Chapter 33 Infections and Infertility
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
Several pathogens associated with STDs are known to cause infertility (Table 33-1) or other syndromes (Table 33-2). In Western society the most common of these pathogens are Neisseria gonorrhoeae and Chlamydia trachomatis. However, other pathogens may influence fertility as well.
Table 33-2 Syndromes Associated with Curable Sexually Transmitted Diseases
| Cervical cancer |
Gonorrhea
Neisseria gonorrhoeae is a gram-negative coccus that grows in pairs, hence the term diplococcus. N. gonorrhoeae mainly infects columnar or cuboidal cells and not the squamous epithelium of a postpubertal girl. After attaching to the mucosal epithelium, it penetrates through the epithelial cells and into the submucosal tissues. Neutrophils respond and cause sloughing of the epithelium, submucosal microabscesses, and formation of pus. If left untreated, neutrophils are eventually replaced by macrophages and lymphocytes.
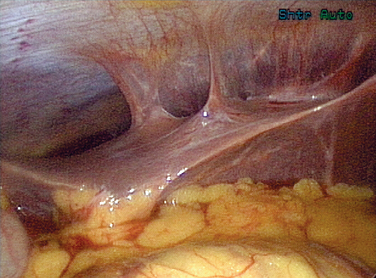
Gonorrhea can also pass beyond the fimbraie of the fallopian tubes and travel up the paracolic gutters and produce an infection around the liver. This is perihepatitis, or Fitz-Hugh–Curtis syndrome (Fig. 33-1). In unusual cases, gonorrhea can produce a disseminated disease with skin rash, infectious arthritis, and rarely endocarditis.
Treatment of a gonococcal infection depends on the location of the infection. If limited to the cervical region, the Centers for Disease Control and Prevention (CDC) recommends treatment with fluoroquinolones, ceftriaxone, or azithromycin (Table 33-3).
Table 33-3 Sexually Transmitted Disease Treatment Guidelines for Adults
| Chancroid | Azithromycin 1 g po × 1 or ceftriaxone 250 mg IM in a single dose or ciprofloxacin 500 mg po BID × 3 d or erythromycin base 500 mg po TID for 7 d |
| Herpes simplex virus — primary | Acyclovir 400 mg po TID for 7–10 d or acyclovir 200 mg po five times a day for 7–10 d or famciclovir 250 mg po TID for 7–10 d or valacyclovir 1 g po BID for 7–10 d |
| Herpes simplex virus recurrences |
* Should not be used in men who have sex with men or in those with a history of recent foreign travel or partners’ travel or infection acquired in California or Hawaii.1
Adapted from CDC Sexually Transmitted Diseases Treatment Guidelines 2006. MMWR 55:1–95, 2006.
Fluoroquinolone resistance in gonococci is becoming common in the Far East and Hawaii. Recently, it has begun emerging as a significant problem in some populations in the mainland United States. In men having sex with men, the prevalence of fluoroquinolone resistance has reached nearly 5%, prompting the CDC in 2004 to recommend that ofloxacin is not the drug of choice in this population.1 The resistance will continue to spread to other populations, and we will undoubtedly see further changes in the recommendations for the general population.
Chlamydia
The National Longitudinal Study of Adolescent Health (Wave III), using ligase chain reaction assays on first-void urine specimens, found chlamydia prevalence of 4% among young adults. The prevalence was greatest among minority women, including African Americans, Native Americans, and Latinos.2 The rate for young African American women was 6 times greater than for white women. From 1987 through 2002, the reported rate of chlamydial infections among all U.S. women increased nearly sixfold, from approximately 78 to 455 cases per 100,000 women. The etiology of this dramatic increase is likely multifactorial and includes increased screening, the use of the more sensitive nucleic acid amplification test (NAT), and better reporting, in addition to an increased infection rate.2
Cervical and upper genital tract chlamydial infections are treated with doxycycline, azithromycin, or erythromycin. The patient and all partners must be treated simultaneously. Treatment options are listed in Table 33-3.
Syphilis
The effect of syphilis on pregnancy has been well documented. Syphilis clearly produces miscarriages, prematurity, stillbirths, neonatal deaths, and congenital disease in the infant. The effects of syphilis on fertility are not clear, but a study out of Senegal did find that women age 40 or older with past or active syphilis were significantly more likely to have no history of gestation than women without evidence of syphilis infection.3
Although syphilis may have devastating long-term consequences if not treated, this disease is relatively easy to treat once diagnosed. Penicillin is the mainstay of therapy (see Table 33-3).
Trichomonas
Several studies have found that women with T. vaginalis infections had an increased risk of tubal infertility (up to 1.9-fold), and that this risk increased sixfold with more than four episodes of T. vaginalis infection.4–6 These organisms have been found in the abdominal cavity of women with acute salpingitis, and there is speculation as to whether or not the motile trichomonads are able to carry bacteria or viruses to the upper genital tract.7
Diagnosis is made most frequently using a wet mount prep of the vaginal discharge and visualizing the motile protozoa. Culture of the trichomonad is the gold standard test but is not widely used in clinical practice. A rapid test utilizing an immunochromatographic strip that can be performed rapidly in a clinical office is being evaluated for clinical use, with reported sensitivities near that of culture.
Treatment is with metronidazole 2 grams orally in a single dose. Tinidazole has also been approved in the United States for treatment of trichomoniasis in a 2-gram single oral dose, but is more costly (see Table 33-3).
Human Papillomavirus
Although HPV has not been shown to directly affect a woman’s fertility, lesions large enough to affect coitus are certainly problematic. The treatments required for localized preneoplastic or neoplastic lesions (i.e., cryotherapy, loop electrosurgical excision; see Table 33-3) can rarely cause scarring or affect the production of cervical mucosa, both of which could hinder the passage of sperm, thus affecting fertility. Invasive cervical neoplasias can require hysterectomy or other significant pelvic surgery.
Treatment does not eradicate the virus; thus prevention becomes especially important for this sexually transmitted infection. Recent advances have shown that a three-dose HPV-16 vaccine prevented the development of persistent HPV-16 infection and HPV-16-related cervical neoplasia in college age women for the first 17 months after vaccination.8 This finding will certainly lead to further vaccine developments for all of the major high-risk HPV types.
Herpes Simplex Virus
Herpes simplex virus cervicitis is found in 90% of women with primary HSV-2 infections and in 70% of women with primary HSV-1 infections. The rate of cervicitis usually decreases to less than 20% with recurrent infections.9 HSV involves the squamous epithelium of the exocervix, and the ulcerative lesions can often be seen on visual examination. In rare cases, both HSV-1 and HSV-2 have been shown to extend locally and cause PID.10 Despite the ability of HSV to cause widely disseminated infection and PID, there has been no evidence that this infection contributes to infertility. Treatment is with acyclovir, valacyclovir, or famciclovir (see Table 33-3).
Mycoplasma
Mycoplasma is a general term often used to refer to the class of more than 120 named species of organisms that are phylogenetically between bacteria and viruses. Of the more than 120 named species, Mycoplasma hominis, Ureaplasma urealyticum, and Mycoplasma genitalium are the most frequently recognized in relation to isolation from the human genitourinary tract. M. hominis has been found in the vagina in two thirds or more of women with abnormal vaginal discharge consistent with bacterial vaginosis, as compared with approximately 10% of women without abnormal discharge.11 Although it is strongly associated with bacterial vaginosis, the pathologic role of M. hominis in the disease is unclear.
M. hominis has also been isolated from the endometrium and fallopian tubes of approximately 10% of women with salpingitis diagnosed by laparoscopy.11 The role of M. hominis as a primary pathogen is unclear because it is usually isolated with other pathologic bacteria, such as gonorrhea or chlamydia.
The role of M. hominis in causing tubal infertility is also unclear. There is serologic evidence that antibodies to M. hominis have been found three times more often in infertile women with a history of PID than in controls.12 X-ray microscopy has been able to show M. genitalium binding directly to human spermatozoa and thus could be carried by motile sperm and cause upper genital tract disease in women.13
Ureaplasmas are clearly a cause of nongonococcal urethritis in men. They may cause an acute urethral syndrome in women as well. Ureaplasmas have been isolated from the fallopian tubes of patients with PID but usually in association with other known pathogens. Thus the direct role in PID is unclear.11
Chancroid
Diagnosis is often made clinically because culture of the organism is difficult. Recommended treatment regimens include azithromycin, ceftriaxone, ciprofloxacin, and erythromycin (see Table 33-3).
Hepatitis
Hepatitis A, B, and C are DNA viruses that can be sexually acquired.
Hepatitis A is usually acquired through the fecal-oral route. Food-borne outbreaks have clearly been reported,14 and outbreaks in patients who practice anal receptive intercourse, mainly men having sex with men, have also been seen. Hepatitis A produces only acute infection that is usually self-limited but can be fulminant and fatal. There is no chronic form of hepatitis A.
Hepatitis C is the most common chronic blood-borne infection in the United States, with nearly 3.9 million persons being infected.15 In an infertility practice, this infection is usually seen in patients who are being screened for an assisted reproductive technology (ART) procedure. The virus is most frequently acquired through contact with blood and blood products, but has been shown in several studies to be sexually transmitted.15 The risk of transmission through sexual contact is very low and far less than for HIV or hepatitis B. It is estimated that the risk of sexual transmission of hepatitis C virus from people with chronic infection varies from 0 to 0.6% if there is only one sex partner to 1% if there are multiple sex partners.16 Approximately 80% of persons exposed will develop chronic infection, with 60% of these developing chronic active liver disease. If a woman develops the chronic form of hepatitis B or C, she can become so significantly ill that ovarian dysfunction such as anovulation or amenorrhea can occur. Chronic carriers of hepatitis B or C can become a logistic issue for the in vitro fertilization laboratory. It is critical to keep viral-contaminated tissue separate from noncontaminated tissue.
Tuberculosis
Tuberculosis has been found in ancient Egyptian remains, but did not become a widespread problem until the 17th and 18th centuries, when urban overcrowding facilitated the ease of spread of the bacteria through respiratory droplets. This development led to the White Plague.17
Although tuberculosis is most commonly a pulmonary disease, it can affect virtually every organ system in the body, including the genitourinary tract. The incidence of genital tuberculosis in infertility clinics in Australia is 0.6% and in India is 19%, but tuberculosis is a rare cause of infertility in the United States.18
Tuberculosis is usually acquired from respiratory droplets. A pulmonary infection can spread hematogeneously to the endosalpinx, from which it may spread to the endometrium (50%), ovaries (30%), cervix (5% to 15%), or vagina (1%).17
The most common presentation of genital tuberculosis is infertility. When other symptoms are present, they are usually local and include menstrual disorders and abdominal pain. Amenorrhea may be present due to complete obliteration of the endometrial cavity, or Asherman’s syndrome, caused by postinfectious adhesions. The cervix may have an ulcerating mass that resembles carcinoma.17
CLINICAL MANIFESTATIONS
Syndromes associated with STDs can range in severity from a mild irritation to a life-threatening illness. Possible consequences include infertility, ectopic pregnancy, hepatitis and cirrhosis of the liver, and cervical cancer (see Table 33-2).
Pelvic Inflammatory Disease
PID is the inflammatory and infectious syndrome caused by the spread of microorganisms from the lower genital tract to the endometrium, fallopian tubes, ovaries, or other pelvic structures. According to the CDC, in the United States more than 1 million women per year experience an episode of acute PID and more than 100,000 women become infertile each year as a result. Annually more than 150 women in the United States die from PID or its complications.19
Pelvic inflammatory disease is responsible for a large proportion of ectopic pregnancies. It is estimated that of women acquiring PID between ages 15 and 44, almost 1% will develop ectopic pregnancies, 1.7% will develop infertility, and almost 2% will develop chronic pelvic pain.20 It is estimated that after one episode of PID there is a 13% incidence of infertility due to tubal occlusion; after two episodes, the incidence is 35%; and after three episodes of PID, infertility approaches 75%.
Pelvic inflammatory disease can be symptomatic or subclinical. Symptoms can consist of lower abdominal tenderness, cervical motion tenderness, adnexal tenderness, mucopurulent cervicitis, and fever. Unfortunately, patients may be asymptomatic and still have enough damage to the upper genital tract to cause infertility.21
Early diagnosis and treatment are imperative to avoid the long-term complications of PID. Women who have symptomatic PID and delay seeking care (present 3 to 9 days after the onset of symptoms) are twice as likely to experience infertility as those who present within 2 days of symptoms. By 9 or more days after symptom onset, the risk for infertility and ectopic pregnancy increase to 3.5 times.22 The threshold for diagnosis must be low, so as to not miss those women with minimal symptoms.
At the other end of the spectrum, some women are clearly ill enough to warrant hospital admission for PID treatment.23 Indications for hospitalization include:
The PID Evaluation and Clinical Health (PEACH) trial evaluated inpatient versus outpatient treatment regimens in 831 women with mild to moderate PID.24 This multicenter, randomized study found no difference in reproductive outcomes between women randomized to inpatient treatment and those randomized to outpatient treatment. Treatment recommendations are listed in Table 33-4.
Adapted from CDC: STD Guidelines 2006. MMWR 55:1–95, 2006.
Anatomic Areas Involved
Various portions of the female genital tract are affected by the organisms described in the first portion of this chapter (Table 33-5). The local pathologic changes in the genital tract that may affect fertility are described.
Table 33-5 Infectious Considerations at Different Anatomic Sites of Infertility
Cervix
Cervical mucus is a healthy medium that, during the periovulatory period, allows sperm to move freely through the cervix to the upper genital tract. It is produced by the secretory cells of the endocervical glands and undergoes quantitative and qualitative changes during the menstrual cycle. The cervix also undergoes anatomic changes during the menstrual cycle. The external os progressively widens during the proliferative phase, reaching maximal width just before ovulation occurs. At that time, the cervical mucus is present in large amounts, facilitating passage of the spermatozoa. After ovulation, the cervical os narrows and the mucus produced decreases in amount and becomes thicker.25
Cervical factor infertility is encountered in approximately 5% of cases in clinical practice. Inflammatory processes of the cervix, such as cervicitis, could alter the physiochemical makeup of the cervical mucus and deny sperm penetration. Pathogens include gonorrhea, chlamydia, trichomonas, and G. vaginalis, streptococci (Group B streptococci), and staphylococci. The staphylococcal and streptococcal infections tend to penetrate more deeply into the cervical wall and involve the gland acini. Gonorrhea usually spreads along contiguous mucous membrane surfaces as a superficial infection.25
Endometrium
Immune cells are part of the normal cellular population of endometrium and subendometrial areas. The cellular type is dependent on the menstrual cycle. Lymphocytes and neutrophils normally appear in the endometrium in the second half of the menstrual cycle. Their presence does not indicate endometritis. Plasma cells, however, are not normal and indicate an immune response, usually to a bacterial infection.25
Salpingitis and endometritis occur together frequently, with 70% to 90% of women with salpingitis also having endometritis.26 Endometrial biopsy can be used as a surrogate marker of tubal involvement and may make laparoscopy less necessary. Clearly, salpingitis has severe implications with regard to future fertility, and therefore accurate diagnosis is important.
The PEACH trial26 found that endometritis itself was not associated with a reduced rate of pregnancy in women with mild to moderate PID. These findings differ from those presented in the landmark study by Westrom27 on PID and fertility on several points. First, the authors concluded that endometritis does not definitively indicate salpingitis. Westrom’s study used laparoscopic evidence of salpingitis as evidence of PID, whereas the PEACH study used endometrial biopsy, not laparoscopy. Second, the antibiotic treatment presently used is more effective than that available during Westrom’s study period, thus likely leading to fewer treatment failures.
Fallopian Tubes
Chlamydia trachomatis is the leading cause of permanent tubal damage worldwide. Chlamydia ascends from the cervix and preferentially invades columnar epithelial cells, which are found in abundance in the densely ciliated ampullary segment of the fallopian tubes.28 Once intracellular, Chlamydia then release a specific heat shock protein, Ch-hsp60, which is capable of inducing a local inflammatory response.29 The immune response keeps the infection from spreading to other cells, but the intracellular Chlamydia can escape immune destruction, thus setting up a chronic infection resulting in localized scarring. This chronic infection leads to the production of Ch-hsp60 antibodies, which can be detected in sera. Ch-hsp60 is similar in some regions to h-hsp60 (human heat shock protein 60). This is expressed on the decidua and embryo in early pregnancy. Thus, a woman who has developed Ch-hsp60 antibodies as a result of a previous significant fallopian tube infection could reactivate her immune system and potentially impede early embryonic events.30
Tuberculosis produces distinct pathology that can be seen on hysterosalpingography. This may reveal a shriveled uterine cavity, calcified tube or ovary, bilateral cornual block, lead pipe appearance with distension of ampullary region, or distal tubal occlusion with jagged fluffiness of tubal outline.18
Tubal obstruction caused by schistosomiasis can result in infertility.31 Inflammation of the cervix, vagina, and vulva from schistosomiasis may occur and interfere with coitus, fertility, and ability to deliver a fetus vaginally.32 Women from areas where schistosomiasis is endemic (Schistosoma mansoni in Africa, South America, and the Caribbean; Schistosoma japonicum in Japan, China, and the Phillipines; Schistosoma mekongi in Southeast Asia; Schistosoma haematobium in Africa and the Middle East; and Schistosoma intercalatum in West and central Africa) should have this included in the differential diagnosis of their infertility.
Actinomyces israelii is a rare cause of pelvic abscesses, but has been associated with intrauterine device use (see Chapter 27).
Other Infertility Causes
Several parasitic infections may impair a woman’s fertility due to maternal debilitation from the disease, leading to a disrupted hypothalamic-pituitary-ovarian axis. These include amebiasis, giardiasis, leishmaniasis, malaria, trypanosomiasis, and helminthic infestations with Ascaris or Trichuria. There may also be impaired fertility due to direct damage to reproductive organs from the following parasitic causes: amebiasis, Ascaris, enterobiasis, filariasis, schistosomiasis, and echinococcosis.33 Researchers in Brussels found an 80% infertility rate in mice actively infected with Trypanosoma cruzi, the protozoa that causes Chagas disease in Latin America.34
EVALUATION OF INFECTIOUS CAUSES OF INFERTILITY
Multiple infectious causes for infertility have been described, with the most common cause currently in the United States being Chlamydia salpingitis. Other causes must be kept in consideration when evaluating a patient with infertility, especially in patients from other parts of the world. Evaluation of the infertile couple for infectious causes should begin with an in-depth history, including episodes of prior STD exposures and treatment, possible exposures to tuberculosis, country of origin, and travel history. The next step is a thorough examination, including evaluation of the cervical mucus for signs of chronic inflammation and inspection of the cervix for signs of lesions caused by HPV, neoplasm, syphilis, or tuberculosis or for scarring from previous procedures. Cervical testing for Chlamydia and gonorrhea should be used liberally. The value of Mycoplasma and Ureaplasma culture of the cervix is less clear for infertility but may be of value in patients with recurrent pregnancy loss.
Chlamydia antibody tests are serologic tests that measure immunoglobulin G antichlamydial antibodies. The usefulness of chlamydia antibody testing in determining the likelihood of tubal damage due to chlamydia has been examined for years. Concerns about the sensitivity of the test are based on several issues. First, the serology does not differentiate between a current chlamydial lower genital tract infection and current or prior tubal involvement. On some assays, cross-reactivity with both Chlamydia pneumoniae and gram-negative bacterial polysaccharides was present. A meta-analysis of 23 studies with a total of 2729 patients undergoing laparoscopy and serum chlamydia antibody tests found that enzyme-linked immunosorbent assays and immunofluorescence assays were the most predictive and were comparable to hysterosalpingography in the diagnosis of any tubal pathology.35
A report on 1009 women who underwent serum immunofluorescence chlamydia antibody tests and laparoscopy for evaluation of infertility found a linear trend between serum antibody tests and the likelihood of tubal damage. Low titers did not definitively rule out tubal damage, but the higher the titer, the more likely significant the tubal damage.36 This lack of sensitivity at the lower titers may be explained by the finding that, after a primary genital chlamydial infection, 18% of patients will have a twofold or greater fall in the chlamydia antibody titer within 4 years.37
HUMAN IMMUNE DEFICIENCY VIRUS (HIV) AND ACQUIRED IMMUNODEFICIENCY SYNDROME (AIDS)
For those HIV-infected patients who desire pregnancy, the most significant risks are vertical transmission to the fetus and transmission to an uninfected partner. There is a low transmission rate to the fetus if the recommended guidelines are rigidly followed. This low risk may make refusing infertility treatment to an HIV-infected woman less valid. The risk of vertical transmission of HIV is approximately 25% without any intervention. With the use of zidovudine (AZT), the transmission decreases to 8.3%.38 The use of highly active antiretroviral treatment (HAART) or combination therapy can decrease the vertical transmission rate in optimally controlled patients to 1% to 2%.39 Optimal control of the mother’s HIV infection is critical and should be managed in conjunction with an HIV expert. If the mother’s viral load is not able to be kept under 1000 copies, then the current recommendation is for cesarean section at 38 weeks to avoid fetal exposure to maternal blood and vaginal fluids during labor and vaginal delivery.39
If the couple has discordant HIV infection status, another goal is to keep the HIV-negative partner from becoming infected. The transmission rate of HIV without the use of condoms is estimated to be 0.1% to 0.5% per sexual act.40 If the man is HIV negative and the woman is HIV positive, semen can be transferred to the vagina via methods that do not involve exposure to the uninfected male, such as insemination. This again is best done at a time when the woman’s HIV infection is optimally controlled, thus decreasing her likelihood to pass the HIV infection to the fetus.
If the male is HIV infected and the female is HIV negative, the situation is much more complicated. The virus is contained within both the seminal plasma and the white blood cells in the semen. Although it has not been conclusively shown, the spermatozoon appears to not be vector for the virus.41 Sperm washing can be accomplished by separating sperm from the seminal fluids and white blood cells, and is usually effective in 90% of samples. After sperm washing, the sample is confirmed to be negative for viral presence by reverse transcription and nested PCR. Throughout the world, several thousand ART cycles using washed sperm have been performed without transmission, but it is too early to state conclusively that this procedure is without risk.42 More clinical trials are necessary to be able to more accurately determine the risk of transmission.
The final issue with all blood-borne viruses and ARTs is the potential of cross-contamination within the laboratory. To achieve the degree of safety to offer this technology to patients with such viruses would probably require separate high-security laboratory areas.43
1 Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men—United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR. 2004;53:335-338.
2 Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2002 Supplement. Chlamydia Prevalence Monitoring Project Annual Report 2002. Atlanta: U.S. Dept. of Health and Human Services, CDC, 2003.
3 Lagarde E, Guyavarche E, Piau JP, et al. Treponemal infection rates, risk factors and pregnancy outcomes in a rural area of Senegal. Int J STD AIDS. 2003;14:208-215.
4 Soper D. Trichomoniasis: Under control or undercontrolled? Am J Obstet Gynecol. 2004;190:281-290.
5 Sherman KJ, Daling JR, Weiss NS. Sexually transmitted disease and tubal infertility. Sex Transm Dis. 1987;14:12-16.
6 Grodstein F, Goldman M, Cramer D. Relation of tubal infertility to history of sexually transmitted diseases. Am J Epidemiology. 1993;137:577-584.
7 Keith LG, Friberg J, Fullan N, et al. The possible role of Trichomonas vaginalis as a “vector” for the spread of other pathogens. Int J Fertil. 1986;31:272-277.
8 Wu TC, Boyd D. HPV-16 vaccine prevented persistent HPV-16 infection and the development of HPV-16 related cervical neoplasia. Evid Obstet Gynecol. 2003;5:42-43.
9 Corey L. Genital herpes. In: Holmes KK, et al, editors. Sexually Transmitted Diseases. 2nd ed. New York: McGraw-Hill; 1990:391-413.
10 Lehtinen M, Rantala I, Teisala K, et al. Detection of herpes simplex virus in women with acute pelvic inflammatory disease. J Infect Dis. 1985;152:78-81.
11 Taylor-Robinson D. Infections due to species of Mycoplasma and Ureaplasma: An update. Clin Infect Dis. 1996;23:671-684.
12 Moller BR, Taylor-Robinson D, Furr PM, et al. Serological evidence that chlamydiae and mycoplasmas are involved in infertility of women. J Reprod Fertil. 1985;73:237-240.
13 Svenstrup HF, et al. Mycoplasma genitalium attaches to human spermatozoa. Hum Reprod. 2003;18:2103-2109.
14 Fiore AE. Hepatitis A transmitted by food. Clin Infect Dis. 2004;38:705-715.
15 Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. 1998;47(RR19):1-39.
16 Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36(Suppl 1):S99-S105.
17 Haas DW, Des Prez RM. Mycobacterium tuberculosis. In: Mandel GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 4th ed. New York: Churchill Livingstone; 1995:2213-2243.
18 Gurgan T, Demirol A. Tuberculosis in assisted reproduction and infertility. Elsevier International Congress Series. 2004;1266:287-294.
19 Centers for Disease Control. Patient Fact Sheet on PID. Available at http://www.cdc.gov/std/PID/STDFact-PID.htm. May 2004. Accessed
20 Veh JM, Hook EW3rd, Goldie SJ. A refined estimate of the average lifetime cost of pelvic inflammatory disease. Sex Transm Dis. 2003;30:369-378.
21 Gump DW, Gibson M, Ashikaga T. Evidence of prior pelvic inflammatory disease and its relationship to Chlamydia trachomatis antibody and intrauterine contraceptive device use in infertile women. Am J Obstet Gynecol. 1983;146:153-159.
22 Hillis SD, Joesoef R, Marchbanks PA, et al. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol. 1993;168:1503-1509.
23 Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2006. MMWR. 2006;55:1-94.
24 Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: Results from the pelvic inflammatory disease evaluation and clinical health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937.
25 Bristow RE, Karlan BY. Disorders of the uterine cervix. In: Scott JR, Di Daia PJ, Hammond CB, Spellacy WN, editors. Danforth’s Obstetrics and Gynecology. 8th ed. Philadelphia: Lippincott Williams &Wilkins; 1999:805-835.
26 Haggerty CL, Ness RB, Amortegui A, et alfor the PID Evaluation and Clinical Health (PEACH) Study. Endometritis does not predict reproductive morbidity after pelvic inflammatory disease. Am J Obstet Gynecol. 2003;188:141-148.
27 Westrom L. Effect of acute pelvic inflammatory disease on fertility. Am J Obstet Gynecol. 1975;121:707-713.
28 Land JA, Evers JLH. Chlamydia infection and subfertility. Best Prac Res Clin Obstet Gynecol. 2002;16:901-912.
29 Paavonen J. Sexually transmitted chlamydial infections and subfertility. Elsevier International Congress Series. 2004;1266:277-286.
30 Witkin SS. Immunological aspects of genital chlamydia infections. Best Prac Res Clin Obstet Gynecol. 2002;16:865-874.
31 Garba M, Almoustapha T, Garba A, Nouhou H. [Extra uterine pregnancy associated with tubal schistosomiasis: Schistosoma haematobium. A case report from Niger][in French]. Bull Soc Pathol Exot. 2004;97:41-42.
32 Bullough CHW. Infertility and bilharziasis of the female genital tract. BJOG. 1976;83:819.
33 Sweet RL, Gibbs RS. Parasitic disease in pregnancy. In: Sweet RL, Gibbs RS, editors. Infectious Diseases of the Female Genital Tract. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2002:570-605.
34 Mjihdi A, Lambot MA, Stewart IJ, et al. Acute Trypanosoma cruzi infection in mouse induces infertility or placental parasite invasion and ischemic necrosis associated with massive fetal loss. Am J Pathol. 2002;161:673-680.
35 Mol BWJ, Dijkman B, Wertheim P, et al. The accuracy of serum chlamydial antibodies in the diagnosis of tubal pathology: A meta-analysis. Fertil Steril. 1997;67:1031-1037.
36 Akande VA, Hunt LP, Cahill DJ, et al. Tubal damage in infertile women: Prediction using chlamydia serology. Hum Reprod. 2003;18:1841-1847.
37 Gijsen AP, Land JA, Goosens VJ, et al. Chlamydia antibody testing in screening for tubal factor significance of IgG antibody decline over time. Hum Reprod. 2002;17:699-703.
38 Connor EM. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. NEJM. 1994;331:1173-1180.
39 U.S. Public Health Service Task Force. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. Perinatal HIV-1 Guidelines Working Group;October 12, 2006:1-65. Published at http://aidsinfo.nih.gov.
40 DeVincenzi I. A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. NEJM. 1994;331:341-346.
41 Williams CD, Finnerty JJ, Newberry YG, et al. Reproduction in couples who are affected by human immunodeficiency virus: Medical, ethical, and legal considerations. Am J Obstet Gynecol. 2003;189:333-341.
42 Englert Y, et al. Medically assisted reproduction in the presence of chronic viral diseases. Hum Reprod Update. 2004;10:149-162.
43 Gilling-Smith C, Emiliani S, Almeida P, et al. Laboratory safety during assisted reproduction in patients with blood-borne viruses. Hum Reprod. 2005;20:1433-1438.