CHAPTER 29 Incisions and Closures
Wound Healing
There are three indistinct, yet overlapping phases of wound healing: the inflammatory, proliferative, and maturation phases. A physical stimulus, such as an incision, initiates a nonspecific inflammatory response in local tissue, which marks the beginning of the inflammatory phase. Factors released from activated platelets, complement components, and prostaglandins induce local changes in the vasculature. The initial transient vasoconstriction establishes hemostasis and is subsequently followed by vasodilation with increased permeability, which further facilitates the influx of leukocytes needed for débridement. Polymorphonuclear leukocytes are the first cells to migrate into the wound, followed by local macrophages and mononuclear leukocytes. Monocytes are essential for normal wound healing by triggering invasion of fibroblasts into the wound and initiating the proliferative or fibroblastic phase of wound healing. Systemic and topical steroids have been demonstrated to retard wound-healing processes, including collagen synthesis, by reducing cell immigration.1 Fibroblasts migrate into the wound at approximately day 4 after injury and deposit disorganized collagen, which produces a scar. Collagen cross-linking prevents degradation of collagen by collagenase and matrix metalloproteinases, thereby increasing wound tensile strength. Vitamin C is a cofactor needed for hydroxylation of lysine residues on collagen, the process by which cross-linking occurs and wounds strengthen. Closed incisional wounds regain about 20% of the original skin tensile strength after 3 weeks and up to 70% at 6 weeks, with the maximal tensile strength of 80% of the original skin strength regained after a year.1 The increase in tensile strength is intimately related to the collagen remodeling that occurs over time. Radiation impairs the ability of fibroblasts to replicate and deposit collagen, which often results in hyperpigmented, ischemic, and sometimes ulcerated wounds vulnerable to infection.1 In contrast, fetuses of certain gestational ages have been shown to heal with complete restoration of original skin tensile strength and epidermal appendages, without scarring. Unique differences in fetal fibroblasts, cellular infiltration, growth factor expression, and other processes have been identified, but little success has been gained in applying these principles to adult patients.1
The protective, moisturizing epidermal layer is restored within 48 hours of closing a wound as marginal basal cells migrate from the wound edge along the extracellular matrix (largely fibrinogen). Epidermal appendages that lie deep within the dermis aid in re-epithelialization of deep wounds that extend beyond the epidermis by contributing basal stem cells capable of division and proliferation. Radiation inhibits the division of basal cells, thereby prolonging re-epithelialization and increasing the risk for bacterial penetration and infection.1 Placing suture to reapproximate wound edges facilitates re-epithelialization of the protective barrier by providing “tracks along which keratinocytes can migrate into the wound.”1 Suture material is also a foreign body, however, and therefore initiates a variable inflammatory response, depending on the suture material involved.
Appropriate suture selection allows the surgeon to have greater control of wound healing and the resultant cosmetic outcome. When selecting suture, the surgeon must take into account the specifics of the wound, in particular, the stress and strain of the wound site, growth potential of the wounded area, the need for permanent mechanical support, and the rate of healing.1 Suture can be categorized as absorbable and nonabsorbable, with further subcategorization depending on suture design. Specific suture characteristics, including tensile strength, rate of infection, and rate of absorption, have been defined to allow surgeons the ability to select the appropriate suture specific for each wound. An open wound after incision is colonized with few bacteria and can therefore be closed primarily with either multifilament or monofilament resorbable suture. Using multifilament suture to close traumatic, heavily contaminated wounds may, however, potentiate a wound infection.
Indications for Antibiotics
The benefit and use of antimicrobials to prevent wound infection have been a controversial subject for years. Infection has been defined quantitatively as greater than 105 bacteria per gram of tissue.1 The time interval between wounding and administration of antibiotics is influential in preventing infection. Three hours from the time of injury to the time of antibiotic administration is critical in preventing infection of contaminated wounds.2 Traumatic wounds benefit from immediate high-pressure irrigation (use of jet lavage or a 35-mL syringe with an 18-gauge needle) and débridement. A 6-hour delay in cleansing and débridement leads to higher rates of infection and is therefore a relative indication for antibiotic administration.2 The anatomic location of the wound has also been shown to have an impact on the incidence of infection, with head and scalp wounds exhibiting the lowest rate of infection and the foot the highest rate. This is probably due to the inherent colonization of bacteria and difference in tissue vascularity that characterizes the foot and the face. Trauma incurred by repeatedly passing the scalpel through tissue has also been shown to limit local defense mechanisms, reduce tissue vascularity, and thereby increase the incidence of infection.2 Studies have demonstrated that stellate lacerations or crush injuries have higher rates of infection than do linear lacerations with less tissue damage, probably secondary to the relative hypovascularity of traumatic wounds. Wounds that have been neglected for more than 6 hours warrant antibiotic administration with or without primary closure in some cases. Wounds in anatomic locations with a higher incidence of infection and wounds contaminated with saliva, feces, or vaginal secretions meet the criteria for the use of antibiotics.2 Infection delays wound healing and often leads to a poor functional and cosmetic outcome.
Preoperative hair removal and its impact on reducing surgical site infection have previously been the subject of much debate. There is little statistically significant evidence supporting hair removal before the day of surgery. However, there is sufficient evidence in the literature and support by the Centers for Disease Control and Prevention in 1999 to advocate clipping hair over shaving or using depilating cream.3
Incision
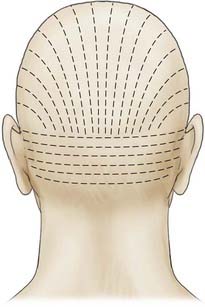
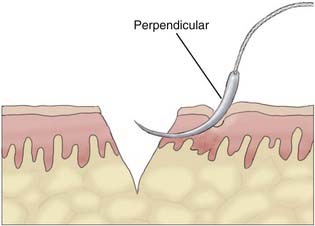
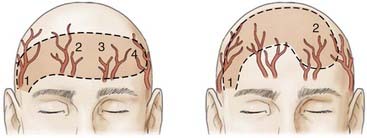
Appropriate technical skill has a significant impact on the functional and cosmetic outcome. Appropriate lighting and equipment, detailed examination of the wound and surrounding tissue, planning of incisions, atraumatic tissue handling, appropriate suture selection, closure technique, and timely suture removal are the basic principles of wound repair that surgeons can manipulate to reduce the possibility of unfavorable healing and scarring. In general, incisions are optimally placed parallel to skin tension lines, referred to as “lines of election” or “Langer’s lines.” These lines lie perpendicular to the direction of underlying muscular contraction. Langer’s lines are directed circumferentially along the occiput and the temporoparietal regions and directed anteroposteriorly on the vertex of the scalp, as displayed in Figure 29-1. Circular scalp defects can be converted to an elliptical shape with the length at least twice as long as the width to avoid skin redundancy or “dog-ears.”4 Elliptical excisions placed along Langer’s lines achieve the greatest width of tissue removal and heal with minimal tension and scarring. Atraumatic tissue handling and appropriate technique also reduce unfavorable healing. Care should be taken when handling the scalpel to make the incision perpendicular to the skin and avoid scything or undermining the adjacent epidermis. The skin edge should be held at the dermal level to avoid damaging the epidermis. The needle should then be inserted perpendicular to the skin edge at an equal depth and distance on each side of the wound, as displayed in Figure 29-2.4 Unequal “bites” create inversion of the wound edges, which results in an inverted scar that casts a shadow and draws more visual attention once healed.4 Unique to the scalp is the ability to conceal the scar within the hairline. The adolescent hairline lies along the superior border of the frontalis muscle, whereas the adult male hairline recedes to about one fingerbreadth superior to the adolescent hairline, or 1.5 cm superior to the upper brow crease.5 The hairline is not a fixed point but rather a transition zone with fine hairs growing anteriorly and coarser hairs growing more posteriorly. The angle of an incision is just as aesthetically important as the location of an incision involving the scalp. Incisions should be placed parallel to hair follicles to avoid transecting the bulb of the follicles and causing cicatricial alopecia.6 The direction of hair growth is typically in the forward direction on the anterior part of the scalp, more radial near the crown, and more inferior along the temples. The direction of hair growth can be used to hide incisions when appropriately placed.5 In general, with scar visibility as the only consideration, the scar line should be oriented perpendicular to the “fall pattern” of the adjacent hair follicles so that the hair covers the scar line.
Surgical Anatomy
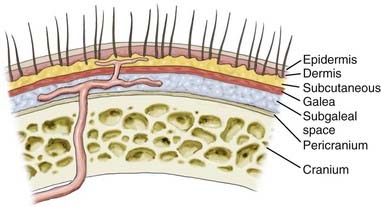
Deciding where to place the incision on the scalp warrants a detailed discussion of the surgical anatomy and blood supply of the scalp. The scalp extends anteroposteriorly from the supraorbital margin to the superior nuchal line and laterally to the zygoma. It is composed of five layers, with the outer three layers—skin, subcutaneous tissue, galea—fixed together as a unit that glides easily over the pericranium, as illustrated in Figure 29-3.
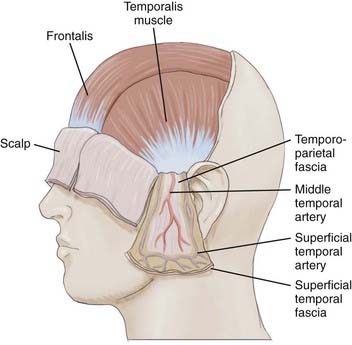
Scalp skin (epidermis and dermis) is the thickest on the body; it ranges from 3 mm at the vertex to 8 mm at the occiput, which is ideal for harvesting split-thickness skin grafts.7 Just deep to the skin is the subcutaneous tissue, where hair follicles, sweat glands, and rich vascular anastomotic networks lie. Dense fibrous septa within the subcutaneous tissue adhere to the adventitia and prevent retraction of arteries when severed. This is important in control of scalp hemorrhage. Manual compression with immediate suturing is a more effective means of controlling bleeding in the scalp than attempting to grasp bleeding points with a hemostat.8,9 Scalp vessels travel within the subcutaneous layer just superficial to the galea. The galea is the aponeurotic layer that connects the frontalis to the occipitalis muscle and is contiguous with the temporoparietal fascia laterally. The superficial temporal fascia is a thin, highly vascular layer that is contiguous with the galea and lies deep to the subcutaneous tissue of the lateral aspect of the scalp.10 A plethora of confusing synonyms have been coined for identification of this layer, including (superficial) temporoparietal fascia, epicranial aponeurosis, superficial muscular aponeurotic system (SMAS), and galeal extension.11 The superficial temporal artery and vein are housed within the fascia, thus rendering this layer vital to the viability of temporoparietal, pericranial flaps.
The superficial temporal fascia is contiguous with the periosteum of the zygoma and inferior to the SMAS of the face. In the temporal region, the galea becomes adherent to the underlying subgaleal fascia, but the subgaleal fascia can easily be detached from the deep temporalis fascia or the layer covering the temporalis muscle, as depicted in Figure 29-4.12 The deep temporal fascia is a thick white fascial layer on the external surface of the temporalis muscle. Similar to the galea or superficial temporal fascia, the deep temporal fascia also has multiple synonyms in the literature: temporal fascia, middle temporal fascia, and superficial deep temporal fascia. Loose areolar tissue, often referred to as subgaleal fascia, allows the galea and overlying layers to glide as a unit over the pericranium. This subaponeurotic space is a potential pathway for the ingress of bacteria intracranially via emissary veins, which can result in meningitis or septic vein thrombosis.7
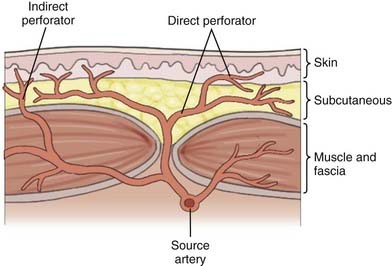
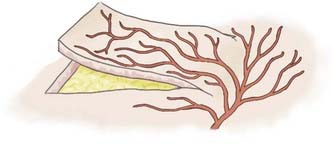
Over the past 50 years, understanding of human anatomy has changed dramatically. Appreciating the interrelationships and three-dimensional nature of regional anatomy replaced the traditional teaching of independent body systems and tissues. The erroneous concept that the skin circulation was derived from a vascular network independent of the deeper structures has been replaced with the concept of angiosomes, or composite blocks of tissue supplied by source arteries.13 Source arteries are described as musculocutaneous, septocutaneous, and fasciocutaneous or “axial” vessels, depending on the path that the vessel takes from the regional artery to the subdermal plexus, as illustrated in Figure 29-5. The anterior aspect of the face is supplied by musculocutaneous perforators, whereas the scalp is supplied by fasciocutaneous perforators.13 The musculocutaneous and fasciocutaneous perforators pierce the deep fascia at fixed skin margins—the supraorbital rim, tragus, mastoid process, masseter, parotid, and superior nuchal line. They then radiate from these fixed regions into areas of mobile skin intimately related to the SMAS and its galeal extension.13 Within these areas of mobile skin, the vessels become superficial and connect to the subdermal plexus. The subdermal plexuses of multiple perforators are connected to one another via small-caliber “choke” vessels. The size, length, direction, and connections of these perforators provide the basis for reliably viable lengths of skin flaps. Clinical studies have demonstrated that an adjacent vascular territory can be included when raising a flap on one to two perforators, but problems arise when attempts are made to include more than two successive angiosome territories. This concept is illustrated by the reliability of the Converse scalp flap, which captures two vascular territories, the superficial temporal and the supraorbital, as opposed to the McGregor flap, which often results in tip necrosis as it attempts to capture four adjacent angiosome territories, as seen in Figure 29-6.9 Conversely, “random-pattern” skin flaps are not dependent on named perforators but rather on the “random” subdermal plexus and can often provide viable local flaps for small defects as long as the flap dimensions remain within a length-to-width ratio of approximately 2 : 1, as seen in Figure 29-7.9 To appropriately plan for adequate exposure and therefore reliable closure, the vascular territories of the scalp and the precise location of their perforation sites must be appreciated.
The blood supply of the scalp is composed of a unique anastomotic network of five paired vessels derived from both the internal and external carotid arteries. These fasciocutaneous perforators—supraorbital, supratrochlear, superficial temporal, posterior auricular, and occipital arteries—originate from the periphery of the scalp and run medially as they branch; they become more tenuous as they reach the midline (particularly after the age of 60).9 Marty and colleagues described the scalp as being divided into three circumferential areas based on the number of anastomoses. The innermost area originated at the vertex, extended 10 cm in diameter, and had the greatest density of anastomoses, with one anastomosis every 5 to 7 mm.14 In fact, the scalp vascular network is so robust that it has been described in numerous case reports to have survived on a single vessel.10 Given the extensive interconnected network that exists between angiosomes, one can appreciate how surgical transection of a single perforating vessel could potentially compromise the viability not only of adjacent but also of distant tissue. A midline incision at the vertex of the scalp incurs the least neurovascular trauma because the vessels begin to merge into the subdermal plexus superficially, and such an incision is therefore preferred when possible.8
Cadaveric studies have detailed four vascular territories of the scalp—anterior, lateral, posterior, and posterolateral.8,9,15 The anterior scalp territory is supplied by the supraorbital and supratrochlear arteries, which are end vessels of the ophthalmic artery, a branch of the internal carotid artery. The supraorbital artery exits the orbit with the supraorbital nerve through the supraorbital notch and then branches and becomes superficial to the galea roughly 1.5 cm superior to the supraorbital rim and 3 cm lateral to the midline. The supratrochlear artery exits the orbit with the supratrochlear nerve and ascends on the forehead, where it pierces through the posterior surface of the frontalis muscle approximately 1 cm superior to the medial palpebral ligament and 1.5 cm lateral to the midline. Terminal branches of both anterior scalp vessels anastomose with each other and with the corresponding paired vessels on the contralateral side.16,17
The posterior territory is supplied by two medial and two lateral occipital arteries, one for each muscle belly of the occipitalis. The occipital artery is derived from the external carotid artery as it branches just opposite the facial artery. It then courses deep to the posterior belly of the digastric and stylohyoid muscles; across the internal carotid artery, jugular vein, and vagus nerve; between the transverse process of the atlas and the mastoid process; and horizontally along the temporal bone to reach the posterior aspect of the cranium. Here, the occipital artery is covered by the sternocleidomastoid, splenius capitis, and longissimus capitis muscles.16–18 It then ascends vertically and pierces through the fascia of the cranial attachment of the trapezius and sternocleidomastoid, about 5 cm lateral to the occipital protuberance, to lie superficially atop the galea. The tortuous path of the occipital artery renders it difficult to use for pericranial flaps despite its long vascular pedicle.16 The greater and lesser occipital nerves arise from the dorsal rami of C2 and lie within the posterior portion of the scalp. The greater occipital nerve exits between the inferior capitis oblique and semispinalis and pierces through the trapezius roughly 1 cm inferior to the nuchal line and 4 cm lateral to the midline. It then ascends parallel and medial to the occipital artery and courses superficially to provide sensation to the posterior portion of the scalp.17 The lesser occipital nerve runs along the posterior border of the sternocleidomastoid until it nears the cranium, where it pierces through to anastomose with the greater occipital nerve in supplying sensation.
The posterolateral territory is supplied by the posterior auricular artery, a derivative of the external carotid. It branches superior to the digastric muscle and courses posteriorly while remaining tightly adherent to the underlying mastoid process of the temporal bone. It can be encountered roughly 2 cm inferior to the auditory canal.16–18
The lateral scalp territory is supplied by the frontal and parietal branches of the superficial temporal artery, an end vessel of the external carotid artery commonly used in pericranial flaps for its length and mobility.16 The superficial temporal artery originates deep to the superficial pole of the parotid and ascends roughly 1 cm anterior to the auditory canal, where it courses superficially over the root of the zygoma and divides into the frontal and parietal branches roughly 5 cm superior to the zygoma.11,19 The ipsilateral temporal artery does not cross the midline to anastomose directly with the contralateral superficial temporal artery; rather, it courses superficially toward the midline and anastomoses with the subdermal plexus at the midline of the scalp.16 A reliable pericranial flap based on the superficial temporal artery can be elevated up to 1 cm proximal to the ipsilateral sagittal suture when care is taken to preserve the subdermal plexus in the subcutaneous tissue.19 A reliable length of the temporoparietal galeal flap based on the superficial temporal artery has been shown to extend up to 10 to 14 cm superior to the zygomatic arch.19 The superficial temporal artery gives rise to the middle temporal artery, the blood supply to the temporalis muscle and deep temporal fascia, at or before the zygomatic arch.8–10 The auriculotemporal nerve is a branch of the mandibular nerve that courses posterior to the superficial temporal artery to provide sensation to the temporal aspect of the scalp.18
The facial nerve lies within a small fat bed between the layers of the deep temporal fascia, posterior to the superficial temporal artery and vein, at the level of the zygomatic arch.15 The facial nerve lies deep to the superficial temporal fascia in the temporoparietal region within the subgaleal tissue. It courses 2.5 cm anterior to the tragus and 1.5 cm lateral to the orbital rim within the deep temporal fascia and innervates the frontalis from its lateral aspect.18 Therefore, when elevating a pericranial flap, care should be taken to preserve the frontal branch of the facial nerve as it runs along the undersurface of the subgaleal fascia on the lateral border of the frontalis muscle.
Cadaveric studies have revealed that the superficial temporal artery does contribute to the blood supply of the periosteum and skull in the frontoparietal region whereas the deep temporal artery supplies the calvaria in the temporal region. The outer table of the posterior cranial vault is supplied by the occipital artery.20,21 The frontoparietal bone survives by its attachment to the middle meningeal artery, a branch of the maxillary artery; however, one study has depicted it perforating between the diploic spaces and the superficial temporal artery.20
Clinical Considerations
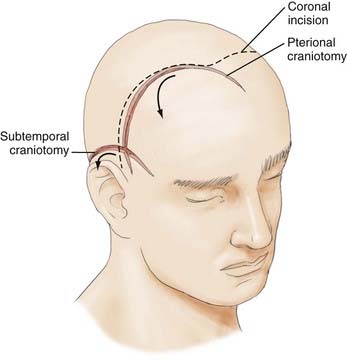
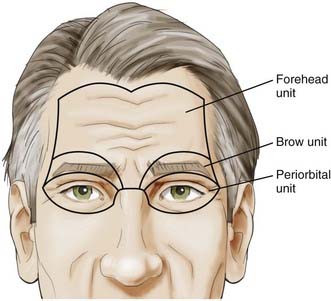
With this understanding of the pertinent regional anatomy, one can now choose the incision that affords the greatest exposure and reliable closure. In general, the most appropriate craniotomy approach for a particular lesion is often the one that provides the shortest traversal through brain tissue. The frontosphenotemporal or pterional craniotomy, commonly used for access to anterior aneurysms and parasellar, sphenoid, and anterior skull base tumors, was traditionally described as involving an incision from the root of the zygoma to the linea temporalis and anteriorly to the center of the forehead, as seen in Figure 29-8.22 The forehead is the most aesthetic part of the calvaria; it is visible in its entirety and forms the medial and lateral walls of the orbit.10 It is described as containing five aesthetic units: two temporal, two brow, and one central component, as illustrated in Figure 29-9. Standard reconstructive principles advocate replacement of an entire aesthetic subunit when 50% or more of the subunit is altered to avoid the “patchwork” appearance that results when only a portion of an aesthetic unit is replaced.10 Use of a coronal incision is oftentimes superior to a pterional incision in providing better exposure and cosmetic result because it preserves all the aesthetic units of the forehead (see Fig. 29-8). The coronal incision is longer, however, and necessitates additional operative time. When elevating the coronal flap, care should be taken to preserve the frontal branch of the facial nerve in the galeal or subgaleal plane (depending on the anatomic location). The skull is exposed either through elevation of periosteum with the flap or by incising the periosteum after a galeal flap is raised. In the temporoparietal region, however, the temporalis muscle overlies the skull. Numerous techniques have been described to decrease potential injury to the temporal branch of the facial nerve when dissecting in this region.23 Elevation of the temporalis muscle with the scalp incision is a reliable means of protecting the facial nerve; however, detachment of the muscle from its superior insertion results in retraction of the muscle inferiorly and an unfavorable cosmetic outcome of temporal wasting. One study proposed leaving a cuff of temporalis muscle superiorly attached to the skull to provide an adequate scaffold for reapproximating the muscle fibers and consequently reducing resultant temporal wasting while protecting the facial nerve.23
A subtemporal craniotomy involves the use of a horseshoe-shaped flap to approach tentorial, clivus, and basilar artery lesions, as depicted in Figure 29-8.22 It is based on the superficial temporal and posterior auricular pedicles, with the former providing the majority of the blood supply. Design and use of this flap imply persistence and flow through these arteries. Incisions in the periphery of the scalp are at risk of transecting vital neurovascular trunks and may result in hypoesthetic, poorly vascularized tissue. Landmarks described earlier should be used to predict where the superficial temporal and posterior auricular arteries, as well as the regional cutaneous nerves, are located to avoid complications.
A midline suboccipital craniotomy imparts an inconspicuous scar and takes advantage of the avascular raphe between the two bellies of the occipitalis muscle to optimize wound healing and closure.22 The region of the posterior scalp territory that lies inferior to the superior nuchal line is not supplied by the centripetal vascular network but rather by direct musculocutaneus perforators from the splenius and trapezius muscles.16
Closure
With understanding of the considerations necessary for planning the most appropriate incision, discussion of the various factors that the surgeon can manipulate to optimize closure is warranted. Management of a simple, atraumatic, noncontaminated wound is best achieved with primary closure, with the goal being to obliterate potential dead space, distribute tension evenly along deep suture lines, and maintain suture tensile strength until tissue tensile strength is adequate.24 Monofilament suture is a single-strand suture with proven resistance to the ingress of bacteria as compared with multifilament suture.25 Monofilament suture also has decreased drag when passed through tissues, which may contribute to a decreased inflammatory response when compared with multifilament suture. Although infection is minimized, monofilament suture exhibits less tensile strength than multifilament suture does.25 For purposes of galea and scalp closure, infection plays a larger role in surgical management and therefore supports the use of monofilament over multifilament suture in most circumstances. Absorbable suture provides temporary wound support, with final resorption being due to enzymatic or hydrolytic degradation of the suture material. Absorption is affected by the local environment, which must be taken into consideration when choosing the type of suture. As the wound heals, however, the absorption and relative loss of suture strength over time should be slower than the gain in tissue tensile strength.25 Nonabsorbable suture provides constant tensile strength. One can select the optimal suture by choosing the smallest suture capable of achieving the desired “tension-free” closure.25
Tension-free closure is highly desirable for wound closure. In general, scalp defects of 2 cm or less can be closed primarily.9 Simple primary closure of the scalp can be performed in two layers with approximation of the galea first, followed by epidermal closure. Deeper tissues bear the majority of the tension and therefore require suture material with appropriate tensile strength and minimal reactivity, such as longer lasting absorbable monofilament suture. An interrupted stitch is the preferred closure technique because it avoids compromising the vessels within the galea supplying the scalp. The detraction of this approach is that it requires longer operating time. Vertical mattress, continuous, and locking sutures are discouraged because they impair the blood supply to the flap. Well-vascularized skin is typically closed with continuous nonabsorbable monofilament suture to provide wound support with only slight tissue reaction. Metallic staples may be used in regions of compromised vascularity to improve the potential for uncomplicated wound healing.
Although some degree of undermining is often performed in most scalp closures, extensive undermining in the subgaleal plane predisposes to scar formation, hematoma, and vascular compromise with potential flap necrosis. Even though quantitative studies using tensiometric measurements have demonstrated no significant correlation between undermining and reduction in surface tension, clinically, the effect is more appreciated.26 Deep-plane fixation is another surgical technique used to help disperse tension-vector forces away from the skin into deeper planes capable of sustaining such forces.6 By placing a series of sutures several centimeters lateral to the wound edge, tension force on superficial tissue is minimized. In theory, deep-plane fixation augments wound healing; however, there is no statistically significant difference in the amount of resection afforded by these means.6,26 Although surgeons continue to design techniques to limit tension in tissues, the most important determinant of optimal scalp closure is skin laxity, an inherent property of skin elastin.
When tension-free closure is not possible with primary closure, one should consider the options depicted in the reconstructive ladder presented in Figure 29-10.4 The reconstructive ladder offers a systematic approach to wound closure with the goal of restoring form and function. Defect size, anatomy, and the quality of the surrounding tissue limit the options for reconstruction. The easiest form of skin replacement is an autogenous skin graft. The advantage of a split-thickness graft is the abundance of donor sites (thigh, buttock, abdomen); however, the disadvantage is that the graft is thin and usually hairless and requires a well-vascularized bed for survival and ingrowth. Full-thickness grafts have the advantage of being thicker, contain hair follicles, and often serve as more homogeneous color matches; however, they also require a well-vascularized bed and produce a more morbid donor site than split-thickness grafts do.
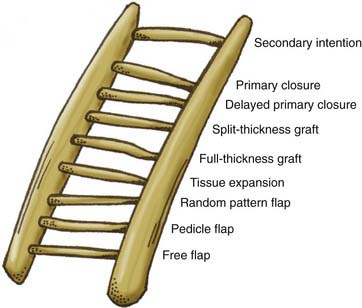
FIGURE 29-10 Reconstructive ladder for a systematic approach to choosing the most appropriate reconstructive option.
Small defects of the scalp can be reconstructed with “random-pattern” advancement flaps based on the subdermal plexus, with care taken to avoid transecting hair follicles, as previously depicted in Figure 29-7.4 Larger defects may necessitate the use of a skin graft or local flaps, such as the bilobed flap used to cover defects less than 25 cm2 or fasciocutaneous flaps such as the Orticochea three- and four-flap technique.4,10 Local techniques require the area surrounding the defect and donor tissue to be healthy, which can be of particular concern in patients with resected cancer, infection, or radiation exposure. Pectoralis major, latissimus dorsi, and trapezius musculocutaneous flaps have been used for reconstruction of the temporal area.4 The ideal free flap contributes well-vascularized tissue with a pedicle of sufficient length to allow the anastomoses to be distanced from the original zone of injury.27 Free flap reconstruction requires advanced surgical skill but offers a viable, single-stage reconstruction of large cranial defects in wounds with vascular compromise, such as those with radiation exposure, previous surgery, or infection.
Abul-Hassan HS, von Drasek Ascher G, Acland RD. Surgical anatomy and blood supply of the fascial layers of the temporal region. Plast Reconstr Surg. 1986;77:17-23.
, Argenta LC, Friedman R, Dingman RO, et al. The versatility of pericranial flaps. Plast Reconstr Surg. 1985;76:695-702.
, Browning J. Surgical pearl: the use of petroleum jelly in performing scalp surgery. J Am Acad Dermatol. 2006;55:515-516.
, Buchfelder M, Ljunggren B. Part 2. The osteoplastic flap. Surg Neurol. 1988;30:428-433.
, Easwer HV, Rajeev A. Cosmetic and radiological outcome following the use of synthetic hydroxyapatite porous-dense bilayer burr-hole buttons. Acta Neurochir (Wien). 2007;149:481-486.
Edlich RF, Kenney JG, Morgan RF, et al. Antimicrobial treatment of minor soft tissue lacerations: a critical review. Emerg Med Clin North Am. 1986;4:561-580.
, Frechet P. Minimal scars for scalp surgery. Dermatol Surg. 2007;33:45-56.
, Goel A. Tenting stitches for the scalp. Br J Neurosurg. 1992;6:357-358.
Horowitz JH, Persing JA, Nichter LS, et al. Galeal-pericranial flaps in head and neck reconstruction. Am J Surg. 1984;148:489-497.
, Horowitz JH, Persing JA, et al. Galeal-pericranial flaps in head and neck reconstruction. Am J Surg. 1984;148:489-497.
, Houseman ND, Taylor GI, Ran WR. The angiosomes of the head and neck: anatomic study and clinical applications. Plast Reconstr Surg. 2000;105:2287-2313.
Inaba Y, Inaba M. Prevention and treatment of linear scar formation in the scalp: basic principles of the mechanism of scar formation. Aesthet Plast Surg. 1995;19:369-370.
, Kahn DS, Pritzker PH. The pathophysiology of bone infection. Clin Orthop Relat Res. 1973;96:12-19.
, Kolt J. Use of adhesive surgical tape with the absorbable continuous subcuticular suture. ANZ J Surg. 2003;73:626-629.
Lorenz PH, Longaker MT. Wound healing: repair biology and wound and scar treatment. In: Mathes SJ, editor. Plastic Surgery. Philadelphia: Elsevier; 2006:209-234.
Marty F, Montandon D, Gumener R, et al. Subcutaneous tissue in the scalp: anatomical, physiological, and clinical study. Ann Plast Surg. 1986;16:368-376.
, Mathes SJ, Hansen SL. Flap classification and applications. In: Mathes SJ, editor. Plastic Surgery. Philadelphia: Elsevier; 2006:365-482.
, Mehrara BJ, McCarthy JG. Repair and grafting of bone. In: Mathes SJ, editor. Plastic Surgery. Philadelphia: Elsevier; 2006:639-718.
, Orchard DC, McColl D. The subgaleal pulley suture. Aust J Dermatol. 1999;40:118-119.
, Rodeheaver G, Edgerton MT. Antimicrobial prophylaxis of contaminated tissues containing suture implants. Am J Surg. 1977;133:609-611.
, Seery G. Scalp surgery: anatomic and biomechanical considerations. Dermatol Surg. 2001;27:827-834.
, Taylor GI, Ives A. Vascular territories. In: Mathes SJ, editor. Plastic Surgery. Philadelphia: Elsevier; 2006:317-364.
Tolhurst DE, Carstens MH, Greco RJ, et al. The surgical anatomy of the scalp. Plast Reconstr Surg. 1991;87:603-612.
Tremolada C, Candiani P, Signorini M, et al. The surgical anatomy of the subcutaneous fascial system of the scalp. Ann Plast Surg. 1994;32:8-14.
Tubbs RS, Salter EG, Wellons JC, et al. Landmarks for the identification of the cutaneous nerves of the occiput and nuchal regions. Clin Anat. 2007;20:235-238.
Wells MD. Scalp reconstruction. In: Mathes SJ, editor. Plastic Surgery. Philadelphia: Elsevier; 2006:607-632.
Whetzel TP, Mathes SJ. Arterial anatomy of the face: an analysis of vascular territories and perforating cutaneous vessels. Plast Reconstr Surg. 1992;89:590-603.
, Winn HR, Jane JA, Rodeheaver G, et al. Influence of subcuticular sutures on scar formation. Am J Surg. 1977;133:257-259.
, Wu T. Plastic surgery made easy: simple techniques for closing skin defects and improving cosmetic results. Aust Fam Physician. 2006;35:492-496.
, Yaremchuk MJ. Acquired cranial bone deformities. In: Mathes SJ, editor. Plastic Surgery. Philadelphia: Elsevier; 2006:547-562.
1 Lorenz PH, Longaker MT. Wound healing: repair biology and wound and scar treatment. In: Mathes SJ, editor. Plastic Surgery. Philadelphia: Elsevier; 2006:209-234.
2 Edlich RF, Kenney JG, Morgan RF, et al. Antimicrobial treatment of minor soft tissue lacerations: a critical review. Emerg Med Clin North Am. 1986;4:561-580.
3 Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection, review. Cochrane Database Syst Rev. 2008;2:1-25.
4 Cowan BJ. Twelve technical strategies to the perfect surgical scar. Skin Ther Lett. 2005:10.
5 Bernstein RM, Rassman WR, Rashid N, et al. The art of repair in surgical hair restoration part I. Basic repair strategies. Dermatol Surg. 2002;28:783-794.
6 Seery G. Improved scalp surgery results by controlling tension vector forces in the tissues by galea to pericranium fixation sutures. Dermatol Surg. 2001;27:569-574.
7 Mueller RV. Facial trauma: soft tissue injuries. In: Mathes SJ, editor. Plastic Surgery. Philadelphia: Elsevier; 2006:1-45.
8 Seery GE. Surgical anatomy of the scalp. Dermatol Surg. 2002;28:581-587.
9 Beran SJ. Scalp reconstruction. Select Readings Plast Surg. 1999;9:23-35.
10 Wells MD. Scalp reconstruction. In: Mathes SJ, editor. Plastic Surgery. Philadelphia: Elsevier; 2006:607-632.
11 Abul-Hassan HS, von Drasek Ascher G, Acland RD. Surgical anatomy and blood supply of the fascial layers of the temporal region. Plast Reconstr Surg. 1986;77:17-23.
12 Tremolada C, Candiani P, Signorini M, et al. The surgical anatomy of the subcutaneous fascial system of the scalp. Ann Plast Surg. 1994;32:8-14.
13 Taylor GI. The angiosomes of the body and their supply to perforator flaps. Clin Plast Surg. 2003;30:331-342.
14 Marty F, Montandon D, Gumener R, et al. Subcutaneous tissue in the scalp: anatomical, physiological, and clinical study. Ann Plast Surg. 1986;16:368-376.
15 Tolhurst DE, Carstens MH, Greco RJ, et al. The surgical anatomy of the scalp. Plast Reconstr Surg. 1991;87:603-612.
16 Whetzel TP, Mathes SJ. Arterial anatomy of the face: an analysis of vascular territories and perforating cutaneous vessels. Plast Reconstr Surg. 1992;89:590-603.
17 Tubbs RS, Salter EG, Wellons JC, et al. Landmarks for the identification of the cutaneous nerves of the occiput and nuchal regions. Clin Anat. 2007;20:235-238.
18 Noemi B, Gunnar B, Sjaastad O. Extracranial nerves in the posterior part of the head. Anatomic variations and their possible clinical significance. Spine. 1998;23:1435-1441.
19 Har-Shai Y, Fukuta K, Collares MV, et al. The vascular anatomy of the galeal flap in the interparietal and midline regions. Plast Reconstr Surg. 1990;89:64-68.
20 Casanova R, Cavalcante D, Grotting JC, et al. Anatomic basis for vascularized outer-table calvarial bone flaps. Plast Reconstr Surg. 1986;78:300-308.
21 Cutting CB, McCarthy JG, Berenstein A. Blood supply of the upper craniofacial skeleton: the search for composite calvarial bone flaps. Plast Reconstr Surg. 1984;74:603-610.
22 Clatterbuck RE, Tamargo RJ. Surgical positioning and exposures for cranial procedures. In: Youmans Neurological Surgery. Philadelphia: WB Saunders; 2006:623-630.
23 Spetzler RF, Lee SK. Reconstruction of the temporalis muscle for the pterional craniotomy. J Neurosurg. 1990;73:636-637.
24 MacGregor IA. Fundamental Techniques of Plastic Surgery. Baltimore: Williams & Wilkins; 1968.
25 Atagi TA, Young VL. Alloplastic materials. In: Mathes SJ, editor. Plastic Surgery. Philadelphia: Elsevier; 2006:745-768.
26 Raposio E, Santi P, Nordström RE. Effects of galeotomies on scalp flaps. Ann Plast Surg. 1998;41:17-21.
27 Wang HT, Erdmann D, Olbrich KC, et al. Free flap reconstruction of the scalp and calvaria of major neurosurgical resections in cancer patients: lessons learned closing large, difficult wounds of the dura and skull. Plast Reconst Surg. 2007;119:865-872.