Increased intracranial pressure
Intracranial elastance
Historically, the intracranial pressure-volume relationship has been termed compliance in the medical literature. Compliance is defined as unit or units of volume (e.g., intracranial volume) change per unit or units of pressure (e.g., ICP) change (i.e., ΔV/ΔP). However, the pressure-volume curve presented in Figure 132-1 and most other textbooks actually depicts the reciprocal of compliance, or elastance.
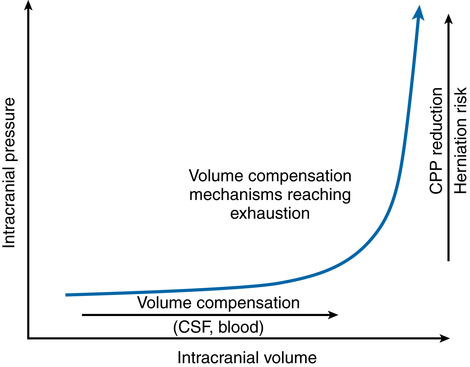
Elastance is defined as ΔP/ΔV. Under normal physiologic conditions, small volume increases in any one of the three intracranial compartments results in little or no change in ICP. The compensatory mechanisms that initially protect against an elevation in ICP are (a) translocation of intracranial CSF through the foramen magnum to the subarachnoid space surrounding the spinal cord, (b) increased CSF absorption through the arachnoid granulations, and (c) translocation of blood out of the intracranial vault. Once these mechanisms are exhausted, abrupt increases in ICP occur in association with small increases in intracranial volume (see Figure 132-1). That is, intracranial compliance is decreased, or more correctly, intracranial elastance is increased.
Anesthetic considerations
The goals of managing a patient with intracranial hypertension include preventing cerebral ischemia and preventing brain herniation (Figure 132-2).
Respiratory
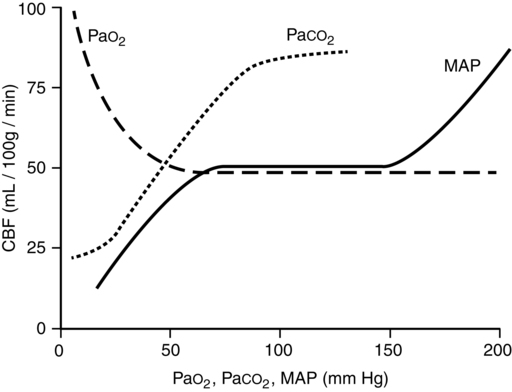
PaCO2 is the single most potent physiologic determinant of CBF (Figure 132-3) and CBV. At a PaCO2 between 20 and 80 mm Hg, CBF decreases 1 mL/100 g brain weight/min and CBV decreases 0.05 mL/100 g brain weight for each 1-mm Hg decrease in PaCO2. Decreasing PaCO2 to 25 to 28 mm Hg should provide near-maximal reductions in ICP, lasting up to 24 h, without adversely affecting acid-base or electrolyte status or decreasing cerebral O2 delivery (i.e., resulting from combined cerebral vasoconstriction and leftward shift in the oxyhemoglobin dissociation curve). Accordingly, in the setting of severe traumatic brain injury, the Brain Trauma Foundation states that aggressive hyperventilation (i.e., PaCO2 ≤ 25 mm Hg) is not recommended, as further reductions in PaCO2 may result in iatrogenic brain injury.