Individuals with Acute Medical Conditions
This chapter describes the physical therapy management of individuals with primary, acute dysfunction of the cardiovascular and pulmonary systems. Such dysfunction may limit participation in life and its related activities in the short- or long-term. Further, such dysfunction can constitute life threat in the absence of limitations to life participation and quality of life (e.g., hypertension and dysrhythmias). Management principles for people with several types of common acute medical conditions are described. Although medical conditions are usually classified as either primary pulmonary disease or primary cardiovascular disease, the heart and lungs work synergistically to effect gas exchange and cardiac output and in series with the peripheral vascular circulation to effect tissue perfusion.1,2 Thus impairment of one organ system invariably has implications for the function of the other. Threat to or impairment of oxygen transport has implications for all other organ systems; thus a multisystem approach is essential for overall management (see Chapters 1 and 5). The primary, acute pulmonary conditions that are presented in this chapter include atelectasis, pneumonia, bronchitis, bronchiolitis, acute exacerbations of chronic airflow limitation, asthma, cystic fibrosis, interstitial pulmonary fibrosis, and tuberculosis. For further epidemiological and pathophysiological detail on these conditions, refer to Mason and colleagues (2010)3 and West4 (2007). The primary, acute cardiovascular conditions presented include hypertension, medically stable angina, and uncomplicated myocardial infarction. For further details on these conditions, refer to Sokolow and Cheitlin5 (2004), Fauci and colleagues6 (2008), and Woods and colleagues7 (2009).
The pathophysiology underlying the medical management of each condition extends the pathophysiology content of Chapter 5 and, in turn, provides a basis for each condition’s physical therapy management. The management principles presented are not intended to serve as treatment prescriptions for any particular patient. The treatment priorities presented are based on the underlying pathology, as well as the potential complexity of its manifestation for a patient. Without discussion of a specific patient and knowledge of other clinically relevant factors (i.e., the effects of restricted mobility, recumbency, and the effects of extrinsic and intrinsic factors on the patient’s presentation including sociocultural context; see Chapter 17); however, the specific parameters of the treatment prescription cannot be completely established. Integration of patient-specific information is essential for treatment to be specific and maximally effective. Chapter 31 extends the principles involved with the management of many of the acute medical conditions described in this chapter, detailing their subacute and chronic stages.
Cardiovascular Pathology
Hypertension
Pathophysiology and Medical Management
Essential hypertension, the “silent killer” (of unknown etiology), is the most common type of hypertension (90% of all reported cases). Although salt sensitivity has been implicated in hypertension in African Americans and increased rennin production in the Hispanic population,8 salt consumption is a serious health concern in the North American population. Widespread campaigns are being aimed at reducing salt consumption in the pediatric as well as adult population.9 Reducing consumption by one-third is estimated to reduce the prevalence of hypertension substantially.
Generally, hypertension is classified as mild, moderate, or severe. It is generally managed pharmacologically with vasodilators (i.e., afterload reducers), diuretics (i.e., volume reducers), and beta-blocking agents (i.e., inotropic agents). Despite a primarily pharmacological orientation to the management of hypertension, a high proportion of individuals with hypertension still have high blood pressure and are at increased risk for its deadly complications. Hypertension is a significant health care concern in that the condition is frequently associated with heart disease, stroke, and renal dysfunction and failure.10 Thus its consequences can be dire. As described in Chapter 1, hypertension often occurs in the presence of obesity and diabetes, which complicates the clinical picture further.
1. To what degree does pharmacological intervention appear warranted, given the patient’s severity of hypertension and overall clinical picture?
2. To what degree could the hypertension be managed with nonpharmacological interventions?
3. To what degree could pharmacological and nonpharmacological interventions be used concurrently? What type of schedule might be anticipated to wean the patient off medication with time or at least minimize it?
Principles of Physical Therapy Management
Physical therapists treat patients with hypertension as a primary or secondary diagnosis. If it is a secondary diagnosis, it is important that the diagnosis is not overlooked. What the physical therapist can do for the hypertension may be clinically more significant than management of the primary diagnosis for which the patient is referred. As with many other lifestyle-related conditions, antihypertensive medication may be perceived by the patient as addressing the problem, whereas in many instances, it only addresses the effect. Lifestyle changes are necessary to normalize blood pressure with the goal of eliminating the need for blood pressure medication.11
Exercise therapy can be an effective intervention for the management of hypertension with the primary goal of eliminating the need for medication.12 Secondarily, physical therapy with a focus on exercise and health education, including smoking cessation and basic nutritional counseling, is aimed at reducing the need for medication or its potency. The foundation of management in a patient with hypertension is a lifestyle review and recommendations in consultation with the patient. Recommendations include nutrition, weight control, exercise, smoking cessation, and stress management.13 Medical management may include beta blockers and diuretics to reduce plasma volume or other antihypertensive medication. A prescription of regular aerobic exercise may control hypertension.14 The prescription is based on a consideration of the patient’s coexistent problems and general health status. If obesity is a concurrent problem, an exercise program is prescribed to address both concerns.
A patient who is being managed acutely with high blood pressure may benefit from relaxation strategies, breathing control, and stress management. Further, complementary therapies, as described in Chapter 27 may have an important role. For noninvasive physical therapy management of hypertension, see Chapter 31.
Angina
Pathophysiology and Medical Management
Principles of Physical Therapy Management
The management of patients with ischemic heart disease who are hemodynamically unstable and require intensive monitoring to assess and to monitor physical therapy treatment is described in Chapter 34. This section addresses management of the patient with a cardiac medical condition who is stable and uncomplicated. Physical therapists must be knowledgeable and proficient in management of the patient with cardiac conditions because these patients are referred with cardiac disease as a primary or secondary problem. With respect to heart disease being a secondary diagnosis, patients often come to the physical therapist for the management of an orthopedic complaint with a history of angina, frank myocardial infarction, or hypertension. The principles for physical therapy in management of patients with acute ischemic heart disease are presented within the principles of phase I cardiac rehabilitation (Table 29-1). Because physical therapy invariably involves physically stressing a patient either with therapeutic exercise or with the application of a therapeutic modality, the physical therapist must address the following questions when managing a patient with ischemic heart disease and its risk factors:
Table 29-1
Phase I of Cardiac and Pulmonary Rehabilitation (Inpatient, <7 to 10 days)*
| Cardiac Rehabilitation | Pulmonary Rehabilitation |
| After anginal attack, myocardial infarction, operative procedures including bypass surgery and valve surgery | After acute exacerbation or thoracic surgery (e.g., admission lung resection) |
| Optimize oxygen transport by directing treatment to the underlying limitations of structure and function (impairments) | Optimize oxygen transport by directing treatment to the underlying limitations of structure and function (impairments) |
| Risk factors assessment | Risk factor assessment |
| Assessment of knowledge deficits and learning style | Assessment of knowledge deficits and learning style |
| Readiness to change assessment | Readiness to change assessment |
| Predischarge submaximal exercise test | Predischarge submaximal exercise test |
| Discharge lifestyle recommendations: | Discharge lifestyle recommendations: |
 Smoking cessation Smoking cessation |
 Smoking cessation Smoking cessation |
 Nutrition and weight control Nutrition and weight control |
 Nutrition and weight control Nutrition and weight control |
 Physical activity and exercise Physical activity and exercise |
 Physical activity and exercise Physical activity and exercise |
 Stress management Stress management |
 Stress management Stress management |
| Plan for follow-up | Plan for follow-up |
*Phases II, III, and IV are related to subacute and chronic care (see Chapter 31).
Modified from Piotrowicz R, Wolszakiewicz J: Cardiac rehabilitation following myocardial infarction. Cardiology Journal 15:481–487, 2008.
1. Does the patient’s cardiac status preclude treatment? Why?
2. Is additional information about the patient necessary before physical therapy assessment and treatment? What information?
3. How should treatment be modified? Why?
4. Is the patient using antianginal medication appropriately? Is the prescription current? Does the patient have the antianginal medication present at all times?
5. Are there other medications that may influence the patient’s cardiovascular and pulmonary status and response to treatment? What are they? How might the patient’s responses to treatment, particularly exercise, be affected?
6. What physiological parameters should be monitored before, during, and after treatment?
7. What is the patient’s knowledge about his or her condition? Can the patient clearly identify what triggers the angina and what makes it worse and better? What lifestyle changes have been made? What should be reinforced and what education is necessary?
Individuals prone to angina may exhibit symptoms in certain body positions.15,16 Usually, this reflects an increased workload and increased work of the heart. Recumbent positions increase the mechanical work of the heart by increasing central blood volume.17 These patients are not encouraged to lie flat. Instead, the head of bed is elevated 10 to 15 degrees. Side-lying positions, particularly left side-lying, increase the work of the heart by compressing the heart and impeding ventricular filling and ejection. Patients with impaired oxygen transport and without prior cardiac disease may exhibit myocardial stress and ischemia in these body positions. Thus patients with impaired or threatened oxygenation must be monitored closely, particularly during turning and activities in which oxygen demand is increased—at these times, oxygen delivery must be increased correspondingly.
The use of medication to minimize the risk for angina and ischemic heart disease warrants review. The need for statins, commonly prescribed lipid-lowering medications, can be reduced through optimal lifestyle choices in many patients. One inherent danger of such a drug, however, in addition to its well documented side effects, is that patients may become complacent, believing that the drug will offset the need to make necessary lifestyle changes. More important, lifestyle changes largely address the cause of the problem, whereas medication such as statins only address an effect. The principles of the physical therapy management of patients with stabilized angina include health education, risk factor reduction, and a long-term health program (see Chapters 24 and 31).
Uncomplicated Myocardial Infarction
Pathophysiology and Medical Management
Myocardial infarction, commonly referred to as a heart attack, refers to insufficient myocardial perfusion resulting in a macroscopic area of damage and necrosis of the heart. Infarction results most frequently from narrowing and occlusion of the coronary blood vessels secondary to atherosclerosis. Other causes include occlusion secondary to a thrombus or embolus, reduced blood pressure, or coronary vasospasm. Angina, or ischemic chest pain, often precedes or accompanies a myocardial infarction. Infarctions vary in severity from being silent (i.e., having no characteristic signs and symptoms and thus going undetected) to being fatal. Most infarctions, when detected, require some hospitalization and monitoring to ensure that the infarction is not evolving further and that the patient is medically stable and in no danger. Chapter 34 describes the management of patients with complicated myocardial dysfunction who are admitted to a coronary care unit. This section focuses on the patient with mild heart disease, the patient with cardiac dysfunction who is discharged from hospital, the patient who has a history of ischemic heart disease, and the patient who is hospitalized for a condition other than heart disease but develops and is being managed for myocardial ischemia. Judicious movement and body positioning are essential elements in the management of the patient with myocardial infarction.18 Because these interventions can place significant demands on cardiovascular and pulmonary function and oxygen transport, they must be prescribed specifically by physical therapists with considerable knowledge and expertise in the area.
Principles of Physical Therapy Management
Table 29-1 shows the primary components of care in the acute phase of management (phase I of cardiac rehabilitation). Physical therapy constitutes a prime hemodynamic stress secondary to exercise and gravitational stress secondary to mobilization/exercise and body position changes. Thus it is essential to establish the adequacy of the patient’s cardiovascular and pulmonary system to effect oxygen transport during and between treatments. The optimal treatment prescription is based on the patient’s overall signs and symptoms of coronary insufficiency and hemodynamic instability. The physical therapist must be knowledgeable in detecting inadequate myocardial tissue perfusion and in reducing and preventing myocardial tissue damage. In addition, acute or chronic impaired heart pump function leads to reduced cardiac output and systemic tissue perfusion. Clinical manifestations include reduced mentation, reduced renal function, fatigue, malaise, and moist, cool, and cyanotic skin.
When selecting body positions for the patient with a myocardial infarction, the therapist selects those that will minimize the work of breathing and of the heart.19 Significant central fluid shifts are minimized by encouraging the upright position as much as possible to reduce the work of the heart20 and by raising the head of the bed 10 to 15 degrees when the patient is recumbent. Patients with elevated intracardiac pressures are less susceptible to orthostatism (see Chapter 20).
Relaxation is central in the management of the cardiac patient who is prone to being anxious and apprehensive. Furthermore, such patients have a high prevalence of sleep-disordered breathing,21 so a sleep assessment is warranted. Relaxation interventions that can be suggested include autogenic relaxation, progressive relaxation, Benson’s relaxation response procedures, biofeedback, and meditation. Also, the patient needs to identify and minimize stress triggers and effective, individual-specific, nonpharmacological relaxants. Relaxation training with or without pharmacological support can be integrated into treatment.7 Patients with ischemic heart disease are often apprehensive and anxious about the intensity of physical activity they can undertake. Thus performing physical activity and exercise while monitored and under the supervision of a physical therapist is often reassuring and gives the patient confidence to perform activity when unsupervised.
Optimal lifestyle habits and a lifelong health plan are central to maximizing recovery and improving an individual’s long-term prognosis. Good nutrition and hydration, good sleep habits, stress management, smoking cessation, and regular physical exercise are all salient to comprehensive physical therapy management (see Chapters 24 and 31). As in the management of patients with other lifestyle-related conditions, the physical therapist needs to reinforce public health policy and health promotion guidelines regarding healthy lifestyle choices, including avoidance of inactivity and regular physical activity.22
Pulmonary Pathology
Atelectasis
Pathophysiology and Medical Management
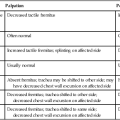
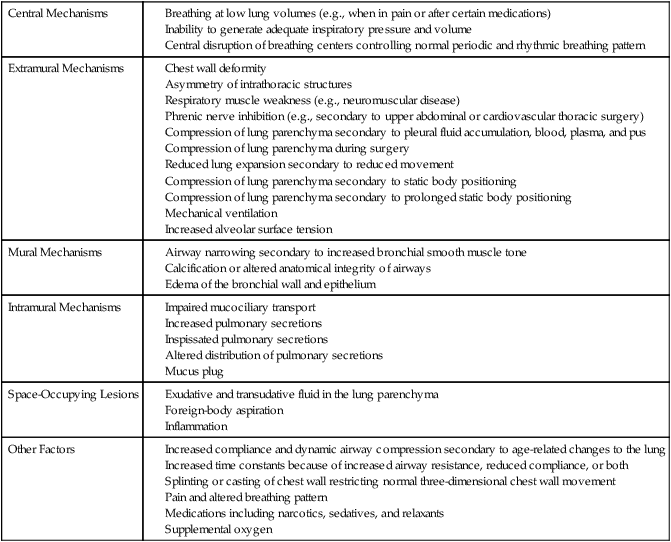
Atelectasis refers to partial collapse of lung parenchyma. The pathophysiological mechanisms contributing to atelectasis are multiple (Table 29-2). These mechanisms include physical compression of the lung tissue (e.g., resulting from increased pleural fluid, pus, pneumothorax, compression during thoracic surgery, or adjacent areas of lung collapse) or obstruction of an airway (e.g., due to secretions or tumor) with subsequent reabsorption of oxygen from the trapped air by the pulmonary capillaries resulting in a collapse of the lung tissue distal to the obstruction (i.e., reabsorption atelectasis).
Table 29-2
Asymmetry of intrathoracic structures
Respiratory muscle weakness (e.g., neuromuscular disease)
Phrenic nerve inhibition (e.g., secondary to upper abdominal or cardiovascular thoracic surgery)
Compression of lung parenchyma secondary to pleural fluid accumulation, blood, plasma, and pus
Compression of lung parenchyma during surgery
Reduced lung expansion secondary to reduced movement
Compression of lung parenchyma secondary to static body positioning
Compression of lung parenchyma secondary to prolonged static body positioning
Increased compliance and dynamic airway compression secondary to age-related changes to the lung
Increased time constants because of increased airway resistance, reduced compliance, or both
Splinting or casting of chest wall restricting normal three-dimensional chest wall movement
Pain and altered breathing pattern
Principles of Physical Therapy Management
Because it can develop instantaneously when respiratory mechanics are disrupted, microatelectasis should be anticipated and prevented. Those factors that contribute to atelectasis for a given patient are countered accordingly with aggressive prophylactic management. Many of the causes of atelectasis outlined in Table 29-2 can be readily reversed. The assessment includes a detailed analysis of the underlying cause(s) and mechanism(s) so that these can be addressed directly for a given patient.
Atelectasis is always treated aggressively because it has the potential to worsen, develop into a severe clinical manifestation, and lead to pneumonia. In turn, overwhelming pneumonia can precipitate acute respiratory distress syndrome (Chapter 36), which is associated with considerably poorer outcomes. Based on long-standing physiological evidence, treatment continues to be primarily directed at reversing the underlying contributing mechanisms whenever possible. For example, atelectasis resulting from restricted mobility is remediated with mobilization. Atelectasis resulting from prolonged static positioning and monotonous tidal ventilation is managed with mobilization, manipulating body position to increase alveolar volume of the atelectatic area, manipulating body position to optimize alveolar ventilation, or some combination of these interventions. Atelectasis arising from reduced arousal is managed by minimizing the causative factors contributing to reduced arousal coupled with frequent sessions of mobilization and the upright position to stimulate arousal, promote greater tidal volumes and alveolar ventilation, increase zone 2 (area of optimal ventilation and perfusion matching), increase FRC, and minimize closing volume. Most often in a given patient, atelectasis results from a combination of these factors, thus necessitating a multipronged approach in its management.
Breathing control and coughing maneuvers augment the cardiovascular and pulmonary physiological effects of mobilization and body positioning. Coordinating these interventions distributes ventilation more uniformly rather than directing gas to already open alveoli, which overdistends these units. The distribution of ventilation has long been known to be altered primarily by body positioning rather than deep breathing.23 Sustained maximal inspiratory efforts may augment alveolar ventilation; however, the parameters necessary for such efforts to be maximally therapeutic remain to be elucidated.
If impaired mucociliary transport or excessive secretions are obstructing airways and contributing to atelectasis, mobilization of pulmonary secretions is the goal. Mobilization and a physiological stir-up regimen24 are instituted as soon as possible for multiple reasons in patients who are acutely ill to augment oxygen transport and minimize reduction in aerobic capacity (Chapter 18). In the event of excessive secretions, mobilization may need to be more vigorous to stimulate eucapnic deep breaths and inspiratory efforts and, hence, effective coughs. Stir-up, coined 70 years ago by Dripps and Waters25 aptly describes the clinical role of physiologically perturbing a patient to reduce risk and improve outcomes.
Pneumonia
Pathophysiology and Medical Management
Routes of Infection
A patient who has impaired or ineffective defense mechanisms of the respiratory tract becomes susceptible to a variety of organisms. The major routes of infection include airborne organisms, circulation, contiguous infection, and aspiration. In the past 20 years, hospitalized patients whose immunity is compromised are susceptible to superbug infections, which necessitates aggressive medical management and isolation techniques.26
Viral Pneumonias
Principles Of Physical Therapy Management
Patients may respond to mobilization coordinated with breathing control exercises and positional rotation for enhancing alveolar ventilation, mucociliary transport, and gas exchange overall.27 Extreme body positions have long been known to enhance alveolar volume and ventilation as well as ventilation and perfusion matching.28–31 Vigorous treatment should be initiated at the first sign of a superimposed bacterial infection, which is often accompanied by a productive cough. The appropriate devices should be prescribed at this time (e.g., ultrasonic or medication nebulizers to loosen secretions). Postural drainage may be indicated in addition to mobilization for airway clearance. Treatments, particularly mobilization, must be paced to avoid unduly tiring the patient or increasing oxygen demand beyond the patient’s capacity to adequately deliver oxygen. Increasing oxygen demands excessively may compromise the patient’s gas exchange. Patient education is also fundamental to the treatment that is to be instituted between treatments (i.e., mobilization and positional rotation coordinated with breathing control and coughing maneuvers).
Principles of Physical Therapy Management
The goals of management of bacterial pneumonia include reversing alveolar hypoventilation, increasing perfusion, reducing right-to-left shunt, increasing ventilation and perfusion matching, minimizing the effects of impaired mucociliary transport, minimizing the effects of increased mucous production, and optimizing lymphatic drainage of the lungs. Bacterial pneumonia is frequently associated with increased mucus production. With respect to airway clearance, management focuses on augmenting mucociliary clearance overall, reducing excess mucus accumulation, and reducing mucus stasis. The role of traditional manual physical therapy techniques remains equivocal in the management of adults with pneumonia.32 Patients are often mobile and should be encouraged to be so to promote lung expansion, augment flow rates, stimulate deep tidal volumes (breaths), and augment mucociliary transport and lymphatic drainage.27,33,34 The oxygen demands of mobilization and exercise, however, must be within the patient’s capacity to delivery oxygen. These interventions are prescribed such that they avoid jeopardizing this balance and unduly fatiguing the patient. Deep breathing and effective coughing are important maneuvers for clearing airways with special attention to the avoidance of airway closure. Prescriptive body positioning can be used to optimize ventilation and perfusion matching,29,35–37 which may be more uniform in the prone position38 (see Chapter 20). A secondary goal is offsetting aerobic deconditioning from this episode of acute illness, particularly in older adults or patients who are debilitated with chronic conditions.
Acute Exacerbation of Chronic Bronchitis
Pathophysiology and Medical Management
Principles of Physical Therapy Management
Patients with chronic bronchitis can benefit from a comprehensive rehabilitation program designed for patients with chronic pulmonary disease, and such a program is best initiated within 1 month of an exacerbation.39 Even modest exercise has long been known to improve walking capacity and subjective well-being.40 Components of acute management are shown in Table 29-1 and are comparable to cardiac rehabilitation phase I. Specific details of exercise prescription in the management of chronic obstructive pulmonary disease and of a comprehensive pulmonary rehabilitation program are described in Chapters 24 and 31. Maximizing health and risk factor reduction are principal goals to minimize further pulmonary pathological changes and damage including irreversible emphysema. High-priority strategies include targeted education, smoking cessation, and nutritional and exercise counseling. Individuals who do not have access to such formal structured pulmonary programs (the majority of people), can be managed by the physical therapist, who applies the same principles of practice on an individual basis.
Bronchiolitis
Pathophysiology and Medical Management
Bronchiolitis results from peripheral airway inflammation. Although bronchiolitis is more often seen in babies and small children because of the small caliber of their airways (Chapter 37), adults may be afflicted with this condition, often secondary to some other condition. In severe bronchiolitis, the exudate in the peripheral airways becomes organized into a connective tissue plug extending into the peripheral airway. The inflammatory process resembles that in other tissues (i.e., an inflammatory stage followed by a proliferative healing stage). Such inflammation is associated with vascular congestion, increased vascular permeability, formation of exudate, mucus hypersecretion, shedding of the epithelium, and narrowing of the bronchioles. Fluid is exuded out of the circulation onto the alveolar surfaces, replacing the surfactant. This, in turn, increases the surface tension and promotes airway closure. The secretion production associated with airway irritants and inflammation results from the excess mucus production in combination with the inflammatory exudate, consisting of fluid protein and cells of the exudate. The underlying pathophysiological cascade contributes to both a restrictive and an obstructive component of pulmonary limitation. Airway obstruction results if these exudative substances are not removed. The airway epithelium has the capacity to repair and reline the lumen. A rapid turnover of cells may contribute to cell sloughing and further airway obstruction and a thickened basement membrane. The obstruction associated with bronchiolitis leads to ventilation and perfusion abnormalities and diffusion defect. Clinically, the patient presents with a productive cough. Obliterative bronchiolitis has been reported to be the most significant long-term complication of heart-lung transplantation.3 Medical management is directed at inflammation control with medications, fluid management, and oxygen administration if necessary. Prevention of infection is a priority.
Principles of Physical Therapy Management
Bronchiolitis is common in babies and young children. The effects of inflammation and obstruction in small children are always serious because the anatomical and physiological components of the cardiovascular and pulmonary systems are smaller, respiratory muscle tone is less well developed, the anatomical configuration of the chest wall is cylindrical, breathing is less efficient under 2 years of age, spontaneous movement and body positioning are more restricted (infants in particular spend more time in non-upright positions), and children are at greater risk for infection (see Chapter 37).
Acute Exacerbation of Chronic Airflow Limitation
Pathophysiology and Medical Management
Chronic airflow limitation (chronic obstructive lung disease) is a leading cause of preventable death from smoking. There are two principal types of emphysema: centrilobular and panlobular. Both types can coexist; however, centrilobular emphysema is 20 times more common than panlobular emphysema. Centrilobular emphysema is characterized by destruction of respiratory bronchioles (see Figure 5-1), as well as edema, inflammation, and thickened bronchiolar walls. These changes are more common and more marked in the upper lung fields. This form of emphysema is found more often in men than women, is rare in nonsmokers, and is common among individuals with chronic bronchitis. Panlobular emphysema is characterized by destructive enlargement of the alveoli distal to the terminal bronchioles (see Figure 5-1). This type of emphysema is also found in individuals with alpha1 antitrypsin deficiency. Airway obstruction in these individuals is caused by loss of elastic recoil or radial traction on the bronchioles. When individuals with normal lungs inhale, the airways are stretched open by the enlarging elastic lung, and during exhalation the airways are narrowed as a result of the decreasing stretch of the lung. The lungs of individuals with panlobular emphysema, however, have decreased elasticity because of disruption and destruction of surrounding alveolar walls. In turn, this leaves the bronchioles unsupported and vulnerable to collapse during exhalation. This form of emphysema can be local or diffuse. Lesions are more common in the bases than the apices and tend to be more prevalent in older adults.
Bullae, emphysematous spaces larger than 1 cm in diameter, may be found in patients with emphysema (see Figure 5-2). They appear to develop from an obstruction of the conducting airways that permits the flow of air into the alveoli during inspiration but does not allow air to flow out during expiration. This causes the alveoli to become hyperinflated and eventually leads to destruction of the alveolar walls with a resultant enlarged air space in the lung parenchyma. These bullae can be more than 10 cm in diameter and, by compression, can compromise the function of the remaining lung tissue (see Figure 5-3). If this happens, surgical intervention to remove the bulla is often necessary. Pneumothorax, a serious complication, can result from the rupture of bullae.
The most common complaint of the patient with emphysema is dyspnea. Physically, these individuals appear thin and have an increased anteroposterior chest wall diameter. Depending on disease severity, they breathe using the accessory muscles of inspiration (Chapter 5). These patients may be observed leaning forward, resting their forearms on their knees, or sitting with their arms extended at their sides, pushing down against the bed or chair to elevate their shoulders and improve the effectiveness of the accessory muscles of inspiration.
Patients with emphysema have increased respiratory work to maintain relatively normal blood gases. On auscultation, decreased breath sounds can be noted throughout most or all of the lung fields. Radiologically, the emphysema patient has overinflated lungs, flattened hemidiaphragms, and a small, elongated heart (see Figure 5-4). Pulmonary function tests show a decreased vital capacity, FEV1, maximum voluntary ventilation, and a greatly reduced diffusing capacity. The total lung capacity, the residual volume, and the FRC are increased. Arterial blood gases reflect a mildly or moderately lowered PaO2, a normal or slightly raised PaCO2, and a normal pH. Patients with emphysema, unlike patients with chronic bronchitis, tend to develop cardiac insufficiency with progression of the condition, leading to failure at the end stage of the disease. At this stage, cardiac hypertrophy may be evident.
As emphysema becomes chronic, the hemidiaphragms become more horizontally positioned, placing the muscle fibers at a less efficient position on their length tension curve.41 When the muscle fibers of respiration are mechanically disadvantaged, the work of breathing is increased, thereby also increasing energy demands and oxygen cost. Respiratory muscle weakness and fatigue are serious complications of chronic lung disease that predispose the patient to respiratory muscle failure (Chapters 26 and 34).
Principles of Physical Therapy Management
Standards of practice for chronic obstructive lung disease have been well documented globally.42,43 Priorities are minimizing exacerbations and reducing overuse of antibiotics.44 Physical therapy has a primary role in helping to prevent this condition and its sequelae (including premature death), as well as in managing it without drugs and surgery as much as possible.
Table 29-1 shows the primary components of care in the acute phase of management, which can be compared with acute cardiac care (phase I of cardiac rehabilitation). Specific treatments are prescribed for the patient based on the specific clinical findings (i.e., the type and severity of the cardiovascular and pulmonary dysfunction and the presence of infection). Therefore treatments include mobilization coordinated with breathing control and coughing maneuvers, which are effective in enhancing alveolar ventilation, mobilizing secretions, and facilitating ventilation and perfusion matching. Body positioning can be prescribed to alter the distribution of ventilation, to aid mucociliary transport, and to remove pulmonary secretions. Although “pure” emphysema is typically dry, postural drainage positions can facilitate the removal of pulmonary secretions from specific bronchopulmonary segments if indicated. In any given body position, alveolar volume is augmented in the uppermost lung fields and alveolar ventilation is augmented in the lowermost lung fields. The type and extent of pathology determine the degree of benefit these physiological effects will have on oxygen transport.
In addition, body positioning is essential to optimize respiratory mechanics and enhance pulmonary gas exchange, thereby reducing the work of breathing and the work of the heart. The assessment defines the parameters of the treatment prescription that will be effective in relieving the work of breathing and the work of the heart. This information is essential not only for prescribing beneficial positions but also for avoiding deleterious positions. A sitting, leaned-forward position will assist ventilation secondary to the gravitational effects of the upright position on cardiovascular and pulmonary function. If the arms are supported, this position stabilizes the upper chest wall and rib cage, thereby facilitating inspiration. Some patients, in respiratory distress and with horizontally positioned hemidiaphragms, benefit from recumbent positions in which the hemidiaphragms are elevated within the chest wall by the viscera falling against the underside of the hemidiaphragms in this position (i.e., viscerodiaphragmatic breathing).45 The muscle fibers of the diaphragm are mechanically placed in a more favorable position with respect to their length-tension characteristics. This effect may further be augmented in the head-down position in some patients.46 Other patients, however, cannot tolerate recumbent positions; in fact, respiratory distress may be increased. If optimal treatment outcome has not been achieved with mobilization and body positioning coordinated with breathing control and coughing maneuvers, conventional physical therapy procedures may offer additional benefit in some patients (e.g., postural drainage and manual techniques if a refractory purulent infective process is present).
As with the individual who has chronic bronchitis, people with chronic airflow limitation due to emphysema should be prescribed a lifelong health program that includes smoking cessation, basic nutritional counseling, and exercise counseling (see Chapters 24 and 31). Based on the assessment, the physical therapist makes a decision with respect to whether professional counseling may augment outcomes (e.g., smoking cessation counselor), whether the patient may benefit from pharmacological support for smoking cessation, and whether the general practitioner needs to be consulted. A decision is also made as to whether a professional nutritionist should be involved. Improved aerobic conditioning can reduce the frequency and severity of subsequent acute exacerbations.47 Quality of life is associated with fewer clinical signs and symptoms.48
Acute Exacerbation of Asthma
Pathophysiology and Medical Management
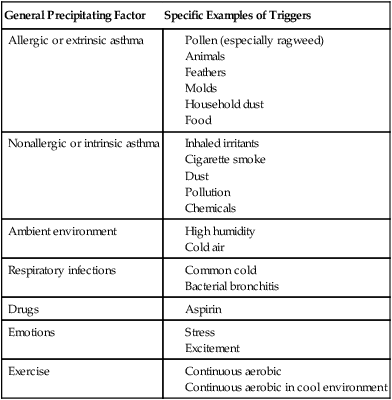
Asthma that begins in patients under the age of 35 years is usually allergic or extrinsic. These asthma attacks are precipitated when an individual comes into contact with a given substance to which he or she is sensitive, such as pollens or household dust (see Table 29-1). Patients with asthma can be allergic to a number of substances rather than only one or two.
If a patient’s first asthma attack occurs after the age of 35, often there is evidence of chronic airway obstruction with intermittent episodes of acute bronchospasm. These individuals, whose attacks are not triggered by specific substances, are referred to as having nonallergic or intrinsic asthma (Table 29-3). Chronic bronchitis is commonly found in this group, and this is the type of asthma most often seen in the hospital setting.
Table 29-3
Factors That Precipitate Asthmatic Symptoms
| General Precipitating Factor | Specific Examples of Triggers |
| Allergic or extrinsic asthma | |
| Nonallergic or intrinsic asthma | |
| Ambient environment | |
| Respiratory infections | |
| Drugs | |
| Emotions | |
| Exercise |
The patient with asthma presents with the following picture during an attack. Lung volumes and expiratory flow rates are reduced, and the distribution of ventilation is less homogeneous.24 The patient has a rapid rate of breathing and uses the accessory respiratory muscles (see Figure 5-6). The expiratory phase of breathing is prolonged, with audible wheezing. The patient may cough frequently, but unproductively, and may complain of tightness in the chest. Radiologically, the lungs may appear hyperinflated or show small atelectatic areas (reabsorption atelectasis). Early in the attack arterial blood gases reflect slight hypoxemia and a low PCO2 (from hyperventilation). If the attack progresses, the PO2 continues to fall as the PCO2 increases above normal. As obstruction becomes severe, deterioration of the patient is evidenced by a high CO2, a low PO2, and a pH of less than 7.3.
An asthmatic attack that persists for several hours and is unresponsive to medical management is referred to as status asthmaticus. This condition constitutes a medical emergency, necessitating admission to the intensive care unit (see Chapter 34).
Principles of Physical Therapy Management
Table 29-1 shows the primary components of care in the acute phase of management, which are comparable to acute cardiac care (phase I of cardiac rehabilitation). Physical therapy is directed at improving gas exchange without aggravating bronchospasm and other symptoms and reversing these when possible. In one systematic review, the role of breathing exercises for people with acute asthma was not substantiated.49 Thus relaxing the patient’s overbreathing by whatever method works best is recommended. Patients experiencing an acute asthmatic attack may reduce their respiratory rate with a CO2 rebreathing bag; the benefits may be augmented by reducing a hyperventilatory response with optimal positioning.50 Overall oxygen demand, including that associated with an increased work of breathing, needs to be reduced during an exacerbation of asthma. This may require reduced activity, body positioning that improves breathing efficiency, judicious rest and sleep periods, altered diet or restricted diet, adequate hydration, maintenance of a thermoneutral environment, rest, reduced arousal, reduced social interaction and excitement, and reduced environmental stimulation. Although general relaxation does not directly relax bronchial smooth muscle, it will assist breathing control, reduce arousal and metabolic demands, and promote more efficient breathing.
Treatment outcome is assessed with indices of oxygen transport overall and of the function of the individual steps in the pathway.51 Bedside spirometry, including peak expiratory flow rate, is a sensitive indicator of ensuing compromise in oxygen transport. Some patients use a peak expiratory flow rate meter at home to detect such changes and as an early indicator of the need for medical attention.
Individuals with asthma can often learn to control their condition effectively with optimal health education and an overall lifelong health program (see Chapters 24 and 31).
Acute Exacerbation of Cystic Fibrosis
Pathophysiology and Medical Management
Cystic fibrosis (CF) is a complex multisystem disorder transmitted by an autosomal-recessive gene that affects the exocrine glands. CF involves all of the major organ systems in the body and is characterized by increased electrolyte content of the sweat, chronic airflow limitation, ventilation inhomogeneity, and pancreatic insufficiency. Definitive diagnosis of CF includes positive family history, clinical symptoms of poor digestion, growth or recurrent pulmonary infection, and most important, a positive sweat chloride test. Survival has increased dramatically since 1940 when survival was reported to be approximately 2 years. Thus, although CF is congenital and manifests in childhood, this condition has now become an adult disorder. Adult patients with CF often have an upper lobe infiltrate, with evidence of atelectasis and bronchiectasis, and chronic staphylococcal infections. The beat frequency of the cilia is often slowed to approximately 3 mm per minute, compared with 20 mm per minute in age-matched, healthy, control subjects.52 Patients can be categorized into three general groups: those with no significant pulmonary signs, those with pulmonary signs and occasional cough and sputum, and those with pulmonary signs and constant cough and sputum. Those patients in the last group tend to have significantly impaired pulmonary function test results, reduced diffusing capacity, and increased hemoptysis, particularly in the presence of an abnormal chest x-ray and hyperinflation. Airway hyperreactivity appears to be variable.
Peripheral airways are often abnormal either anatomically or functionally because of mucus plugging. The lungs of patients with CF may be excessively stiff at maximal lung capacity, with a loss of elastic recoil at low lung volumes. Regional ventilation is nonuniform and contributes to ventilation and perfusion mismatch and hypoxemia.24
Principles of Physical Therapy Management
Table 29-1 shows the primary components of care in the acute phase of management, which can be compared generally with acute cardiac care (phase I of cardiac rehabilitation). The clinical deficits related to oxygen transport include impaired mucociliary transport, increased mucus production, increased difficulty clearing mucus, impaired ventilation and perfusion matching, right-to-left shunt, a diffusion defect, respiratory muscle weakness or fatigue, and reduced cardiovascular and pulmonary conditioning. The increased production of mucus and the difficulty removing mucus increase the risk for bacterial colonization and chronic respiratory infections. These manifestations of CF are worsened with recumbency. Significant postural hypoxemia has been reported in patients with CF when moving from sitting to a supine position.53 Thus the object of treatment is to optimize oxygen transport and pulmonary gas exchange. Given the pathophysiological deficits in an acute exacerbation of CF, the specific goals are to enhance mucociliary transport, promote airway clearance, optimize alveolar ventilation and therefore gas exchange, maximize the efficiency of oxygen transport overall, and prevent and minimize infection.
Although the degree to which patients with chronic lung conditions, and in particular CF, can respond to aerobic training may be limited, it is essential that their capacity to transport oxygen overall is optimized to compensate for deficits in specific steps of the oxygen transport pathway.54 Deconditioning severely impairs oxygen transport. Improved aerobic capacity and cardiovascular and pulmonary conditioning are central in the management of patients with CF. Prescriptive aerobic exercise enhances the efficiency of oxygen transport overall by reducing airway resistance by mobilizing secretions, improving the homogeneity of ventilation in the lungs and therefore ventilation and perfusion matching, optimizing oxygen extraction at the tissue level, and increasing respiratory muscle endurance.55 If optimal conditioning is maintained, oxygen transport is not as compromised during acute exacerbations given the improved efficiency of oxygen transport overall. These effects will be lost, however, as the patient deconditions secondary to restricted mobility and recumbency during the acute episode. Thus it is important that mobility is minimally restricted during an exacerbation (based on the clinical assessment and morbidity) and that exercise conditioning is a mainstay of management between exacerbations. An important additional effect of long-term exercise, which has particular importance for patients with CF, is improved immunity.56 This may minimize the risk for infection and perhaps minimize the severity of an infection once acquired. Patients with CF can have an abnormal hemodynamic response to exercise and myocardial adaptation; thus appropriate cardiac assessment and monitoring is indicated.57
In severe exacerbations the patient is extremely stressed physiologically and has significantly increased oxygen consumption because of the increased work of breathing and for the heart. In addition, the patient is prone to arterial desaturation. Thus minimizing undue oxygen demand and fatigue guides the selection of treatment interventions and their parameters in conjunction with stringent monitoring. These patients become hypoxemic and distressed readily. Treatment interventions are selected based on the assessment and the patient’s ability to tolerate the treatment and derive optimal benefit. Gradual, paced, low-intensity mobilization and frequent body positioning enhance mucociliary transport and airway clearance and maximize the efficiency of the steps in the oxygen transport pathway. Postural drainage can offer additional benefit in these patients if further clearance is required. The addition of manual techniques may be indicated; however, stringent monitoring must be carried out given their potential deleterious effects on gas exchange.58
Over the past decade, other interventions aimed at secretion clearance in the treatment of patients with CF have included autogenic drainage, the use of the positive expiratory pressure (PEP) mask, and the Flutter valve.2,59 One study of patients with CF hospitalized for acute exacerbations compared the short-term efficacy of three regimens: postural drainage (PD), positive expiratory pressure physiotherapy (PEP), and high-frequency chest compression (HFCC) physical therapy.60 The authors reported no change in lung function or in sputum production when the number of coughs was considered.
Autogenic drainage may be a useful adjunct for facilitating airway clearance in patients with CF. It is a procedure that patients may use independently and can be applied relatively unobtrusively. It helps optimize the patient’s coughing efforts and minimizes the potential for airway closure in these patients. This procedure is designed to minimize exhaustive, metabolically costly coughing and preserve energy to expectorate most effectively.61
The PEP mask and Flutter® valve device are believed to reduce airway closure in conjunction with exercise, thus optimizing alveolar ventilation and enhancing mucociliary clearance in patients with CF.62 Individuals with CF can often learn to control their condition effectively with optimal health education and overall lifelong health program (see Chapters 24 and 31).
Acute Exacerbation of Interstitial Pulmonary Fibrosis
Pathophysiology and Medical Management
Principles of Physical Therapy Management
Table 29-1 shows the primary components of care in the acute phase of management, which are similar to those of acute cardiac care (phase I of cardiac rehabilitation). In mild cases mobilization increases the homogeneity of ventilation and ventilation and perfusion matching. Between treatments and in the management of the severely affected patient, body positioning is used to reduce the work of breathing and arousal, maximize alveolar ventilation, maximize ventilation and perfusion matching, and optimize coughing.
Patients with moderate-to-severe interstitial lung disease may desaturate during sleep and readily desaturate on physical exertion.63 Thus they warrant close monitoring during and between treatments. Increased pulmonary vascular resistance secondary to hypoxic vasoconstriction contributes to increased work of the right heart and potential cardiac insufficiency.
General debility and deconditioning warrant an exercise program that can optimize the conditioning and function of all of the steps in the oxygen transport pathway.64 Individuals with interstitial pulmonary fibrosis require health education and an overall health program to help manage their symptoms (see Chapters 24 and 31).
Tuberculosis
Pathophysiology and Medical Management
Maintenance of good general health is particularly important in the management, control, and prevention of tuberculosis (e.g., sanitation, balanced diet, sleep, regular exercise, and stress control). Finally, smoking has been attributed to a disproportionate number of deaths due to pulmonary tuberculosis, thus smoking cessation education and counseling is essential.65
Principles of Physical Therapy Management
Although the acute presentation of tuberculosis is comparable with acute pneumonia (refer to pneumonia section), there are some important differences with respect to physical therapy management.66 First, tuberculosis is particularly infectious; thus special precautions should be taken by the physical therapist to prevent its spread during its infectious stage. Second, patients may be prone to fatigue; treatments should be selected to promote improved oxygen transport without exceeding the patient’s capacity to deliver oxygen and without contributing to excessive fatigue. Stimulation of the oxygen transport system with exercise is necessary to avoid the deleterious effects of deconditioning and further compromise of oxygen transport. The patient warrants being monitored closely.