CHAPTER 338 Indications and Techniques for Cranial Decompression after Traumatic Brain Injury
Reduction of death and disability from traumatic brain injury (TBI) is the goal of cranial decompressive surgery. Secondary insults and injury, including brain edema, elevated intracranial pressure (ICP), and decreased cerebral perfusion pressure (CPP), are the leading causes of in-hospital deaths after TBI.1 The primary goals of treatment of TBI are therefore to control brain edema and maintain adequate blood flow and delivery of oxygen to the injured brain tissue. Adherence to published prehospital, intensive care, and surgical guidelines for the management of severe TBI has resulted in improved survival and outcomes by mitigating the deleterious effects of secondary insults and injury. ICP monitoring guides the use of specific therapies such as drainage of cerebrospinal fluid (CSF), sedation, mild hyperventilation, or hyperosmolar euvolemia with the administration of hypertonic saline or mannitol (or both) for control of ICP and CPP. Although these therapies may provide adequate treatment for many patients, there is a cohort of patients in whom cerebral edema will continue to propagate despite “maximal medical management” and culminate in increased cellular injury and death and ultimately in poorer outcomes. These patients are possible candidates for decompressive surgery to assist in the control of ICP.
Background
The concept of cranial decompression is by no means new and perhaps dates as far back as Neolithic times when trephination is thought to have been practiced. In the modern medical era, decompressive surgery has been used intermittently with variable success since Bergmann first described the technique in 1880, followed by Cushing’s descriptions of subtemporal decompressive craniectomy for control of ICP.1–3 Reports of high morbidity and mortality rates were common in the 1960s to 1980s, but a variety of techniques were used, primarily after clinical deterioration.4–16 With the development of aggressive intensive care management of TBI patients, decompressive surgery has garnered renewed interest in the past decade or so because of improved results.1,2,17–31 The setting in which decompressive craniotomy or craniectomy (DC) is used has evolved tremendously in recent years, most notably with respect to prehospital care, neuroanesthesiology, neuroradiology, neurointensive care, and rehabilitation. Advances in these areas have contributed to a reduction in mortality and improved outcomes for patients with severe TBI.32,33
DC has been used in nontrauma settings as well. Uncontrolled ICP after aneurysmal subarachnoid hemorrhage has been treated with DC in a variety of settings, including signs of cerebral edema during aneurysm clipping; increased ICP and epidural, subdural, or intracerebral hematoma after aneurysm surgery; and cerebral edema and elevated ICP without radiologic evidence of cerebral infarction after aneurysm surgery.34,35 DC has also been reported for the treatment of cerebral edema after cerebral infarction.36–44 Cerebral edema and mass effect after an infarction further compromise CPP, oxygenation, and metabolism and lead to refractory intracranial hypertension and eventually transtentorial herniation, the leading cause of death in these patients.35 In experimental models, DC has been shown to improve CPP, survival, and neurological outcome, as well as reduce the volume of infarction after extensive ischemic stroke.45 These beneficial effects have been attributed to increased collateral circulation, reduction in tissue edema, and improvements in oxygenation and energy metabolism in the ischemic penumbra.35 DC for other indications such as brain tumor, meningitis, acute encephalitis, toxoplasmosis, encephalopathy secondary to Reye’s syndrome, subdural empyema, and cerebral venous and dural sinus thrombosis has also been described.35,46–52
The rationale for DC is straightforward. The incidence of elevated ICP requiring therapy after severe TBI is 65%, and half of the patients who die of severe TBI die with uncontrolled ICP.53,54 The total time that ICP is elevated greater than 20 mm Hg correlates directly with outcome, and mortality is increased when ICP is not controlled.55–59 Therapeutic approaches for decreasing ICP include reduction of the volume of intracranial contents (blood, brain, or CSF), reduction of cerebral metabolic demands, or an increase in cranial volume via DC. DC has been proved to decrease ICP.19,60–62 Decompressive surgery also leads to a rightward shift of the pressure-volume curve and therefore to massive increases in compliance, as well as a decrease in the amplitude of ICP waves and an increase in compensatory reserve.25,63,64 This results in an increase in cerebral blood flow and subsequently CPP and cerebral microcirculation, which allows rebalancing of cerebral inflow-outflow regulation.61 Brain tissue oxygenation (PbtO2) also improves with durotomy at DC.61,62 Because reduction of ICP is associated with improved outcome and DC is associated with reduction of ICP, it stands to reason that DC should also be associated with improved outcome, given an acceptable risk-benefit ratio and appropriate patient selection.
Indications
Most authors agree that patients with bilaterally fixed and dilated pupils, a Glasgow Coma Scale (GCS) score of 3, brainstem injury, and central herniation are universally poor candidates for DC because of the known association of these findings with poor outcomes.16,65 The postresuscitation GCS score, especially the motor score, is one of the most important factors to consider in patient selection.63,66 Care should be taken to exclude possible influences on GCS scores such as intoxication, hypoxia, hypotension, and paralytics or sedatives. Younger patients generally have better outcomes; however, age alone should not be used as an exclusion criterion.65,18,67 The presence of midline shift on computed tomography (CT) of the brain is highly predictive prognostically. The degree of shift is inversely related to outcome, and elevated ICP is presumed.68,69 Absent or compressed cisterns are also predictive of elevated ICP and a poor outcome.68,70 The decision to proceed to DC must therefore take into account the preoperative CT appearance, clinical factors, and measured neuromonitoring trends.
The timing of decompressive surgery is important prognostically; once ICP becomes unmanageable and signs of brainstem compression are noted, DC may be lifesaving, but at the expense of severe neurological impairment. Early decompression (within 4 hours of injury) results in profound decreases in mortality and improvement in functional outcome at 6 months.18 Decompressive surgery should be considered very shortly after failure of maximal medical therapy to control ICP. Initial management includes elevation of the head to 30 degrees, use of sedation with or without paralytics, adjustment of ventilation parameters to maintain Paco2 at 30 to 35 mm Hg, maintenance of normothermia, maintenance of hyperosmolar euvolemia (with the administration of mannitol and hypertonic saline), avoidance of hyperglycemia, support of CPP with volume or pressors, and drainage of CSF. Occasionally, pentobarbital therapy or hypothermia may be used before proceeding to surgery. ICP in the low twenties may be tolerated without proceeding to DC if CPP or PbtO2 (or both) is judged to be adequate. However, ICP elevations or CPP or PbtO2 depressions in the face of maximization of the aforementioned parameters are not tolerated for long periods before surgery.
Technique
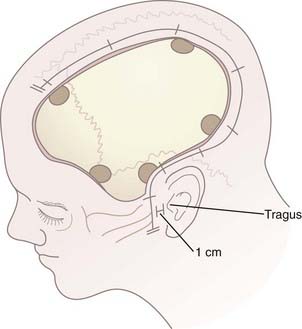
After hair clipping extending just across midline and as far posteriorly as possible, the hemicranium is prepared, marked, and injected with 1% lidocaine with epinephrine to facilitate hemostasis before draping. For a unilateral craniotomy, a standard large question mark or reverse question mark incision is used. The skin incision should start 1 cm in front of the tragus at the zygomatic arch and extend posteriorly above the auricle, upward over the parieto-occipital area, and forward to the frontal region to the hairline. The superior limb should approach the midline, and the posterior limb should be sufficiently posterior to allow creation of an adequately sized bone flap. Although the exact dimensions of the bone flap may vary according to the size and shape of the cranium, the scalp exposure should allow access to specific bony landmarks. For example, the inferior exposure at the temporal region must allow the temporal craniectomy to be extended to the floor of the temporal fossa after the bone flap has been removed (Fig. 338-1; see later also). Scalp hemostasis is facilitated with the use of Raney clips. Bovie cautery is then used to divide the temporalis fascia and muscle in line with the scalp incision. The temporalis muscle, which is often quite edematous, may be reflected anteriorly and inferiorly with the cutaneous flap and both secured with fishhooks after protecting the musculocutaneous flap with rolled sponges underneath. An epinephrine-soaked laparotomy sponge is frequently used on the galeal surface of the cutaneous and muscle flaps to aid in hemostasis. For bifrontal DC, a standard Souttar incision is used.
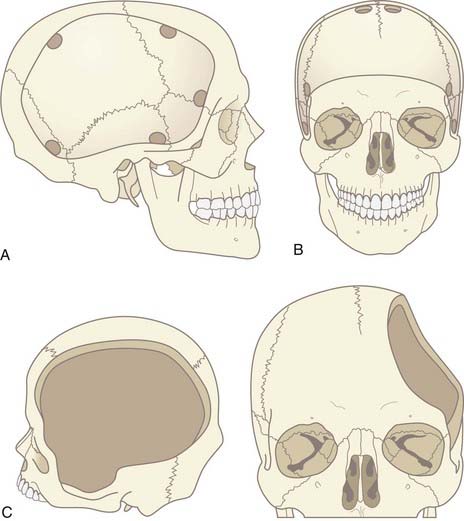
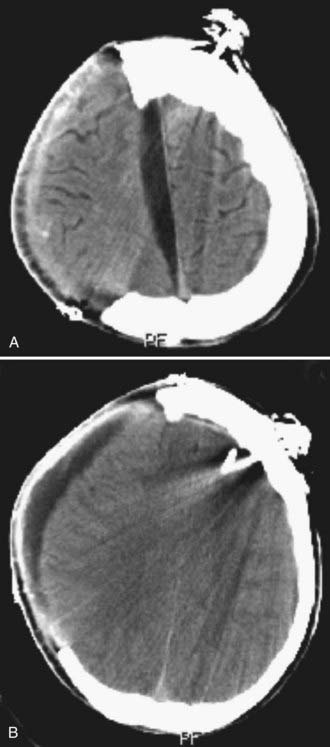
The craniotomy itself for unilateral DC must encompass a large enough area to prevent brain herniation and strangulation, typically from just lateral to the superior sagittal sinus, frontally to the midpupillary line, inferiorly to the floor of the temporal fossa, and posteriorly to the parieto-occipital area (Fig. 338-2A). Bifrontal openings should span from the anterior cranial fossa floor to the coronal suture posteriorly and to the temporal fossa floor bilaterally (Fig. 338-2B). The frontal sinus may be entered, and if so, it should be cranialized. Visibility of the mesencephalic cisterns on postoperative CT correlates with the distance from the craniectomy to the base of the cranium,18 and the size of the bone flap correlates with the degree of reduction of ICP19 (Fig. 338-2C).
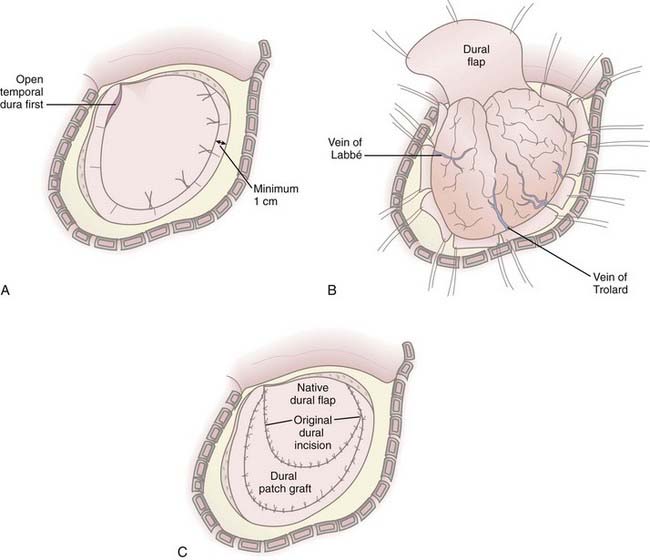
In cases in which it is determined that the bone flap will be left out after evacuation of a mass lesion or after primary DC, durotomy must be performed; otherwise, the operation is ineffective in reducing ICP.61,66,71 Durotomy is akin to fasciotomy in other body compartments. A variety of dural openings have been described. For unilateral operations we use a “C-shaped” dural opening mirroring the scalp incision, with the dural base along the frontal fossa floor (Fig. 338-3A and B). Some have advocated slit dural openings, stellate openings, or other curvilinear openings. For bifrontal procedures, ligation and division of the anterior sagittal sinus, as well as the falx at its most anterior extent, are advocated to allow maximal relief of constriction on the frontal lobes. Whatever the dural opening technique, it is imperative that the temporal dura be opened to decompress the temporal lobe or lobes.
Duraplasty using autograft or allograft may be performed or the dura left open. Several options for dural graft material are available: fascia lata, pericranium, bovine pericardium, collagen matrix products, and synthetic dural substitutes. We perform duraplasty with a collagen matrix or bovine pericardium dural substitute to provide brain protection and avoid adherence of the galea to the underlying brain tissue because return to surgery will be mandated for survivors (Fig. 338-3C). Duraplasty also restores more normal CSF circulation and may prevent or minimize the development of postoperative hygromas and leakage of CSF from the wound. The duraplasty is constructed such that a wide patch graft is created over the herniating cerebral hemisphere or hemispheres, but the following must be avoided: constriction of underlying brain tissue by dural folds and wrinkles and redundant dura or patch graft material resulting in excess graft tissue volume (remembering that in cases of profound cerebral edema, loss of autoregulation, and poor compliance, even the smallest excess volume in the intracranial compartment can contribute to significant increases in ICP). We have used an additional layer of nonbiologic, nonincorporated, synthetic dural substitute as a subtemporalis barrier at the time of DC, which can be readily identified and removed during reimplantation of the bone flap. This has dramatically decreased the dissection time at reoperation and reduces the associated risks of traction on the underlying brain.
A vascular tunneling technique has been described by Csokay and colleagues to alleviate venous congestion (and therefore evolution of brain edema) after decompressive surgery.72 In this technique, small supporting pillars made of hemostatic sponge are placed around the superficial vessels supplying the protruding portion of the brain, thus preventing them from being compressed by the pressure between the dural edge and the brain tissue. In our experience, performing a large craniectomy and durotomy with dural slits along the perimeter of the dural opening perpendicular to the dural incision alleviates the need to perform such a maneuver (Fig. 338-3B).
Partial or anatomic lobectomies have also been performed in conjunction with DC for intractable intracranial hypertension; however, the need for this intervention is significantly lessened when DC is performed early after failure of medical management. If performed, it is often used in conjunction with evacuation of cortical contusions or intraparenchymal hematomas and is limited to the frontal and anterior temporal lobes, preferably on the nondominant side. Lobectomy does not preclude a good outcome.73 However, in our experience, lobectomy is seldom necessary and is reserved for instances of a “pulped” or necrotic lobe with malignant cerebral edema at surgery, pulselessness of the hemisphere, vessel occlusion or infarction (which can be related to a blunt vascular injury), profound coagulopathy in the face of multiple intracranial hemorrhages, or any combination of such conditions. There is a high correlation of lobectomy with poor initial GCS score and emergency surgery in our institution.
The bone flap may be stored in an abdominal subcutaneous pocket or in a certified tissue bank. Abdominal storage has been associated with resorption, rhabdomyolysis,74 and infection and of course requires an additional incision and procedure at both storage and retrieval. Contaminated flaps should not be stored in the abdominal wall.
The interval between decompressive surgery and secondary bone flap replacement in the literature varies from 4 weeks up to 12 months, during which time the unprotected brain is exposed to the risk of injury.75 We recommend the use of a soft helmet when out of bed until the bone flap is replaced to reduce the risk for secondary brain injury. The helmet is removed when the patient is safely in bed or in a chair to prevent scalp sweating and friction on the incision. Our typical approach to the timing of staged secondary bone replacement is to replace the flap at around 3 months. When patients with severe TBI arrive for their first neurosurgical follow-up after discharge from the hospital or acute inpatient rehabilitation, elective surgery for replacement of the bone flap is scheduled if no contraindications exist, such as ongoing active infection, ongoing advanced-stage decubitus ulcers, or extremely poor nutritional status. This approach allows in-hospital and rehabilitative treatment of associated injuries, resolution of cerebral edema, and reduction in the risk for infection related to intensive care unit interventions or nosocomial infections. At the same time, avoidance of longer intervals before bone replacement reduces the incidence of scalp contraction (which can contribute to wound dehiscence), temporalis atrophy (which can contribute to temporomandibular joint complaints and cosmetic asymmetry), and development of the syndrome of the trephine (which can cause severe headache). Furthermore, some patients do exhibit neurological improvement once the flap is replaced, especially improvement in motor and speech deficits, reduction in seizure frequency, and cognitive improvements. Early replacement before the development of severe encephalomalacia also eliminates the potential dead space under the flap postoperatively, thereby decreasing the postoperative risk for epidural hematoma, pneumocephalus, and fluid collections. Finally, early replacement provides cerebral protection during the phases when patients are most at risk for further cerebral injury, such as after they are beginning to be actively transferred and are starting to walk again but are still likely to experience imbalance, incoordination, and orthostatic hypotension.
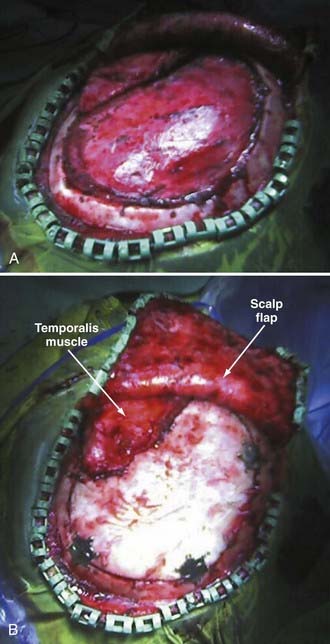
Reimplantation surgery requires meticulous wide sterile preparation of the scalp incision. We use a wide hair clip to identify potential problems from lacerations, abrasions, and other injuries and to facilitate preparation and postoperative wound care. Perioperative antibiotics are administered. The previous incision is used, and care is taken to avoid direct trauma to the underlying brain tissue from local anesthetic injection, scalp incision, and dissection of the cutaneous flap away from the dura. Care must be taken to avoid transfer of heat to the underlying brain from monopolar cautery or pressure on the brain from digital dissection or traction on the cutaneous flap. We use sharp dissection with Metzenbaum scissors and bipolar cautery for hemostasis. The cutaneous flap is gently and consistently lifted upward and gradually retracted as the dissection proceeds, and the bone edges are identified and dissected free of scar tissue with a Penfield No. 1 dissecting tool. Removal of previously placed epidural tack-ups is often necessary to achieve anatomic reseating of the bone flap. There is frequently a ridge of scar tissue at the perimeter of the bone flap that may be shrunken back with bipolar cautery (Fig. 338-4A). The border of the temporalis muscle is identified by sharp dissection, and the muscle is dissected off the underlying dura, to which it is frequently quite adherent unless a barrier has been placed as previously described. It is often not necessary to dissect the temporalis entirely because the bone flap may not fill the cranial defect completely if portions of the temporal bone were rongeured and discarded at the original surgery. Central epidural tack-ups are used to aid in postoperative hemostasis.
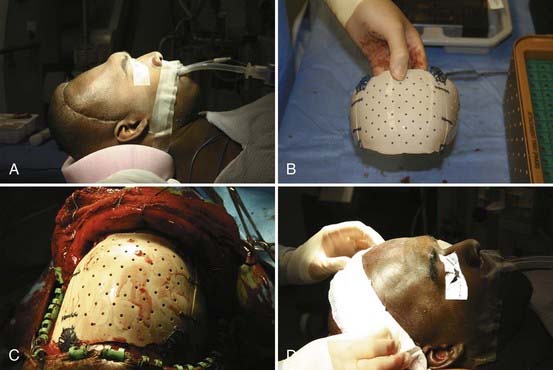
The bone flap is replaced with a standard cranial miniplating system (Fig. 338-4B). In patients with large temporal bone or other defects, this procedure may be combined with mesh cranioplasty with or without the use of organic bone putty or methyl methacrylate. We tend to prefer the former over a titanium mesh framework for large remaining defects. In patients with fragmented bone flaps or grossly contaminated wounds, a customized synthetic implant may be used instead of autograft (Fig. 338-5A to D). These implants actually confer the additional advantage of filling in the temporal craniectomy defect and providing a strut for the temporalis muscle, which often results in better cosmesis.
Complications
The most frequent complications seen with decompressive surgery are hygromas (26%) and hydrocephalus (14% to 29%).75 Other complications include wound infection and dehiscence, seizures, syndrome of the trephine, and secondary brain injury.
In the acute phase after DC, a postoperative increase in brain edema is a potential concern. After pressure is relieved by the operation, the injured brain will have a high demand for oxygen, and the previously compressed vessels will refill and dilate maximally as a result of metabolic changes, thus putting the brain in a hyperemic state.61 Although an increase in brain edema may be noted on postoperative CT scans, the reduced ICP from the DC and duraplasty allows adequate reperfusion of the brain and establishes sufficient brain oxygenation (as noted by continuous monitoring) to prevent further secondary injury.61 Brain herniation and strangulation at the bone defect may occur, particularly with inadequately sized bone flaps. Focal cerebral infarction can result from direct brain compression, venous compression, or diminished regional blood flow.
Hygromas frequently occur on the ipsilateral side after decompressive surgery,63,76 probably because of altered CSF dynamics (Fig. 338-6A and B). Most resolve spontaneously without intervention. They may be treated by observation alone, isolated or serial lumbar puncture, temporary continuous lumbar drainage, lumboperitoneal shunting, or ventriculoperitoneal shunting.63,76 Percutaneous drainage of associated epidural CSF collections may also be considered. Diagnostic lumbar puncture with measurement of opening and closing pressure can assist in distinguishing between simple hygromas and so-called external hydrocephalus. Frequently, replacement of the bone flap will lead to resolution of the hygromas.
DC patients are susceptible to infection (2%63 to 6%76) as a result of extensive scalp incisions; subgaleal fluid accumulations; lengthy hospitalizations; colonization with nosocomial and resistant bacteria; invasive supportive procedures such as tracheostomies, central lines, and bladder catheterizations; and frequent comorbid conditions of lung injury and pneumonia, intra-abdominal injuries, and orthopedic injuries, all of which may require invasive procedures or surgery. Aggressive fever evaluation, including CSF testing, is recommended for patients with signs or symptoms of infection in the acute phases after DC.77 Patients seen later with new or enlarging subdural or epidural/subgaleal fluid collections should undergo CT of the head with and without contrast enhancement, a complete blood count with differential, and determination of the erythrocyte sedimentation rate and C-reactive protein level. Some will require reopening of the incision and fluid drainage to assess for empyema and débridement and irrigation if it is present.
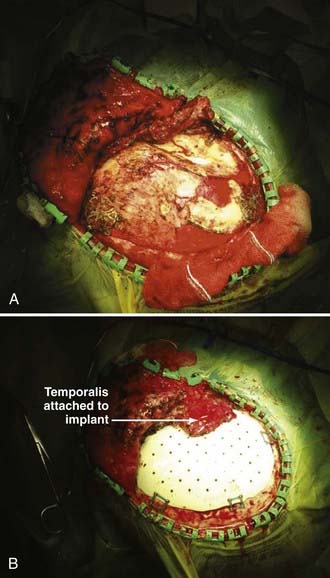
Bone flap resorption has been reported in up to 12% of patients after secondary bone flap replacement, with approximately half requiring removal and replacement with a synthetic implant (Fig. 338-7A and B).63 In our experience, the incidence of bone flap resorption is increased when contaminated bone flaps have been irradiated and with fragmented bone flaps. Others have reported increased resorption rates with abdominal bone flap storage.
Seizures can occur in both the acute and chronic phases after DC, with persistent seizure activity requiring medical treatment developing in up to 5% of patients.76 Early in our series, we noted a high rate of immediate postoperative seizures. This was reduced to essentially nil with the avoidance of Bovie cautery for dissecting the scalp flap away from the underlying dura (thereby reducing potential exposure of the underlying brain tissue to heat) and with intraoperative loading of anticonvulsants, which are then continued for 7 days postoperatively. Patients receiving ongoing antiepileptic therapy for seizures are administered bolus therapy if their levels are subtherapeutic or are maintained on their therapeutic regimen.
Grant and Norcross described the syndrome of the trephine in 1939 as a condition characterized by headaches, dizziness, mood changes, or seizures in patients who had undergone DC.78 As the underlying cerebral edema resolves and encephalomalacia progresses, a sunken flap develops. In some patients, symptoms of the syndrome of the trephine may be alleviated by recumbency. In nearly all, the symptoms resolve with replacement of the bone flap.
The lateral ventricle can migrate toward the craniectomy defect. This may represent a localized hydrocephalus ex vacuo effect, altered CSF dynamics, or a combination of the two.77 The potential for neurological injury as a result of direct trauma from falls is a postoperative consideration. Anesthesia risks from the second procedure must also be taken into account, although rare.
Outcomes
During the past 3 decades, more than 20 centers have published retrospective or nonrandomized prospective case studies (class II and III evidence) on outcomes in patients undergoing DC for multiple causes, with a mean mortality rate of 20% to 30% as opposed to 70% to 80% in patients treated conservatively.35
The literature on DC demonstrates wide variability in outcome but can be divided grossly into studies done before 1991 and studies after 1997. Less favorable outcomes were demonstrated in the earlier years,4–1679 but these studies were hampered by their retrospective nature, by lack of standardization of technique (craniotomy, duraplasty, and unilateral versus bilateral approaches), by heterogeneity in patient groups (TBI and nontraumatic causes and mixing of trauma causes), by lack of a control or comparison group, by lack of standardization of other therapies (including even ICP monitoring), and by use as a salvage maneuver either very late in the course of uncontrolled ICP or only in the most severely injured patients (e.g., those with decerebrate posturing or no pupillary reactivity, or both). Furthermore, the older DC studies were performed in settings quite different from current therapeutic standards. More recent studies have used DC as an earlier treatment option for control of ICP and reduction of the therapeutic intensity level. Several authors have demonstrated improved outcomes with DC for severe TBI.1,2,17–31,63,70 Specifically, earlier intervention has resulted in significantly better results.17,18,22,23,63
A randomized prospective pilot study done in the United States showed encouraging early results.22 There are currently two ongoing multicenter prospective randomized controlled trials of DC in patients with severe diffuse TBI: the Australian Decompressive Craniectomy (DECRA) study35 and the international RESCUEicp trial.35,80 Surgical guidelines for the treatment of severe TBI indicate that DC should be considered for evacuation of subdural hematomas and may be the treatment of choice for patients with posttraumatic edema, hemispheric edema, or diffuse parenchymal injury, depending on the clinical context.81,82
Bullock MR, Chesnut RM, Clifton GL, et al. Management and prognosis of severe traumatic brain injury. J Neurotrauma. 2000;17:449-597.
Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58:S25.
Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58:S16.
Eisenberg HM, Frankowski RF, Contant CF, et al. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J Neurosurg. 1988;69:15.
Marmarou A. Increased intracranial pressure in head injury and influence of blood volume. J Neurotrauma. 1992;9(Suppl 1):S327.
Marshall LF, Becker DP, Bowers SA, et al. The National Traumatic Coma Data Bank. Part 1:Design, purpose, goals, and results. J Neurosurg. 1983;59:276.
Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006;1:CD003983.
Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg. 1982;56:498.
Toutant SM, Klauber MR, Marshall LF, et al. Absent or compressed basal cisterns on first CT scan: ominous predictors of outcome in severe head injury. J Neurosurg. 1984;61:691.
Valadka AB, Gopinath SP, Robertson CS. Midline shift after severe head injury: pathophysiologic implications. J Trauma. 2000;49:1.
1 Kontopoulos V, Foroglou N, Patsalas J, et al. Decompressive craniectomy for the management of patients with refractory hypertension: should it be reconsidered? Acta Neurochir (Wien). 2002;144:791.
2 Meier U, Zeilinger FS, Henzka O. The use of decompressive craniectomy for the management of severe head injuries. Acta Neurochir Suppl. 2000;76:475.
3 Cushing HI. Subtemporal decompressive operations for the intracranial complications associated with bursting fractures of the skull. Ann Surg. 1908;47:641.
4 Clark K, Nash TM, Hutchison GC. The failure of circumferential craniotomy in acute traumatic cerebral swelling. J Neurosurg. 1968;29:367.
5 Shigemori M, Syojima K, Nakayama K, et al. The outcome from acute subdural haematoma following decompressive hemicraniectomy. Acta Neurochir (Wien). 1980;54:61.
6 Britt RH, Hamilton RD. Large decompressive craniotomy in the treatment of acute subdural hematoma. Neurosurgery. 1978;2:195.
7 Kerr FW. Radical decompression and dural grafting in severe cerebral edema. Mayo Clin Proc. 1968;43:852.
8 Ransohoff J, Benjamin MV, Gage ELJr, et al. Hemicraniectomy in the management of acute subdural hematoma. J Neurosurg. 1971;34:70.
9 Ransohoff J, Benjamin V. Hemicraniectomy in the treatment of acute subdural haematoma. J Neurol Neurosurg Psychiatry. 1971;34:106.
10 Venes JL, Collins WF. Bifrontal decompressive craniectomy in the management of head trauma. J Neurosurg. 1975;42:429.
11 Da Silva JA, Da Silva CE, Sousa MB. Decompression craniotomy in acute brain edema. Apropos of 30 operated cases. Arq Neuropsiquiatr. 1976;34:232.
12 Pereira WC, Neves VJ, Rodrigues Y. Bifrontal decompressive craniotomy as the treatment for severe cerebral edema. Arq Neuropsiquiatr. 1977;35:99.
13 Gerl A, Tavan S. Bilateral craniectomy in the treatment of severe traumatic brain edema. Zentralbl Neurochir. 1980;41:125.
14 Karlen J, Stula D. Decompressive craniotomy in severe craniocerebral trauma following unsuccessful treatment with barbiturates. Neurochirurgia (Stuttg). 1987;30:35.
15 Gower DJ, Lee KS, McWhorter JM. Role of subtemporal decompression in severe closed head injury. Neurosurgery. 1988;23:417.
16 Gaab MR, Rittierodt M, Lorenz M, et al. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir Suppl. 1990;51:326.
17 Polin RS, Shaffrey ME, Bogaev CA, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84.
18 Munch E, Horn P, Schurer L, et al. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47:315.
19 Skoglund TS, Eriksson-Ritzen C, Jensen C, et al. Aspects on decompressive craniectomy in patients with traumatic head injuries. J Neurotrauma. 2006;23:1502.
20 Kunze E, Meixensberger J, Janka M, et al. Decompressive craniectomy in patients with uncontrollable intracranial hypertension. Acta Neurochir Suppl. 1998;71:16.
21 De Luca GP, Volpin L, Fornezza U, et al. The role of decompressive craniectomy in the treatment of uncontrollable post-traumatic intracranial hypertension. Acta Neurochir Suppl. 2000;76:401.
22 Coplin WM. Intracranial pressure and surgical decompression for traumatic brain injury: biological rationale and protocol for a randomized clinical trial. Neurol Res. 2001;23:277.
23 Coplin WM, Cullen NK, Policherla PN, et al. Safety and feasibility of craniectomy with duraplasty as the initial surgical intervention for severe traumatic brain injury. J Trauma. 2001;50:1050.
24 Whitfield PC, Kirkpatrick PJ, Czosnyka M, et al. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2001;49:225.
25 Whitfield PC, Patel H, Hutchinson PJ, et al. Bifrontal decompressive craniectomy in the management of posttraumatic intracranial hypertension. Br J Neurosurg. 2001;15:500.
26 Hejazi N, Witzmann A, Fae P. Unilateral decompressive craniectomy for children with severe brain injury. Report of seven cases and review of the relevant literature. Eur J Pediatr. 2002;161:99.
27 Schneider GH, Bardt T, Lanksch WR, et al. Decompressive craniectomy following traumatic brain injury: ICP, CPP and neurological outcome. Acta Neurochir Suppl. 2002;81:77.
28 Soukiasian HJ, Hui T, Avital I, et al. Decompressive craniectomy in trauma patients with severe brain injury. Am Surg. 2002;68:1066.
29 Figaji AA, Fieggen AG, Peter JC. Early decompressive craniotomy in children with severe traumatic brain injury. Childs Nerv Syst. 2003;19:666.
30 Albanese J, Leone M, Alliez JR, et al. Decompressive craniectomy for severe traumatic brain injury: evaluation of the effects at one year. Crit Care Med. 2003;31:2535.
31 Messing-Junger AM, Marzog J, Wobker G, et al. Decompressive craniectomy in severe brain injury. Zentralbl Neurochir. 2003;64:171.
32 Fakhry SM, Trask AL, Waller MA, et al. Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges. J Trauma. 2004;56:492.
33 Patel HC, Menon DK, Tebbs S, et al. Specialist neurocritical care and outcome from head injury. Intensive Care Med. 2002;28:547.
34 Fisher CM, Ojemann RG. Bilateral decompressive craniectomy for worsening coma in acute subarachnoid hemorrhage. Observations in support of the procedure. Surg Neurol. 1994;41:65.
35 Hutchinson P, Timofeev I, Kirkpatrick P. Surgery for brain edema. Neurosurg Focus. 2007;22(5):E14.
36 Rengachary SS, Batnitzky S, Morantz RA, et al. Hemicraniectomy for acute massive cerebral infarction. Neurosurgery. 1981;8:321.
37 Kondziolka D, Fazl M. Functional recovery after decompressive craniectomy for cerebral infarction. Neurosurgery. 1988;23:143.
38 Delashaw JB, Broaddus WC, Kassell NF, et al. Treatment of right hemispheric cerebral infarction by hemicraniectomy. Stroke. 1990;21:874.
39 Kalia KK, Yonas H. An aggressive approach to massive middle cerebral artery infarction. Arch Neurol. 1993;50:1293.
40 Rieke K, Schwab S, Krieger D, et al. Decompressive surgery in space-occupying hemispheric infarction: results of an open, prospective trial. Crit Care Med. 1995;23:1576.
41 Carter BS, Ogilvy CS, Candia GJ, et al. One-year outcome after decompressive surgery for massive nondominant hemispheric infarction. Neurosurgery. 1997;40:1168.
42 Bendszus M, Mullges W, Goldbrunner R, et al. Hemodynamic effects of decompressive craniotomy in MCA infarction: evaluation with perfusion CT. Eur Radiol. 2003;13:1895.
43 Fandino J, Keller E, Barth A, et al. Decompressive craniotomy after middle cerebral artery infarction. Retrospective analysis of patients treated in three centres in Switzerland. Swiss Med Wkly. 2004;134:423.
44 Oro J, Amiridze N, Boyer R. Decompressive craniotomy in medically uncontrollable malignant infarction. Mo Med. 2000;97:17.
45 Doerfler A, Engelhorn T, Heiland S, et al. Perfusion- and diffusion-weighted magnetic resonance imaging for monitoring decompressive craniectomy in animals with experimental hemispheric stroke. J Neurosurg. 2002;96:933.
46 Schwab S, Junger E, Spranger M, et al. Craniectomy: an aggressive treatment approach in severe encephalitis. Neurology. 1997;48:412.
47 Perin A, Nascimben E, Longatti P. Decompressive craniectomy in a case of intractable intracranial hypertension due to pneumococcal meningitis. Acta Neurochir (Wien). 2008;150:837.
48 Keller E, Pangalu A, Fandino J, et al. Decompressive craniectomy in severe cerebral venous and dural sinus thrombosis. Acta Neurochir Suppl. 2005;94:177.
49 Ohya M, Sato O. A case of cerebral venous and superior sagittal sinus thrombosis, treated with decompressive craniectomy (author’s transl). No Shinkei Geka. 1980;8:803.
50 Stefini R, Latronico N, Cornali C, et al. Emergent decompressive craniectomy in patients with fixed dilated pupils due to cerebral venous and dural sinus thrombosis: report of three cases. Neurosurgery. 1999;45:626.
51 Agrawal D, Hussain N. Decompressive craniectomy in cerebral toxoplasmosis. Eur J Clin Microbiol Infect Dis. 2005;24:772.
52 Ausman JI, Rogers C, Sharp HL. Decompressive craniectomy for the encephalopathy of Reye’s syndrome. Surg Neurol. 1976;6:97.
53 Marshall LF, Maas AI, Marshall SB, et al. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J Neurosurg. 1998;89:519.
54 Hukkelhoven CW, Steyerberg EW, Farace E, et al. Regional differences in patient characteristics, case management, and outcomes in traumatic brain injury: experience from the tirilazad trials. J Neurosurg. 2002;97:549.
55 Marmarou A. Increased intracranial pressure in head injury and influence of blood volume. J Neurotrauma. 1992;9(Suppl 1):S327.
56 Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg. 1982;56:498.
57 Narayan RK, Kishore PR, Becker DP, et al. Intracranial pressure: to monitor or not to monitor? A review of our experience with severe head injury. J Neurosurg. 1982;56:650.
58 Eisenberg HM, Frankowski RF, Contant CF, et al. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J Neurosurg. 1988;69:15.
59 Marshall LF, Becker DP, Bowers SA, et al. The National Traumatic Coma Data Bank. Part 1: Design, purpose, goals, and results. J Neurosurg. 1983;59:276.
60 Timofeev I, Kirkpatrick PJ, Corteen E, et al. Decompressive craniectomy in traumatic brain injury: outcome following protocol-driven therapy. Acta Neurochir Suppl. 2006;96:11.
61 Reithmeier T, Speder B, Pakos P, et al. Delayed bilateral craniectomy for treatment of traumatic brain swelling in children: case report and review of the literature. Childs Nerv Syst. 2005;21:249.
62 Stiefel MF, Heuer GG, Smith MJ, et al. Cerebral oxygenation following decompressive hemicraniectomy for the treatment of refractory intracranial hypertension. J Neurosurg. 2004;101:241.
63 Aarabi B, Hesdorffer DC, Ahn ES, et al. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469.
64 Hase U, Reulen HJ, Meinig G, et al. The influence of the decompressive operation on the intracranial pressure and the pressure-volume relation in patients with severe head injuries. Acta Neurochir (Wien). 1978;45:1.
65 Yamaura A, Uemura K, Makino H. Large decompressive craniectomy in management of severe cerebral contusion. A review of 207 cases. Neurol Med Chir (Tokyo). 1979;19:717.
66 Woertgen C, Erban P, Rothoerl RD, et al. Quality of life after decompressive craniectomy in patients suffering from supratentorial brain ischemia. Acta Neurochir (Wien). 2004;146:691.
67 Ucar T, Akyuz M, Kazan S, et al. Role of decompressive surgery in the management of severe head injuries: prognostic factors and patient selection. J Neurotrauma. 2005;22:1311.
68 Bullock MR, Chesnut RM, Clifton GL, et al. Management and prognosis of severe traumatic brain injury. J Neurotrauma. 2000;17:449-597.
69 Valadka AB, Gopinath SP, Robertson CS. Midline shift after severe head injury: pathophysiologic implications. J Trauma. 2000;49:1.
70 Toutant SM, Klauber MR, Marshall LF, et al. Absent or compressed basal cisterns on first CT scan: ominous predictors of outcome in severe head injury. J Neurosurg. 1984;61:691.
71 Jourdan C, Convert J, Mottolese C, et al. [Evaluation of the clinical benefit of decompression hemicraniectomy in intracranial hypertension not controlled by medical treatment.]. Neurochirurgie. 1993;39:304.
72 Csokay A, Nagy L, Pentelenvi T. Vascular tunnel formation to improve the effect of decompressive craniectomy in the treatment of brain swelling caused by trauma and hypoxia. Acta Neurochir (Wien). 2001;143:173.
73 Oncel D, Demetriades D, Gruen P, et al. Brain lobectomy for severe head injuries is not a hopeless procedure. J Trauma. 2007;63:1010.
74 Wrobel CJ, Taubman K. Unusual cause of rhabdomyolysis in a head injury patient: case report. J Trauma. 2001;50:939.
75 Chibbaro S, Tacconi L. Role of decompressive craniectomy in the management of severe head injury with refractory cerebral edema and intractable intracranial pressure. Our experience with 48 cases. Surg Neurol. 2007;68:632.
76 Guerra WK, Gaab MR, Dietz H, et al. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90:187.
77 Akins PT, Guppy KH. Sinking skin flaps, paradoxical herniation, and external brain tamponade: a review of decompressive craniectomy management. Neurocrit Care. 2008;9:269.
78 Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939;110:488.
79 Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006;1:CD003983.
80 Hutchinson PJ, Corteen E, Czosnyka M, et al. Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEICP study. (www.RESCUEicp.com). Acta Neurochir Suppl. 2006;96;:17.
81 Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58:S16.
82 Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58:S25.