CHAPTER 34 Indications for surgery
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide, affecting an estimated 60 million people in 20101. More than 50% of sufferers in developed countries are unaware of their condition and this figure increases to over 90% in the developing world. The economic burden is substantial at almost $3 billion in the US in 20042. A recent estimate of the risk of progressing to blindness in patients with diagnosed glaucoma attending a specialist glaucoma clinic in the US is around 14% for unilateral blindness and 6% for bilateral blindness after 15 years3.
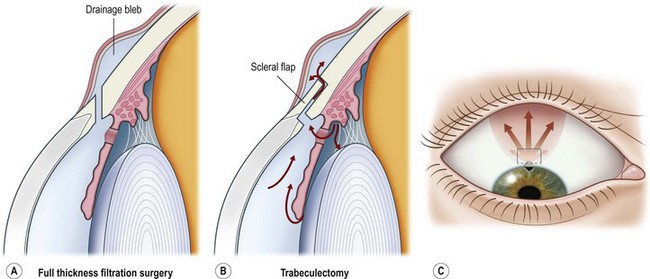
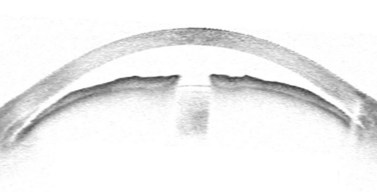
Surgery for primary open angle glaucoma (POAG) has evolved in the past 50 years from full-thickness filtration surgery, in which a hole in the scleral wall at the corneo-scleral limbus drained aqueous from the anterior chamber to a subconjunctival ‘drainage bleb’. As this procedure carried a high risk of ocular hypotony, and bleb-related endophthalmitis4,5, it evolved to a partial-thickness scleral flap which provided an extra flow resistor (Fig. 34.1)6, prevented hypotony, and consequently lowered aqueous pressure within the subconjunctival drainage bleb lowering the risk of conjunctival breakdown and infection.
As a guarded filtration procedure, trabeculectomy was safer than the full-thickness procedure. However, as satisfactory long-term IOP control could not be achieved in a significant proportion of patients, pharmacological enhancement with anti-proliferative agents (e.g. 5-fluorouracil (5FU)7 and later, mitomycin C (MMC)) was introduced in the late 1980s. While these improved success rates some safety concerns, previously reduced by the evolution of trabeculectomy from full-thickness filtration surgery, reappeared8,9. The main concerns are hypotony10 and infection, especially as trabeculectomy-related infection has a predilection for more aggressive organisms than do other types of intraocular infections11. The alteration in conjunctival surface healing that resulted from the use of 5FU, and especially MMC, also resulted in prominent drainage blebs that could cause chronic discomfort or dysesthesia12. These and a higher incidence of cataract after trabeculectomy have promoted further evolution in glaucoma surgical techniques in four main directions.
General considerations
Medical versus surgical treatment
While medical and laser treatment are usually the first lines of therapy, there are some situations where early surgery is advisable. Surgery is the principal treatment for angle closure, developmental glaucomas, and a number of secondary glaucomas. Surgery is recommended for POAG in three circumstances (Box 34.1):
Which procedure first?
Traditionally trabeculectomy has been the procedure of choice in patients who have not had prior glaucoma surgery. This has been challenged in a number of ways. Advocates of both non-penetrating glaucoma surgical procedures (NPGS) and GDDs promote these as better options to trabeculectomy. Both cite safety issues. GDDs do not rely on aqueous drainage to the perilimbal subconjunctival space, whereas NPGS produce less limbal drainage than trabeculectomy. Bleb-related infection is therefore less likely with both. The risk of hypotony is less with NPGS. Contact lens wear is also safer after both than after trabeculectomy. Advocates of GDDs also claim better efficacy. Recently a large scale randomized trial has supported the use of GDDs15. While there have also been randomized trials comparing deep sclerectomy with trabeculectomy, they have been relatively small in size, one suggesting a significantly lower IOP was reported with trabeculectomy18 while another reported a slightly lower but statistically insignificant mean IOP with trabeculectomy19. As neither study used MMC for either procedure, nor was an implant (e.g. collagen) used for deep sclerectomy, it is questionable whether they are representative of current surgical methodology.
Indications for surgery
Primary open angle glaucoma
Surgery is offered in POAG if there is advanced glaucoma (e.g. fixation-threatening visual field defects) at presentation, especially in younger patients, or if glaucoma is progressing despite 2–3 classes of drugs or after laser trabeculoplasty. Other indications include poor adherence to the prescribed medical regimen or inability to tolerate medical therapy. The United Kingdom National Institute for Health and Clinical Excellence (NICE) guidelines on management of primary open angle glaucoma recommend that surgery is offered to those with advanced glaucoma (MD < –12 dB or sensitivity < 15 dB in the central degrees in both hemifields) at presentation20, as these patients are at highest risk of loss of central vision from glaucoma.
Surgery achieves lower mean IOP and less diurnal fluctuations than medical treatment. The Collaborative Initial Glaucoma Treatment Study (CIGTS) group recently reported that, 9 years after randomization, patients with higher visual field loss at baseline (–10 dB) had less visual field loss over time when treated with initial surgery as compared with initial medical treatment, except in patients with diabetes21,22.
However, glaucoma surgery differs from other types of intraocular surgery in that there is no immediate tangible visual benefit. The aim of surgery is preventive. Initially the vision may be worse after surgery and in some cases, e.g. after a ligated GDD, the IOP may be higher initially than before surgery, at least for the initial postoperative period; this is evident from early results of the Ahmed Baerveldt Comparison Study (ABC), comparing16,17 the Ahmed glaucoma valve with the Baerveldt glaucoma implant. In the early postoperative period, the patients with the occluded non-valved Baerveldt implant had significantly higher IOP levels than those with the valved Ahmed valve. Patients must be aware of this possibility and that it does not imply failure. Usually these factors will not influence the decision to proceed with surgery, but are important to manage patient expectations once the decision to operate is made.
Ultimately, surgical trials with visual fields and patient-reported outcome measures as endpoints would give a clearer indication of the relative benefits of surgery, but such trials are costly. Most patients prefer to avoid surgery if possible, though interestingly a recent conjoint analysis of factors most import to patients found they were less concerned about the mode of treatment than the risk of visual loss23. In the CIGTS study, the impact on quality of life was similar in both medically and surgically treated arms of the study 5 years after initial treatment24.
In other situations where there is higher risk of infection (e.g. significant posterior lid margin disease), or where an anterior bleb is not desirable (e.g. committed soft contact lens wearer), trabeculectomy is better avoided and a GDD may be the preferred option. For many years, trabeculectomy has been the standard surgical procedure in previously unoperated eyes in primary glaucomas, whereas shunts have been preferred for several secondary and refractory glaucomas or where further ocular surgery is planned (e.g. penetrating keratoplasty, vitreoretinal surgery, etc). Had it not been for the advent of the newer ocular hypotensive drugs, such as prostaglandin agonists, one might speculate that trabeculectomy would have been displaced by non-penetrating procedures performed relatively early in the disease process. However, as surgery is still performed relatively late, in patients with significant visual field defects, many surgeons argue that definitive IOP control is required, tipping the balance in favor of the greater perceived efficacy of trabeculectomy. However, randomized trial evidence that the Baerveldt 101-350 implant (Abbott Medical Optics, Abbott Park, Illinois, USA) is able to achieve IOP levels in the low teens25 was the first to substantiate a growing body of surgical experience15,17 that contradicts the popular belief that GDD (glaucoma drainage devices) cannot achieve low target IOP levels in the average patient, and as a result shunts are beginning to displace trabeculectomy in some centers.
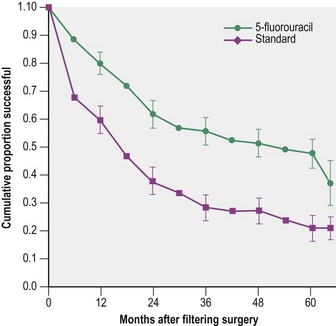
The safety and efficacy of trabeculectomy surgery, both as a primary treatment21,26 and in higher risk eyes7,15,27, has been reported in a number of trials and a number of risk factors for trabeculectomy failure have been identified. These include type of glaucoma, race, young age, previous filtration surgery, pseudophakia, other prior surgery (penetrating keratoplasty, vitreoretinal surgery), anterior segment disease (fibrous/epithelial ingrowth, ICE syndrome), ocular surface disease (e.g. atopy, rosacea), and postoperative inflammation. The fluorouracil filtering surgery study, performed in the late 1980s investigated the potential benefits of pharmacological augmentation of trabeculectomy with an intensive regimen of 5FU. While 5FU almost doubled trabeculectomy success, the study highlighted the poor likelihood of trabeculectomy success in high failure risk eyes7. After 5 years’ follow-up, 51% of 105 5FU eyes and 74% of 108 control eyes were classified as failures (P < 0.001) (Fig. 34.3). 5FU augmentation clearly improved success, but did not provide the answer in higher risk cases. The use of 5FU was rapidly superseded by MMC, which improved the success rate in these cases, albeit at a higher risk of complications from hypotony and infection. Randomized clinical trials comparing MMC with 5FU trabeculectomy have reported better efficacy in terms of IOP control with MMC28, but there has been no study of an equivalent size and follow-up, until the Tube Versus Trabeculectomy study (TVT).
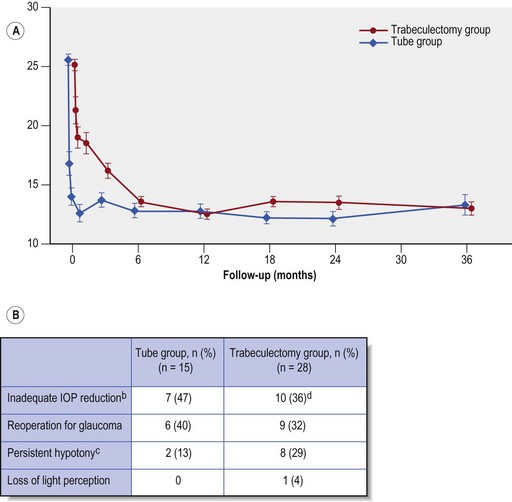
At three years both trabeculectomy with MMC and Baerveldt 350 tube surgery showed similar reductions in IOP (mean IOP 13.3 ± 6.8 mmHg in the trabeculectomy and 13.0 ± 4.9 mmHg in GDD group, P = 0.78) with similar numbers of glaucoma medications (1.1 ± 1.5 in trabeculectomy and 1.3 ± 1.3 in tube group, P = 0.30) (Fig. 34.4A). However the cumulative probability of failure was higher in the trabeculectomy group as compared with the GDD group (30.7% vs. 15.1%, P = 0.01).While more patients in the trabeculectomy group failed from inadequate IOP reduction, the same is also true of those failing from hypotony and the overall impression is that the Baerveldt GDD produces more predictable pressure control at 3 years than trabeculectomy i.e. both fewer high and fewer low IOP failures (Fig. 34.4B).
The TVT investigators also stratified enrolled patients according to perceived failure risk:
The relative efficacy profile of the two procedures was anticipated to differ between these strata, but surprisingly at 3 years this was not so. An identical trend was reported in each stratum, i.e. a slightly higher complete success (i.e. IOP <22 mmHg and at least 20% drop in IOP off medications) in the trabeculectomy group, which was outweighed by a greater qualified success (the same as above, but with ocular hypotensive medications) in the tube group, and a greater failure rate in the trabeculectomy group. Overall trabeculectomy patients were significantly more likely to be free of ocular hypotensive medications, although only 40% of trabeculectomy patients achieved this. Overall the TVT study results suggest better predictability for the Baerveldt GDD than MMC-augmented trabeculectomy: while mean IOP levels and numbers of glaucoma medications were similar at 3 years, the trabeculectomy group had more failures, from both high and low IOPs (see Fig. 34.4). There was a higher incidence of early postoperative complications in the trabeculectomy group, but significantly the difference between the two groups was largely due to early conjunctival wound leak and hyphema. There was no difference in the rate of vision loss from complications in the two study groups.
With improved surgical technique, complications that appeared to be relatively frequent after the introduction of MMC, such as bleb-related endophthalmitis8,29, bleb-dysesthesia12, and hypotony maculopathy11 have become less of a concern, though a lingering worry is the influence of trabeculectomy on cataract creation27,30 and the detrimental influence of subsequent cataract surgery on long-term trabeculectomy function31. When considering a GDD, these must be balanced against the 5–10% risk of diplopia17,32 and possible longer-term risk of corneal damage and erosion33–35.
Primary angle closure
Most patients with occludable angles or angle closure in clinical practice have not had an acute episode, and are rather more challenging to diagnose than those with acute primary angle closure. Careful slit-lamp examination and especially gonioscopy is essential to detect those at risk and to differentiate pupil block from other mechanisms of angle closure, such as plateau iris or forward movement of the lens–iris diaphragm. As the level of slit-lamp and room illumination has a large influence on angle configuration, perform gonioscopy with minimal illumination, especially when attempting to diagnose an occludable angle in a patient without angle closure. Generally, in a patient without IOP elevation or symptoms of intermittent angle closure (blurring of vision and colored rings around lights late at night or early morning) or evidence of glaucomatous optic neuropathy, the indication for laser iridotomy is an occludable angle, diagnosed as either failure to visualize trabecular meshwork through at least 180°, or the presence of irido-trabecular contact. Anterior segment optical coherence tomography is helpful to differentiate occludable from non-occludable angle narrowing (Fig. 34.5), having the advantage that it can be performed in the dark.
After relief of pupil block, persistent anterior synechiae (chronic primary angle closure) or trabecular meshwork damage, from an episode of treated angle closure, may result in chronic IOP elevation that is treated in the long term similarly to POAG, with the exception that is often appropriate to perform lens extraction earlier in an eye with chronic angle closure and chronic IOP elevation (Fig. 34.6). If medical treatment fails to control the IOP, lens extraction alone may be required to reduce the IOP. In eyes with significant glaucomatous optic neuropathy and chronic synechial angle closure, lens extraction may be beneficial in improving pressure control, but ultimately filtration surgery may also be required. EAGLE (Effectiveness in Angle-closure Glaucoma, of Lens Extraction) is a multicenter randomized surgical trial designed to establish whether lens extraction for newly diagnosed primary angle closure glaucoma results in better patient reported health, vision, lower IOP, and other outcomes compared with standard treatment and should help guide management of these patients.
Surgical indications in secondary glaucoma
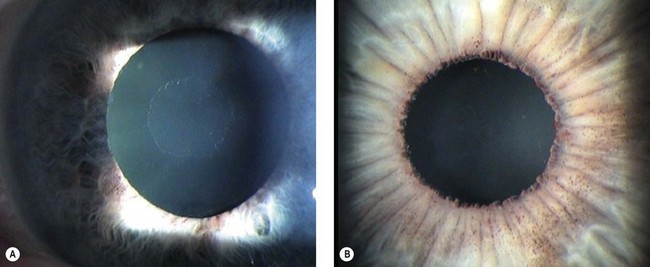
Secondary glaucomas are often asymmetrical or unilateral and cause vision loss more commonly than primary glaucomas. When first examining a patient with glaucoma look for signs of the common secondary glaucomas. Exfoliation syndrome (pseudoexfoliation) (Fig. 34.7) and pigmentary glaucoma may be missed if the pupillary area of the iris, drainage angle, and anterior lens surface are not examined carefully. These usually present in less dramatic fashion than other secondary glaucomas and can be managed with the same medical regimens as POAG, though treatment failure is more common. Both respond well to trabeculectomy, though cataract surgery has been reported to cause a greater IOP reduction in exfoliation than in POAG. Pigmentary glaucoma patients are often young, male, and myopic and therefore at a higher risk of maculopathy should hypotony develop after surgery; take care to avoid trabeculectomy over-drainage at the time of scleral flap closure. Patients with exfoliation more commonly develop cataract and cataract surgery is more often complicated, though trabeculectomy success appears to be comparable with POAG.
Be aware of the many known risk factors for failure when considering trabeculectomy in secondary glaucomas as such eyes often have multiple risk factors for failure and GDDs are more often indicated (Box 34.2). In single chamber eyes, anterior segment proliferative conditions (e.g. neovascularization, fibrous/epithelial ingrowth, ICE syndrome), prior anterior chamber or subconjunctival silicone oil, or severe ocular surface scarring or thinning (scleral buckles, scleritis, chemical burns) greatly diminish prospects for successful trabeculectomy. In other conditions such as uveitic glaucoma, trabeculectomy may be successful if inflammation is well controlled, sometimes aggressively.
Box 34.2
Risk factors for filtration (trabeculectomy failure)
1 Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262-267.
2 Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754-1760.
3 Chen PP. Blindness in patients with treated open-angle glaucoma. Ophthalmology. 2003;110:726-733.
4 Hattenhauer JM, Lipsich MP. Late endophthalmitis after filtering surgery. Am J Ophthalmol. 1971;72:1097-1101.
5 Sugar HS, Zekman T. Late infection of fltering conjunctival scars. Am J Ophthalmol. 1958;46:155-170.
6 Cairns JE. Trabeculectomy: Preliminary report of a new method. Am J Ophthalmol. 1968;66:673-679.
7 The Fluorouracil Filtering Surgery Study Group. Five-year follow-up of the fluorouracil filtering surgery study group. Am J Ophthalmol. 1996;121:349-366.
8 Greenfield DS, Suñer IJ, Miller MP, et al. Endophthalmitis after filtering surgery with Mitomycin. Arch Ophthalmol. 1996;114:943-949.
9 Suner IJ, Greenfield DS, Miller MP, et al. Hypotony maculopathy after filtering surgery with mitomycin C. Incidence and treatment [see comments]. Ophthalmology. 1997;104:207-214.
10 Zacharia PT, Deppermann SR, Schuman JS. Ocular hypotony after trabeculectomy with Mitomycin C. Am J Ophthalmol. 1993;116:314-326.
11 Mandelbaum S, Forster RK, Gelander H, et al. Late onset endophthalmitis associated with filtering blebs. Ophthalmology. 1985;92:964-972.
12 Budenz DL, Hoffman K, Zacchei A. Glaucoma filtering bleb dysesthesia. Am J Ophthalmol. 2001;131:626-630.
13 Wells AP, Cordeiro MF, Bunce C, et al. Cystic bleb formation and related complications in limbus- versus fornix-based conjunctival flaps in pediatric and young adult trabeculectomy with mitomycin C. Ophthalmology. 2003;110:2192-2197.
14 Gedde SJ, Schiffman JC, Feuer WJ, et al. The tube versus trabeculectomy study: design and baseline characteristics of study patients. Am J Ophthalmol. 2005;140:275.
15 Gedde SJ, Schiffman JC, Feuer WJ, et al. Three-year follow-up of the Tube Versus Trabeculectomy Study. Am J Ophthalmol. 2009;148:670-684.
16 Barton K, Gedde SJ, Budenz DL, et al. The Ahmed Baerveldt Comparison Study: Methodology, baseline patient characteristics, and intraoperative Complications. Ophthalmology 2011;118:435-42.
17 Budenz DL, Barton K, Feuer WJ, et al. Treatment outcomes in the Ahmed Baerveldt Comparison Study after one year of follow-up. Ophthalmology 2011;118(3):443-52. Epub 2010 Oct 8.
18 Chiselita D. Non-penetrating deep sclerectomy versus trabeculectomy in primary open-angle glaucoma surgery. Eye. 2001;15:197-201.
19 El Sayyad F, Helal M, El Kholify H, et al. Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open angle glaucoma. Ophthalmology. 2000;107:1671-1674.
20 National Institute for Health and Clinical Excellence. Glaucoma: diagnosis and management of chronic open angle glaucoma and ocular hypertension: methods, evidence and guidance. London: National Collaborating Center for Acute Care; 2009.
21 Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943-1953.
22 Musch DC, Gillespie BW, Niziol LM, et al. Factors associated with intraocular pressure before and during 9 years of treatment in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2008;115:927-933.
23 Bhargava JS, Patel B, Foss AJ, et al. Views of glaucoma patients on aspects of their treatment: an assessment of patient preference by conjoint analysis. Invest Ophthalmol Vis Sci. 2006;47:2885-2888.
24 Janz NK, Wren PA, Lichter PR, et al. The Collaborative Initial Glaucoma Treatment Study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology. 2001;108:1954-1965.
25 Britt MT, LaBree LD, Lloyd MA, et al. Randomized clinical trial of the 350-mm2 versus the 500-mm2 Baerveldt implant: longer term results: is bigger better? Ophthalmology. 1999;106:2312-2318.
26 Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology. 1994;101:1651-1656.
27 Gedde SJ, Herndon LW, Brandt JD, et al. Surgical complications in the Tube Versus Trabeculectomy Study during the first year of follow-up. Am J Ophthalmol. 2007;143:23-31.
28 Skuta GL, Beeson CC, Higginbotham EJ, et al. Intraoperative mitomycin versus postoperative 5-fluorouracil in high-risk glaucoma filtering surgery. Ophthalmology. 1992;99:438-444.
29 Higginbotham EJ, Stevens RK, Musch DC, et al. Bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmology. 1996;103:650-656.
30 Jampel H. Trabeculectomy: more effective at causing cataract surgery than lowering intraocular pressure? Ophthalmology. 2009;116:173-174.
31 Chen PP, Weaver YK, Budenz DL, et al. Trabeculectomy function after cataract extraction. Ophthalmology. 1998;105:1928-1935.
32 Rauscher FM, Gedde SJ, Schiffman JC, et al. Motility disturbances in the tube versus trabeculectomy study during the first year of follow-up. Am J Ophthalmol. 2009;147:458-466.
33 Topouzis F, Coleman AL, Choplin N, et al. Follow-up of the original cohort with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1999;128:198-204.
34 Hau S, Barton K. Corneal complications of glaucoma surgery. Curr Opin Ophthalmol. 2009;20:131-136.
35 Hau S, Scott A, Bunce C, et al. Corneal endothelial morphology in eyes implanted with anterior chamber aqueous shunts. Cornea. 2011;30(1):50-55.