4 Infections and antibiotics
Importance of infection
In the latter half of the 19th century Louis Pasteur hypothesized that bacteria caused infection by being carried through the air (germ theory of disease). Aware of Pasteur’s work, in 1865, Joseph Lister first used carbolic acid (phenol) as a spray in the operating theatre to successfully prevent and treat infection in compound fractures. In the early part of the 20th century, with the advent of sterilized instruments, surgical gowns and the first rubber gloves, antisepsis was replaced by modern aseptic surgical techniques which were championed by Birmingham surgeon Robert Lawson Tait. Penicillin was discovered by Alexander Fleming in 1928 and first used clinically in 1940 by Howard Florey. The prevention and treatment of surgical infection was further transformed by the many different classes of antibiotics that were discovered through the latter part of the 20th century. Nevertheless, control of infection in surgical practice remains an important and challenging issue due to the emergence of antibiotic-resistant organisms and the rise in the numbers of elderly, co-morbid and immunocompromised patients undergoing increasingly complex surgical interventions that frequently involve the use of implants. The risk of infection is related to the type of surgery (Table 4.1). Postoperative infections impact on patient outcomes and increase the length of hospital stay, which in turn increases the cost of surgery. In the UK, there is now a legal duty on hospitals to do all they can to minimize the risk of healthcare associated infections (HCAI) in patients.
Table 4.1 Classification of operative wounds and infection risk with prophylaxis
| % Infection rate with prophylaxis* | |
|---|---|
| Clean (e.g. non-traumatic wound, respiratory / gastrointestinal /genitourinary tracts intact) |
0.8 |
| Clean-contaminated (e.g. non-traumatic wound, respiratory / gastrointestinal /genitourinary tracts entered but insignificant spillage) |
1.3 |
| Contaminated (e.g. fresh traumatic wound from dirty source, gross spillage from gastrointestinal tract or infected urine/bile or major break in aseptic technique) |
10.2 |
* Based on data from: Olson M, O’Connor M, Schwartz ML. Surgical wound infections. A 5-year prospective study of 20,193 wounds at the Minneapolis VA Medical Center. Ann Surg. 1984 Mar;199(3):253–259.
Biology of infection
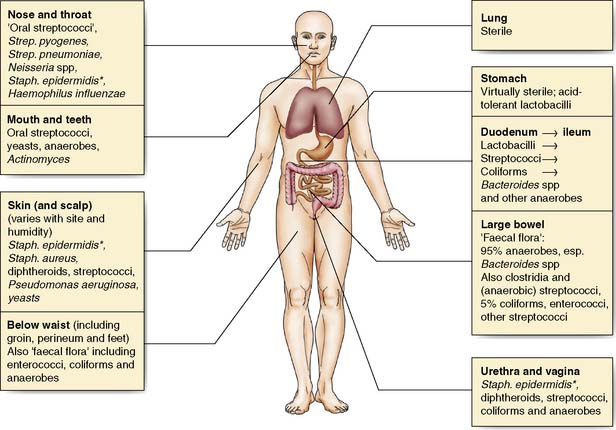
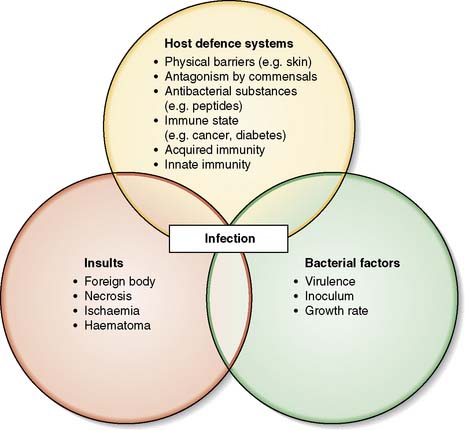
Many body surfaces are colonized by a wide range of micro-organisms, called commensals, with no ill effects (Fig. 4.1). However, once the normal defences are breached in the course of surgery, such as skin (e.g. Staphylococcus aureus) and bowel (e.g. Bacteroides spp. and Escherichia coli) commensals can then cause infection. Infection is defined as the proliferation of micro-organisms in body tissue with adverse physiological consequences. The factors involved in the evolution of infection are shown in Figure 4.2.
Host defence systems
A number of host factors make infection more likely:
• Old age, obesity, malnutrition, cancer and immunosuppressive agents (e.g. steroids) and diabetes
• The presence of dead tissue; for example, burned flesh or haematoma provide a rich source of nutrients for bacteria and hamper the local immune response
• Poor vascularity; in the leg this is often associated with peripheral arterial disease and diabetes
• Foreign material present in tissues either as a result of trauma (e.g. broken glass, clothing, shrapnel) or surgical procedure (e.g. joint replacements, heart valves, vascular prostheses).
Preventing infection in surgical patients
Preoperative MRSA screening
Since 2008 hospitals in England have been required to screen all elective surgical patients for methicillin-resistant Staphylococcus aureus (MRSA). Carriers receive decolonization treatment (nasal mupirocin cream and antiseptic skin wash) and appropriate antibiotic prophylaxis, usually a glycopeptide antibiotic (e.g. teicoplanin) prior to surgery. This policy reduces MRSA transmission in surgical wards (EBM 4.1). Screening for nasal carriage of Staphylococcus aureus followed by decolonization also reduces surgical wound infection (EBM 4.2). These hospitals now screen emergency admissions although the timing of available results will determine whether this has an impact on management and outcomes.
Hardy K, et al. Reduction in the rate of methicillin-resistant Staphylococcus aureus acquisition in surgical wards by rapid screening for colonization: a prospective, cross-over study. Clin Microbiol Infect. 2010; 16:333-9.
4.2 Preventing surgical site infections in nasal carriers of Staphylococcus aureus
Bode LG, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010; 362:9-17.
Hand decontamination
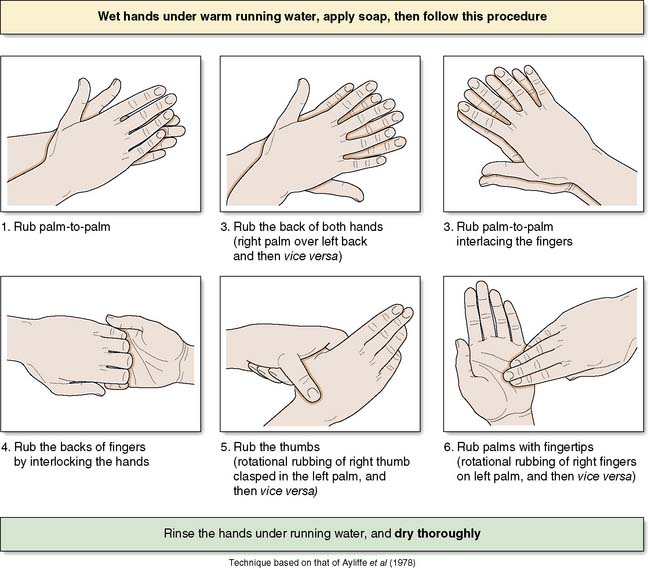
The operating team should wash their hands prior to each operation on the list using an aqueous antiseptic surgical solution, with a single-use brush for the nails. The ‘six-step hand hygiene technique’ is now widely adopted (Fig. 4.3). Hospitals will have policies for which antiseptic agents are used. Where hands are not soiled, alcohol hand gel is a suitable alternative for decontamination on the wards.
Surgical instruments
Terminology
• Decontamination: a process which removes or destroys infectious or unwanted material
• Cleaning: the physical removal of soil and organic matter
• Disinfection: the removal or destruction of some micro-organisms but not bacterial spores
• Sterilization: the complete destruction of all micro-organisms including spores.
Creutzfeldt–Jakob disease (CJD) and other prion diseases
Summary Box 4.1 Prevention of infection
• Preoperative screening of patients for MRSA, and subsequent decolonization of carriers, is now an integral part of surgical care in UK hospitals
• The routine practices of hand washing, surgical scrub, skin preparation of the patient and maintaining a sterile field are collectively known as ‘aseptic technique’
• The practice of aseptic technique is an important component in preventing surgical site infections
• Sterility of surgical instruments is critical to preventing cross-infection. This may be achieved by decontamination of instruments in SSDs or by using sterile, disposable instruments.
Prophylactic use of antibiotics
Antibiotic prophylaxis is defined as their use before, during, or after a diagnostic, therapeutic, or surgical procedure to prevent infectious complications. The evidence base and guidance can be found at www.sign.ac.uk/pdf/sign104.pdf and in the British National Formulary (BNF).
Prophylaxis for immunosuppressed patients
• commenced on lifelong antibiotic prophylaxis with penicillin or amoxicillin
• immunized against pneumococcus, Haemophilus influenzae type b (Hib), Group C meningococcus.
Summary Box 4.2 Prophylactic antibiotics
• Antibiotic prophylaxis in surgical practice aims to prevent infection by achieving high concentrations of antibiotic at the incision and site of operation during surgery
• The choice of antibiotic must cover the likely pathogens for the operation site
• A single dose of antibiotic is usually adequate for prophylaxis, although during prolonged procedures or where there is excessive blood loss, a second dose may be required
• In some circumstances, e.g. colonization with multi-resistant bacteria or immunocompromised patients, the antibiotic choice may need to be modified and expert advice should be sought.
Management of surgical infections
Surgical infections are of two types; those that occur in patients who:
• have undergone a surgical procedure
• present with sepsis and require surgery as part of their management.
Diagnosis
Infections in the early postoperative period (< 48 hours) are most likely to be respiratory or urinary; wound infections usually becoming evident later. Implant-related infections may not be evident for weeks, months or even years. Leakage of a gastrointestinal anastomosis usually presents after 5–6 days with low grade pyrexia and abdominal symptoms and signs; there may also be leakage of bowel content from surgical drains. Questioning for cough, dysuria and abdominal pain is important. Tachycardia, tachypnoea and pyrexia are all indicators of infection. Should hypotension and signs of septic shock be present, urgent resuscitation and assessment by the critical care unit outreach team is required. Whenever possible, the focus of infection should be identified (e.g. plain x-ray, ultrasound, computed tomography, (CT), or magnetic resonance imaging, (MRI)) and cultures taken (e.g. urine, sputum) before commencing antibiotic treatment. Aspiration of pus from deep seated infections (e.g. subphrenic abscess) followed by Gram staining to guide empirical therapy is helpful. Intravenous lines should be removed and cultured together with blood in any patient suspected of having bacteraemia. If indicated, urine and sputum should also be cultured. Serious sepsis in the surgical patient often arises from intra-abdominal infections (IAI). Approximately 30% of patients admitted to the ICU with IAI die, and if peritonitis develops mortality rises to 50%. Early diagnosis and treatment is essential but clinical examination is often unreliable, even misleading. CT, or MRI, preferably with contrast, should be performed to detect peritoneal leaks and collections of pus and can be life-saving. An integrated and logical approach to patient management should be followed as described in the surviving sepsis guidelines which are summarized in Tables 4.2 and 4.3.
| Are any two of the following present? | |
SEVERE SEPSIS CARE PATHWAY
(Table 4.3)
WCC, white cell count; MAP, mean arterial pressure, SBP systolic blood pressure; INR, international normalized ratio; APTT, activated partial thromboplastin time.
http://www.survivingsepsis.org/
Table 4.3 Severe sepsis care pathway
Antibiotic therapy
• Antibiotic policies – each hospital has its own antibiotic formulary and this should be consulted. The principles behind such policies are shown in Table 4.4.
• Specimens for culture and sensitivity testing should always be obtained if possible and then specific antibiotics used as suggested in Table 4.5. It is not always possible to await these results if the patient is seriously ill and empirical therapy should be started immediately according to Table 4.6.
• When using some antibiotics such as gentamicin and vancomycin, therapeutic drug monitoring is needed to (i) establish adequate serum concentrations and (ii) identify toxic concentrations before renal or neurological damage develops. Specific protocols are available from microbiology/pharmacy departments at individual hospitals.
• Advice should be sought early about antibiotic treatment regimens from microbiologists/infectious diseases specialists, particularly when the diagnosis is not certain and/or the patient is critically ill.
Table 4.5 Antibiotics in surgery: suggestions for specific therapy
| Organism | First choice | Alternative |
|---|---|---|
| Methicillin-sensitive Staphylococcus aureus (MSSA) | Flucloxacillin | Clarithromycin |
| Methicillin-resistant Staphylococcus aureus (MRSA)* | Vancomycin | Linezolid Daptomycin |
| Coagulase-negative staphylococci | Vancomycin | Linezolid Daptomycin |
| Streptococcus pneumoniae | Benzylpenicillin | Clarithromycin |
| Streptococcus pyogenes (group A β-haemolytic streptococcus) | Benzylpenicillin Clindamycin |
Clarithromycin |
| Enterococci | Amoxicillin | Vancomycin |
| Bacteroides species | Metronidazole | Co-amoxiclav |
| Escherichia coli | Piperacillin-Tazobactam Trimethoprim |
Meropenem Co-amoxiclav |
| Haemophilus influenzae | Amoxicillin | Co-amoxiclav |
| Klebsiella spp | Co-amoxiclav | Meropenem |
| Proteus species | Co-amoxiclav | Meropenem |
| Pseudomonas aeruginosa | Piperacillin-Tazobactam | Meropenem |
| Clostridium spp | Benzylpenicillin + metronidazole | Metronidazole |
| Clostridium difficile | Stop predisposing antibiotic Metronidazole |
Vancomycin, oral, re-treat relapse |
These suggestions should be considered in the light of local epidemiology, sensitivities, drug availability, site and severity or infection.
* Gould FK et al. Guideline (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J. Antimicrobial Chemotherapy 2009;63:849-861
Table 4.6 Empirical therapy for acute infections
| Type of Infections | Antimicrobial | Alternative |
|---|---|---|
| Chest infection Uncomplicated Community-acquired pneumonia ‘Aspiration’ pneumonia Hospital-acquired/postoperative |
Amoxicillin Benzyl penicillin + clarithromycin Co-amoxiclav Piperacillin-tazobactam |
Clarithromycin Levofloxacin + clarithromycin Levofloxacin + metronidazole Meropenem + vancomycin |
| Urinary tract infection ‘Lower’ infection Acute pyelonephritis Prostatitis |
Trimethoprim Co-amoxiclav Ciprofloxacin |
Amoxicillin Gentamicin |
| Wound infection Cellulitis Abscess |
Penicillin + flucloxacillin Drain collection |
Clarithromycin Flucloxacillin |
| Intra-abdominal sepsis | Amoxicillin + metronidazole + gentamicin | Meropenem |
| Cholecystitis-cholangitis | Co-amoxiclav | Meropenem |
| Pelvic inflammatory disease | Azithromycin + metronidazole + gentamicin | Doxycycline + piperacillin-tazobactam |
| Amputations and gas gangrene | Benzylpenicillin + metronidazole | Metronidazole |
| Septicaemia and septic shock | Amoxicillin + metronidazole + gentamicin/ciprofloxacin | Piperacillin-tazobactam, meropenem |
| Severe Pseudomonas infections | Piperacillin-tazobactam + gentamicin | Meropenem ± gentamicin |
| Candida sepsis | Fluconazole | Caspofungin |
Note The suggestions are for occasions when immediate treatment is necessary. Amendments may be necessary in the light of local epidemiology.
Specific infections in surgical patients
Surgical site infection (SSI)
All surgical wounds are contaminated by microbes but in most cases infection does not develop because of innate host defences. A complex interplay between host, microbial, and surgical factors ultimately determines whether infection takes hold and how it progresses (Fig. 4.2, EBM 4.3 and see Table 4.1).
Horan TC, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992; 13:606-8.
Urinary tract infections (UTI)
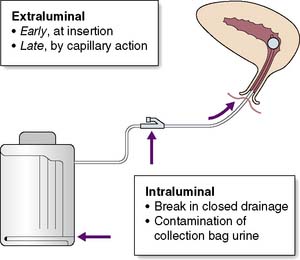
These are common and may range from simple cystitis to pyelonephritis or even perinephric abscess. Catheterized patients are at increased risk of infection. The most common organisms are Escherichia coli, Klebsiella species, Enterococcus faecalis and Pseudomonas aeruginosa. Multi-resistant organisms such as ESBL-producing E. coli and MRSA are increasingly being seen and can be difficult to treat. Symptoms include dysuria, fever and, in patients who are not catheterized, frequency and nocturia. Cystitis may not give rise to any clinical signs. Pyelonephritis is typically associated with rigors, renal angle pain and tenderness. Urine samples must be sent for microscopy and culture. In catheterized patients the urine frequently contains organisms but not white cells. This does not require antibiotics unless there are signs of systemic illness. The urine will not become sterile until the catheter is removed. Trimethoprim, gentamicin and co-amoxiclav are reasonable antibiotic choices until sensitivities become available. Fluoroquinolones (e.g. ciprofloxacin) may be used although C. difficile infection is a risk particularly in elderly patients. Expert advice should be sought in the case of multi-drug resistant pathogens. Aseptic introduction and meticulous care of the urinary catheter helps to prevent bacteria entering the urinary tract (Fig. 4.4).
Clostridium difficile infection (CDI)
This occurs when the normal colonic microflora is disturbed by the administration of antibiotics in patients either pre-colonized with or exposed after antibiotic treatment to C. difficile (an anaerobic spore-forming bacillus). Some antibiotics are particularly prone to cause CDI: clindamycin (the first identified in 1978), cephalosporins and fluoroquinolones. The disease is much more common in the elderly and in hospitals with poor cleaning. The bacterium produces two cytotoxins A and B (some strains only produce B) that destroy the colonic mucosal cell cytoskeleton. A spectrum of disease is seen ranging from abdominal discomfort to profuse watery diarrhoea (one of the commonest features), severe abdominal cramps and rarely toxic dilatation of the colon leading to rupture. At colonoscopy characteristic yellow plaques, bleeding mucosa and islands of normal tissue are seen, which is called pseudomembranous colitis. Surgical patients can acquire CDI as a consequence of antibiotic treatment or prophylaxis. Infrequently patients with severe CDI may require urgent surgical referral. Emergency colectomy in patients with fulminant colitis can be life saving although mortality is high. Diagnosis of CDI is by identification of the toxins in faeces by enzyme immunoassay (EIA) or the more sensitive and specific PCR detection of the toxin genes. Treatment of mild/moderate disease is with oral metronidazole, with severe disease responding better to oral vancomycin. Control is aimed at isolating patients with diarrhoea, reducing the environmental burden of spores by cleaning with bleach solutions and reducing the selective pressure from high risk antibiotics by antibiotic stewardship. Current UK guidelines are available in the document ‘Clostridium difficile – how to deal with the problem’ (www.dh.gov.uk); (www.hpa.org.uk).
Infections of prosthetic devices
Summary Box 4.3 Management of surgical infection
• The risk of surgical site infection rises in direct proportion to the degree of microbial contamination of the wound
• Whenever possible, the focus of infection should be identified by careful history-taking, clinical examination, imaging and microbiological culture
• Collections of pus should be drained
• In many surgical infections, antibiotics are often an adjunct to surgical treatment, e.g. drainage of abscesses, debridement, excision of infected tissue or lavage of a serous cavity
• Tetanus immunization status of patients must be determined prior to elective surgery or following trauma.
Infections primarily treated by surgical management
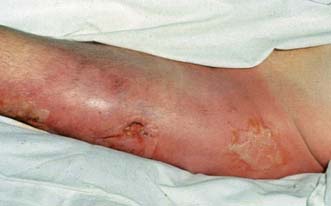
Necrotizing fasciitis
This is an uncommon but severe, life-threatening infection of skin and subcutaneous tissues characterized by necrosis of deep fascia (Fig. 4.5). There are two main types depending on causative organisms.
• Type I: Polymicrobial aetiology which is also known as synergistic bacterial gangrene; Fournier’s gangrene is a special type affecting the perineal area
• Type II: Single organism infection, usually by β-haemolytic Group A streptococci (Streptococcus pyogenes).
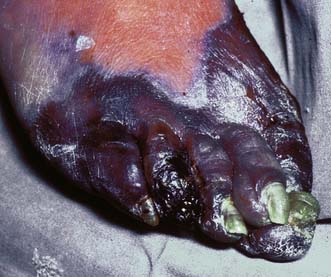
Gas gangrene
This is rarely seen in civilian practice and is typically associated with the battlefield. Clostridium perfringens, a spore-forming anaerobic bacterium normally found in soil and faeces, is the main cause; other species include Clostridium novyi and Clostridium septicum. Patients become rapidly and profoundly septic as exotoxins lead to rapidly spreading muscle necrosis with overlying skin discolouration, oedema and crepitus (Fig. 4.6). Even with urgent wide surgical excision of all necrotic tissue and high-dose antibiotics (penicillin and metronidazole) the disease still carries a high mortality.
Tetanus
This is caused by Clostridium tetani, a spore-forming anaerobic organism which enters the body through soil or animal faecal contamination of a wound, injury or burn and then multiplies anaerobically in tissues, if the wound is not adequately cleaned or debrided. The incubation period varies from 4 to 21 days. Tetanospasmin (a neurotoxin) spreads along nerves from the site of infection and causes generalized rigidity and spasm of skeletal muscles. The muscle stiffness usually involves the jaw (lockjaw) and neck and then becomes generalized. The mortality ranges from 10 to 90%, being highest in infants and the elderly. Antibiotic treatment is with penicillins or, for penicillin allergic patients, clarithromycin but is only an adjunct to correct surgical care of wounds and further specialized medical treatment. Tetanus can be prevented by immunization. In the UK, all young children are offered the tetanus vaccine as part of the routine NHS childhood vaccination programme (www.nhs.uk/conditions/Tetanus); current advice is to have five doses over a life time. For non-immune individuals who have suffered a tetanus-prone injury, Human Tetanus Immunoglobulin (HTIG) is given to provide immediate protection together with wound debridement, active immunization and antibiotic treatment.
Healthcare associated infections (HCAI)
In 2006 a survey of 190 acute hospitals in England showed that 8.2% of patients had developed a HCAI (previously known as a nosocomial infection), most commonly SSI, GI infections, UTI, and pneumonia. The UK Health Act of 2006 (revised 2008) places a legal duty on hospitals to do all they can to minimize the risk of HCAI. The hospital infection control team are most closely involved in the design and delivery of the HCAI programme and will liaise with the microbiology laboratory to ensure that infections caused by important pathogens are identified at an early stage and that trends in antibiotic resistance are monitored. However, all staff members and students have a duty to take responsibility for this very important aspect of patient care. In recent years, there has been a national focus on reducing MRSA and C. difficile infections in England using a multi-faceted approach; Figure 4.7 shows the successful reduction in England of MRSA bloodstream infections (bacteraemia) from 2006 to 2010 but continuing high levels of MSSA bacteraemia. Monitoring SSI is also an important quality indicator. The Nosocomial Infection National Surveillance Service (NINSS), a national programme of SSI surveillance, was established in the UK in 1997. Participation in the scheme is voluntary (except for orthopaedic surgery) but provides hospitals with useful benchmarking data for the main types of surgery. This systematic collection of infection data (surveillance) is by nurse follow-up of all patients who have undergone surgery during a given period. Surveillance nurses will inspect surgical wounds for any signs of infection and often also follow-up the patient once discharged home to detect infection. This enables the early identification of increased incidences of infection so that measures can be taken to prevent further infections. These measures could include suspension of further surgery; deep cleaning of theatres; change in antibiotic treatments and isolation of infected patients.
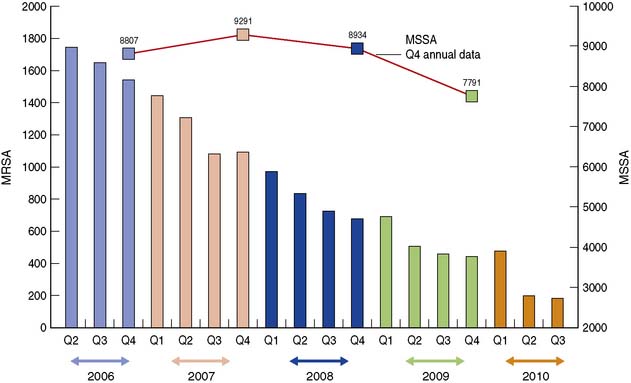
Fig. 4.7 Numbers of MRSA and MSSA bacteraemia (quarterly) in England, derived from HPA Surveillance Data.
Summary Box 4.4 Healthcare associated infection (HCAI)
• All hospitals must have effective programmes for prevention and control of HCAI
• The hospital infection control team design and deliver the HCAI programme, but all staff working in healthcare must take responsibility for preventing HCAI
• Monitoring for trends in surgical site infection is an important indicator of the quality of patient care.