Chapter 10. Infection
Congenital infections with organisms that may have little effect on an adult or older child can have a devastating effect on the fetus and infant, resulting in profound long-term damage. The commonest cause of congenital infection is cytomegalovirus affecting 0.5–3% of live births worldwide. In very low birth weight infants who need prolonged intensive care, rates of culture-proven infection of up to 30% have been quoted, with mortality rates that are equally high. Neonatal units are busy and as they become busier both time and staffing constraints lead to an increase in nosocomial infections.
Questions in this chapter will cover different aspects of congenital infections, the presentation of sepsis, prevention of infection and other common situations such as contact with chickenpox on the neonatal unit.
QUESTION 1
Which of the following statements about cytomegalovirus (CMV) are correct (answer true or false)?
i) CMV is an RNA virus
ii) It is the most common intra-uterine infection
iii) Congenital CMV can be caused by maternal primary infection or reactivation and both are of the same severity
iv) The incidence of primary CMV infection acquired during pregnancy is 5–10%
v) Primary CMV infection is associated with a 40% risk of congenital CMV infection
vi) At least 50% of infected infants will show clinical signs at birth
vii) There is a 20% mortality associated with symptomatic congenital CMV
viii) Infants can become infected by breast feeding if the mother is seropositive.
QUESTION 2
You are called to counsel a mother who is 31 weeks gestation. The baby was noted to be small on scans. Karyotyping from the amniotic fluid is normal and the fluid is positive for CMV by PCR. The fetus is noted to have unilateral ventriculomegaly.
i) What other abnormalities should be looked for on the antenatal scans?
ii) Which of the following symptoms could be present in the newborn period? Choose six correct answers.
a. Seizures
b. Hepatosplenomegaly
c. Thrombocytopenia
d. Unconjugated hyperbilirubinaemia
e. Chorioretinitis
f. Cataracts
g. Defective enamelisation
h. Inguinal hernia.
iii) What is the likely prognosis if the baby shows symptoms at birth?
iv) If the baby is asymptomatic at birth, what is the likely prognosis?
v) Which is the preferred method for testing for congenital CMV? Choose one answer.
a. Detection of pp65 antigen in white blood cells
b. Virus isolation from placenta
c. PCR assay of urine
d. Serology testing for IgG and IgM
e. Histology of skin biopsy.
vi) What action would you take after birth to confirm the diagnosis of congenital CMV and over what time scale?
The baby’s investigations support the diagnosis of congenital CMV. On examination, the baby has a large liver and spleen, thrombocytopenia and imaging reveals intracranial calcification.
vii) What treatment options are available?
QUESTION 3
Regarding parvovirus B19, which of the following statements are correct?
i) It is a large double stranded DNA virus
ii) It is a small single stranded DNA virus
iii) It replicates in rapidly dividing cells
iv) Approximately 25% of adults show evidence of a previous infection
v) A rash and arthralgia occur in the second phase of the illness. At this point, the child is still infectious
vii) If parvovirus B19 infection occurs during pregnancy, the majority of fetuses will be affected
viii) The outcome of the fetus depends on the point at which the infection occurred in pregnancy.
QUESTION 4
The following features are typically associated with which intrauterine infection – hydrocephalus, epilepsy, cerebral calcification and chorioretinitis. Choose the best answer.
i) Congenital CMV
ii) Congenital toxoplasmosis
iii) Congenital Epstein–Barr virus
iv) Congenital varicella
v) Congenital rubella
vi) Congenital herpes
vii) Congenital TB.
QUESTION 5
Which of the following statements are true of toxoplasmosis?
i) The risk of congenital infection remains constant throughout pregnancy
ii) The risk of congenital infection is up to 90% in the third trimester
iii) Congenital infection is more severe if maternal infection is acquired in the third trimester
iv) In the UK, about 60% of women are sero-positive
v) Congenital infection occurs in 1 in 100 exposed babies
vi) Increasing gestational age at seroconversion is associated with an increased risk of mother-to-child transmission
vii) CSF will show a lymphocytosis
viii) Toxoplasmosis cannot be treated
ix) If diagnosed antenatally in the first trimester the mother can be treated with pyrimethamine and sulfadiazine to decrease the risk of fetal infection
x) If there are no eye signs within the first year of birth, the infant is unlikely to develop ocular problems.
QUESTION 6
You are called to the postnatal ward to review a baby who is 8 hours old.
The midwife reports that the baby is not feeding well. His mother says he tried to feed after birth but now is not interested. On examination, the infant looks pale, and feels slightly floppy. He is mottled with cool peripheries and has a heart rate of 160 bpm and mild recession. He has normal heart sounds, and both femoral pulses can be felt.
i) What is your first management step?
ii) What questions if any do you ask the mother?
iii) List five investigations you should carry out.
iv) What other investigations may be useful?
v) What is your next step in managing this baby?
The results of the initial investigations are obtained and shown below:
| Hb | 14.7 g/dL |
| WBC | 21.4×10 9/L |
| Neut | 1.7×10 9/L |
| Platelets | 104 × 10 9/L |
| CRP | 94 mg/L |
Urine SPA sample – no cells, no organisms seen
Chest x-ray shows a diffuse, fine, reticulogranular pattern, much like that seen in RDS.
The infant is breathing without ventilatory support with some low flow oxygen to maintain his saturations. He has had his first dose of antibiotics and is currently on maintenance fluid.
vi) What do you do now?
A lumbar puncture is carried out and the result is below:
| RBC | 18,000/mm 3 |
| WCC | 12/mm 3 |
| Protein | 2.5 g/L |
| Glucose | 1.4 mmol/L |
CSF is moderately blood stained
vii) What does this result mean?
Six hours later the infant is more symptomatic having frequent apnoeas and desaturations. He is intubated and ventilated. Repeat bloods show the following results:
| CRP | 120 mg/L |
| Platelets | 45×10 9/L |
| Neutrophils | 0.8×10 9/L |
There is a phone call from the microbiology department the following day saying the blood cultures are growing Group B Streptococcus. The infant’s CRP is now 40 mg/L, and his platelets have been stable above 100 after the platelet transfusion.
ix)
a. How will you modify your treatment, if at all?
b. How long will you treat the baby?
c. What will you tell the parents?
QUESTION 7
A baby is born at term by normal vaginal delivery with good Apgars requiring no resuscitation. Membranes were ruptured just prior to delivery, and the mother was well throughout pregnancy and labour. The mother is a primigravida. You receive a phone call from the midwife looking after the mother, when the baby is 12 hours old to say group B Streptococcus is growing from a maternal high vaginal swab. The mother wishes to go home.
What do you do? Choose the best answer.
i) Admit the baby to the NICU for observation
ii) Admit the baby to the NICU for observation and routine surface swabs
iii) Admit the baby to the NICU for a septic screen (blood cultures, FBC and CRP) and await results before considering antibiotics
iv) Admit the baby to NICU, perform septic screen and start antibiotics
v) Reassure and discharge home
vi) Allow to go home with instructions if the baby becomes unwell to seek medical advice
vii) Discuss with the mother and say that she must stay in and the baby must be observed for a further 24 hours
viii) Observe the baby for another 12 hours; allow to go home with instructions if the baby becomes unwell to seek medical advice.
QUESTION 8
You are working on the Neonatal Unit when one of the mothers tells you that her other child has been sent home from nursery with suspected chickenpox. The mother is unsure whether she has had chickenpox. The baby is now 5 days old, corrected gestational age 35 weeks and is well. The mother is also well.
What do you do? Choose the correct answer.
i) Reassure the mother that no treatment is needed
ii) Reassure mother but isolate baby in side room
iii) Check the mother and baby’s immune status and then give VZIG to both
v) Check the baby’s immune status and if negative give the baby VZIG
vi) Give the baby and mother VZIG
vii) Give the baby VZIG and aciclovir.
QUESTION 9
Which of the following organisms are known to commonly cause osteomyelitis? Choose four answers.
i) Bacillus fragilis
ii) Bacillus cereus
iii) Bacteroides fragilis
iv) Campylobacter jejuni
v) Haemophilus influenzae
vi) Proteus mirabilis
vii) Salmonella typhi
viii) Group B streptococcus (GBS)
ix) Staphylococcus aureus
x) Klebsiella
xi) Serratia marcescens.
QUESTION 10
Which of the following babies should receive hepatitis B immunoglobulin?
i) Mother is HBsAg negative and HBeAg negative
ii) Mother is HBsAg negative and HBeAg positive
iii) Mother is HBsAg positive and HBeAg negative
iv) Mother is HBsAg positive and HBeAg positive
v) Mother had acute hepatitis during pregnancy
vi) Mother where eAg and eAb status are unknown.
QUESTION 11
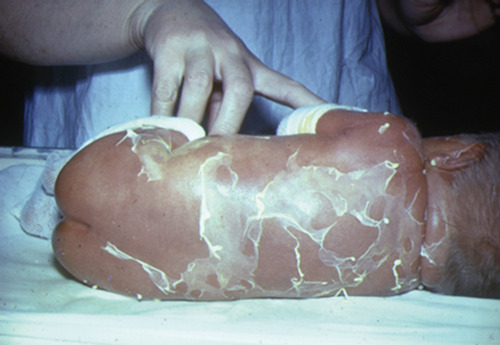
A baby presents with the following skin lesions.
QUESTION 12
Which of the following are true of congenital rubella syndrome?
i) It is a notifiable disease
ii) Causes hydrocephalus
iii) Causes thymic hypoplasia
iv) Causes corneal opacity
v) Causes bony abnormalities
vi) Causes IUGR
vii) Causes microphthalmia
viii) Causes conductive deafness
ix) Causes PDA
x) Causes aortic stenosis
xi) Infection during the first 12 weeks of pregnancy results in congenital infection and/or miscarriage in all cases
xiii) The infant is extremely infectious in the immediate postnatal period but this is no longer a risk after the first 4 weeks of life
xiv) Rubella is increasing in the immigrant population.
QUESTION 13
You are called to see a baby after delivery. The mother has had routine booking bloods and serology. The results are as follows:
RPR reactive
TPHA reactive
Rubella immune
Hepatitis negative
i) What do these test show?
ii) What test do you carry out to confirm this?
The confirmatory test is also reactive.
iii) What do you do now? Name two things.
iv) Which one test would confirm a diagnosis of congenital syphilis?
v) If the test is negative, what does this show?
vi) What information do you need from the mother?
vii) What other test might be helpful in assessing the risk to the baby?
On examination the baby looks normal, the non-treponemal serologic titre is less than four times the maternal titre, and the mother had treatment 2 weeks ago.
viii) What do you do?
QUESTION 14
On your neonatal unit, three babies are found to be growing an Enterobacter with identical antibiotic resistance. It is the opinion of the microbiologists that these must have been transmitted by staff contact. It is their recommendation that the three infants are isolated and barrier nursed and that there is a serious review of infection control procedures.
Which of the following statements are true?
i) Infected infants should remain in the cots they currently occupy; all staff involved in the care of the baby should adopt barrier nursing procedures
ii) Wearing of sterile gloves avoids the need for repeated hand washing
iii) Infected babies may be nursed by the same member of staff as uninfected infants
v) Topical antibiotic ointment should always be used
vi) Use of separate gowns for each baby is an extremely important element of infection control
vii) Screening of all babies and all staff may be implicated
viii) Staffing levels are closely related to the incidence of nosocomial infection, as is the availability of hand washing facilities
ix) Units should be closed to all admissions as soon as the possibility of an outbreak is considered
x) Environmental screening is of no value
xi) Meticulous attention to hand hygiene is by far the most important element in reducing the spread of infection
xii) There is good evidence that standards of hand hygiene are normally very high in neonatal intensive care units
xiii) Soap and water hand wash is more effective than the use of alcohol-based hand rub
xiv) Topical cord care at birth has a major role in reducing the incidence of infection.
ANSWER 1
i) False – CMV is a DNA virus of the herpes virus group.
ii) True – it affects up to 2.5% of live born infants a year worldwide. In the UK the incidence is approximately 0.2%.
iii) False – fetal infection is more likely to occur and is more severe after primary maternal infection than after reactivation of latent infection. The earlier the infection occurs in pregnancy the worse the outcome. 1
iv) False – the incidence is thought to be 1–4%.
v) True.2
vi) False – overall, only 10–15% of infected infants show clinical signs at birth but up to 90% of these will develop long-term sequelae. 3
vii) True – reports state mortality ranges between 10% and 30% although much higher in premature infants.
viii) True – in the postpartum period, mother to infant transmission can occur as up to 88% of seropositive mothers shed the virus into their milk. Approximately 50% of these infants will become infected. CMV excretion is usually the result of reactivation in a seropositive mother; most infants will not develop clinical signs due to the presence of maternally derived transplacental antibodies. This is usually a benign and asymptomatic infection.
ANSWER 2
i) Echogenic bowel, intracranial calcification, cortical dysplasia.
ii) Answers a, b, c, e, g and h are correct. Hepatosplenomegaly and petechiae secondary to thrombocytopenia are relatively common manifestations of congenital CMV in the neonatal period. Jaundice is also common but this is usually a conjugated hyperbilirubinaemia as CMV hepatitis can cause both intrahepatic and extrahepatic bile duct destruction. 4 Intracranial manifestations of congenital CMV are microcephaly and periventricular calcification where the infection has caused brain necrosis. Lissencephaly and polymicrogyria can be present depending on the timing of the infection, lissencephaly occurring with an earlier infection. Defective enamelisation occurs in 40% of symptomatic infants at birth and 5% of asymptomatic infants. Inguinal hernias can occur with congenital CMV in male infants although the reason for this is unclear.
iii) Up to 80% of symptomatic newborns will develop sequelae such as mental retardation, cerebral palsy, visual defects and sensorineural hearing loss. Symptoms at birth are associated with a worse prognosis. 5
iv) Up to 15% of infants who are asymptomatic at birth will have neurodevelopmental problems and sensorineural (SN) deafness. SN deafness is the most common abnormality seen in congenital CMV infection, occurring in 30–65% of symptomatic infants and up to 15% of asymptomatic infants. It usually develops after the newborn period and tends to be progressive. Cochlear implants have been used successfully.
v) Answer c is correct.
a. Detection of the pp65 antigen has been used to detect active infection in immunocompromised people but due to the large quantity of blood required this test is less useful in neonates. However a recent paper showed that high levels of pp65 antigen in the blood were seen in newborns that developed sequelae. 5 This test is very rarely used in the UK as it has been superseded by PCR.
b. High viral load in the urine is highly predictive of audiological impairment. 6
c. PCR is the gold standard for testing for congenital CMV.
d. A positive IgG reflects passage of maternal antibodies and therefore does not diagnose infection in the baby. Maternal IgG can persist up to 12 months. A positive IgM test does suggest congenital infection but both false positive and false negative results can occur. The test has a sensitivity of approximately 75%. With the availability of PCR, viral serology should not be used to diagnose congenital infection.
e. Skin biopsy – this is not used for CMV testing.
vi) PCR testing on a urine sample needs to be done within the first 3 weeks after birth. If the test is carried out after 3 weeks it is difficult to distinguish between a congenital, perinatal or postnatal infection. Infants infected at birth after exposure to infected secretions during delivery may start shedding virus by 3 weeks of age.
vii) There are currently a few antiviral chemotherapeutic agents, such as ganciclovir and foscarnet, that are available for treatment of serious, life-threatening or sight-threatening CMV in the immunocompromised patients. Cidofovir is also licensed for CMV retinitis. A randomised, controlled multicentre clinical trial investigated the use of ganciclovir for the treatment of infants with congenital CMV infection with evidence of CNS involvement. 7 Ganciclovir (or placebo) was given intravenously as an infusion every 12 hours for 6 weeks. The ganciclovir group were significantly more likely to have improved or normal hearing at 6 months of age compared to the placebo group. They also showed improvement in their liver function tests and head circumference. No change in mortality was demonstrated and currently there are no published data on long-term outcome. Ganciclovir is not thought to be beneficial for infants who show severe intracranial pathology antenatally. Several centres in the UK are using a six-week course of intravenous ganciclovir and then switching over to oral valganciclovir, with close monitoring of levels and viral load to assess response. The main side effects of ganciclovir are secondary to bone marrow suppression. Nigro8 described a study using CMV specific hyperimmune globulin and found that pregnant women whose amniotic fluid was CMV positive, and who were given passive immunisation, had a lower rate of congenital CMV when compared to a control group. There are currently no randomised trials looking at this preventative treatment.
ANSWER 3
i) False.
ii) True – it was discovered in 1975 and is a small, singled stranded DNA virus consisting of about 5600 base pairs.
iii) True – it has a predilection for rapidly dividing cells, hence the affinity for erythrocyte precursor cells. A characteristic haemolytic anaemia can occur in the fetus.
iv) False – about 50% of adults show serological evidence of a previous infection with p antigen and are therefore immune.
v) False – there is an incubation period of about 10 days when flu-like symptoms and viraemia occur; 7–10 days after this, the second phase occurs with joint pain and the characteristic rash, but no viraemia. 30% of infections may be asymptomatic.
vi) False – there is no correlation between the severity of maternal infection and fetal infection.
vii) False – the incidence of parvovirus infection in pregnancy is 0.3–3.7%. 9 The overall risk of fetal infection is 30–50%. Most fetal infections are self-limited and do not result in adverse outcome. In 1–2% of fetuses with serological evidence of infection, parvovirus will cause abortion, stillbirth or hydrops. Between 10% and 25% of cases of non-immune hydrops are related to parvovirus.
viii) True – around 1 in 10 women infected before 20 weeks of gestation will suffer a fetal loss due to B19. The risk of an adverse outcome of pregnancy after this stage is remote. Infected women can be reassured that the maximum possible risk of a congenital abnormality due to B19 is under 1% and that long-term development will be normal. 10
ANSWER 4
The combination of hydrocephalus, epilepsy, cerebral calcification and chorioretinitis is the classic presentation of congenital toxoplasmosis. Infection in the central nervous system causes extensive cortical and periventricular necrosis which then calcifies. The periventricular damage can cause obstruction to the aqueduct of the midbrain and cause hydrocephalus. Although the classic ophthalmological presentation is chorioretinitis, cataracts and microphthalmia can also occur.
ANSWER 5
i) False.
ii) True – the risk of congenital disease is lowest (10–25%) when maternal infection occurs during the first trimester and highest (60–90%) when maternal infection occurs during the third trimester. The overall risk of congenital infection from acute T. gondii infection during pregnancy is approximately 20–50%. 11. and 12.
iii) False – congenital disease is more severe when infection is acquired in the first trimester.
iv) False – about 30% of women are sero-positive in the UK.
v) False – congenital infection occurs in less than 1 in 1000 infants. 13
vi) True – increasing gestational age at seroconversion is strongly associated with increased risk of mother-to-child transmission (OR 1.15, 95% CI 1.12–1.17) and decreased risk of intracranial lesions (0.91, 0.87–0.95), but not with eye lesions (0.97, 0.93–1.00). 14
vii) True – the CSF will show raised protein, lymphocytosis and tachizoites (cysts of T. gondii). PCR on the CSF will confirm the diagnosis.
viii) False – infants with congenital toxoplasmosis should be treated for a year to avoid adverse outcomes. They should be given spiramycin for 4–6 weeks, alternating with 3 weeks of pyrimethamine and sulfadiazine, beginning shortly after birth and continuing for 12 months. The medications are synergistic against toxoplasma. A recent study15 showed that in infants with moderate or severe neurological disease at birth, more than 72% had normal neurological and/or cognitive outcomes, if treated, and none had sensorineural hearing loss. 91% of children without substantial neurological disease and 64% of those with moderate or severe neurological disease at birth did not develop new eye lesions. All children without substantial neurological disease at birth who received treatment had normal cognitive, neurological and auditory outcomes.
ix) False – the mother can be treated antenatally if infection is diagnosed by PCR of the amniotic fluid. The treatment is with spiramycin as pyrimethamine and sulfadiazine are contraindicated in the first trimester as pyrimethamine is a folate antagonist. Spiramycin is a macrolide antibiotic.
x) False – in a recent study 24% of children developed retinal lesions over a six-year follow-up period. In only 11% was a diagnosis of retinochoroiditis made during the first month of life and half of the lesions were diagnosed after 1 year of age. The delay in onset of lesions is an important consideration when counselling families. 16
ANSWER 6
i) The baby should be resuscitated using an ABC approach and taken to the neonatal unit for ongoing care and management.
ii) Ask about possible events that may make infection more likely. Was there prolonged rupture of membranes? Did the mother have any episodes of raised temperature? If there have been any previous pregnancies were any infants treated for infection? Ask about UTIs (these are important risk factors for neonatal sepsis) as well as increasing the risk of premature birth, pre-eclampsia and low birth weight infants. 17
iii) Blood culture, urine culture, FBC, CRP and CXR. Blood culture is the definitive test. The majority of blood cultures taken will grow in 48 hours if they are going to be positive. The majority of neonatal infections are associated with a bacteraemia and thus a positive blood culture. Urine culture is an important investigation if sepsis is suspected. The drawback with urine analysis and culture is obtaining the specimen. If a baby is unwell and presumed septic, the easiest way to obtain a sample is by suprapubic aspiration of urine. This avoids the contamination which happens in the majority of bag urine specimens. If a bag urine specimen is thought to be positive, it must have at least 150 white cells/mm 3 and a pure growth of more than 10 5 organisms/mL urine. Be wary of any organisms other than E. coli or a mixed growth. If this is the case, an SPA will have to be obtained to confirm the infection.
A full blood count itself is not very useful. However a differential white count is of help. A low neutrophil count, <2.0–2.5×10 9/L in the first 2 days of life, suggests that there is a bacterial infection. After this time, either neutropenia or neutrophilia (>7.5–8.0×10 9/L) may occur. Other helpful markers of infection may be the ratio of immature to total number of neutrophils. The maximum acceptable ratio for excluding sepsis during the first 24 hours is 0.16. In most newborns, the ratio falls to 0.12 within 60 hours of life. An I/T ratio of >0.2 has been shown to be a good marker for infection. As well as being used in early onset sepsis, an I/T ratio >0.16 differentiated between Gram-positive, Gram-negative and fungal infections ( p=0.007) in late-onset infections. The sensitivity of the I/T ratio is in the range 60–90%, and elevations may be observed with other physiological events, limiting the positive predictive value of these ratios. An elevated I/T ratio should be used in combination with other signs when diagnosing sepsis. 18
C-reactive protein is a good indicator of infection if serial measurements are made and the trend analysed. It is better than white blood cell indices as an infection marker. It takes a few hours to rise and therefore should not be used to decide when to start antibiotics. Culture-proven sepsis is unlikely if the CRP does not rise within 48 hours of the onset of the illness, and thus it is generally safe to stop antibiotics if the cultures are negative and the CRP is normal at 48 hours. 19
All infants with suspected sepsis should have a chest X-ray.
iv) There has been much research and interest into procalcitonin. Procalcitonin (PCT) and C-reactive protein (CRP) concentrations in umbilical cord blood of 197 neonates were measured to evaluate their value as markers of infection. Sixteen of the neonates were infected. The sensitivity and specificity were 87.5% and 98.7% for PCT, and 50% and 97%, for CRP. It seems therefore that serum PCT in cord blood could be a useful and early marker of antenatal infection. 20 Other groups have made similar observations and suggest that a procalcitonin level >2.3 ng/mL or a CRP >30 mg/L indicates a high likelihood for neonatal sepsis, and antibiotic therapy should be continued even in the presence of sterile cultures. 21 Research has also shown that a raised interleukin-6 and TNF-alpha are good indicators of early sepsis. 22
v) After resuscitation, stabilisation and investigations, antibiotics should be given immediately. Most units have policies for which antibiotic to use, but in general for early onset sepsis (which is most likely in this infant’s case) the antibiotics need to cover group B Streptococcus, Listeria and Gram-negative organisms such as E. coli. A combination of a penicillin and gentamicin would be a good choice as there is synergistic action between the two against GBS. Cephalosporins alone will have no coverage for E. coli or Listeria.
vi) Most people in this situation with such a high CRP would carry out a lumbar puncture. If the platelet count was below 50, then a platelet transfusion should be given before the lumbar puncture.
vii) It is unlikely that this baby has meningitis. Generally in meningitis a polymorphonuclear count is >30/mm 3. When blood-stained CSF is obtained, the ratio of red to white cells should be calculated and if there is no infection, the ratio of red to white cells should be more than 500 to 1 (this can be most accurately calculated from the peripheral white cell to red cell ratio). The protein is difficult to interpret as the CSF is heavily blood stained. Without a blood glucose level, it is difficult to interpret the glucose of 1.4 mmol/L in the CSF. The CSF glucose should be at least 50% of the blood glucose level and a level <1.0 mmol/L suggests bacterial meningitis. Ideally a lumbar puncture should be performed prior to starting antibiotics. The one dose of antibiotics that has been given pre lumbar puncture may mean that cultures are less likely to be positive, but a lumbar puncture should still be performed in any baby who is sick with presumed sepsis as the cell count may be highly informative.
viii)
a. Fresh frozen plasma is often given when infants are septic to try and boost their immune response. It has been shown in adults to improve neutrophil chemotaxis, but this is not the case in neonates. In fact, the only indications for FFP, as described in the document by the British Committee for Standards in Haematology, Haemostasis and Thrombosis task force, in 2002, are DIC, vitamin K dependent bleeding and inherited deficiencies of coagulation. It should therefore not be used in this situation.
b. Intravenous immunoglobulin infusions have been studied as a possible therapy for neonatal sepsis to provide type-specific antibodies to improve opsonisation and phagocytosis of bacterial organisms and to improve complement activation and chemotaxis of neonatal neutrophils. Cochrane Reviews in 2004 showed that prophylactic IVIG led to a 3% reduction in sepsis and a 4% reduction in any serious infection but is not associated with reductions in other important outcomes such as sepsis, NEC, IVH or length of hospital stay. 23 The use of IVIG in proven sepsis, as part of a treatment regime, has been less well researched. A meta-analysis showed a significant decrease in mortality in proven sepsis, however results were not as convincing in the suspected sepsis group. There are difficulties with IVIG therapy for neonatal sepsis – the effect can be transient, and problems associated with the infusion of any blood product can occur. Dose-related problems with this therapy decrease the usefulness in neonatal populations. At present, data do not support the routine use of IVIG in neonatal sepsis. Results from a large multicentre trial are awaited.
c. Exchange transfusions with fresh whole blood have been used in neonatal sepsis to remove toxins and cytokines. The available data show that exchange transfusion may improve survival, but there are only a small number of reports with small patient numbers. There are currently no randomised trials to confirm these findings. 24. and 25.
d. 50% of infants with sepsis will develop a platelet count of <100×10 9/L and it would be prudent to transfuse these infants with platelets. Thrombocytopenia occurs as a combination of increased platelet consumption, often without evidence of DIC, and reduced platelet production.
e. It would be sensible to continue with the first choice antibiotics for at least 24 hours or until you have identified the bacteria from blood culture. If, after 48 hours, the infant’s condition is deteriorating with continuing rise in infective markers, consider other causes such as abscesses, fungal infection and osteomyelitis and treat appropriately with early discussion and involvement from microbiology colleagues.
f. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF), naturally occurring cytokines, are used to induce granulocyte production, as their names suggest. There are many trials which show an increase in neutrophil counts and enhanced functional activity as seen by C3bi expression. Mortality rates have been shown to be decreased by up to a third with treatment with rhGM-CSF in critically ill septic neutropenic neonates. However, the Cochrane Review concludes that there is currently insufficient evidence to support the introduction of either G-CSF or GM-CSF into neonatal practice, either as treatment of established systemic infection to reduce resulting mortality or as prophylaxis to prevent systemic infection in high risk neonates. 26.27. and 28.
g. Heliox has no place in the treatment of neonatal sepsis. It can be used in bronchiolitis or other obstructive airway disease as it decreases respiratory resistance and enhances carbon dioxide removal. 29
h. Pentoxifylline is a methylxanthine derivative with a broad spectrum of activity that modulates the inflammatory response, including the inhibition of TNF production. In animal models it has been shown to preserve micro-vascular blood flow, prevent circulatory failure and intestinal vaso-constriction, and improve survival. Two randomised controlled trials of pentoxifylline recruited 140 preterm infants with clinical sepsis. In the 107 with positive blood cultures, pentoxifylline was associated with 86% reduction in risk of mortality (RR 0.14, 95% CI 0.03–0.76). The recent Cochrane Review concludes that the use of pentoxifylline as an adjunct to antibiotics in neonatal sepsis reduces mortality without any adverse effects. 30.31. and 32.
ix)
a. Therapy can be simplified to intravenous benzyl penicillin alone as GBS is very sensitive to this.
b. Without meningitis, GBS bacteraemia can be treated with antibiotics for a total of 10 days. If meningitis was also present, antibiotics should be continued for at least 21 days.
c. Up to 30% of pregnant women are colonised with GBS. The majority of neonatal infections present within the first 4–6 hours of life and about 90% of cases will present within the first 24 hours of life. His infection was picked up early and treatment commenced promptly. His infective markers are improving showing he is responding to treatment. The baby has no signs of meningitis and is on the correct antibiotics with maximum supportive treatment. Previously, mortality from early-onset GBS sepsis was approaching 50% with a mortality rate up to 100% for babies weighing less than 1.5 kg.Currently in the UK, the mortality rate is 10%. At this stage we would be cautiously optimistic with the parents. 33
ANSWER 7
Answer viii would be the most appropriate plan of action. Most cases of GBS present within 4–6 hours after delivery and the majority of cases by 24 hours. This baby has no other risk factors for infection (e.g. prolonged rupture of membranes, prematurity, maternal infection, bacteriuria or previous sibling being affected) and therefore close observation without treatment for 24 hours is indicated. It is imperative the you inform the mother about the test result, and should the infant become unwell after discharge, for example becoming less interested in feeds or poor handling, that she seeks medical advice.
The incidence of early-onset GBS disease in term infants without antenatal risk factors in the UK is 0.2 cases/1000 births. This infant has none of the risk factors associated with GBS infection which are prematurity, rupture of the membranes more than 18 hours before delivery, rupture of the membranes before the onset of labour and intrapartum fever. If postnatal antibiotic treatment was completely effective and there were no adverse effects, 5000 infants would need to be treated to prevent a single case and at least 80,000 infants would have to be treated to prevent a single death from early-onset GBS disease. Most infants who develop early-onset GBS disease present with illness soon after birth and 90% have presented clinically by 12 hours of age, before culture results become available. This figure is the same for infants born to mothers who have received intrapartum antibiotics. Postnatal antibiotic treatment has not been shown to eradicate carriage of GBS or to influence the risk of late-onset GBS disease. It is therefore unnecessary to perform routine surface cultures or blood cultures on well infants, whether they received antibiotic prophylaxis or not. 34.35. and 36.
ANSWER 8
Answer v is correct.
Chickenpox is an extremely common infection, such that 90% of the antenatal population are seropositive for VZV IgG antibody. Contact with chickenpox is common, especially in mothers with other children, so primary VZV infection is rare – 3 in 1000 pregnancies.
If the mother is immune, there is no need to do anything as the baby will be protected by the antibodies. If the mother is susceptible and the baby is less than 7 days old, the newborn should be given VZIG. The mother does not require VZIG as she is no longer at risk of serious complications since she has delivered. Aciclovir is used with VZIG if the mother develops chickenpox between 7 days pre and 7 days post delivery as it appears to provide some protection and may reduce the chance of transmission to the newborn infant.
This baby should have his immune status checked using a varicella zoster enzyme-linked immunosorbent assay (ELISA). If it is negative and thus the baby has not acquired antibodies from the mother, he needs to be given a dose of zoster immune globulin, 100 mg. After significant nursery exposure to VZV, ZIG should be given to seronegative babies and to all babies born before 28 weeks gestation. 37.38. and 39.
ANSWER 9
Answers i, v, viii and ix are correct.
i) Yes – Bacillus fragilis is a Gram-positive bacillus that does cause osteomyelitis.
ii) No – Bacillus cereus is another Gram-positive bacilli that causes meningitis and pneumonia.
iii) No – Bacteroides fragilis is a Gram-negative bacillus that causes meningitis.
v) Yes – Haemophilus influenzae is a Gram-negative rod that can cause not only osteomyelitis but pneumonia and meningitis.
vi) No – Proteus mirabilis is a Gram-negative bacillus that causes meningitis and abscesses.
vii) No – Salmonella typhi classically causes gastroenteritis and typhoid fever. This organism is much more common in the developing world.
viii) Yes – group B streptococcus is otherwise known as Streptococcus agalactiae. It is a Gram-positive coccus that can cause infection in most tissues – osteomyelitis, empyema, brain abscess, endocarditis, cellulitis and UTI. It is a commensal of the gut and the vagina and colonises up to a third of pregnant women in the UK. There are nine serotypes, but types I, II and III are responsible for most neonatal infections. The osteomyelitis seen with GBS can be a subtle presentation with only one site being affected. Commonly hip, knee, pelvis and humerus.
ix) Yes – Staphylococcus aureus, a Gram-positive coccus, is associated with a high mortality and morbidity. Some strains are more virulent than others, due to extracellular factors and toxins. It is the primary cause of infection in bones, joints and skin. Pustular skin lesions are classic and there is rapid seeding to bones, joints and lungs. It is a relatively rare cause of meningitis and very rarely a UTI. A clear association has been shown between the severity of bacteraemia and mortality. 40 The systemic infection leads to marked neutropenia and thrombocytopenia with a rapidly increasing CRP.
x) No – Klebsiella is a Gram-negative bacillus that causes urinary tract infections and meningitis. Gram-negative bacilli are thought to account for about 20% of cases of late-onset neonatal sepsis and even higher numbers in the developing world. The mortality from systemic infections is extremely high, especially in preterm infants. There is a link between Gram-negative bacilli and the severity of bronchopulmonary dysplasia. 41
xi) No – Serratia marcescens is an opportunistic Gram-negative bacillus which is often seen in the developed world and typically infects compromised hosts. It commonly causes a septicaemia with associated meningitis, with a high mortality. It has been reported as a causative organism in infected ventriculo-peritoneal shunts. It occurs sporadically in the NICU, primarily in premature infants requiring support, and appears late in their hospital course. There are very occasional cases of neonatal osteomyelitis being caused by Serratia marcescens but affected infants had underlying immunodeficiency disorders. 42. and 43.
ANSWER 10
Answers iv, v and vi are correct.
HBsAg is a marker of the carrier state of hepatitis B and its incidence varies from 0.1% in parts of Europe to up to 20% in Africa and Asia. The presence of the e antigen greatly increases the risk of infection to the baby. Therefore the baby in part iv of the question is at greatest risk of infection and must receive both hepatitis B vaccination and immunoglobulin. The expression of the e antigen seems to be genetically determined with more Chinese women carriers being HBeAg positive than African carriers. It is recommended that babies born to mothers with acute hepatitis should also receive hepatitis B immunoglobulin as the risk of transmission is significantly increased. Where e antigen and e antibody status are unknown immunoglobulin should be considered.
ANSWER 11
i) The baby’s skin shows desquamation which has been provoked by rubbing the skin’s surface. This is called Nikolsky’s sign. This occurs after an initial scarlatiniform rash, which is usually present on the face and in the flexures. If denudation occurs, this is followed by drying of the skin and a further desquamation phase that lasts for a few days. The mucosal surfaces are unaffected.
ii) The diagnosis is staphylococcal scalded skin syndrome, previously known as Ritter’s disease.
iii) The responsible organism is Staphylococcus aureus. The commonest source of infection is an infected umbilical stump.
iv) The infant should be treated with intravenous flucloxacillin.
v) Infants respond extremely well to intravenous antibiotics and the skin heals with no sequelae.
ANSWER 12
i) Yes – rubella was made a notifiable disease in 1988, and is monitored through clinical and laboratory reports. 44
ii) No – toxoplasmosis causes hydrocephalus. Rubella does not. It does cause microcephaly and delayed developmental milestones.
iii) Yes – although this is not present at birth, infants may develop this along with reduced cellular immunity and thyroid autoantibodies. Some infants with congenital rubella do not present in the neonatal period with the well described rubella syndrome including jaundice, thrombocytopenia and hepatosplenomegaly. They present in later life with neurological problems, heart disease, eye defects and deafness.
iv) Yes – corneal opacities are present in congenital rubella.
v) Yes – irregular lucencies in the bone and abnormal trabecular pattern in the long bones. The X-ray appearance is said to resemble a stick of celery.
vi) Yes – one-third of affected babies are growth restricted.
vii) Yes – micro-ophthalmia does occur as well as glaucoma. One study reported that up to 78% of affected infants developed ocular abnormalities and no significant association was found between gestational age at time of maternal infection and the incidence of individual ocular conditions. 45
viii) No – conductive deafness does not occur in congenital rubella, it is usually sensorineural deafness and commonly bilateral. These deficits occur in up to 66% of affected infants. 46
ix) Yes – cardiac abnormalities, especially PDA, occur in up to 58% of infants.
x) No – aortic stenosis does not occur. Peripheral pulmonary stenosis is a frequent finding and due to viral damage to endothelium of large blood vessels.
xi) No – although the risk of congenital infection is highest in the first trimester, not all infants are affected; approximately 10–20% are not.
xii) Yes – although the incidence decreases with increasing gestation. Congenital rubella is rare after 20 weeks’ gestation.
xiii) No – the infants can continue to shed the virus up to months after delivery and therefore appropriate precautions should be taken with female nursing and medical personnel.
xiv) Yes – despite the incidence of congenital rubella dramatically falling with the introduction of the MMR immunisation programme, there are still cases occurring. The majority of the mothers are from the immigrant population and there is a drive by the World Health Organization to improve the global control of rubella and CRS. 45. and 47.
ANSWER 13 48. and 49.
i) RPR (rapid plasma reagin test) is a non-treponemal serological test and TPHA ( Treponema pallidum haemagglutination assay) is a treponemal test. Both being reactive show that the mother may have syphilis.
ii) You must confirm with a treponemal test that is different from that used in screening such as the treponemal ELISA for IgG and IgM.
iii) You must:
a. Examine the baby for any signs of congenital syphilis such as thrombocytopenia, jaundice, hepatosplenomegaly, rhinitis, periostitis (visible on long bone X-ray) and IUGR.
b. Take blood for serology – from infant not from cord blood as the latter can become contaminated from maternal blood and give a false positive result.
iv) A positive anti-treponemal EIA IgM is consistent with a diagnosis of congenital infection. It should be repeated to confirm.
v) Serology can be negative in babies infected late in pregnancy. A negative IgM should be repeated at 1, 2 and 3 months of age as the IgM response may be suppressed.
vii) Quantitative non-treponemal serological titres (VDRL/RPR) are useful to assess risk to the baby. If the titres are four-fold higher than the mother’s titres, the infant is at high risk and needs treating. If the titres are the same or less than four-fold the maternal titre, treatment of the infant depends on the adequacy of maternal treatment.
viii) Due to inadequate maternal treatment, the infant needs treating with benzyl penicillin 30 mg/kg IV (50,000 units/kg) 12-hourly for 7 days and then 8-hourly for 10 days treatment in total. Follow-up at three and sixth months and one year with VDRL/RPR is required.
ANSWER 14
i) False – if several infants are affected by the same organisms, it is more sensible to cohort infants, separating them from uninfected infants. Although this is dependent on available space and staffing, it should always be attempted where highly resistant or highly infective organisms are spreading. Once cohorted, all staff involved in the care of the baby should adopt barrier nursing procedures.
ii) False – the use of gloves may have a role in the reduction of infection, but there is good evidence that scrupulous attention to hand hygiene is of paramount importance.
iii) False – nursing staff should care for segregated cohorts in this situation.
iv) False – there was a vogue for regular skin disinfection in the early years of neonatal care. This policy led to widespread damage of babies, including significant brain damage with the use of hexachlorophene. 50
v) False – topical antibiotic therapy may be indicated but should always be targeted toward a specific organism (e.g. Staphylococcal aureus cord infection) and should never be routine and generalised.
vi) False – although the wearing of gowns may contribute to reducing the spread of infection, there is some evidence to suggest that the impact is minimal and other infection control measures are more important. 51
vii) True – the source of infection often remains unclear but it is advisable to screen other babies to identify asymptomatic carriers. If the means of spread is uncertain, staff screening may sometimes be of value, but must be managed tactfully and diplomatically.
viii) True – staff numbers and the thoroughness of their hand washing have been shown to be very important elements in reducing infection rate. 52
ix) False – although this may be indicated on rare occasions, such a decision should only be taken following advice from microbiology and infection control specialists. Proper barrier nursing and meticulous attention to hygiene should allow a unit to function without having to resort to closure.
xi) True.
xii) False – there are published data which suggest that effective hand washing of an appropriately high standard may be performed in little more than 20% of patient contacts. Even more worryingly, there are incidents where hands are not cleansed adequately (or at all) between patients – even when there has been contact with body fluids or excreta. 53
xiii) False – there is now good evidence that the proper use of alcohol-based hand rub between patients is more effective in infection control than the use of soap and water.
xiv) False – there is no evidence that application of sprays, creams or powders is any better than keeping the baby’s cord clean and dry at birth. 54
REFERENCES
1. Yow, MD; Williamson, DW; Leeds, LJ; et al., Epidemiologic characteristics of cytomegalovirus infection in mothers and their infants, Am J Obstet Gynecol 158 (1988) 1189–1195.
2. Demmler, GJ, Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease, Rev Infect Dis 13 (1991) 315–329.
3. Lipitz, S; Achiron, R; Zalel, Y; et al., Outcome of pregnancies with vertical transmission of primary cytomegalovirus infection, Obstet Gynecol 100 (2002) 428–433.
4. Hart, MH; Kaufman, SS; Vanderhoof, JA; et al., Neonatal hepatitis and extrahepatic biliary atresia associated with cytomegalovirus infection in twins, Am J Dis Child 145 (1991) 302–305.
5. Lanari, M; Lazzarotto, T; Venturi, V; et al., Neonatal cytomegalovirus blood load and risk of sequelae in symptomatic and asymptomatic congenitally infected newborns, Pediatrics 117 (2006) 76–83.
6. Rivera, LB; Boppana, SB; Fowler, KB; et al., Predictors of hearing loss in children with symptomatic congenital cytomegalovirus infection, Pediatrics 110 (2002) 762–767.
7. Kimberlin, D; Lin, C; Sanchez, P; et al., Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial, J Pediatr 143 (2003) 16–25.
8. Nigro, G; Adler, SP; La Torre, R; et al., Congenital Cytomegalovirus Collaborating Group, Passive immunization during pregnancy for congenital cytomegalovirus infection, N Engl J Med 353 (2005) 1350–1362.
9. Gay, NJ; Hesketh, LM; Cohen, BJ; et al., Age specific antibody prevalence to parvovirus B19: how many women are infected in pregnancy?Commun Dis Rep 4 (1994) 104–107.
10. Miller, E; Fairley, CK; Cohen, BJ; et al., Immediate and long term outcome of human parvovirus B19 infection in pregnancy, Br J Obstet Gynaecol 105 (1998) 175–178.
11. Dunn, D; Wallon, M; Peyron, F; et al., Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counseling, Lancet 353 (1999) 1829–1833.
12. In: (Editors: Remington, JS; Klein, JO) Infectious diseases of the fetus and newborn infant5th edn ( 2001)Saunders, Philadelphia, pp. 205–346.
13. Hohlfeld, P; Daffos, F; Thulliez, P; et al., Fetal toxoplasmosis: outcome of pregnancy and infant follow-up after in utero treatment, J Pediatr 115 (1989) 765–769.
14. Thiebaut, R; Leproust, S; Chene, G; et al., SYROCOT (Systematic Review on Congenital Toxoplasmosis) study group, Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data, Lancet 369 (2007) 115–122.
15. Mcleod, R; Boyer, K; Karrison, T; et al., Outcome of treatment for congenital toxoplasmosis, 1981–2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study, Clin Infect Dis 42 (2006) 1383–1397.
16. Wallon, M; Kodjikian, L; Binquet, C; et al., Long-term ocular prognosis in 327 children with congenital toxoplasmosis, Pediatrics 113 (2004) 1567–1572.
17. Schieve, LA; Handler, A; Hershow, R; et al., Urinary tract infection during pregnancy: its association with maternal morbidity and perinatal outcome, Am J Public Health 84 (3) ( 1994) 405–410.
18. Gonzalez, BE; Mercado, CK; Johnson, L; et al., Early markers of late-onset sepsis in premature neonates: clinical, hematological and cytokine profile, J Perinat Med 31 (1) ( 2003) 60–68.
19. Bomela, HN; Ballot, DE; Cory, BJ; et al., Use of C-reactive protein to guide duration of empiric antibiotic therapy in suspected early neonatal sepsis, Ped Inf Dis J 19 (2000) 531–535.
20. Joram, N; Boscher, C; Denizot, S; et al., Umbilical cord blood procalcitonin and C reactive protein concentrations as markers for early diagnosis of very early onset neonatal infection, Arch Dis Child Fetal Neonatal Ed 91 (1) ( 2006) F65–F66.
21. Turner, D; Hammerman, C; Rudensky, B; et al., The role of procalcitonin as a predictor of nosocomial sepsis in preterm infants, Acta Paediatr 95 (12) ( 2006) 1571–1576.
22. Mishra, UK; Jacobs, SE; Doyle, LW; et al., Newer approaches to the diagnosis of early onset neonatal sepsis, Arch Dis Child Fetal Neonatal Ed 91 (3) ( 2006) F208–F212.
23. Ohlsson, A; Lacy, JB, Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants, Cochrane Database Syst Rev ( 1) ( 2004); CD000361.
24. Mathur, NB; Subramanium, BKM; Sharma, YK; et al., Exchange transfusion in neutropenic septicemic neonates: effect on granulocyte functions, Acta Paediatr 82 (1993) 939–943.
25. Vain, NE; Mazlumian, JR; Swarner, OW; et al., Role of exchange transfusion in the treatment of severe septicemia, Pediatrics 66 (5) ( 1980) 693–697.
26. Bilgin, K; Yaramis, A; Haspolat, K; et al., A randomized trial of granulocyte-macrophage colony-stimulating factor in neonates with sepsis and neutropenia, Pediatrics 107 (1) ( 2001) 36–41.
27. Miura, E; Procianoy, RS; Bittar, C; et al., A randomized, double-masked, placebo-controlled trial of recombinant granulocyte colony-stimulating factor administration to preterm infants with the clinical diagnosis of early-onset sepsis, Pediatrics 107 (1) ( 2001) 30–35.
28. Carr, R; Modi, N; Dore, C, G-CSF and GM-CSF for treating or preventing neonatal infections, Cochrane Database Syst Rev ( 3) ( 2003); CD003066.
29. Myers, TR, Use of heliox in children, Respir Care 51 (6) ( 2006) 619–631.
30. Lauterbach, R; Pawlik, D; Tomaszczyk, B; et al., Pentoxifylline treatment of sepsis of premature infants: preliminary clinical observations, Eur J Pediatr 153 (9) ( 1994) 672–674.
31. Haque, K; Mohan, P, Pentoxifylline for neonatal sepsis, Cochrane Database Syst Rev ( 4) ( 2003); CD004205.
32. Lauterbach, R; Pawlik, D; Kowalczyk, D; et al., Effect of the immunomodulating agent, pentoxifylline, in the treatment of sepsis in prematurely delivered infants: a placebo-controlled, double-blind trial, Crit Care Med 27 (4) ( 1999) 807–814.
33. Heath, P; Nicoll, A; Efstratiou, A, Group B streptococcal disease in UK and Irish infants younger than 90 days, Lancet 363 (9405) ( 2004) 292–294.
34. Lin, FY; Brenner, RA; Johnson, YR; et al., The effectiveness of risk-based intrapartum chemoprophylaxis for the prevention of early-onset neonatal group B streptococcal disease, Am J Obstet Gynecol 184 (2001) 1204–1210.
35. Escobar, GJ; Li, DK; Armstrong, MA; et al., Neonatal sepsis workups in infants≥2000 grams at birth: a population-based study, Pediatrics 106 (2000) 256–263.
36. Bromberger, P; Lawrence, JM; Braun, D; et al., The influence of intrapartum antibiotics on the clinical spectrum of early-onset group B streptococcal infection in term infants, Pediatrics 106 (2000) 244–250.
37. Heuchan, AM; Isaacs, D, The management of varicella-zoster virus exposure and infection in pregnancy and the newborn period. Australasian Subgroup in Paediatric Infectious Diseases of the Australasian Society for Infectious Diseases, Med J Aust 174 (6) ( 2001) 288–292.
38. Royal College of Obstetricians and Gynaecologists, Chickenpox in pregnancy. 2nd edn. ( 2001)RCOG, London; Clinical Green-top Guideline No. 13..
39. Ng, PC; Lyon, DJ; Wong, MY, Varicella exposure in a neonatal intensive care unit: emergency management and control measures, J Hosp Infect 32 (3) ( 1996) 229–236.
40. Schonheyder, HC; Gottschau, A; Friland, A; et al., Mortality rate and magnitude of Staphylococcus aureus bacteremia as assessed by a semiquantitative blood culture system, Scand J Inf Dis 27 (1995) 19–21.
41. Cordero, L; Ayers, L; Davis, K, Neonatal airway colonization with Gram-negative bacilli: association with severity of bronchopulmonary dysplasia, Ped Inf Dis 16 (1997) 18–23.
42. Mayer, CW; Bangash, S; Bocchini, JA; et al., Serratia marcescens osteomyelitis in an infant, Allergy Asthma Proc 27 (6) ( 2006) 544–548.
43. Bizzarro, MJ; Dembry, LM; Baltimore, RS, Case-control analysis of endemic Serratia marcescens bacteremia in a neonatal intensive care unit, Arch Dis Child Fetal Neonatal Ed 92 (2) ( 2007) F120–F126.
44. Tookey, P, Rubella in England, Scotland and Wales, Euro Surveill 9 (4) ( 2004) 21–23.
45. Best, JM; Castillo-Solorzano, C; Spika, JS; et al., Reducing the global burden of congenital rubella syndrome: report of the World Health Organization Steering Committee on Research Related to Measles and Rubella Vaccines and Vaccination, June 2004, J Infect Dis 192 (11) ( 2005) 1890–1897.
46. Givens, KT; Lee, DA; Jones, T; et al., Congenital rubella syndrome: ophthalmic manifestations and associated systemic disorders, Br J Ophthalmol 77 (6) ( 1993) 358–363.
47. Robinson, JL; Lee, BE; Preiksaitis, JK; et al., Prevention of congenital rubella syndrome – what makes sense in 2006?Epidemiol Rev 28 (2006) 81–87.
48. Screening Guidelines Steering Committee, Sexually transmitted infections: UK screening and testing guidelines. ( 2006)British Association for Sexual Health and HIV, London.
49. British Association for Sexual Health and HIV. UK National Guidelines on the Management of Early Syphilis, Clinical Effectiveness Group (Association for Genitourinary Medicine and the Medical Society for the Study of Venereal Diseases), 2002, www.bashh.org/guidelines
50. Anderson, JM; Cockburn, F; Forfar, JO; et al., Neonatal spongioform myelinopathy after restricted application of hexachlorophane skin disinfectant, J Clin Pathol 34 (1) ( 1981) 25–29.
51. Pelke, S; Ching, D; Easa, D; et al., Gowning does not affect colonization or infection rates in a neonatal intensive care unit, Arch Pediatr Adolesc Med 148 (10) ( 1994) 1016–1020.
52. Parry, GJ; Tucker, JS; Tarnow-Mordi, WO; , UK Neonatal Staffing Study Group, Relationship between probable nosocomial bacteraemia and organisational and structural factors in UK neonatal intensive care units, Qual Saf Health Care 14 (4) ( 2005) 264–269.
53. Cohen, B; Saiman, L; Cimiotti, J; et al., Factors associated with hand hygiene practices in two neonatal intensive care units, Pediatr Infect Dis J 22 (6) ( 2003) 494–499.
54. Zupan, J; Garner, P, Topical umbilical cord care at birth, Cochrane Database Syst Rev ( 3) ( 2004); CD001057.