RSV). The bacterium Bordetella pertussis is often associated with bronchitis in infants and preschool children (Table 69-1). The best specimen for diagnosis of pertussis is a deep nasopharyngeal specimen collected with a calcium alginate swab (see Chapter 37).
TABLE 69-1
Major Causes of Acute Bronchitis
| Bacteria | Viruses |
| Bordetella pertussis, B. parapertussis, Mycoplasma pneumoniae, Chlamydia pneumoniae |
Influenza virus, adenovirus, rhinovirus, coronavirus (other less common viruses: respiratory syncytial virus, human metapneumovirus, coxsackie A21 virus) |
Bronchiolitis
Bronchiolitis, the inflammation of the smaller diameter bronchiolar epithelial surfaces, is an acute viral lower respiratory tract infection that primarily occurs during the first 2 years of life. Characteristic clinical manifestations include an acute onset of wheezing and hyperinflation as well as cough, rhinorrhea (runny nose), tachypnea (rapid breathing), and respiratory distress. The disease is primarily caused by viruses including a recently discovered virus, human metapneumovirus. RSV accounts for 40% to 80% of cases of bronchiolitis and demonstrates a marked seasonality; the etiologic agents of bronchiolitis are listed in Box 69-3. Like other viral infections, bronchiolitis shows a marked seasonality in temperate climates with a yearly increase in cases during winter to early spring.
Initially, the virus replicates in the epithelium of the upper respiratory tract, but in the infant it rapidly spreads to the lower tract airways. Early inflammation of the bronchial epithelium progresses to necrosis. Symptoms such as wheezing may be related to the type of inflammatory response to the virus as well as other host factors. For the most part, patients are managed based on clinical parameters, with the laboratory having a role in cases that require hospitalization; a specific viral etiology can be identified in a large number of infants by viral isolation from respiratory secretions, preferably from a nasal wash (see Chapter 65).
Pneumonia
Epidemiology/Etiologic Agents
As previously mentioned, there are two major categories of pneumonias: those considered community-acquired pneumonias and hospital-, ventilator-, or health care–associated pneumonias. Because the epidemiology and etiologies can differ, these two categories are discussed separately. Pneumonia in the immunocompromised patient is addressed separately in this chapter. Emerging viral infections associated with severe acute respiratory syndrome (SARS) and influenza outbreaks (H1N1) are typically associated with upper respiratory infections but may lead to serious lower respiratory infections in the young, elderly, or immunocompromised patient. See Chapter 66 for detailed information related to these emerging viral infectious diseases and diagnostic recommendations.
Adults (Viral pneumonia).
Adults may suffer from an estimated 100 million cases annually of community-acquired viral pneumonia cased by influenza, adenovirus, enteroviruses (coxsackieviruses and rhinoviruses), coronaviruses, human metapneumovirus, parainfluenza, varicella, rubeola or RSV, particularly during epidemics. Influenza associated viral pneumonia poses an increased risk for pregnant women of approximately 4-9 times greater than the general public, with the greatest risk associated with the third trimester. RSV is considered the third most common cause of community-acquired pneumoniae with 78% of the deaths occurring in patients over the age of 65. Similarly to RSV, human metapneumovirus has been associated with outbreaks in long-term care facilities. Following viral pneumonia, secondary bacterial disease caused by beta-hemolytic streptococci, S. aureus, M. catarrhalis, H. influenzae, and Chlamydia pneumoniae. Other agents may be considered depending on the geographic location and clinical presentation are viruses in the Hantavirus group, the most common of which is sin nombre virus as well as severe acute respiratory syndrome (SARS). (See Chapter 65.)
Immunocompromised Patients.
Patients with Neoplasms.
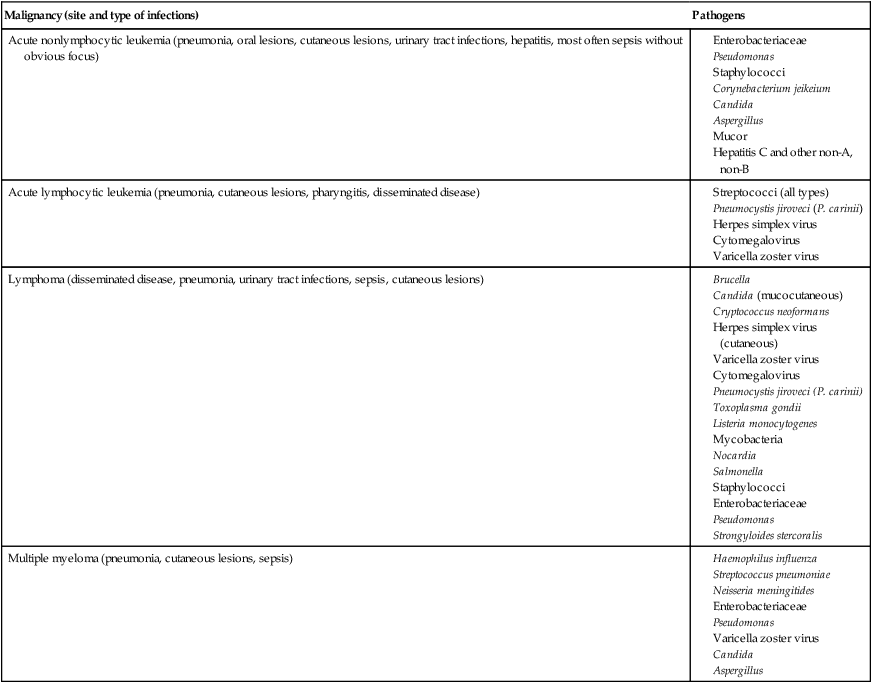
Patients with cancer are at high risk to become infected because of either granulocytopenia or other defects in phagocytic defenses, cellular or humoral immune dysfunction, damage to mucosal surfaces and the skin, and various medical procedures such as blood product transfusion. In these patients, the nature of the malignancy often determines the etiology (Table 69-2) and pneumonia is a frequent clinical manifestation.
TABLE 69-4
Examples of Infectious Agents Frequently Associated with Certain Malignancies
| Malignancy (site and type of infections) | Pathogens |
| Acute nonlymphocytic leukemia (pneumonia, oral lesions, cutaneous lesions, urinary tract infections, hepatitis, most often sepsis without obvious focus) |
Laboratory Diagnosis of Lower Respiratory Tract Infections
Specimen Collection and Transport
Although rapid determination of the etiologic agent is of paramount importance in managing pneumonia, the responsible pathogen is not identified in as many as 50% of patients, despite extensive diagnostic testing. Unfortunately, no single test is capable of identifying all potential lower respiratory tract pathogens. Refer to Table 5-1 for an overview of the method used to collect, transport, and process specimens from the lower respiratory tract.
Endotracheal or Tracheostomy Suction Specimens
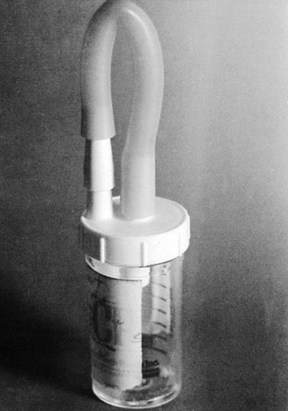
Patients with tracheostomies are unable to produce sputum in the normal fashion, but lower respiratory tract secretions can easily be collected in a Lukens trap (Figure 69-2). Tracheostomy aspirates or tracheostomy suction specimens should be treated as sputum by the laboratory. Patients with tracheostomies rapidly become colonized with gram-negative bacilli and other nosocomial pathogens. Such colonization per se is not clinically relevant, but these organisms may be aspirated into the lungs and cause pneumonia. Culture results should be correlated with clinical signs and symptoms.
Bronchoscopy.
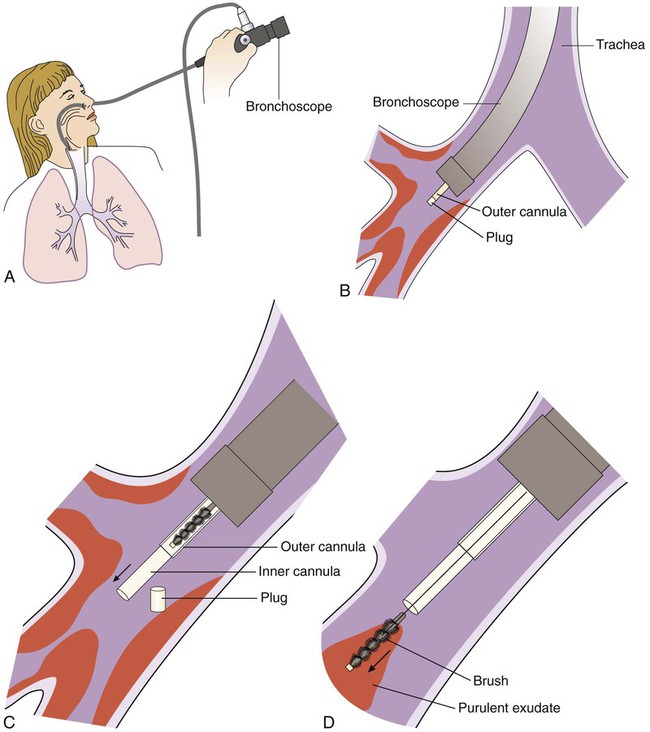
Another type of respiratory specimen is obtained via a protected catheter bronchial brush as part of a bronchoscopy examination. Specimens obtained by this moderately invasive collection procedure are suited for microbiologic studies, particularly in aspiration pneumonia. Protected specimen brush bristles collect from 0.001 to 0.01 mL of material. An overview of the collection process is shown in Figure 69-3. Upon receipt, contents of the bronchial brush may be suspended in 1 mL of broth solution with vigorous vortexing and inoculated onto culture media using a 0.01-mL calibrated inoculating loop. Some researchers have indicated that specimens obtained via double-lumen–protected catheters are suitable for both anaerobic and aerobic cultures. Colony counts of greater than or equal to 1000 organisms per milliliter in the broth diluent (or 106/mL in the original specimen) have been considered to correlate with infection. All facets of the bronchoscopic procedure—such as order of sampling, use of anesthetic, and rapidity of plating—should be rigorously standardized.
Other Invasive Procedures.
When pleural empyema is present, thoracentesis may be used to obtain infected fluid for direct examination and culture. This constitutes an excellent specimen that accurately reflects the bacteriology of an associated pneumonia. Laboratory examination of such material is discussed in Chapter 77. Blood cultures, of course, should always be obtained from patients with pneumonia.
Specimen Processing
Direct Visual Examination
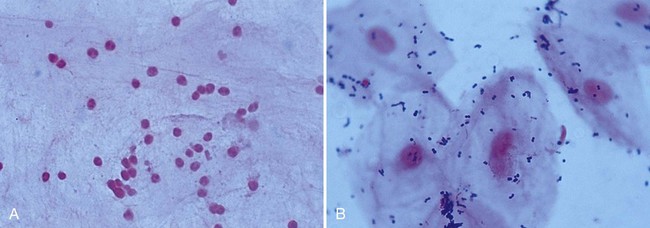
For most other evaluations, the specimen must be fixed and stained. Bacteria and yeasts can be recognized on Gram stain. One of the most important uses of the Gram stain, however, is to evaluate the quality of expectorated sputum received for routine bacteriologic culture. A portion of the specimen consisting of purulent material is chosen for the stain. The smear can be evaluated adequately even before it is stained, thus negating the need for Gram stain of specimens later judged unacceptable. An acceptable specimen yields fewer than 10 squamous epithelial cells per low-power field (100×). The number of white blood cells may not be relevant, because many patients are severely neutropenic and specimens from these patients will not show white blood cells on Gram stain examination. On the other hand, the presence of 25 or more polymorphonuclear leukocytes per 100× field, together with few squamous epithelial cells, implies an excellent specimen (Figure 69-4). Samples that contain predominantly upper respiratory tract material should be rejected. Previously, only expectorated sputa were suitable for rejection based on microscopic screening. However, endotracheal aspirates (ETAs) from mechanically ventilated adult patients can be screened by Gram stain. Criteria used to reject ETAs from adult patients include greater than 10 squamous epithelial cells per low-power field or no organisms seen under oil immersion (1000×). In Legionella pneumonia, sputum may be scant and watery, with few or no host cells. Such specimens may be positive by direct fluorescent antibody stain and culture, and they should not be subjected to screening procedures. Conversely, sputum from patients with CF should be screened. A throat swab is an acceptable specimen from patients with CF in selected clinical settings and should be processed in a similar manner as CF sputum. Staining of respiratory samples is useful and should be compared to culture results to reveal errors in procedures, specimen collection, and transport or specimen identification.
In addition to the Gram stain, respiratory specimens may be stained for acid-fast bacilli with either the classic Ziehl-Neelsen or the Kinyoun carbolfuchsin stain. Auramine or auramine-rhodamine is also used to detect acid-fast organisms. Because they are fluorescent, these stains fluorescent superior already here comment only are more sensitive than the carbolfuchsin formulas and are preferable for rapid screening. Slides may be restained with the classic stains directly over the fluorochrome stains as long as all of the immersion oil has been removed carefully with xylene. All of the acid-fast stains will reveal Cryptosporidium spp. if they are present in the respiratory tract, as may occur in immunosuppressed patients. These patients are often at risk of infection with P. jiroveci. Although the modified Gomori methenamine silver stain has been used traditionally to recognize Nocardia, Actinomyces, fungi, and parasites, it takes approximately 1 hour of the technologist’s time to perform, is technically demanding, and is not suitable as an emergency procedure. A fairly rapid stain, toluidine blue O, has been used in many laboratories with some success. Toluidine blue O stains Pneumocystis, Nocardia asteroides, and some fungi. A monoclonal antibody stain is the optimum stain for Pneumocystis (see Chapter 59) for less invasive specimens such as BAL and induced sputa.
Direct fluorescent antibody (DFA) staining has been used to detect Legionella spp. in lower respiratory tract specimens. Sputum, pleural fluid, aspirated material, and tissues are all suitable specimens. Because there are so many different serotypes of legionellae, polyclonal antibody reagents and a monoclonal antibody directed against all serotypes of Legionella pneumophila are used. Because of low sensitivity (50% to 75%), DFA results should not be relied on in lieu of culture. Rather, Legionella culture, DFA or urinary antigen, and serology should be performed for optimum sensitivity. See Chapter 35 for details regarding detection of Legionella spp.
Commercially available DFA reagents are also used to detect antigens of numerous viruses, including herpes simplex, cytomegalovirus, adenovirus, influenza viruses, and RSV (see Chapter 65). Commercial suppliers of reagents provide procedure information for each of these tests. Monoclonal and polyclonal fluorescent stains for Chlamydia trachomatis are available and may be useful for staining respiratory secretions of infants with pneumonia. A number of molecular amplification techniques (see Chapter 8) for the direct detection of respiratory pathogens have been described; however, the sensitivity and specificity of these assays vary greatly from one study to another. Amplification assays are also available for the direct detection of Mycobacterium tuberculosis on smear-positive specimens (see Chapter 43).
Routine Culture
Numerous bacterial agents that cause lower respiratory tract infections are not detected by routine bacteriologic culture. Mycobacteria, Chlamydia, Nocardia, Bordetella pertussis, Legionella, and Mycoplasma pneumoniae require special procedures for detection; this also applies to viruses and fungi. Optimal recovery for Mycobacterium tuberculosis requires multiple specimens for acid-fast staining culture, and at least one sample for molecular testing as recommended by the Centers for Disease Control. Refer to the appropriate chapter section for more information regarding these organisms. Finally, one must keep in mind those potential agents for bioterrorist attack, such as Bacillus anthracis, Francisella tularensis, and Yersinia pestis, that might be recovered from respiratory specimens (see Chapter 80).
1. Which of the following lung conditions are a major cause of illness and death?
2. What is the most frequent cause of community-acquired pneumonia in adults?
3. Psittacosis is a lower respiratory infection in humans caused by contact with what animal?
4. What organism may be recovered as a subacute illness in respiratory disease caused by M. tuberculosis?
5. All of the acid-fast stains will reveal which of the following organisms from respiratory specimens?
6. Which of the following stains is used to detect Legionella in lower respiratory specimens?
7. Which of the following media is used in addition to normal respiratory culture media in suspected cases of Legionnaires’ disease?
8. Which of the following organisms could potentially be isolated from a respiratory specimen and is not also considered a potential agent for bioterrorism?
9. Which of the following procedures has dramatically affected the evaluation and management of pneumonia, particularly in HIV-infected and immunocompromised patients?
_____ An acceptable sputum specimen when visualized by Gram stain must show significant white blood cells and fewer than 10 squamous epithelial cells per low-power field.
_____ Respiratory specimens first stained for acid-fast bacilli, with auramine or auramine-rhodamine, can be restained with the classic stains directly over the fluorochrome stain as long as all the immersion oil has been removed with xylene.
_____ A single culture is able to identify all potential pathogens that cause lower respiratory tract infections.
_____ Sputum is the most clinically relevant specimen received for culture in the microbiology laboratory for the diagnosis of respiratory infection.
_____ A moderate amount of time at room temperature will not affect the quality of a sputum sample.
_____ Patients with tracheostomies are unable to produce sputum in the normal fashion.
_____ The modified Gomori methenamine silver stain has been used traditionally to recognize Norcardia, Actinomyces, fungi, and parasites but is technically and time demanding.
_____ Pneumonia patients that are assessed to be risk classes II and III generally require hospitalization.
_____ The production of extracellular toxin was one of the first pathogenic mechanisms discovered in bacteria.
_____ The areas of the lungs most affected by pneumonia are the alveolar spaces and the interstitium, the supporting structure of the alveoli, and the terminal bronchioles.
a. needle puncture of the pleural cavity used to obtain pleural fluid for direct examination and culture in patients with pleural empyema
b. used exclusively for isolation of acid-fast bacilli and particularly used for young children
c. used to obtain a wedge of lung tissue to diagnose severe viral infection or other hard-to-diagnose or life-threatening pneumonia
d. good method for detecting Pneumocystis cysts and fungal elements
e. useful for detecting anaerobes such as Actinomyces but rarely still used
f. useful for isolating agents of mycobacterial or fungal disease; provides a high diagnostic yield in cases of Pneumocystis jiroveci pneumonia
g. good for microbiology studies, particularly in aspiration pneumonia
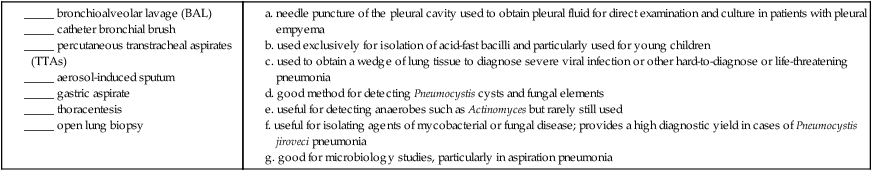
Match the term with the appropriate definition.

1. Explain the benefits of using the Binax NOW S. pneumoniae urinary antigen test for presumptive diagnosis of pneumococcal pneumonia.
2. Why are enrichment broths or anaerobe plates not used in the culture of routine sputum specimens, specimens obtained by bronchial washing, and lavage tracheal aspirates?
3. What specimens are suited for anaerobic culture with regard to respiratory culture?
4. What characteristics do the following bacteria share that enable the organisms to resist engulfment by phagocytic host cells: S. pneumoniae, N. meningitides, H. influenzae, K. pneumoniae, P. aeruginosa, and Cryptococcus neoformans.
5. Define the pathogenesis of acute bronchitis.
6. When is thoracentesis used to obtain a sample for direct examination and culture?
7. What is an important epidemiologic variable and risk factor in hospital- or ventilator-associated pneumonia?
8. What virus infection effect may predispose patients to a secondary bacterial infection?
9. What hinders diagnosing the causative agent in pediatric pneumonia in children younger than 5 years of age?