Chapter 58 Indications for Spine Fusion for Axial Pain
Low back pain (LBP) is among the leading reasons for individuals to seek medical attention. One out of 17 patients seen by a family practitioner presents primarily with LBP, with approximately 31 million patient visits for back pain occurring in the United States annually.1 It is estimated that 70% to 85% of individuals will suffer an acute episode of LBP in their lifetime. Most people experience a benign course with near complete resolution of symptoms within a few months of onset.2 Unfortunately, a small percentage, approximately 5% to 10%, continue to develop persistent or chronic LBP.3
Persistent LBP is often a significant source of personal anxiety, distress, and disability. In addition, chronic LBP weighs heavily on society. It is estimated that 28% of the working population in the United States will be disabled by LBP at some time during their professional lives, with approximately 8% of the entire work force disabled in a given year.3 Acute LBP occurs across a wide range of ages. Chronic LBP, however, is the primary cause of disability in individuals less than 50 years of age, when most people expect to be at their peak productivity. The total socioeconomic burden of LBP, including both health-care costs and lost wages, is estimated at $100 billion to $200 billion annually, with two thirds of this cost due to work-related disability.4 Of alarming concern is that while the incidence of diagnosed chronic LBP has been stable for 30 years, the rate of LBP-related disability claims has increased by 14 times that of population growth.5
Controversy regarding axial LBP is due to a number of factors. First, there is a lack of definitive scientific evidence identifying the disc as the pathoanatomic source of pain. The validity and reliability of current diagnostic modalities for discerning which disc or discs are the primary pain generators are unresolved. Second, general medical journals and popular media have generated controversy by suggesting that surgeons are overtreating axial LBP without any proven basis for diagnosis and intervention. Subsequently, many people have made the claim that current trends for operative management of axial LBP have been propagated by ulterior interests from the surgical community and medical device industry. In fact, survey and national inpatient data sample results have indicated that lumbar fusion surgery for degenerative spinal conditions has been steadily increasing. Approximately 300,000 spine fusions are performed annually in the United States, which is a relative increase of 220% between 1990 and 2001. Of these fusion operations, about 75% are performed for degenerative disc disorders and spinal stenosis. A national inpatient data sample identifies degenerative disc disease as the diagnosis that accounts for the largest increase in the number of lumbar fusions during this period.6
Spine fusion for axial LBP is predicated on the theory that pain is related to the degenerated disc’s causing abnormal movement across a motion segment. Painful disc degeneration may be analogous to other arthritic joint pathology involving the hips or knees, in which the degenerated joint causes altered local mechanical and chemical processes that generate pain. Arthrodesis across these degenerated joints in the appendicular skeleton is known to eliminate pain successfully. Spine surgeons have adopted fusion across a degenerated disc as a method for stabilizing the abnormal motion segment to similarly relieve pain.
Unfortunately, the spine surgical literature has failed to demonstrate consistent successful clinical outcomes after fusion surgery in patients with axial LBP. Critics of fusion surgery for axial LBP again raise the issue that current imaging and functional diagnostic modalities do not accurately identify the source of LBP in most patients that lack evidence of nerve compression or neurologic deficit.7 Therefore, difficulties with patient selection and determining which levels to fuse may account for suboptimal rates of clinical success. Furthermore, the relationship between solid arthrodesis and LBP relief remains equivocal. This uncertainty is accentuated by an overall lack of improvement in successful outcomes despite increasing fusion rates with advancing surgical technique, fixation devices, and osteobiologic agents.
The controversy regarding fusion surgery for axial LBP continues today. In 1989, Nachemson issued an editorial stating that for the majority of patients, basic science has yet to demonstrate the true origin of back pain.8 He further stated that our present-day treatments are mostly ineffective, as evidenced by the epidemic increase in back pain related disability in all industrialized societies. Twenty years later, these issues remain subjects for debate, and several recent highly contested studies suggest that there is a lack of clinical evidence to support the surgical treatment for a number of degenerative lumbar disorders.6,9 To gain a better understanding of axial LBP and the indications for spine fusion, this chapter provides an overview of the pathophysiology and current literature with regard to diagnosis, surgical treatment, and outcomes for axial LBP.
Pathophysiology
With aging, the disc gradually becomes less hydrated, and the concentration of proteoglycans decreases. Normal disc metabolism shifts toward catabolic processes, which further deplete proteoglycans and lead to increasing matrix degeneration. As a result, the disc becomes progressively dysfunctional as the nuclear material is replaced by desiccated fibrocartilaginous material. Loss of fluid results in decreased hydrostatic pressure as a mechanism for effective load transference. Thinning or microfracture of the end plates can occur, and subsequent loss of end-plate vascularity reduces transport of nutrients and waste products out of the disc. Eventually, with cyclic loading of the degenerated disc, radial fissures or cracks propagate through the anulus, with migration of nuclear material peripherally. With complete anular disruption, disc material can herniate into the central canal, lateral recess, or foramen. These degenerative processes are estimated to occur in 90% of normal individuals by 50 years of age.10
In 1970, Crock first associated back pain with the pathophysiologic process of disc desiccation and subsequent radial fissure formation of the anulus.11,12 He termed this entity internal disc disruption, which was characterized by the progressive disruption of the internal architecture of the disc while essentially maintaining the external shape such that nerve root compression did not occur. Crock hypothesized that pain is generated when degradation of the disc matrix causes release of inflammatory cytokines, which then migrate through the disrupted inner anular fibers to irritate the high concentration of sensory nerve endings in the outer anulus. His conclusion was supported radiographically by the relative absence of any nerve root compression but the high correlation of concordant pain in discs that exhibited severe radial fissures with intradiscal contrast injection.
Since then, several theories regarding the relationship between degenerative disc disease and pain generation have developed. The mechanical theory suggests that degeneration results in alteration in the biomechanical properties of the disc. As the disc degenerates and the anulus becomes disrupted, increasing instability occurs at the motion segment. Therefore, with normal physiologic loading, the motion segment responds with excessive compression, bending, or rotation, which can trigger pain transmission in surrounding nociceptors. CT and MRI studies have quantified the response of the lumbar spine to rotatory torque and have correlated increased axial rotation in degenerated discs with pain provocation on discography.13,14 Also as the disc desiccates, it loses hydrostatic pressure, so with normal physiologic loading, more stress is transferred to the anulus and the end plate, where pain-sensitive nerve fibers are in high concentration. Increased stress to the end plate can lead to end-plate fracture and disc herniation into the vertebral body, which may further propagate pain generation.
The chemical theory suggests that catabolism of the disc results in release of proinflammatory chemical mediators. Nitric oxide, phospholipase A2, prostaglandin E, matrix metalloproteinases, and other cytokines have been implicated as chemical agents that infiltrate through radial fissures to irritate nociceptors that are present in the outer aspect of the anulus and the end plate. Proteoglycan breakdown is known to have a high concentration of the neurotransmitter glutamate, which may in turn stimulate glutamate-specific receptors in the dorsal root ganglion, resulting in back or radicular pain in the absence of nerve root compression. Nociceptors are also known to be present in high concentrations elsewhere within the spinal canal, such as the posterior longitudinal ligament, dura, and blood vessels.
Plain Radiographs
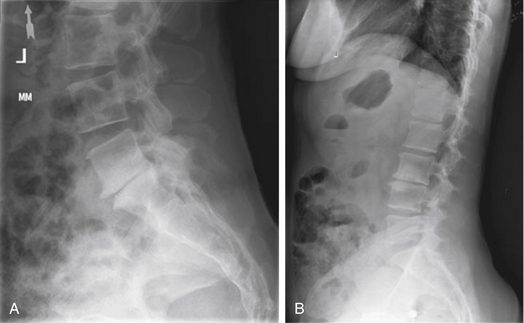
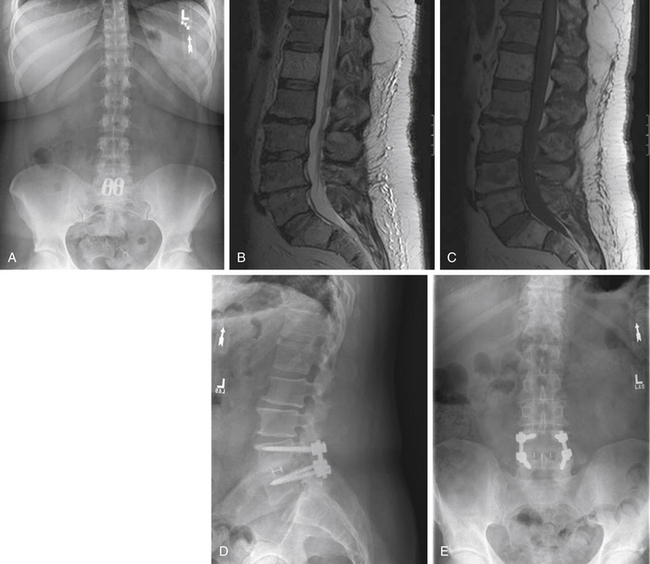
Plain radiographic findings in patients with axial LBP may demonstrate characteristics consistent with degenerative disc disease. While radiography does not visualize the soft tissue disc, plain films may reveal decreased disc height consistent with a collapsed or dehydrated disc (Fig. 58-1A). Sclerotic end plates or bone-on-bone appearance are commonly seen with severely degenerated discs (Fig. 58-1B). Plain radiographs can be performed with patients in weight bearing, flexion, or extension to demonstrate the alignment of the spine and the nature of the motion segments under normal physiologic loading. The presence of hypermobility or malalignment under such stresses may help to identify which levels are symptomatic or may suggest other pathologic processes.
In evaluating lumbar plain radiography in symptomatic patients, Scavone et al. observed that radiographs alone were uniquely diagnostic in only 2.4% of patients.15 Liang and Komaroff found that lumbar radiographs did not provide diagnostic value in differentiating patients with acute versus chronic LBP.16 Coste et al. reported that there was a high degree of variability in interpretation of plain films performed in LBP patients, underscoring the poor diagnostic value of these studies for this condition.17 Many clinicians conclude that the degenerative findings that are seen on lumbar plain radiographs may in fact represent normal age-related changes and as such provide little information with regard to differentiating symptomatic degenerated discs from asymptomatic age-appropriate discs. Plain radiographs, however, are useful in the assessment of axial LBP for effectively ruling out other etiologies of back pain. Fractures, osteomyelitis, tumor, spondylolisthesis, and deformity can often be quickly assessed with lumbar weight-bearing or 36-inch standing plain films. Therefore, plain radiographs are indicated in LBP patients who are of pediatric age, are at high risk for osteoporosis, have a history of prior surgery, present with neurologic deficit or gross deformity, or have clinical signs suggestive of trauma, infection, or malignancy.
Discography
Several studies provide evidence to support discography as an effective method for identifying degenerated, symptomatic discs. Cadaveric studies have shown a strong correlation between discographic contrast distribution and the severity of degeneration on gross examination. There is also a good correlation between disc morphology on discography and intraoperative findings of disc protrusions and herniations. Importantly, studies have identified morphologic findings on discography that correlate with concordant pain provocation. As expected, pain provocation is most commonly observed in discs that demonstrate dorsal anular tears (65.3%) as compared to simply degenerated discs (36.6%), internal anular disruption (20%), or intraosseous disc herniations (0%).18 The use of axial CT imaging provides even further morphologic assessment of the disc. Using the Dallas Discogram Description (DDD) for CT-based discography, severe and moderately graded degenerated discs show a strong correlation with exact reproduction of pain, anular disruption being the best predictor of concordant pain.19 McCutcheon and Thomas found that contrast tracking to the periphery of the anulus suggesting radial fissures and anular disruption has an 87% correlation with concordant pain.20
Subsequent studies, however, have failed to consistently reproduce these positive correlations. Several studies have demonstrated pain production with discography in otherwise normal-appearing discs in asymptomatic individuals. Carragee et al. studied discography in a group of patients who did not have back pain but had chronic pain related to iliac crest bone harvest for non–lumbar spine surgery. Of these patients, 37.5% had similar or exact reproduction of donor site pain with lumbar discography. Some have proposed that high-pressure injection is responsible for false-positive pain production in normal discs. However, even with low-pressure discography, pain was produced in the same number of asymptomatic volunteers as LBP patients.21 Vanharanta et al. found that morphologically normal discs on DDD grading still provoked some pain response in 24% of individuals.22 Also, they observed that severely degenerated discs resulted in exact reproduction of pain in only 22% of individuals. In their study, even discs with severe anular disruption had exact pain reproduction in only 36% of subjects. The reverse association demonstrated slightly better correlation. Patients who had a positive pain response were also more likely to have evidence of disc degeneration. However, discs with exact reproduction of pain were distributed among a range of DDD grades from slight (20%) to moderate (39%) to severe (37%). The best correlation among patients with exact reproduced pain was for severe anular disruption (77%). Overall, the investigators found that painful discs tend to have higher degrees of degeneration and anular disruption compared to painless discs. However, all discs as they deteriorated were more likely to provoke pain, although often the pain was not similar to the presenting back pain and therefore the degenerating disc could not be conclusively diagnosed as the symptomatic disc. Further adding to diagnostic uncertainty, studies have shown that nonspinal factors such as abnormal psychometric findings, chronic pain states, and disputed compensation claims also strongly correlate with positive discography.21
Certain studies have demonstrated that positive discography reliably predicts good surgical outcomes. Simmons and Segil studied patients who had discography prior to undergoing lumbar discectomy, discectomy and fusion, or fusion alone. They found that preoperative discography demonstrated 82% diagnostic accuracy in identifying the symptomatic level.23 This study, however, represented a heterogeneous patient population that included not only back pain patients, but also those suffering from herniated discs and nerve root compression. Colhoun et al. found that among patients undergoing lumbar fusion, 89% of those with a positive preoperative discogram had significant improvement postoperatively, including decreased pain, return to work, and cessation of analgesics.18 Patients with nondiagnostic preoperative discography had a lower rate of success after lumbar fusion, only 52% of patients reporting a similar satisfactory postoperative outcome.
Varying degrees of success with preoperative discography have been observed. Good clinical outcomes have been demonstrated in 64% to 86% of patients with positive discography who undergo anterior lumbar interbody fusion (ALIF).24–26 Other studies have shown that more than 90% of patients with positive discography improve after posterior lumbar fusion.27,28 Derby et al. argue that better correlation is observed when chemically sensitive discs are identified on preoperative discography.29 Chemically sensitive discs provoke concordant pain under particularly low-pressure injection, suggesting that pain is generated by the displacement of biochemical agents that then stimulate sensory nerve endings in the outer anulus. Therefore, Derby et al. hypothesize that patients with chemically sensitive discs require complete discectomy with thorough removal of the offending disc for pain relief. Among patients with chemically sensitive discs, successful clinical outcome was observed in 89% of patients who underwent discectomy and interbody fusion, compared to only 20% of patients who underwent dorsolateral fusion alone and 12% of patients who were treated nonoperatively. Similarly, Weatherly et al. used discography to identify painful, symptomatic discs within a fused segment in patients with persistent LBP after posterior lumbar fusion.30 Subsequent ventral discectomy and interbody fusion of the positive discographic levels resulted in complete resolution of pain.
The positive predictive value of discography for success after lumbar fusion, however, has not been borne out through multiple repeated studies. Of particular concern is that the potential for a high false-positive rate may lead to an inappropriate rise in fusion surgery and consequently an unacceptable rate of unsatisfactory outcomes. Carragee et al. evaluated patients with positive single-level discography using low-pressure injection who then underwent lumbar fusion of the abnormal disc.31 They observed that only 27% of patients had a highly effective outcome as defined by a visual analogue score (VAS) of 2 or less, Oswestry Disability Index (ODI) of 15 or less, full return to work, and cessation of narcotics and analgesics. A minimal acceptable outcome of VAS of 4 or less, ODI of 30 or less, no narcotic use, and at least some gainful employment was reached in only 43% of patients. The authors concluded that in the best case calculation, the adjusted positive predictive value for a minimal acceptable outcome was only 55%. Other studies have similarly found less promising results, with successful outcomes in only 35% to 46% of patients who had undergone lumbar fusion with discography as the primary diagnostic tool.32 It should be noted, however, that in one of these studies, a particularly low arthrodesis rate was observed (47.9%), which may account for the unexpected poor outcomes.
Magnetic Resonance Imaging
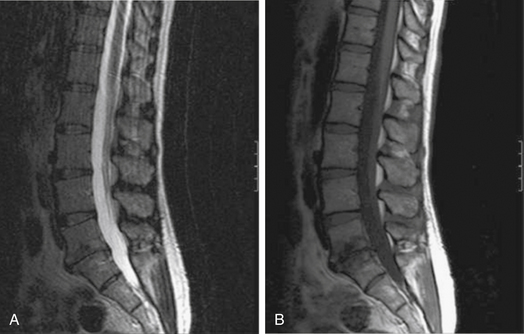
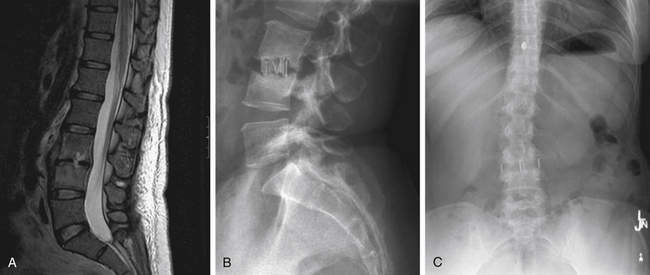
MRI is the radiologic study of choice for visualizing the soft tissue structures of the spine, including the discs, ligaments, joints, and neural elements. As a result, MRI is the preferred test for nerve root compression from disc herniation or lumbar stenosis. MRI is also well equipped for clearly visualizing the intervertebral disc. Besides the ability to image in multiple planes, MRI, with good-quality spin-echo T2-weighted images, provides excellent characterization of the morphology of the disc and superb differentiation between the nucleus pulposus and anulus. The signal intensity within the nucleus is related to the concentration of water in the proteoglycan matrix. Therefore, a reduction in signal intensity correlates with matrix degradation and disc degeneration (Fig. 58-2A). The ability of MRI to detect loss of water content and disc desiccation has prompted consideration of MRI as a sensitive measure of degenerative disc disease.
MRI also well characterizes the effect of disc degeneration on the adjacent end plates and vertebral bodies. With advanced degeneration of the discs, greater load is transferred to the end plates. In the early phase, the normal vertebral body bone marrow is replaced with vascularized fibrous tissue as a reparative response to injury.33 This appears on T1-weighted MRI as decreased signal (Fig. 58-2B), and conversely increased signal on T2-weighted images. With chronic degeneration, the normally red bone marrow is converted to yellow marrow as the marrow elements are replaced by fat cells, a situation that appears to represent a chronic, stable state. The prevalence of fat is represented by increased signal on T1-weighted MRI.
Because MRI adeptly characterizes the various stages of disc degeneration and its effects on the discovertebral complex, it is often performed as the initial study in evaluating patients who present with axial LBP. However, it is unclear whether findings of disc degeneration on MRI are in fact age-related changes or abnormal processes that are related to pain generation. Several studies have evaluated MRI in asymptomatic volunteers and found that more than 30% of individuals without back pain have evidence of degenerated lumbar discs.5,34 These include significant findings of herniated discs (24%) and anular defects (14%) in individuals who are asymptomatic. Boden et al. observed that 28% of asymptomatic individuals had an MRI of the lumbar spine that was characterized as abnormal.34 The most common finding was bulging discs, which appeared in 79% of individuals older than 60 years of age and in 54% of those younger than 60 years of age. Degenerated discs were seen in all but one subject older than 60 years of age, many having multiple degenerated discs. Boden et al. concluded that given the high incidence of bulging and degenerated discs in asymptomatic subjects, these findings in part represent the normal process of aging.
Several studies, however, have shown that MRI findings correlate well with abnormal discography that is suggestive of symptomatic degenerative disc disease.35 Horton et al. performed both MRI and lumbar discography in 25 patients presenting with discogenic LBP.36 They observed that normal-appearing discs on MRI with a high fluid content rarely had concordant pain or abnormal morphology on discography. Alternatively, discs that demonstrated a dark nuclear pattern on MRI, suggesting severe disc dehydration and anular disruption, strongly correlated with pain provocation on discography. The more common finding, however, of a dark or speckled nucleus on MRI, which was interpreted as some degree of disc degeneration, had poor correlation with discographic findings. Subsequent studies observed that painful discs on discography tend to show more evidence of degeneration such as anular fissures, disc prolapse, and decreased disc height on MRI. However, there is no significant difference in pattern or frequency of these degenerative findings between asymptomatic individuals and those with LBP to suggest that MRI can effectively screen for painful, symptomatic discs.37
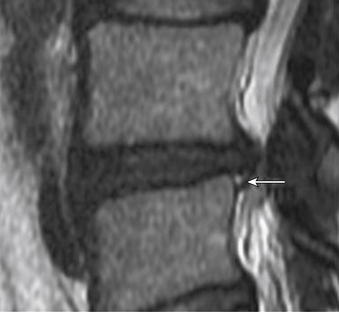
Aprill and Bogduk describe a specific finding on MRI that appears to correlate particularly well with concordant pain on discography.38 The high-intensity zone (HIZ) is an area in the anulus fibrosus that can appear on T2-weighted MRI and has high signal intensity in an otherwise degenerated dark-appearing disc (Fig. 58-3). The HIZ was found in 28% of 500 patients undergoing lumbar MRI for chronic LBP. All discs with an HIZ on MRI were also noted to have a grade 3 or 4 anular disruption on the DDD scale. The HIZ appears to be related pathologically to injury to the anulus. Importantly, the presence of an HIZ correlated strongly with exact reproduction of pain. The sensitivity and specificity of the HIZ for exact pain reproduction on discography were 82% and 89%, respectively. The positive predictive value of the HIZ for concordant pain was determined to be 95%, suggesting that the MRI finding of an HIZ may be a reliable noninvasive measure for anular disruption and pain provocation on discography.
Ultimately, however, there does not appear to be a clear relationship between the mere presence of degenerative changes on MRI and axial LBP. Therefore, one must be careful in interpreting MRI findings and assigning symptomatology to discs that demonstrate radiologic signs of degeneration. Particularly, when evaluating patients for potential surgery, one must consider that the same degenerative changes occur with similar frequency in normal, asymptomatic individuals. Therefore, definitive clinical significance cannot be attributed to abnormal-appearing discs on MRI in the absence of nerve root compression and neurologic symptoms. Modic et al. found that MRI does not appear to have any measurable value in terms of management and outcome of patients with acute LBP.39 With regard to patient selection for surgery, it does appear that MRI is effective for ruling out normal-appearing discs as the source of pain. The decision whether to treat discs that appear abnormal on MRI, however, warrants either further evaluation with provocative discography and/or the experienced assessment of a spine care provider taking into account the patient’s clinical history and symptomatology.
Summary of the Diagnostic Evaluation of Axial Low Back Pain
In 2005, the Joint Section of the American Association of Neurological Surgeons/Congress of Neurological Surgeons published guidelines for the performance of fusion surgery for lumbar degenerative conditions and made recommendations regarding the diagnostic evaluation of axial LBP.35 MRI allows for the assessment of multiple levels simultaneously and noninvasively. Therefore, one can gain an appreciation of the disease pattern of the discs and determine which discs may have evidence of advanced degeneration, disc prolapse, anular disruption, or disc herniation. Once the degenerated levels have been identified on MRI, discography can be performed to further subselect which discs demonstrate abnormal morphology and concordant pain provocation. In their assessment of MRI and discography, they recommended that MRI be performed initially instead of discography in the evaluation of chronic LBP. They also recommended that normal-appearing discs on MRI should not undergo fusion. Conversely, surgery should not be offered on the basis of discography alone, as patients with positive discography but normal MRI had poor outcomes after fusion surgery.40 The use of discography to determine which levels to fuse in patients with an abnormal MRI should demonstrate both concordant pain provocation and morphologic abnormalities.
Nonsurgical Treatment
The first line of treatment for axial LBP remains conservative, nonsurgical therapy. While the majority of Americans will suffer an acute episode of LBP at some point in their life, only about 5% to 10% will then continue to develop chronic LBP.3 Basic symptomatic treatment consists of oral analgesics such as nonsteroidal anti-inflammatory agents, narcotics, and muscle relaxants. Injection therapies with caudal epidural steroid injections, selective nerve blocks, direct injections into the peripheral anulus, facet blocks, and trigger point injections may provide significant long-term benefit for some individuals. Alternatively, they may provide enough short-term relief to allow the patient to engage in a physical therapy program and adaptive lifestyle modification, which may afford better long-term benefits. Spinal injections, in addition to being therapeutic, may also help to diagnose which structure is the primary source of pain. Temporary relief of symptoms after selective nerve root injection may help to predict whether decompression will provide more lasting benefit. Pain relief after facet block has not proven to similarly predict long-term success after fusion, although it can be useful in selecting patients who may benefit from medial dorsal rhizotomy. Ohtori et al. observed that preoperative pain relief after intradiscal injection of bupivicaine correlated well with successful improvement in VAS and ODI after lumbar fusion.41
Bracing has a role in the short-term treatment of acute LBP. In general, however, bracing is not recommended for the treatment of chronic LBP, because there is no evidence to suggest that it provides any long-term benefit. Brace therapy has not been shown to decrease the incidence of LBP or lost work days when used as a preventive strategy.42
Patient Predictors of Treatment Outcome
Patient age has been identified as a risk factor for the occurrence of LBP; however, it is not an individual risk factor for developing chronic disabling LBP. Studies have revealed conflicting results regarding the influence of patient age on outcome after treatment for chronic LBP. Buchner et al. performed a 6-month follow-up on chronic LBP patients who had undergone a multidisciplinary biopsychosocial therapy approach.43 The treatment regimen consisted of cognitive behavioral therapy, lumbar exercises, relaxation training, work-related training, medical training, and therapy directed at developing adaptive coping skills. Significant improvement was observed in all age groups compared to pretherapy except that older patients did not demonstrate significant improvement in functional capacity. Overall, younger patients had significantly better results for both functional capacity and pain level compared to older patients.
Elevated body mass index (BMI) has been associated with LBP symptoms, obesity representing an individual risk factor for the development of chronic LBP.44 The relationship between the BMI and the occurrence of LBP is becoming better clarified. Khoueri et al. evaluated morbidly obese patients with chronic axial LBP who were undergoing elective bariatric surgery.44 They found a statistically significant improvement in both VAS and ODI in patients who had a decrease in BMI after weight-reduction surgery, despite not undergoing any specific LBP therapy. Obesity is also associated with increased risk for surgical procedures of the spine. A logistic regression analysis demonstrated a positive correlation between risk of significant complication after spine surgery and increasing BMI. While other studies argue that even complex spine surgery can be performed in obese patients with an acceptable morbidity that is comparable to that of nonobese patients, it stands to reason that preoperative weight reduction may improve various aspects of clinical outcome. Overall cardiovascular conditioning, aspects of patient positioning, length of surgery, blood loss, wound healing, and earlier postoperative mobilization and return to activities are potential advantages for patients with an optimal BMI prior to treatment.
Psychological and emotional factors are known to significantly contribute to chronic pain disorders. Anxiety, depression, and maladaptive coping skills that can occur when individuals suffer from longer pain duration result in less improvement in daily activity and work-leisure activity after treatment. Patients with back pain for less than 2 years have significantly better pain relief after treatment than do those with more than 2 years of chronic back pain. The Minnesota Multiphasic Personality Inventory has been well established as a presurgical instrument for predicting outcome after lumbar surgery. Various other measures of psychological and emotional well-being have also been correlated with surgical outcome. Andersen et al. found that the Dallas Pain Questionnaire grading for emotional distress predicted likelihood of significant disability after lumbar fusion surgery.45 Wasan et al. observed that psychiatric conditions, particularly depression or anxiety, affected the likelihood of positive outcome from medial branch block injections for LBP.46 Carragee et al. reported that absolute psychometric scores, particularly fear avoidance characteristics, were significant predictors of poor outcome.31 Derby et al. studied the effect of preoperative psychological and emotional status on patients undergoing single-level lumbar fusion for discogenic LBP.47 They found that patients with a normal preoperative mental component summary (MCS) score had significantly better surgical outcomes at 1 and 2 years compared to those with abnormal MCS scores. Regression analyses have also shown that tobacco use, depression, and ongoing litigation are the most consistent presurgical predictors for poor patient outcome.
Work status and pending worker’s compensation claims prior to treatment appear to be significant predictors of outcome. Beals and Hickman demonstrated that work disability for longer than 6 months was a strong predictor of poor outcome.48 Only 50% of patients that are disabled from work for more than 6 months eventually return to full work capacity, with only 20% still employed at 1 year and 0% at 2 years after attempting to return to work. Alternatively, patients who are preoperatively employed are more likely to demonstrate good clinical outcomes and return to work function. In a study of patients undergoing dorsolateral fusion for discogenic LBP, all patients who had good or excellent outcomes were employed preoperatively or had been disabled for less than 3 months.49 Interestingly, they found that preoperative work status was a better predictor of outcome than was positive discography. Anderson et al. observed similar findings in patients undergoing ALIF for discogenic LBP.50 Patients who were employed preoperatively were 10.5 times more likely to be working at follow-up. Only 43% of patients who were unemployed before surgery were employed at follow-up. Alternatively, 90% of patients who were working before surgery were still working at follow-up. Preoperative work status also predicted posttreatment pain relief. Patients who were employed before surgery demonstrated greater improvement in pain scores, while those with worker’s compensation claims had less pain relief. These findings were independent of number of levels treated or other patient demographics. For patients with musculoskeletal injuries in general, it has been shown that continuing even modified work activities prior to surgery had a significant impact on the likelihood of returning to work after treatment.51,52 Therefore, patients should be encouraged to remain active and continue to work in some capacity, even if work accommodations or restrictions are necessary. Limiting preoperative work disability to less than 3 months may improve outcome and the likelihood of returning to full work capacity postoperatively.
Lumbar lordosis and sagittal balance have recently come to light as independent predictors of LBP and overall health status. An association between LBP and loss of lumbar lordosis has been observed. Jackson et al. characterized lumbar lordosis in asymptomatic individuals and patients with mechanical-type LBP who were matched for age, gender, and size.53 They observed that two thirds of total lumbar lordosis occurs at L4-5 and L5-S1 and that LBP patients have significantly different segmental lordosis for each motion segment, with particularly less distal segmental lordosis. Significant conclusions regarding the effect of decreased lumbar lordosis on back pain must be considered carefully, as it has also been shown that all people, including asymptomatic individuals, demonstrate progressive positive sagittal balance with normal aging. The absence of back pain in individuals with sagittal imbalance may reflect effective compensatory mechanisms, the development of pain representing eventual failure of compensation.
The relationship between sagittal balance and outcome after surgery may potentially be extrapolated from the spinal deformity literature. Glassman et al. investigated predictors of poor health status in adult scoliosis patients.54 They found that among both surgical and nonsurgical patients, positive sagittal balance was the most reliable predictor of clinical symptoms. Worse self-assessments in pain, function, and self-image were correlated with positive sagittal balance as compared to other patient factors. They also noted that relative kyphosis of the lumbar spine was correlated with more significant disability than was the case in patients with a normal or lordotic lumbar spine.55 Takayama et al. reported the incidence of LBP in patients who had previously undergone spinal deformity surgery.56 They observed that LBP was strongly correlated with sagittal balance, more so than latest Cobb angle or evidence of degenerative changes at the adjacent segments. Therefore, when considering operative intervention, one should carefully assess the overall lumbar alignment, particularly the segmental lordosis at L4-5 and L5-S1, as disc degeneration and collapse at these levels factor significantly in the global sagittal balance. Relative straightening of the distal lumbar segments may help to dictate a surgical plan not only to fuse the abnormal motion segment but also to increase lumbar lordosis and improve sagittal balance.
Surgical Outcome
The surgical treatment of axial LBP due to degenerative disc disease remains controversial. Axial back pain is thought to be due to alterations in the biomechanical and biochemical environment created by the degenerated disc. Pathologic motion or stress transference to pain-generating structures under normal physiologic loads can lead to pain. Disruption of the normal architecture of the disc may result in extravasation of biochemical agents that stimulate surrounding nociceptors. Lumbar fusion surgery, by immobilizing the motion segment, is believed to counteract these pathologic processes to reduce pain generation and decrease disability.
The concern with fusion surgery stems from a lack of consensus regarding the appropriate indications for surgical treatment of axial LBP. Because patients commonly present with generalized complaints, a normal neurologic examination, and imaging studies that are often unreliable in identifying the pain generator, determining which patients are candidates for fusion surgery is challenging at best. Unclear surgical indications likely result in a heterogeneous patient population undergoing spine fusion for symptoms of back pain. Many of these patients may in fact have varying underlying etiologies of back pain. Therefore, it is not surprising that surgeons and institutions demonstrate a broad spectrum of management practices for axial LBP, which consequently is reflected in a wide range of patient outcomes in the surgical literature. To compound the issue, the actual correlation between bony fusion, clinical outcome, and pain relief has yet to be definitively established.57–59 Studies of lumbar spondylolisthesis–related instability, which is commonly treated with arthrodesis, have failed to demonstrate a clear correlation between successful fusion and outcome. In a randomized, prospective study of patients with lumbar spondylolisthesis and stenosis, dramatically increased fusion rates in patients with pedicle screw fixation did not, however, result in a concomitant improvement in clinical outcomes.58
There are a number of clinical studies that have examined outcomes after fusion surgery for axial LBP and degenerative disc disease. Drawing reasonable conclusions from these studies, however, must be done cautiously. The majority of studies are retrospective case series, with only four prospective, randomized controlled studies comparing fusion to nonsurgical treatment for back pain to date. As previously stated, many of these studies employ wide-ranging practices with regard to diagnostic workup, indications for surgery, procedure performed, and method for assessing outcome. Differences with regard to diagnostic evaluation and patient selection vary in the use of discography, MRI, or relying on clinical judgment for determining which patients and what levels to fuse. A broad spectrum of surgical procedures is also described. Currently, there are a number of ways to arthrodese a motion segment, including anterior interbody fusion (ALIF) (Fig. 58-4), posterior interbody fusion (PLIF), transforaminal interbody fusion (TLIF) (Fig. 58-5), lateral interbody fusion (Fig. 58-6), intertransverse fusion (PLF), and facet fusion. Preferences differ with regard to choice of bone graft material, such as autogenous iliac crest, local autograft, allograft, and synthetic osteobiologic agents. The use of segmental instrumentation and interbody devices is also variable. The specific techniques, nuances, advantages, and disadvantages of these surgical procedures are beyond the scope of this chapter. The literature supporting these general fusion techniques in the treatment of axial LBP will be presented separately; however, ultimately, the common singular objective is to achieve solid arthrodesis of the motion segment, and, therefore, the specific surgical measures to perform the fusion are presumed to be secondary.
Clinical Series: ALIF, PLIF, PLF, 360-Degree Fusion
Studies evaluating outcome after ALIF for axial LBP demonstrate high rates of clinical improvement. Newman et al. evaluated 36 patients who underwent ALIF with autogenous iliac crest bone graft for degenerated discs diagnosed with MRI and provocative discography.24 Twenty-eight patients underwent single-level fusion, and eight patients had two-level fusions. Overall, 86.1% of patients had a successful clinical outcome, with a fusion rate of 88.9%. Unsuccessful results were observed in 13.9% of patients. Chow et al. similarly reported promising outcomes in 97 patients undergoing ALIF for degenerative disc disease.60 Complete relief of symptoms occurred in 60%, with an additional 29% having marked improvement in symptoms. Of note, the primary diagnosis of degenerative disc disease and which levels were symptomatic was made by using only the clinical history and plain radiographs in the majority of patients. Only four patients had preoperative provocative discography to facilitate assessment of the symptomatic discs. Blumenthal et al. found a slightly lower rate of successful outcome after ALIF.26 They observed that 74% of patients with single-level discogenic disease had a good outcome as defined by return to work and cessation of narcotic use.
Lee et al. studied 62 patients undergoing uninstrumented PLIF with autogenous iliac crest bone graft for chronic disabling LBP.28 Symptomatic levels for fusion were determined on the basis of provocative discography and corresponding morphologic abnormalities on MRI. Overall, 59.2% of patients were free of narcotic use at last follow-up. Eighty-one percent of patients had completely returned to work, with over 92% of patients employed in at least some partial capacity. Seventy-two percent of patients reported that they were completely satisfied with their surgical outcomes, with 80.5% stating that they would undergo the operation again.
Parker et al. assessed 23 patients who underwent instrumented PLF with iliac crest autograft for discogenic LBP.49 All patients underwent both MRI and discography preoperatively; however, the fusion levels were ultimately determined by which discs demonstrated concordant pain on provocative discography. Overall, only 39% of patients had a good or excellent outcome as defined by a VAS of 4 or less, no analgesic use, and return to at least 75% of premorbid work status. Poor outcomes were observed in 48% of patients, who had a VAS of more than 6, daily narcotic use, and less than 25% of previous work capacity. They noted that pseudarthrosis (22%) and unemployment for more than 3 months preoperatively were associated with poor outcomes. Overall, only 56% of patients reported that they were extremely satisfied with the surgical results.
Combined anterior-posterior or 360-degree fusion for degenerative disc disease has also been reported. Moore et al. studied 58 patients who underwent ALIF with instrumented dorsolateral fusion for discogenic pain.61 With a minimum of 2 years of follow-up, they observed a 95% arthrodesis rate. At final follow-up, 86% of patients were improved in comparison to their situation preoperatively, with 88% of patients having returned to work. Linson et al. similarly studied patients undergoing anterior-dorsal fusion and reported that 80% of patients had measurable improvement in LBP symptoms.25
Leufven and Nordwall studied 29 patients undergoing combined instrumented PLIF and PLF for axial LBP of greater than 2 years’ duration.62 Fusion levels were identified by positive discography. Sixty-nine percent of patients reported either no back pain or improvement of back pain symptoms, with 31% describing complete relief of symptoms, being off all analgesics, and return to full activities. Forty-eight percent of patients had a suboptimal outcome consisting of continued moderate or severe pain, some or total activity restriction, and continued daily use of analgesics. Only 62% of patients were employed at follow-up, and only 76% of patients stated that they would have the surgery again.
Recently, studies have also evaluated surgical outcome for the treatment of disease on three or more levels, challenging the presumption that surgical fusion for degenerative disc disease is best limited to single-level or two-level fusions. Suratwala et al. assessed 360-degree fusion for three or more levels in patients with lumbar degenerative disc disease.63 Surgical procedures consisted of either ALIF or TLIF with dorsal instrumentation. Diagnostic workup consisted of MRI in all patients, with 60 of 80 patients undergoing discography. Overall, the authors reported a 29.5% improvement in ODI and 30.7% improvement in Roland Morris score at a minimum of 2 years of follow-up.
Lettice et al. compared patients undergoing lumbar fusion for single-level or two-level discogenic disease with those with three or more affected levels.64 Surgical procedures include PLF plus ALIF or PLIF. Symptomatic discs were identified by using provocative pressure-controlled discography. They found significant improvement in SF-36 physical component scores in both groups at 1-year and 2-year follow-up. The longer-segment group did have a higher pseudarthrosis rate and reoperation rate; however, the number of levels that were treated did not have a significant effect on overall clinical outcome as measured by SF-36.
In 2004, Geisler et al. performed a meta-analysis of the literature to compare various fusion techniques for the treatment of axial LBP.65 They reviewed 25 papers that had a minimum of 2 years of follow-up for the standardized outcome measures ODI and VAS. They found that patients undergoing ALIF had a mean 45.5% improvement in VAS and a mean 27.9-point reduction in ODI. Similarly, for 360-degree fusion (PLF plus ALIF, TLIF, or PLIF), patients demonstrated a mean 49.1% improvement in VAS and a mean 20.6-point reduction in ODI. In a prospective, randomized controlled trial, Fritzell et al. compared patients who had undergone uninstrumented PLF, instrumented PLF, and instrumented 360-degree fusion.66 At 2 years of follow-up, there was no significant difference in pain, disability, depressive symptoms, or overall rating between the three surgical groups. While they did observe that the use of instrumentation resulted in increased fusion rates, no correlation between fusion rate and clinical outcome was demonstrated. More complications, lengthier hospitalization, increased blood loss, and new incidence of postoperative leg pain were identified in the patients who had undergone pedicle screw instrumentation.
Prospective, Randomized Studies
The Oxford Centre for Evidence-Based Medicine classifies studies investigating medical treatment effect based on the soundness of their scientific method and the value of their clinical relevance.67 On the basis of this classification, the majority of existing studies supporting lumbar fusion for the treatment of axial LBP provide only class III or IV evidence (retrospectively collected data; case-control study or case series). To date, there have been only four prospective, randomized controlled trials comparing lumbar fusion to nonsurgical therapy for the treatment of chronic LBP (class I evidence). In 2001, the Swedish Lumbar Spine Study Group published their findings comparing lumbar fusion with nonoperative treatment in patients with chronic LBP.68 A total of 294 patients were randomized, 222 patients undergoing surgery and 72 patients treated nonoperatively. Inclusion criteria were patients with chronic disabling LBP with disc degeneration. Exclusion criteria were leg pain, spondylolisthesis, spinal stenosis, fracture, infection, or tumor. Surgical patients were then randomized to either uninstrumented dorsolateral fusion, instrumented dorsolateral fusion, or 360-degree instrumented fusion. Nonsurgical therapy was not standardized, with the patient’s treating physician deciding from a range of interventions including physical therapy, education, transcutaneous electrical nerve stimulation, epidural steroid injections, cognitive and functional training, and coping strategies.
Although this study by Fritzell et al.68 received the 2001 Volvo Award for clinical studies, it also sparked considerable debate. The primary criticism stems from the nonsurgical group and the lack of a standardized nonsurgical treatment strategy. Because the nonsurgical arm consisted of whichever intervention was offered by the individual treating physician, these patients were subjected to any variety of possible therapies, which are not well detailed in the report. It is certainly possible that the members of the nonsurgical group that had already failed conservative treatment for an average duration of 8 years were potentially being randomized to a treatment arm that consisted of essentially the same continued failed therapy. Therefore, it is not surprising that the nonsurgical group demonstrated modest (if at all) improvement in pain and disability compared to any benefit seen in the surgical group. Additional criticism arises from an unclear algorithm for diagnosing the etiology of back pain and determining the symptomatic levels. Taken a step further, a reasonable concern is that the study could be misconstrued as wholesale advocating of lumbar fusion surgery as a better treatment for chronic LBP than nonsurgical therapy.
In response to these issues, Brox et al. performed a prospective, randomized controlled trial comparing instrumented lumbar fusion and a standardized nonsurgical treatment protocol, which had been shown to be more effective than conventional conservative care.69 A total of 64 patients with more than 1 year of LBP and evidence of disc degeneration at L4-5 or L5-S1 were enrolled. Nonsurgical treatment consisted of an intensive 3-week program in which patients stayed at a “back hotel” and participated in physical therapy sessions three times per day, with an average total of 25 hours per week of exercises, cognitive intervention, education, and peer counseling.
In a subsequent study, the same research group performed a prospective, randomized clinical trial comparing lumbar fusion versus the same nonsurgical treatment protocol in patients with chronic LBP after prior disc herniation surgery.70 A total of 60 patients were enrolled. The investigators found that the surgical group had a mean ODI reduction of 9 points compared to a 13-point decrease in the nonsurgical group. These differences were not statistically significant, and the overall success rate of the surgical group was 50% compared to 48% of the nonsurgical group. Again, the authors concluded that at 1-year follow-up, lumbar fusion failed to demonstrate any significant benefit over cognitive intervention and exercises in the treatment of chronic LBP.
The primary criticism of these two studies is that the follow-up duration was only 1 year and that both studies were significantly underpowered. Fairbank et al. investigated lumbar fusion versus an intensive rehabilitation program.71 Three hundred forty-nine patients were randomly assigned, with 176 patients undergoing surgical treatment and 173 undergoing rehabilitation. The surgical procedures varied and were dependent on surgeon and institutional practices. The nonsurgical treatment was a standardized protocol that involved 5 days per week for 3 weeks of outpatient therapy. A total of 75 hours of intervention were performed consisting of cognitive behavioral therapy and various exercises directed toward strengthening, flexibility, endurance, and aerobic conditioning. The surgical group demonstrated an average reduction in ODI of 12.5 points compared to an 8.7-point decrease in the nonsurgical group. This difference was marginal although statistically significant. The authors concluded that the modest advantage of fusion surgery did not provide clear evidence that surgery is more beneficial than intensive rehabilitation for the treatment of chronic LBP. Alternatively, they suggest that their study provides evidence that intensive rehabilitation with cognitive behavioral principles is a viable treatment option and alternative to fusion surgery. Critics of this study point out that 28% of nonsurgical patients crossed over to the surgical arm. Therefore, the patients who were demonstrating the worst outcomes with nonsurgical therapy ultimately had surgery. An intent-to-treat analysis was performed that was inherently biased against surgery, as these patients who were failing nonsurgical therapy may have gained benefit after crossing over, although this was not reflected in the final outcomes assessment.
In 2008, Mirza and Deyo performed a Cochrane summary of these three prospective, randomized controlled studies (Fritzell et al.,68 Brox et al.,69 and Fairbank et al.71).72 Mirza and Deyo concluded that in all three studies, surgical treatment demonstrated similar functional improvement with an 8.9 to 15.6 decrease in ODI, which corresponded to a 19% to 37% change from baseline. The nonsurgical group, which ranged from a nonstandardized variety of treatments to standardized protocols for intensive cognitive therapy and exercise, showed an improvement in ODI of 2.8 to 13.3 points and a 5.8% to 30.1% improvement from baseline. Their conclusion was that surgery provided a fairly modest increase in improvement compared to nonsurgical treatment for discogenic back pain. However, none of the observed differences in average back-specific disability outcome were sufficiently large to meet FDA criteria, as defined by a 15-point or more improvement in ODI, for a clinically meaningful difference between treatments.
Analysis of Lumbar Fusion Outcomes in Studies of Total Lumbar Disc Replacement
Over the last 20 years, there has been considerable interest in exploring lumbar disc replacement as a surgical treatment for degenerative disc disease. For a full discussion regarding disc arthroplasty, see Chapter 161. However, heightened enthusiasm for this device technology has led to several FDA-approved investigational device exemption (IDE) studies comparing total disc replacement to lumbar fusion for degenerative disc disease. As part of the FDA IDE protocol, study centers maintained meticulous high standards with regard to subject enrollment, preoperative evaluation, standardization of surgical procedure, prospective outcomes assessment, duration of follow-up, and controlling for confounding variables. Although these studies were intended to evaluate the effectiveness of disc replacement compared to fusion, they also provide valuable information about lumbar fusion outcomes in a highly controlled patient population with axial LBP. Therefore, evaluating only the lumbar fusion treatment arm of these FDA IDE studies provides prospective, controlled data regarding lumbar fusion for the treatment of axial LBP. Based on the Oxford Centre for Evidence-Based Medicine, these studies provide class II evidence regarding the use of lumbar fusion to treat axial LBP.
Blumenthal et al. performed a prospective, randomized controlled trial comparing treatment of degenerative disc disease with the Charité total disc replacement versus ALIF with a BAK cage packed with autogenous iliac crest bone graft.73 Study enrollment included patients suffering from back pain without leg pain, positive discography, single-level disease, failure with conservative therapy, and no prior lumbar fusion surgery. The conclusions of the study were that the Charité total disc replacement demonstrated noninferiority compared to ALIF for the treatment of degenerative disc disease with 2 years of follow-up.
Focusing specifically on only the lumbar fusion group, 99 patients underwent ALIF with outcomes assessment at 24 months. The fusion patients demonstrated a mean 42.4% reduction in ODI and an average 34.1% reduction in VAS at 2 years. However, at 2 years, 80% of patients still required narcotic pain medications. A small increase in employment rate was observed from 57.6% preoperatively to 65% at 2 years. As part of the FDA IDE protocol, a standardized criteria for meaningful clinical success was determined prior to initiating the study. The FDA criteria for overall clinical success were defined as a 25% or greater improvement in ODI, no device failure, no major complications, and no neurologic deterioration. At 2 years, 56.8% of patients undergoing ALIF for degenerative disc disease met FDA criteria for clinical success. A subsequent study that followed a portion of these same patients at 5 years after surgery demonstrated that the employment rate dropped to 46.5% and the overall clinical success rate decreased to 51.2%.74 Overall, the study by Blumenthal et al. provides class II evidence that slightly more than half of patients undergoing ALIF for single-level degenerative disc disease meet FDA criteria for clinical success at 2 years.
Zigler et al. studied the Prodisc-L total disc replacement compared to 360-degree fusion for patients with back pain due to single-level degenerative disc disease.75 Patients were prospectively, randomized to either disc replacement or ALIF with femoral ring allograft and dorsolateral fusion with iliac crest autograft and pedicle screw fixation. At 24 months of follow-up, patients who were treated with the Prodisc-L had statistically significant better reduction in ODI than did fusion patients, with an improvement in VAS that trended toward significance. Again focusing specifically only on the fusion group, 75 total patients underwent 360-degree fusion for debilitating back pain due to single-level degenerative disc disease. Of these patients, 84.5% reported some improvement in ODI, with 54.9% demonstrating a 25% or greater improvement in ODI and 64.8% having a minimum 15% or greater improvement in ODI. A mean 32-point reduction in VAS was observed. At 24 months, 70% of fusion patients had improvement in their composite SF-36 physical and mental score compared to baseline. A modest improvement in employment rate was noted, with 78.1% working preoperatively and 85.1% employed at 24 months postoperatively. The FDA criteria for success for this study included a 15-point or higher ODI improvement; improvement in SF-36; device success as defined by absence of reoperation to modify, remove implants, or supplemental fixation; radiographic evidence of fusion without graft subsidence, migration, radiolucency, or loss of disc height; and no evidence of neurologic deterioration. Only 40.8% of patients undergoing 360-degree fusion for single-level degenerative disc disease met FDA criteria for overall clinical success.
Together, these two studies provide class II evidence that lumbar fusion provides meaningful clinical success in 56.8% and 40.8% of patients undergoing ALIF or 360-degree fusion for axial back pain, respectively. The strength of these studies is that they are prospective, controlled, multicenter studies with rigorous follow-up using validated outcome assessment measures. Strict inclusion and exclusion criteria were maintained with a well-defined medical condition for study enrollment. Importantly, they also evaluated for clinically meaningful success as determined by FDA criteria. On the basis of these findings, one can conclude that lumbar fusion may significantly benefit approximately half of patients with single-level degenerative disc disease who have failed conservative therapy.
Biyani A., Andersson G.B. Low back pain: pathophysiology and management. J Am Acad Orthop Surg. 2004;12(2):106-115.
Brox J.I., Sorensen R., Friis A., et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine (Phila Pa 1976). 2003;28(17):1913-1921.
Fairbank J., Frost H., Wilson-MacDonald J., et al. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. BMJ. 2005;330(7502):1233.
Fritzell P., Hagg O., Wessberg P., Nordwall A. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine (Phila Pa 1976). 2002;27(11):1131-1141.
Mirza S.K., Deyo R.A. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine (Phila Pa 1976). 2007;32(7):816-823.
Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 6: magnetic resonance imaging and discography for patient selection for lumbar fusion. J Neurosurg Spine. 2005;2(6):662-669.
Zigler J., Delamarter R., Spivak J.M., et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976). 2007;32(11):1155-1162. discussion 63
1. Rosomoff H.L., Rosomoff R.S. Low back pain. Evaluation and management in the primary care setting. Med Clin North Am. 1999;83(3):643-662.
2. Biyani A., Andersson G.B. Low back pain: pathophysiology and management. J Am Acad Orthop Surg. 2004;12(2):106-115.
3. Manchikanti L., Singh V., Datta S., et al. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12(4):E35-E70.
4. Katz J.N. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg [Am]. 2006;88(Suppl 2):21-24.
5. Jensen M.C., Brant-Zawadzki M.N., et alObuchowski N. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69-73.
6. Deyo R.A., Gray D.T., Kreuter W., et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976). 2005;30(12):1441-1445. discussion 6–7
7. Manchikanti L., Singh V., Pampati V., et al. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician. 2001;4(4):308-316.
8. Nachemson A. Lumbar discography: where are we today? Spine (Phila Pa 1976). 1989;14(6):555-557.
9. Deyo R.A., Nachemson A., Mirza S.K. Spinal-fusion surgery: the case for restraint. N Engl J Med. 2004;350(7):722-726.
10. Miller J.A., Schmatz C., Schultz A.B. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine (Phila Pa 1976). 1988;13(2):173-178.
11. Crock H.V. A reappraisal of intervertebral disc lesions. Med J Aust. 1970;1(20):983-989.
12. Crock H.V. Internal disc disruption: a challenge to disc prolapse fifty years on. Spine (Phila Pa 1976). 1986;11(6):650-653.
13. Haughton V.M., Rogers B., Meyerand M.E., Resnick D.K. Measuring the axial rotation of lumbar vertebrae in vivo with MR imaging. AJNR Am J Neuroradiol. 2002;23(7):1110-1116.
14. Blankenbaker D.G., Haughton V.M., Rogers B.P., et al. Axial rotation of the lumbar spinal motion segments correlated with concordant pain on discography: a preliminary study. AJR Am J Roentgenol. 2006;186(3):795-799.
15. Scavone J.G., Latshaw R.F., Weidner W.A. Anteroposterior and lateral radiographs: an adequate lumbar spine examination. AJR Am J Roentgenol. 1981;136(4):715-717.
16. Liang M., Komaroff A.L. Roentgenograms in primary care patients with acute low back pain: a cost-effectiveness analysis. Arch Intern Med. 1982;142(6):1108-1112.
17. Coste J., Paolaggi J.B., Spira A. Reliability of interpretation of plain lumbar spine radiographs in benign, mechanical low-back pain. Spine (Phila Pa 1976). 1991;16(4):426-428.
18. Colhoun E., McCall I.W., Williams L., Cassar Pullicino V.N. Provocation discography as a guide to planning operations on the spine. J Bone Joint Surg [Br]. 1988;70(2):267-271.
19. Sachs B.L., Vanharanta H., Spivey M.A., et al. Dallas discogram description: a new classification of CT/discography in low-back disorders. Spine (Phila Pa 1976). 1987;12(3):287-294.
20. Zucherman J., Derby R., Hsu K., et al. Normal magnetic resonance imaging with abnormal discography. Spine (Phila Pa 1976). 1988;13(12):1355-1359.
21. Carragee E.J., Alamin T.F., Carragee J.M. Low-pressure positive discography in subjects asymptomatic of significant low back pain illness. Spine (Phila Pa 1976). 2006;31(5):505-509.
22. Vanharanta H., Sachs B.L., Spivey M.A., et al. The relationship of pain provocation to lumbar disc deterioration as seen by CT/discography. Spine (Phila Pa 1976). 1987;12(3):295-298.
23. Simmons E.H., Segil C.M. An evaluation of discography in the localization of symptomatic levels in discogenic disease of the spine. Clin Orthop Relat Res. 1975;108:57-69.
24. Newman M.H., Grinstead G.L. Anterior lumbar interbody fusion for internal disc disruption. Spine (Phila Pa 1976). 1992;17(7):831-833.
25. Linson M.A., Williams H. Anterior and combined anteroposterior fusion for lumbar disc pain: a preliminary study. Spine (Phila Pa 1976). 1991;16(2):143-145.
26. Blumenthal S.L., Baker J., Dossett A., Selby D.K. The role of anterior lumbar fusion for internal disc disruption. Spine (Phila Pa 1976). 1988;13(5):566-569.
27. Schechter N.A., France M.P., Lee C.K. Painful internal disc derangements of the lumbosacral spine: discographic diagnosis and treatment by posterior lumbar interbody fusion. Orthopedics. 1991;14(4):447-451.
28. Lee C.K., Vessa P., Lee J.K. Chronic disabling low back pain syndrome caused by internal disc derangements: the results of disc excision and posterior lumbar interbody fusion. Spine (Phila Pa 1976). 1995;20(3):356-361.
29. Derby R., Howard M.W., Grant J.M., et al. The ability of pressure-controlled discography to predict surgical and nonsurgical outcomes. Spine (Phila Pa 1976). 1999;24(4):364-371. discussion 71–72
30. Weatherley C.R., Prickett C.F., O’Brien J.P. Discogenic pain persisting despite solid posterior fusion. J Bone Joint Surg [Br]. 1986;68(1):142-143.
31. Carragee E.J., Lincoln T., Parmar V.S., Alamin T. A gold standard evaluation of the “discogenic pain” diagnosis as determined by provocative discography. Spine (Phila Pa 1976). 2006;31(18):2115-2123.
32. Wetzel F.T., LaRocca S.H., Lowery G.L., Aprill C.N. The treatment of lumbar spinal pain syndromes diagnosed by discography. Lumbar arthrodesis. Spine (Phila Pa 1976). 1994;19(7):792-800.
33. Modic M.T., Steinberg P.M., Ross J.S., et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193-199.
34. Boden S.D., Davis D.O., Dina T.S., et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg [Am]. 1990;72(3):403-408.
35. Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 6: magnetic resonance imaging and discography for patient selection for lumbar fusion. J Neurosurg Spine. 2005;2(6):662-669.
36. Horton W.C., Daftari T.K. Which disc as visualized by magnetic resonance imaging is actually a source of pain? A correlation between magnetic resonance imaging and discography. Spine (Phila Pa 1976). 1992;17(Suppl 6):S164-S171.
37. Buirski G. Magnetic resonance signal patterns of lumbar discs in patients with low back pain: a prospective study with discographic correlation. Spine (Phila Pa 1976). 1992;17(10):1199-1204.
38. Aprill C., Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65(773):361-369.
39. Modic M.T., Obuchowski N.A., Ross J.S., et al. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology. 2005;237(2):597-604.
40. Gill K., Blumenthal S.L. Functional results after anterior lumbar fusion at L5-S1 in patients with normal and abnormal MRI scans. Spine (Phila Pa 1976). 1992;17(8):940-942.
41. Ohtori S., Kinoshita T., Yamashita M., et al. Results of surgery for discogenic low back pain: a randomized study using discography versus discoblock for diagnosis. Spine (Phila Pa 1976). 2009;34(13):1345-1348.
42. Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 14: brace therapy as an adjunct to or substitute for lumbar fusion. J Neurosurg Spine. 2005;2(6):716-724.
43. Buchner M., Neubauer E., Zahlten-Hinguranage A., Schiltenwolf M. Age as a predicting factor in the therapy outcome of multidisciplinary treatment of patients with chronic low back pain: a prospective longitudinal clinical study in 405 patients. Clin Rheumatol. 2007;26(3):385-392.
44. Khoueir P., Black M.H., Crookes P.F. Prospective assessment of axial back pain symptoms before and after bariatric weight reduction surgery. Spine J. 2009;9(6):454-463.
45. Andersen T., Christensen F.B., Bunger C. Evaluation of a Dallas Pain Questionnaire classification in relation to outcome in lumbar spinal fusion. Eur Spine J. 2006;15(11):1671-1685.
46. Wasan A.D., Jamison R.N., Pham L., et al. Psychopathology predicts the outcome of medial branch blocks with corticosteroid for chronic axial low back or cervical pain: a prospective cohort study. BMC Musculoskelet Disord. 2009;10:22.
47. Derby R., Lettice J.J., Kula T.A., et al. Single-level lumbar fusion in chronic discogenic low-back pain: psychological and emotional status as a predictor of outcome measured using the 36-item Short Form. J Neurosurg Spine. 2005;3(4):255-261.
48. Beals R.K., Hickman N.W. Industrial injuries of the back and extremities. Comprehensive evaluation—an aid in prognosis and management: a study of one hundred and eighty patients. J Bone Joint Surg [Am]. 1972;54(8):1593-1611.
49. Parker L.M., Murrell S.E., Boden S.D., Horton W.C. The outcome of posterolateral fusion in highly selected patients with discogenic low back pain. Spine (Phila Pa 1976). 1996;21(16):1909-1916. discussion 16–17
50. Anderson P.A., Schwaegler P.E., Cizek D., Leverson G. Work status as a predictor of surgical outcome of discogenic low back pain. Spine (Phila Pa 1976). 2006;31(21):2510-2515.
51. Crook J., Moldofsky H. The probability of recovery and return to work from work disability as a function of time. Qual Life Res. 1994;3(Suppl 1):S97-S109.
52. Crook J., Moldofsky H., Shannon H. Determinants of disability after a work related musculoskeletal injury. J Rheumatol. 1998;25(8):1570-1577.
53. Jackson R.P., McManus A.C. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size: a prospective controlled clinical study. Spine (Phila Pa 1976). 1994;19(14):1611-1618.
54. Glassman S.D., Berven S., Bridwell K., et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976). 2005;30(6):682-688.
55. Glassman S.D., Bridwell K., Dimar J.R., et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976). 2005;30(18):2024-2029.
56. Takayama K., Nakamura H., Matsuda H. Low back pain in patients treated surgically for scoliosis: longer than sixteen-year follow-up. Spine (Phila Pa 1976). 2009;34(20):2198-2204.
57. Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 12: pedicle screw fixation as an adjunct to posterolateral fusion for low-back pain. J Neurosurg Spine. 2005;2(6):700-706.
58. Fischgrund J.S., Mackay M., Herkowitz H.N., et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997;22(24):2807-2812.
59. Bjarke Christensen F., Stender Hansen E., Laursen M., et al. Long-term functional outcome of pedicle screw instrumentation as a support for posterolateral spinal fusion: randomized clinical study with a 5-year follow-up. Spine (Phila Pa 1976). 2002;27(12):1269-1277.
60. Chow S.P., Leong J.C., Ma A., Yau A.C. Anterior spinal fusion or deranged lumbar intervertebral disc. Spine (Phila Pa 1976). 1980;5(5):452-458.
61. Moore K.R., Pinto M.R., Butler L.M. Degenerative disc disease treated with combined anterior and posterior arthrodesis and posterior instrumentation. Spine (Phila Pa 1976). 2002;27(15):1680-1686.
62. Leufven C., Nordwall A. Management of chronic disabling low back pain with 360 degrees fusion: results from pain provocation test and concurrent posterior lumbar interbody fusion, posterolateral fusion, and pedicle screw instrumentation in patients with chronic disabling low back pain. Spine (Phila Pa 1976). 1999;24(19):2042-2045.
63. Suratwala S.J., Pinto M.R., Gilbert T.J., et al. Functional and radiological outcomes of 360-degree fusion of three or more motion levels in the lumbar spine for degenerative disc disease. Spine (Phila Pa 1976). 2009;34(10):E351-E358.
64. Lettice J.J., Kula T.A., Derby R., et al. Does the number of levels affect lumbar fusion outcome? Spine (Phila Pa 1976). 2005;30(6):675-681.
65. Geisler F.H., Blumenthal S.L., Guyer R.D., et al. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: results of a multicenter, prospective, randomized investigational device exemption study of Charite intervertebral disc. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(2):143-154.
66. Fritzell P., Hagg O., Wessberg P., Nordwall A. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine (Phila Pa 1976). 2002;27(11):1131-1141.
67. . Oxford Centre for Evidence-Based Medicine Levels of Evidence and Grades of Recommendation. 2009. http://www.cebm.net/index.aspx?o=1025. Accessed April 21, 2010
68. Fritzell P., Hagg O., Wessberg P., Nordwall A. 2001 Volvo Award winner in clinical studies. Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976). 2001;26(23):2521-2532. discussion 32–34
69. Brox J.I., Sorensen R., Friis A., et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine (Phila Pa 1976). 2003;28(17):1913-1921.
70. Brox J.I., Reikeras O., Nygaard O., et al. Lumbar instrumented fusion compared with cognitive intervention and exercises in patients with chronic back pain after previous surgery for disc herniation: a prospective randomized controlled study. Pain. 2006;122(1–2):145-155.
71. Fairbank J., Frost H., Wilson-MacDonald J., et al. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. BMJ. 2005;330(7502):1233.
72. Mirza S.K., Deyo R.A. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine (Phila Pa 1976). 2007;32(7):816-823.
73. Blumenthal S., McAfee P.C., Guyer R.D., et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine (Phila Pa 1976). 2005;30(14):1565-1575. discussion E387–E391
74. Guyer R.D., McAfee P.C., Banco R.J., et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: five-year follow-up. Spine J. 2009;9(5):374-386.
75. Zigler J., Delamarter R., Spivak J.M., et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976). 2007;32(11):1155-1162. discussion 63