Incision and Drainage
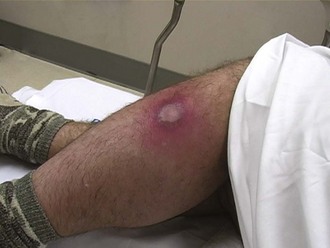
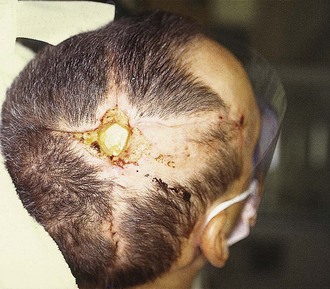
Incision and drainage (I&D) procedures in the emergency department (ED) are most commonly performed for soft tissue abscesses (Fig. 37-1).1,2 The total number of ED visits increased from 90 million to 115 million over a 10-year period, with visits for abscess-related complaints increasing faster than overall ED visits.3
Abscess Etiology and Pathogenesis
An abscess typically starts as a local superficial cellulitis. Various organisms that colonize normal skin can cause necrosis and liquefaction with subsequent accumulation of leukocytes and cellular debris. Loculation and subsequent walling off of these products lead to abscess formation. The cause of localized abscesses depends on the anatomic location, and they are usually caused by the flora indigenous to that area. Different organisms cause disease based on environmental exposure. For example, direct inoculation of extraneous organisms may occur during a mammalian bite (e.g., Eikenella, Pasteurella), exposure to saltwater (e.g., various Vibrio strains) or freshwater (e.g., Aeromonas), or meat or fish exposure (e.g., Erysipelothrix rhusiopathiae). Pseudomonas folliculitis has been associated with the use of whirlpools.4
Staphylococcal strains, which are normally found on the skin, produce rapid necrosis, early suppuration, and localized infections with large amounts of creamy yellow pus—the typical manifestation of an abscess. Conversely, group A β-hemolytic streptococcal infections tend to spread through tissues and cause a more generalized infection characterized by erythema, edema, a serous exudate, and little or no necrosis—typical manifestations of cellulitis. Anaerobic bacteria, which proliferate in the oral and perineal regions, produce necrosis with profuse brownish, malodorous pus5 and may cause both abscesses and cellulitis.
Normal skin is extremely resistant to bacterial invasion, and few organisms are capable of penetrating intact epidermis. In a normal healthy host with intact skin, the topical application of even very high concentrations of pathogenic bacteria does not result in infection. The requirements for infection usually include a high concentration of pathogenic organisms, such as in hair follicles in the adnexa; occlusion of glands or other structures that prevent desquamation and normal drainage; a moist environment; adequate nutrients; and trauma to the corneal layer, which allows organisms to penetrate into deeper tissues.6 Tissue perfusion may also play a role in the ability to prevent infection. Trauma may be the result of abrasions, shaving, insect bites, hematoma, injection of chemical irritants, incision, or occlusive dressings that macerate the skin. The presence of a foreign body can potentiate skin infections by enabling a lower number of bacteria to establish an infection. For example, abscesses occasionally develop at suture sites in otherwise clean wounds. In addition, abscesses can develop at any site used for body piercing. “High” ear piercings (through the cartilage of the pinna) seem to be at particular risk for infection because of the avascularity of auricular cartilage.7
When favorable factors are present, the normal flora that colonize cutaneous areas flourish and infect the skin and deeper structures. In persons performing manual labor, the arms and the hands are infected most frequently. In women, the axilla and submammary regions are frequently infected because of minor trauma from shaving, contact with garments, a moist environment, and an abundance of bacteria in these areas. Infections may develop anywhere on the body in intravenous (IV) drug users, although the upper extremities are most commonly affected.8,9 Deep soft tissue abscesses can be caused by an addict’s attempts to access deep venous structures when peripheral venous access sites are exhausted.10 In addition, areas with compromised blood supply are more prone to infection because normal host defenses, including cell-mediated immunity, are less available.7
Bacteriology of Cutaneous Abscesses
Although most abscesses contain bacteria, 5% of abscesses are sterile, especially those associated with IV drug use. Clinically, sterile abscesses cannot be differentiated from those caused by bacteria. Somewhat atypical abscesses develop in parenteral drug users. Injection of a cocaine-heroin mixture (“speedball”) may predispose users to abscesses by inducing soft tissue ischemia.11 Bergstein and coworkers9 found anaerobes in 143 of 243 isolates from 57 drug-abusing patients. Abscesses at the site of injection tend to contain predominantly staphylococcal and streptococcal species. However, some drug users lubricate their hypodermic needles with saliva, which potentially explains the isolation of oral pathogens such as Eikenella corrodens from injection site abscesses.
In 2002, Brook12 compiled the findings from more than 15 bacteriologic studies of 676 polymicrobial abscesses. S. aureus and group A β-hemolytic streptococci were the most prevalent aerobes in skin and soft tissue abscesses and were isolated in specimens from all body sites. Gastrointestinal and cervical flora (enteric gram-negative bacilli and Bacteroides fragilis) were found most often in intraabdominal, buttock, and leg lesions. Group A β-hemolytic streptococci, pigmented Prevotella, Porphyromonas species, and Fusobacterium species—all normal residents of the oral cavity—were most commonly found in lesions of the mouth, head, neck, and fingers.
In a study of the bacteriology of cutaneous abscesses in children, Brook and Finegold13 found aerobes (staphylococci and group A β-hemolytic streptococci) to be the most common isolates from abscesses of the head, neck, extremities, and trunk, with anaerobes predominating in abscesses of the buttocks and perirectal sites. Mixed aerobic and anaerobic flora was found in the perirectal area, head, fingers, and nail bed. This study noted an unexpectedly high incidence of anaerobes in nonperineal abscesses. Anaerobes were found primarily in areas adjacent to mucosal membranes (e.g., the mouth), where these organisms tend to thrive, and in areas that are easily contaminated (e.g., by sucking fingers, which causes nail bed and finger infections or bite injuries).
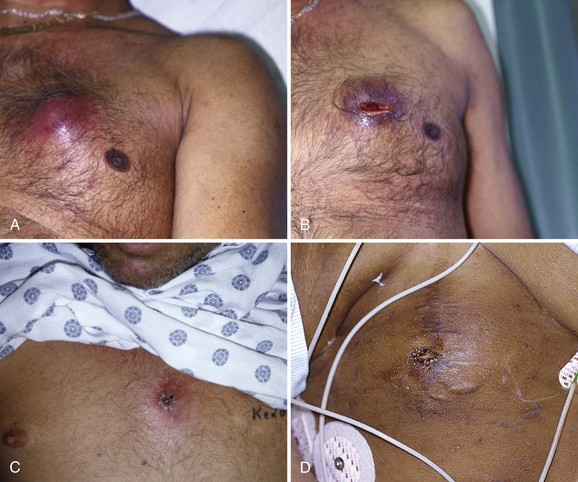
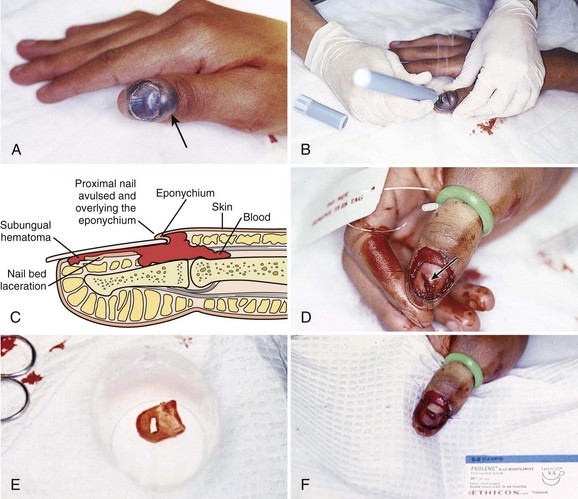
If an unexpected or atypical organism is found in an abscess culture, the clinician should consider an underlying process not readily apparent from the history or physical examination. For example, tuberculosis or fungal isolates are sometimes found in immunocompromised patients (e.g., those with diabetes or acquired immunodeficiency syndrome). Finding Escherichia coli suggests an enteric fistula or even self-inoculation of feces in some patients with a psychiatric illness such as Munchausen’s syndrome. Recurrent abscesses without an obvious underlying cause could indicate clandestine drug use. What appears to be a typical recurrent abscess may be a manifestation of an underlying septic joint, osteomyelitis, or rarely, metastatic or primary cancer (Fig. 37-2).
Special Considerations
Parenteral drug users, insulin-dependent diabetics, hemodialysis patients, cancer patients, transplant recipients, and individuals with acute leukemia have an increased frequency of abscess formation when compared with the general population. At initial evaluation the patient may emphasize an exacerbation of the underlying disease process or an unexplained fever, with symptoms of an abscess being a secondary complaint. In this situation, abscesses tend to have exotic or uncommon bacteriologic or fungal causes and typically respond poorly to therapy.14–17 Patients with diabetes-induced ketoacidosis (DKA) should be evaluated extensively for an infectious process; a rectal examination should be included with the physical examination to rule out a perirectal abscess as the infectious trigger of DKA. This is also true for patients who are immunocompromised. There are several reasons why patients with diabetes and parenteral drug users are at increased risk for abscess formation: intrinsic immune deficiency, an increased incidence of staphylococcal carriage, potentially compromised tissue perfusion, and frequent needle punctures, which allow a mode of entry for pathogenic bacteria.18
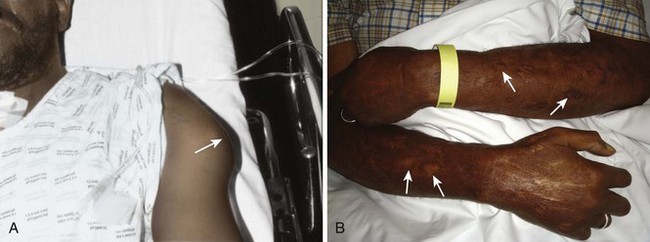
Drug users frequently use veins in the neck and in the femoral areas, which can produce abscesses and other infectious complications at these sites.19 Any abscess near a vein of the antecubital fossa or dorsum of the hand should alert the clinician to possible IV drug use; however, substance users may also inject directly into the skin (“skin popping”), which can cause cutaneous abscesses distant from veins (Fig. 37-3).
A foreign body may serve as a nidus for abscess formation. IV drug users frequently break needles off in skin that has been toughened by multiple injections, so the clinician should maintain a high index of suspicion for retained needle fragments. If an abscess is recurrent or if the patient is a known or suspected IV drug user, consider radiographs or other techniques to search for foreign bodies, an underlying septic joint, or osteomyelitis.20 Ultrasound is also a useful adjunct to evaluate for foreign bodies that are not radiopaque.
MRSA
First acknowledged in the 1960s as a cause of infection in patients in health care settings, MRSA has now become the most common identifiable cause of community-acquired skin and soft tissue infections in many metropolitan areas in the United States. The spread of this organism is considered an epidemic, and it is a very virulent and aggressive.21,22
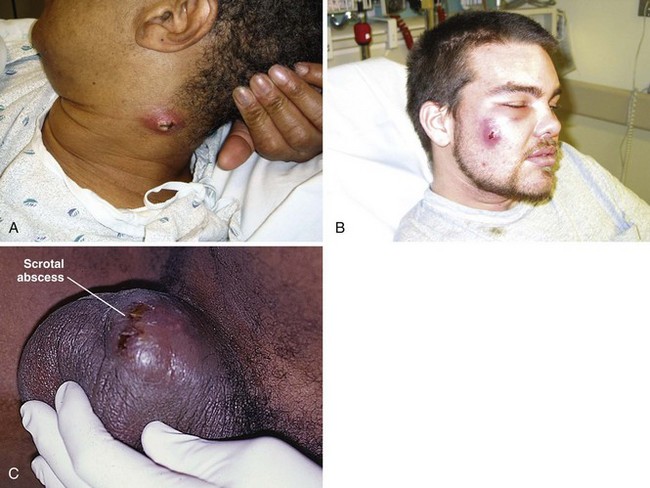
Virulent community-acquired MRSA (CA-MRSA) causes rapid and destructive soft tissue infection because of the presence of two bacterial toxins elaborated by the omnipresent VSA-300 and VSA-400 strains. Panton-Valentine leukocidin enhances tissue necrosis, and phenol-soluble modulin is toxic to neutrophils. A small pustule can become a large abscess in 24 to 48 hours (Fig. 37-4). Such lesions are often mistaken for a spider bite or drug use because of their rapid progression and seemingly spontaneous onset in an otherwise healthy person. Methicillin resistance is mediated by PBP-2a, a penicillin-binding protein encoded by the mecA gene that permits the organism to grow and divide in the presence of methicillin and other β-lactam antibiotics. S. aureus acquires methicillin resistance through a mobile staphylococcal cassette chromosome (SCC) that contains the mecA gene complex (SCCmec). MRSA probably arose as a result of antibiotic selective pressure.23,24
A single clone probably accounted for most MRSA isolates discovered during the 1960s; by 2004, six major MRSA clones had emerged.25 The spread of resistance is thought to be mediated by horizontal transfer of the mecA gene and related regulatory sequences thereon.26
In 1980, the spread of MRSA from hospitals into communities became evident. More recently, community-acquired infections have occurred more frequently, even in people without known risk factors. These observations have led to the identification of some risk factors for CA-MRSA (Box 37-1), including skin trauma (e.g., lacerations, tattoos, IV and intradermal drug use, shaving), incarceration, shared razors or towels, and close contact with others colonized or infected with MRSA.27–35 Animals can also carry MRSA and can function as a source of transmission.36 Importantly, many patients with CA-MRSA have no identifiable risk factors for acquisition of the disease.37
CA-MRSA tends to be more virulent than health care–associated MRSA (HA-MRSA) and is associated with more frequent serious complications such as osteomyelitis, joint infections, sepsis, and death. However, these organisms fortunately tend to be susceptible to a broader array of antibiotics.38
The prevalence of MRSA has increased in both health care and community settings. For example, the prevalence of methicillin resistance among S. aureus isolates in intensive care units in the United States was 60%,21 and more than 90,000 invasive infections by MRSA occurred in the United States in 2005.39
HA-MRSA and CA-MRSA differ with respect to their clinical epidemiology and molecular structures.
HA-MRSA is defined as MRSA infection that occurs following hospitalization (hospital onset, formerly “nosocomial”) or MRSA infection that occurs outside the hospital within 12 months of exposure to a health care setting (e.g., history of surgery, hospitalization, dialysis, or residence in a long-term care facility—community onset instead of community acquired).21
HA-MRSA is usually associated with severe, invasive disease, including skin and soft tissue infection, bloodstream infection, and pneumonia.6,40 In fact, S. aureus continues to be a significant cause of surgical site infections.39 HA-MRSA strains tend to be resistant to multiple drugs. MRSA is one of the few pathogens routinely implicated in nearly every type of hospital-acquired infection. This is probably related in part to the organism’s capacity for biofilm formation on indwelling lines and tubes in hospital settings.24
Biofilm facilitates survival and multiplication of MRSA on these surfaces, thereby prolonging the duration of exposure of the organism to antibiotics, as well as promoting the potential for the development of genetic resistance.27
CA-MRSA is defined as MRSA infection that occurs in the absence of health care exposure. It is often associated with skin and soft tissue infections in young, otherwise healthy individuals.27
Most CA-MRSA strains are sensitive to non–β-lactam antibiotics, although a multidrug-resistant isolate has been described in men who have sex with men.41,42 This strain contains the pUSA03 plasmid and carries resistance genes for β-lactams, fluoroquinolones, tetracycline, macrolides, clindamycin, and mupirocin.
The CA-MRSA and HA-MRSA classifications are no longer distinct since MRSA colonization can develop in one realm and manifestations of infection in another. In the mid-2000s in San Francisco, the annual incidence of CA-MRSA surpassed that of HA-MRSA.43
Furthermore, community-onset HA-MRSA infections have been observed with increasing frequency. This was illustrated in a study of 209 patients discharged from hospitalized care; within 18 months following hospital discharge, 49% of new MRSA infections began outside the hospital.44
In another series of 102 patients with CA-MRSA infections, 29% had molecular typing consistent with HA-MRSA.45
CA-MRSA was initially reported in injection drug users in the early 1980s and has since become the most frequent cause of skin and soft tissue infections seen in U.S. EDs and ambulatory clinics. In an assessment of the prevalence of MRSA across the United States, Moran and colleagues46 compiled data from adults who sought treatment of acute skin and soft tissue infections in EDs in 11 American cities in August 2004. S. aureus was isolated from three fourths of the 422 patients who met the study criteria. Seventy-eight percent of the S. aureus isolates were resistant to methicillin. MRSA was isolated from 59% of patients in the study. The prevalence of MRSA ranged from 15% to 74% in the participating EDs (Table 37-1). MRSA was the most common identifiable cause of skin and soft tissue infections in all but one of the EDs.
TABLE 37-1
Bacterial Isolates from Purulent Skin and Soft Tissue Infections in U.S. EDs*
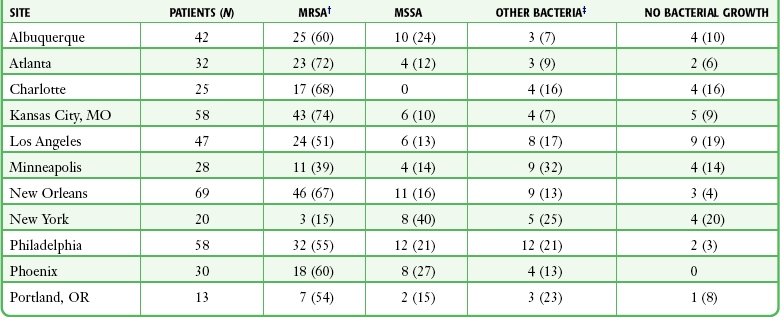
*Thirty-one cultures, including 10 from which MRSA was isolated, were polymicrobial. Because of rounding, percentages may not total 100.
†P < 0.001 for the test for homogeneity of MRSA prevalence across sites.
‡Other bacterial isolates were as follows: MSSA (17%), Streptococcus species (7%), coagulase-negative staphylococci (3%), and Proteus mirabilis (1%).
From Moran GJ, Krishnadasan A, Gorwitz RJ, et al, for the EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
Frazee and associates,38 reporting from an ED in northern California, found that half of the 137 patients in their study were either infected with or colonized by MRSA. Three fourths of all S. aureus isolates were MRSA. In addition, 76% of cases met a strict clinical definition of CA-MRSA.
The incidence of CA-MRSA, genetically unrelated to nosocomial isolates, increased steadily from 1990 to 2001 and then dramatically in 2002 and each year thereafter.47,48
MRSA has also emerged as a potential sexually transmitted disease. Roberts and colleagues described their treatment of two patients who came to their urban ED with abscesses probably transmitted by heterosexual oral-genital contact. Both tested positive for MRSA.22 A 2010 case report drew a similar conclusion. It reported orogenital transmission of MRSA to an immunocompetent 22-year-old man who tested orally positive for MRSA and group B (genital) Streptococcus after oral contact with a female partner in whom MRSA-positive gluteal lesions had previously been diagnosed.48
In a retrospective chart review, Roberts and colleagues found that 18% of the 524 subcutaneous abscesses treated in their urban ED in 2006 were confined to the genital area. Almost three fourths of the 272 outpatient wound cultures performed on that year’s patient population were positive for MRSA.22
Manifestations of Cutaneous Abscesses
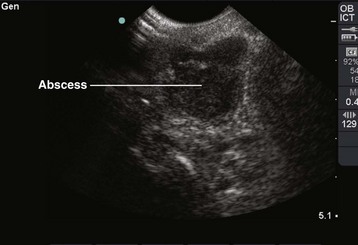
The diagnosis of cutaneous abscess formation is usually straightforward. The presence of a fluctuant mass in an area of induration, erythema, and tenderness is clinical evidence that an abscess exists (see Fig. 37-1). An abscess may appear initially as a definite tender soft tissue mass, but in some cases, a distinct abscess may not be readily evident. If the abscess is deep, as is true of many perirectal, pilonidal, and breast abscesses, the clinician may be misled by the presence of a firm, tender, indurated area without a definite mass. If the findings on physical examination are equivocal, needle aspiration or ultrasound examination may be performed to assist in the diagnosis.49 This approach may also identify a mycotic aneurysm or an inflamed lymph node simulating an abscess. A specific entity commonly mistaken for a discrete abscess is the sublingual cellulitis of Ludwig’s angina (see Chapter 64).
Parenteral injection of illicit drugs can produce simple cutaneous abscesses that unpredictably advance to extensive necrotizing soft tissue infections. The emergency clinician must maintain a high index of suspicion to avoid missing this potentially life-threatening condition.8 Cellulitis and abscess formation can lead to bacteremia and sepsis, especially in immunocompromised patients.
Laboratory Findings
A complete blood count (CBC), blood cultures, and Gram stain are not standard or required for the treatment of straightforward cutaneous abscesses in the ED. Recommendations for culturing abscesses encountered in the ED are confusing, and clinical practice varies. Firm recommendations for the emergency clinician are difficult to standardize, partly because of insufficient data but also because the recommendations promulgated are not confined to ED abscess treatment. In addition, “complicated” and “uncomplicated” criteria are somewhat arbitrary. Traditionally, culturing the contents of a readily drainable cutaneous abscess was not indicated, nor standard. It simply provided no useful information to the clinician under most circumstances. Many clinicians still forgo routine culturing, even in the CA-MRSA milieu. Currently, there is no agreed on standard concerning routine culturing, and reasonable arguments can be made for a culture or no-culture approach to most abscesses treated in the ED. The authors support selective, not routine culturing but acknowledge that some now consider cultures to be indicated for all abscesses drained in the ED. A culture will potentially identify an unusual or resistant organism, especially if I&D is not curative. Culture will also permit identification of antibiotic susceptibility and assist in customization of antibiotic therapy. Cohort results also provide a framework for local epidemiology and resistance patterns. Culturing the abscess contents will distinguish between MRSA and nonresistant abscesses and will provide useful sensitivity information when managing complicated cases. Culturing should be performed for recurrent, unusual, or atypical abscesses. This information could be useful if the patient responds poorly to initial surgical drainage, if secondary spread of the infection occurs, or if bacteremia develops.50,51 It also appears prudent to obtain cultures from abscesses and other purulent skin and soft tissue infections in patients already taking antibiotics, in immunosuppressed patients, in those with signs of systemic illness, in patients who have not responded adequately to initial treatment, if there is concern for a cluster or outbreak of infection, or in patients with severe local infection.51 Severe local infection can be defined as an abscess larger than 5 cm in diameter, multiple lesions, or extensive surrounding cellulitis. However, the degree of surrounding cellulitis qualifying as “extensive” is ill defined.
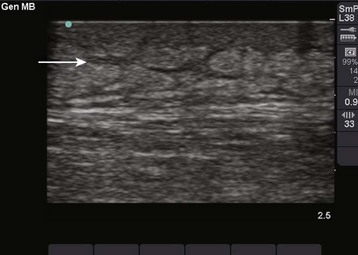
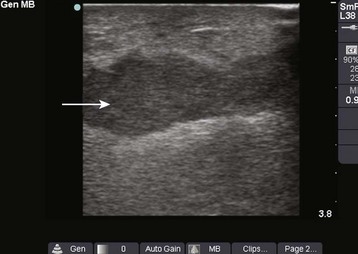
The discovery of solid or suspicious material in an abscess should prompt histologic evaluation since a malignancy may mimic cutaneous abscesses (see Fig. 37-2A and B).
Gram stain is neither indicated nor standard in the care of uncomplicated simple abscesses. However, patients who appear “toxic” or immunocompromised and those who require prophylactic antibiotics (see the section “Prophylactic Antibiotics” later in this chapter) may benefit from Gram stain in addition to cultures. Gram stain results have been shown to correlate well with subsequent culture results, so in compromised hosts the test can be used to direct the choice of antibiotic therapy. Anaerobic infections should be suspected when multiple organisms are noted on Gram stain, when a foul odor is associated with the purulence, when free air is noted on radiographs of the soft tissue, and when no growth is reported on cultures.12
Indications for and Contraindications to I&D
Surgical I&D is the definitive treatment of a soft tissue abscess52; antibiotics alone are often inadequate. Drainage of a suppurative focus generally results in marked resolution of the symptoms in most uncomplicated cases. In the initial stages, only induration and inflammation may be found in an area destined to produce an abscess. Premature incision, before localization of pus, will not be curative and may theoretically be deleterious because extension of the infectious process and, rarely, bacteremia can result from manipulation. In some cases, the application of heat to an area of inflammation may ease the pain, speed resolution of the cellulitis, and facilitate the localization and accumulation of pus. Nonsurgical methods are not a substitute for surgical drainage and should not be continued for more than 24 to 36 hours before the patient is reevaluated.
Prophylactic and Therapeutic Antibiotic Therapy
The duration of therapy for skin and soft tissue infections has not been well defined, although no differences in outcome were observed in adult patients with uncomplicated cellulitis receiving 5 versus 10 days of therapy in a randomized, controlled trial.53 In the Food and Drug Administration licensing trials for complicated skin and soft tissue infections, patients were typically treated for 7 to 14 days. However, in the outpatient setting of uncomplicated infections, 3 to 5 days of antibiotic therapy is reasonable but should be individualized on the basis of the patient’s clinical condition and response to treatment. Accordingly, a return wound inspection or primary care follow-up is an important component of the care plan.
IV drug users with an abscess and fever require parenteral antibiotic therapy after blood has been drawn for culture until bacterial endocarditis can be ruled out.50 Additionally, patients who have extensive cellulitis or are clinically septic require immediate IV antibiotics, as well as aggressive surgical drainage of pus. By administering IV ampicillin/sulbactam (2 g/1 g) every 6 hours, Talan and colleagues51 achieved 100% eradication of pathogens from major abscesses in hospitalized IV drug users and non–drug users.
Therapeutic Antibiotics
In contrast to prophylaxis before surgery, the routine use of therapeutic oral antibiotics after I&D of simple cutaneous abscesses in otherwise healthy patients who are not immunocompromised appears to have no value, and their empirical use cannot be scientifically supported. Llera and Levy52 performed a randomized, double-blind study to compare the outcomes of patients treated with a first-generation cephalosporin after the drainage of cutaneous abscesses in the ED with those who received placebo. They found no significant difference in clinical outcome between the two groups and concluded that antibiotics are unnecessary for abscesses in individuals with normal host defenses. This is in agreement with several previous studies.54–56 It should be noted that high-risk patients were often excluded from these studies. Immunocompromised patients have not been adequately studied in this situation and are therefore often given antibiotics empirically, but this practice, though common, has not been supported by rigorous prospective studies.
Prophylactic Antibiotics
The precise risk for endocarditis after I&D of a cutaneous abscess remains unknown, and it is difficult to predict in which patients an infection will develop and which particular therapeutic procedures subject the patient to the highest risk for infection. However, bacteremia clearly occurs with manipulation of infected tissue, and mortality rates are substantial for MRSA-associated endocarditis (30% to 37%).57,58
Given this risk, it is reasonable that patients at highest risk for cardiac complications related to transient bacteremia be pretreated with appropriate antibiotics within 1 hour preceding the procedure.59–61
Guidelines issued by the American Heart Association (AHA) in 2007 recommend antibiotic prophylaxis for procedures involving the respiratory tract or involving infected skin or musculoskeletal tissue only in patients with cardiac conditions that carry the highest risk for an adverse outcome from infective endocarditis.60 These conditions are listed in Box 37-2. Most skin infections are polymicrobial, but only staphylococci and β-hemolytic streptococci are likely to cause infective endocarditis. Therefore, the therapeutic regimen should include an agent active against these organisms, such as an antistaphylococcal penicillin or a cephalosporin. For patients who cannot tolerate a β-lactam or if MRSA is suspected, vancomycin or clindamycin can be substituted.
Cutaneous abscesses may result from active endocarditis and prophylactic antibiotics may obscure subsequent attempts to identify the causative organism. With this in mind, two or three blood cultures (aerobic and anaerobic) should be considered before antibiotic therapy for those at risk for endocarditis. Patients with a diagnosis of mitral valve prolapse have traditionally been included for treatment with prophylactic antibiotics, but the indication for this is unclear, and antibiotic prophylaxis for uncomplicated mitral valve prolapse is no longer part of the AHA guidelines.62
Kaye63 suggested prophylaxis only for patients who have a holosystolic murmur secondary to mitral valve prolapse.
Prophylaxis for Bacteremia in Other Conditions
Conflicting results have been reported from the few studies investigating the relationship between I&D of cutaneous abscesses and bacteremia. For example, in 1985 Fine and associates64 concluded that I&D of cutaneous abscesses is often accompanied by transient bacteremia. They compared blood culture results from specimens obtained before and at 1, 5, and 20 minutes after I&D procedures in 10 patients with soft tissue infections. None of the cultures of blood obtained before I&D were positive; however, six patients had at least one positive culture after the procedure. Eleven of the 30 postprocedure cultures yielded growth. In contrast, in 1997 Bobrow and coworkers65 concluded that I&D of a localized cutaneous abscess is unlikely to result in transient bacteremia in afebrile adults. Their study included 50 patients with localized cutaneous abscesses. Blood samples were collected before and at 2 and 10 minutes after I&D. In addition, specimens from the wound were collected after drainage. None of the blood cultures were positive, even though 64% of the wound cultures were positive, primarily for S. aureus. Bobrow and coworkers65 noted that prophylactic antibiotics should be given to patients at high risk for bacterial endocarditis. In a discussion of the differences between these findings and those reported by Fine and associates,64 Bobrow and coworkers65 noted that Fine and associates’ cultures were obtained from indwelling, heparinized IV catheters, a practice that allows ample opportunity for contamination. Furthermore, half the patients in Fine’s group had perirectal abscesses, and if these abscesses involved mucosal surfaces, the risk for bacteremia was potentially increased.64
Immunocompromised patients may benefit from the prophylactic administration of antibiotics in preparation for I&D of cutaneous lesions. In contrast to patients with risks for endocarditis, immunocompromised patients are at risk for septicemia secondary to brief bacteremia. IV drug users have a high incidence of diseases associated with human immunodeficiency virus (HIV),61,66,67 and the treating clinician must anticipate various degrees of immunodeficiency among them. Because no specific standard of care exists, clinical judgment must guide the use of antibiotics in these situations.
Recurrent Infections
An approach to individual treatment of recurrent CA-MRSA soft tissue infections is outlined in Box 37-3.
I&d Procedure
Lack of adequate anesthesia is the most common factor limiting I&D in the ED. The current increased use of ED procedural sedation (see Chapter 33) has changed previous OR cases to ones that can be managed well in the ED. If the clinician believes that the abscess cannot be fully incised and drained because of inadequate anesthesia, the patient should be taken to the OR for management under general anesthesia. In addition to limiting proper drainage, it is inhumane and unethical to subject a patient to extreme pain when alternatives are available.
Equipment and Anesthesia
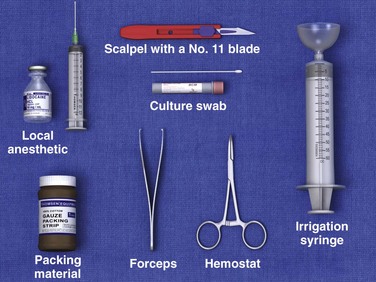
A standard suture tray provides adequate instruments if a scalpel and packing material are added (Fig. 37-5). Although sterility is impossible during the procedure, one should avoid contamination of surrounding tissue. Some clinicians prefer to use an obligatory skin scrub with an antiseptic solution, but the value of this step is dubious. Most clinician use nonsterile gloves while draining pus, but practice varies.
It is often quite difficult to achieve local anesthesia by direct infiltration because of the poor function of local anesthetic agents in the low pH of infected tissue. Furthermore, distention of sensitive structures by a local injection is quite painful and hence poorly tolerated by most patients. Skin anesthesia is usually possible, but total anesthesia of the abscess cavity itself cannot generally be achieved. If a regional block can be performed (see Chapters 30, 31, and 32), this type of anesthesia is preferred. Alternatively, a field block may be used. It should be noted that infected tissue is very vascular and local anesthetics are therefore absorbed quickly. Strict adherence to maximum safe doses of local anesthetics is required.
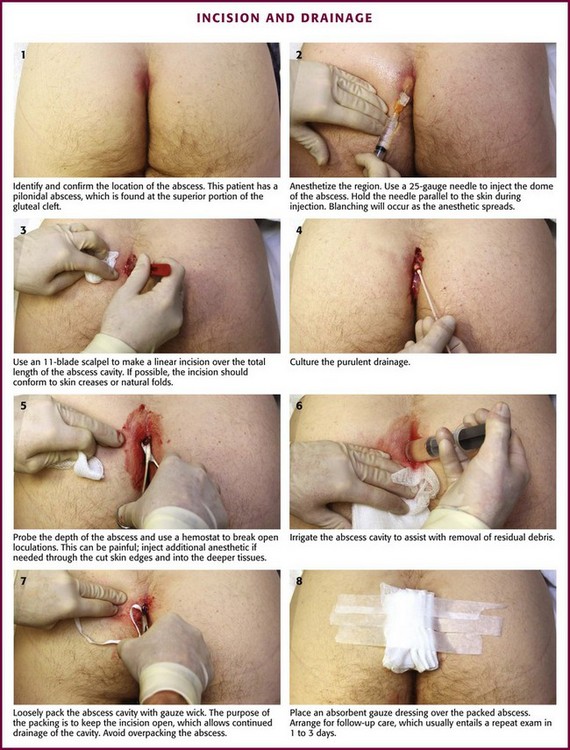
The skin over the dome of an abscess is often quite thin, thus making skin anesthesia difficult. If a 25-gauge needle is used carefully, one can frequently inject the dome of the abscess subcutaneously. Without moving the tip of the needle, the anesthetic solution spreads over the dome through the subcutaneous layers into the surrounding skin and provides excellent skin anesthesia. If the needle is in the proper plane (best accomplished by holding the syringe parallel rather than perpendicular to the skin), the surrounding skin blanches symmetrically during infiltration without having to reposition the needle (Fig. 37-6, step 2). In an extremely anxious or uncomfortable patient, judicious use of preoperative sedation (see Chapter 33) with IV opioids and sedatives or with nitrous oxide makes the procedure easier for both the patient and clinician. Ketamine, propofol, or a combination of these drugs is a popular option in the ED setting.
Incision
One should make all incisions conform with skin creases or natural folds to minimize visible scar formation (Fig. 37-7). Care should be taken in areas such as the groin, the posterior aspect of the knee, the antecubital fossa, and the neck so that vascular and neural structures are not damaged.
A No. 11 or 15 scalpel blade, held perpendicular to the skin, is used to nick the skin over the fluctuant area, and then a simple linear incision is carried the total length of the abscess cavity (see Fig. 37-6, step 3). This will afford more complete drainage and facilitate subsequent breakup of loculations. Attempting to drain an abscess with an inadequate incision is counterproductive and makes packing changes more difficult. A cruciate or X-shaped incision and an elliptical skin excision are to be avoided in the routine treatment of cutaneous abscesses. The tips of the flaps of a cruciate incision may necrose and result in an unsightly scar (Fig. 37-8). A timid “stab” incision may produce pus but is not generally adequate for proper drainage. The scalpel is used only to make the skin incision and is not used deep in the abscess cavity.
Wound Dissection
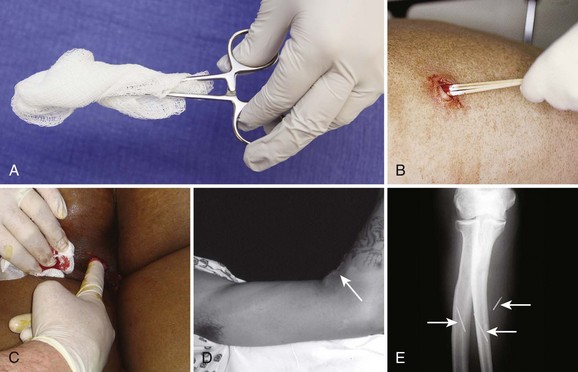
Following a standard incision, the operator should probe the depth of an abscess to assess its extent and ensure proper drainage by breaking open loculations (see Fig. 37-6, step 5). An ideal instrument for this procedure is a hemostat, optionally wrapped in gauze (or a cotton swab for small abscesses), which is placed into the abscess cavity and spread and manipulated throughout the cavity (Fig. 37-9). Traditionally, the operator’s gloved finger has been suggested as an ideal way to assess the depth of the abscess cavity and to break up loculations, but this is a potentially dangerous practice that should be avoided unless it is certain that the abscess contains no sharp foreign body. Of particular concern is an abscess caused by skin-popping of IV drugs. These abscesses occasionally harbor broken needle fragments (see Fig. 37-9D and E). In addition, patients who engage in this practice have a high incidence of hepatitis and HIV infection. Clinicians are often surprised at the depth or extent of abscesses discovered during probing. Sharp curettage of the abscess cavity is not usually required and may produce bacteremia. Although tissue probing is generally the most painful aspect of the technique and total local anesthesia is difficult to attain, this portion of the procedure should not be abbreviated. If pain persists, additional local anesthetic can be administered through the cut skin edges and into deeper tissues to provide additional anesthesia (Fig. 37-10A). If the procedure is limited because of pain, use of appropriate analgesia or anesthesia is mandated. Failure to adequately pack the abscess on the first visit makes follow-up packing changes more problematic.
A blunt-ended suction device can be used to extract copious pus from large or deep abscesses while also assisting in breakup of loculations (see Fig. 37-10B).
Wound Irrigation
Following the breakup of loculations, some clinicians advocate copious irrigation of the abscess cavity with normal saline to ensure adequate removal of debris from the wound cavity (see Fig. 37-6, step 6). Though intuitively beneficial, irrigation of the abscess cavity is not universal and has not been experimentally demonstrated to significantly augment healing or affect outcome. Hyperemic tissue may bleed profusely, but the bleeding usually stops in a few minutes if packing is used. Abscesses of the extremities can be drained with the use of a tourniquet to provide a bloodless field.
Packing and Dressing
When used, a loose packing of gauze or other material is placed gently into the abscess cavity to prevent the wound margins from closing and to afford continued drainage of any exudative material (see Fig. 37-6, step 7). The packing material should make contact with the cavity wall so that on removal, gentle débridement of necrotic tissue will occur spontaneously. A common error is to attempt to pack an abscess too tightly with excessive packing material. In essence, the pack merely keeps the incision open, and its main purpose is not to absorb all drainage—a dressing accomplishes this goal. Care must be exercised to ensure that the packing does not exert significant pressure against the exposed tissue and lead to further tissue necrosis. Some prefer to use plain gauze, some use gauze soaked in saline or povidone-iodine, and some use gauze impregnated with iodine (iodoform). For large abscess cavities, gauze pads (without cotton backing) are ideal packing material (Fig. 37-11). If gauze pads are used, the number of pads placed in the wound should be counted and charted—ideally, the corner of each pad should exit from the wound. The clinician must ensure that all gauze pads will be removed when the packing is changed or discontinued. More commonly, thin (0.6 to 1.2 cm) packing strip gauze, either plain or iodoform, is used. The iodoform gauze may sting the patient for a few minutes after it is inserted. Packing, especially packing strips containing iodine, will be radiopaque on a plain radiograph. If a foreign body is considered, a radiograph should be obtained before packing. The value of antibiotic-impregnated gauze is uncertain.
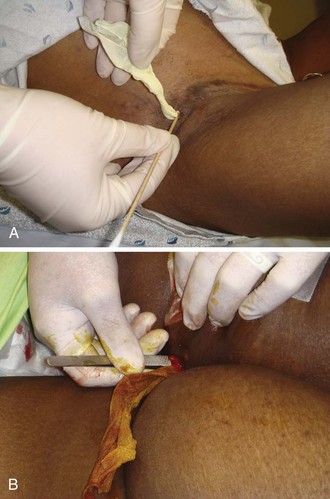
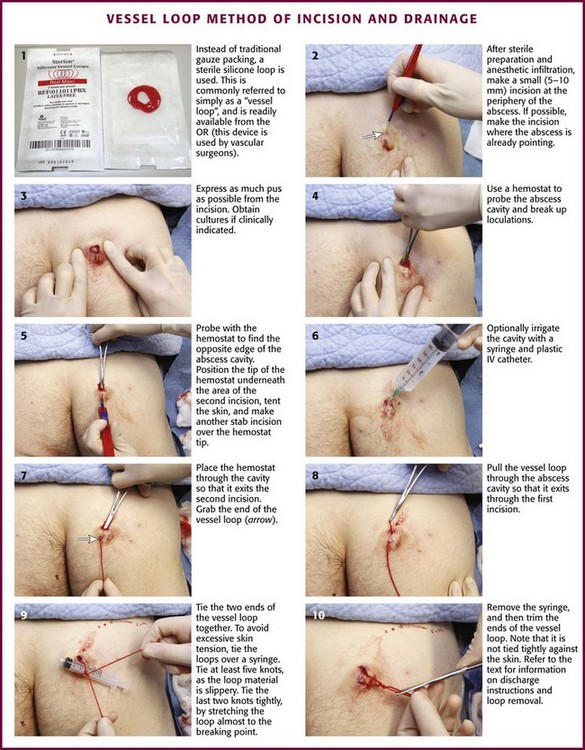
Figure 37-11 Wound packing. A, The traditional packing material is  gauze, plain or with iodoform. B, A 4- × 4-cm gauze pad soaked in povidone-iodine (Betadine) can be used to pack a large abscess, but be careful to avoid losing or forgetting about packing material in the base of a large abscess. Use an instrument to introduce the packing into the bottom of the abscess. Use only enough gauze to keep the incision open, and avoid tight packing. Some clinicians do not use packing at all for easily drained abscesses, and packing is not an absolute standard. See discussion about the vessel loop abscess drainage technique (see Fig. 37-13).
gauze, plain or with iodoform. B, A 4- × 4-cm gauze pad soaked in povidone-iodine (Betadine) can be used to pack a large abscess, but be careful to avoid losing or forgetting about packing material in the base of a large abscess. Use an instrument to introduce the packing into the bottom of the abscess. Use only enough gauze to keep the incision open, and avoid tight packing. Some clinicians do not use packing at all for easily drained abscesses, and packing is not an absolute standard. See discussion about the vessel loop abscess drainage technique (see Fig. 37-13).
An absorbent gauze dressing should be placed over the packed abscess, or if an extremity is involved, a lightly wrapped circumferential dressing should be used (see Fig. 37-6, step 8). Generous amounts of dry gauze are used over the packing to soak up any drainage or blood. The affected part should be splinted if possible, and elevation should be routine. The dressing and splint should not be disturbed until the first follow-up visit. Drainage relieves most of the pain of an abscess, but postoperative analgesics may be required.
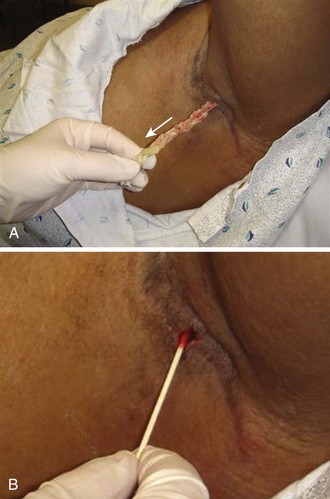
After treatment, packing is often changed periodically (Fig. 37-12A). Most patients require a repeated visit to the clinician for packing change, but if the original packing is to be removed and not replaced (as with a paronychia or hair follicle abscess), selected patients may remove the packing and perform their own wound care totally at home. Motivated patients can provide total follow-up, and a cotton-tipped applicator swirled in the base of the wound for a few days can replace formal packing (see Fig. 37-12B).
Specific Abscess Therapy
Folliculitis, Furuncles, and Carbuncles
Folliculitis, furuncles, and carbuncles can develop in healthy individuals with no predisposing risk factors other than skin, nasal, perineal, or gut colonization with a Staphylococcus bacterium. The umbilicus of neonates is also commonly colonized. Individuals in close contact with others who have active disease in the form of abscesses, furuncles, or carbuncles are at increased risk for similar infections. The pathogenesis of staphylococcal disease is a complex host-bacterium interaction. S. aureus invades the skin by way of the hair follicles or an open wound (abrasions, shaving, or insect bites) and produces local tissue destruction followed by hyperemia of vessels. Subsequently, an exudative reaction occurs, during which polymorphonuclear cells invade. The process then extends along the path of least resistance. The abscess may “point” or form sinus tracts. The process can disseminate by invasion of vessels and thus can infect other distant organs. Most cases of staphylococcal osteomyelitis, meningitis, and endocarditis occur by this mechanism.2,69
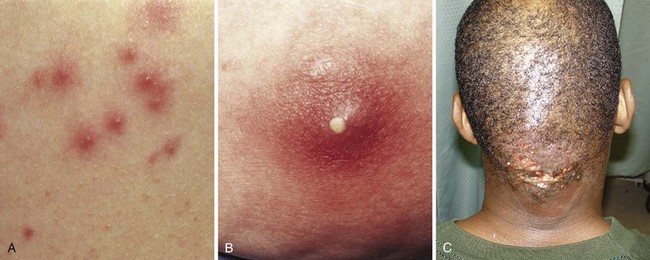
Folliculitis represents inflammation of hair follicles in response to infection, chemical irritation, or skin injury (Fig. 37-14A). An area of purulence often forms after superficial bacterial invasion of the hair follicle and is generally isolated to the epidermis. S. aureus is commonly found, although other bacteria are also responsible for specific follicular infections. Pseudomonas is an important pathogen in cases caused by exposure to hot tubs, pools, or whirlpools that are not adequately chlorinated.4 In persons with broad-spectrum antibiotic use or immunosuppression, consider Candida as well.70 Individuals exposed to whirlpool foot baths at nail salons are at risk for mycobacterial infection. Treatment generally involves local measures, including warm compresses, antibacterial soaps and ointments, and avoidance of the source of contaminated water in the case of hot tub or whirlpool footbath folliculitis. These measures usually suffice, but systemic antibiotics may be required if multiple sites are involved or if the patient is a chronic staphylococcal carrier. Shaving of involved areas should be temporarily avoided.
Furuncles, or boils, are acute circumscribed abscesses of the skin and subcutaneous tissue that usually involve the hair follicle (see Fig. 37-14B). They most commonly occur on the face, neck, buttocks, thigh, perineum, breast, and axilla. Carbuncles are aggregates of interconnected furuncles that frequently occur on the back of the neck (see Fig. 37-14C). In this area the skin is thick, so extension occurs laterally rather than toward the skin surface. S. aureus species, both methicillin susceptible and methicillin resistant, are the usual culprits.40 Carbuncles may become large and can cause systemic symptoms and complications. All patients with a carbuncle should be evaluated for diabetes because this is a common risk factor. Treatment should consist of surgical drainage and administration of systemic antibiotics. Large carbuncles may be difficult to drain adequately in the ED and could require operative management. Carbuncles usually consist of many loculated pockets of pus, so simple I&D is often not curative. Occasionally, wide excision and skin grafting are required.
Most recurrent staphylococcal skin infections are caused by autoinfection from skin lesions or nasal reservoirs. Prevention is directed at eliminating the organism. This is accomplished by the application of bacitracin to the nares, chlorhexidine baths, and good hygiene, including frequent cleansing with antibacterial soap. If these measures are unsuccessful, systemic oral antistaphylococcal treatment is instituted for 2 to 3 weeks. Detection and treatment of infection in family members may be necessary.2,71,72
S. aureus can cause suture abscesses. A suture abscess is often misdiagnosed as a wound infection, but in fact it is a local nidus of inflammation, infection, or both caused and potentiated by suture material. Such an abscess usually appears after sutures have been in place for 3 to 5 days, with single or multiple discrete areas of erythema and tenderness being noted at the site where the suture penetrates the skin. Simply removing the suture (a drop of pus may be expressed) and providing warm compresses and topical antibiotic ointment is generally all that is required. Wide opening of the wound and systemic antibiotics are seldom required. When the suture is buried, a small incision should be followed by probing the wound with a small hook or bent needle (see Chapter 35) to snare the suture for removal.
Hidradenitis Suppurativa
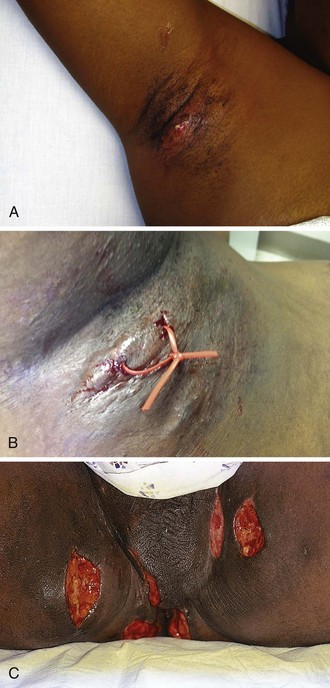
Hidradenitis suppurativa (Greek hidros, or “sweat,” and aden, or “gland”) is a chronic, relapsing, inflammatory disease process that affects the apocrine sweat glands in the axilla, the inguinal region,73 and the perineum (Fig. 37-15). Its prevalence is 0.3% to 4% in industrialized countries; most affected individuals are young women.74 The condition results from follicular inflammation and subsequent occlusion of the apocrine ducts by keratinous debris, which leads to ductal dilation, inflammation, and rupture into the subcutaneous area. Most commonly, the disease is manifested as an episodic appearance of localized pain, edema, pruritus, burning, swelling, and purulence. Dysregulation of the immune response is thought to play a role, with some studies showing a response to immunosuppressive agents.75 Occlusion of the hair follicle predisposes to secondary bacterial infection and subsequent abscess formation. In its early stages it is indistinguishable from a simple furuncle. Progression and recurrence, however, lead to the distinctive appearance of multiple foci coupled with areas of induration and inflammation that are in various stages of healing. This chronic process can create draining fistulous tracts, often involving large areas that are not amenable to simple I&D procedures (see Fig. 37-15C).72
Genetic factors may play some role in hidradenitis suppurativa.76 Fitzsimmons and Guilbert77 proposed a single dominant gene transmission. Some authors have suggested an increased incidence in patients of African descent, although this does not seem to be substantiated by studies.78 A hormonal association has also been postulated. More women than men are affected, and onset after menopause and before puberty is rare.79 Other factors thought to be related include obesity and smoking. A matched-pair case-control study found an odds ratio of 9.4 in smokers versus controls (95% confidence interval, 3.7 to 23.7).80 Environmental factors, including antiperspirants and shaving,81 were implicated in the past, but more recent studies have not found any association.82
Bacterial cultures of specimens from suppurative hidradenitis lesions are frequently sterile or reflect organisms seen in other soft tissue abscesses. Staphylococcus is the most commonly isolated organism,82 with E. coli and β-hemolytic streptococci being other important pathogens. In the perineal region, enteric flora are often found. Many of these abscesses have multiple isolates, and anaerobic bacteria are frequently cultured. CA-MRSA is an increasing cause of such abscesses.
Initial outpatient management of an acute suppurative lesion usually involves ED intervention. Any fluctuant area requires drainage, as described in the section “I&D Procedure.” In patients with extensive cellulitis, a broad-spectrum, antistaphylococcal antibiotic should be used. Unfortunately, hidradenitis suppurativa cannot be cured with localized I&D. If I&D is required for relief of a secondary abscess, it should be made clear to the patient that this procedure does not cure the underlying disease.83 The chronic nature of the disease produces multiple areas of inflammation and subcutaneous fistulous tracts that induce routine recurrences. The patient must be informed of this unfavorable prognosis and should be referred to a dermatologist or surgeon for long-term care.
Milder forms of the disease are initially treated with conservative measures. Many different approaches have been tried, with only limited degrees of success. Few controlled studies of treatment strategies have been performed. Patients are often counseled to lose weight, stop smoking, refrain from shaving, stop using deodorants, and improve personal hygiene. The benefits of these efforts are unknown. Oral antistaphylococcal antibiotics are most commonly used, with varying results.84 Retrospective reviews looking at the efficacy of combination therapy (clindamycin and rifampin) have been promising, with some patients experiencing long-term remission, although diarrhea is a common side effect.85,86 There have been reports of success with topical clindamycin,87,88 hormone therapy in females,89 and laser therapy.90 Finally, immunosuppressive therapy with tumor necrosis factor-α inhibitors (infliximab, adalimumab91,92) has been successful in treating a few intractable cases.
Advanced stages of the disease are managed by wide or local excision. Smaller excisions are more likely to recur than wider excisions.93 Delayed closure94,95 may offer better results, but this has not been directly compared with primary closure. Wide excision may warrant skin grafting.96
Breast Abscess
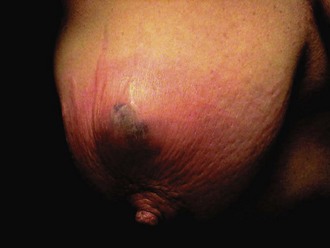
Breast abscesses can occur in lactating (14%) and nonlactating breasts (86%).97 They are caused by normal skin bacteria that enter through a break or crack in the skin, usually the nipple (Fig. 37-16). The infection becomes established in parenchymal tissue and causes pain, swelling, and localized hyperthermia. In its early stages, when cellulitis predominates, a breast abscess can be difficult to diagnose. In equivocal cases, antibiotics may be curative. Cellulitis may progress to frank abscess formation. Women with this condition can be quite ill and appear toxic.
The estimated incidence of inflammatory processes of the breast (mastitis) in lactating women ranges from 2% to 33%.98 In lactating women the infection is usually precipitated by milk stasis after weaning or missed feedings and usually occurs in the first 6 weeks of breastfeeding or during the weaning phase. The cause is usually bacterial invasion through a cracked or abraded nipple by S. aureus, including MRSA, or streptococci originating from the mouth of the nursing child.99–101 Manifestations include redness, heat, pain, fever, and chills. Treatment consists of antistaphylococcal antibiotics, continued breast emptying with a breast pump, and application of heat. It is important to encourage continued breast emptying to promote drainage. Nursing can be continued with the noninfected breast.102
Breast abscesses in nonlactating women are more common. People at risk are smokers and diabetics. These are also risk factors for recurrence. In addition, abscesses may be a complication of nipple piercing or breast implants inserted under appropriate medical care,103 as well as via illicit cosmetic procedures.104,105 In nonpuerperal infections, S. aureus and streptococci are common causative organisms, as are Bacteroides species and anaerobic streptococci.105 Mixed flora are more commonly found in recurrent abscesses (20.5% in those with recurrence versus 8.9% in those with a single episode).106 Recommended antibiotics include amoxicillin and clavulanic acid or, in patients with penicillin allergies, combination therapy with erythromycin and metronidazole. If the community prevalence of MRSA is high, treatment with clindamycin, tetracycline, or TMP-SMX should be considered.107
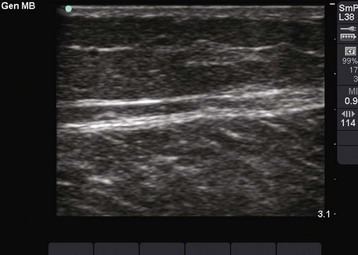
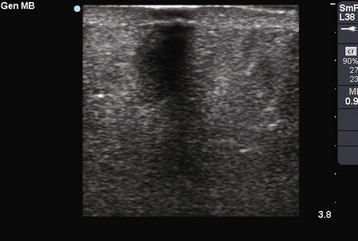
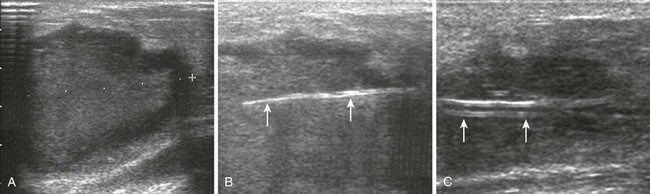
Ultrasound-guided needle aspiration is becoming the standard of care for most breast abscesses. When compared with I&D, aspiration causes less scarring, does not interfere with breastfeeding, and does not require procedural sedation.107 Christensen and associates108 recommended that ultrasound-guided needle drainage replace surgery as first-line treatment of uncomplicated puerperal and nonpuerperal breast abscesses. Emerging treatment parameters are ultrasound-guided needle aspiration for abscesses less than 3 cm in diameter and ultrasound-guided catheter drainage for abscesses 3 cm in diameter or larger.109 Ultrasound images of breast abscesses appear as inhomogeneous, hyperechoic masses (Fig. 37-17). Repeat aspiration every other day to achieve complete resolution, with a mean of 3.5 aspirations being required.109
Recurrent abscesses are a common, troublesome complication after traditional treatment with I&D and antibiotics.110,111 Fortunately, the reported recurrence rate associated with ultrasound-guided aspiration or drainage procedures is quite low.112,113 Patients with persistent recurrences should be managed by a surgeon for total excision of the involved area.
Although a breast abscess is rarely a harbinger of malignancy, it could be the initial manifestation of a metastatic process.112,114 After needle aspiration or catheter drainage, send the aspirate for culture and cytologic examination, and perform a postdrainage mammogram and ultrasound to evaluate for underlying or coexistent malignancy.113
A breast abscess in a male is an unusual occurrence, so consider malignancy and underlying bone or joint infection in these cases (see Fig. 37-2A and B).
Bartholin Gland Abscess
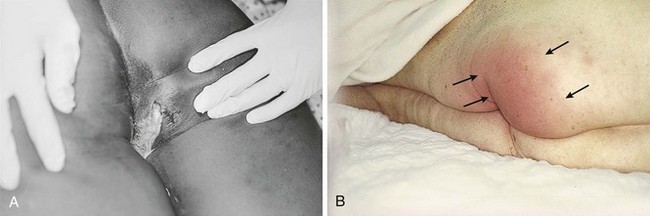
The Bartholin glands (or greater vestibular glands) are located at the 4- and 8-o’clock positions on each side of the vestibule of the vagina (Fig. 37-18A). These mucus-secreting glands maintain the moisture of the vaginal mucosa. When the ostium becomes blocked by inflammation or trauma, a cyst or abscess may form, which occurs in 2% of women.115
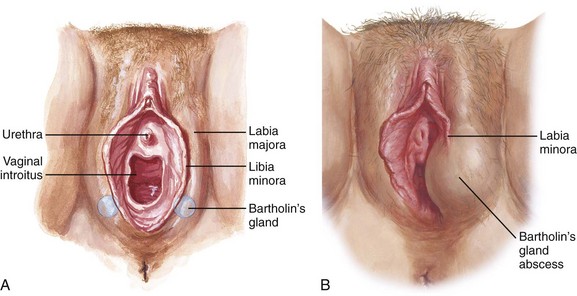
Figure 37-18 Bartholin’s gland and abscess. A, Anatomy of the female external genitalia. Note the location of Bartholin’s glands at 5 and 7 o’clock. B, Bartholin gland duct cysts and abscesses are recognized by the presence of a fluctuant mass of variable size within the posterior vestibule, with the labia minora transecting the cyst. (Netter illustration from www.netterimages.com. © Elsevier Inc. All rights reserved.)
Asymptomatic cysts (no pain, no discharge) frequently occur as a result of duct blockage and retention of secretions. Symptomatic cysts (vulvar pain, dyspareunia, and discomfort while walking or sitting) can be managed with warm sitz baths and compresses. Abscesses involve an acute inflammatory reaction within the stroma of the duct and are usually accompanied by pain, rubor, and occasionally discharge. The majority of cases (80%) are caused by mixed vaginal flora (Bacteroides, E. coli, S. aureus).116 Neisseria gonorrhea and, less commonly, Chlamydia can also be involved. Examination reveals a tender, fluctuant mass in the posterior of the labia that can be palpated between the thumb and index finger (see Fig. 37-18B).
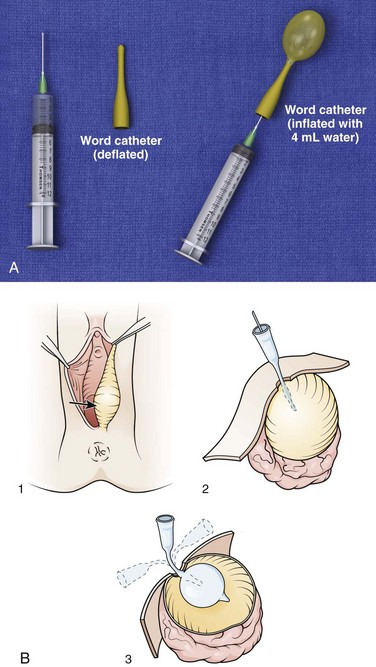
The most common procedure for treating a Bartholin abscess is insertion of a drainage catheter, as described by Word in 1968.117 This single-barreled, sealed-stopper, balloon-tipped catheter serves as initial and long-term therapy (Fig. 37-19A). In his original description, Word reported only 2 recurrences in 72 lesions, both of which were treated successfully with a second catheter. None of the patients required marsupialization. The Word catheter procedure involves fistulization of the duct cavity by a 1-inch catheter with an inflatable balloon tip.118 Though not a traditional I&D procedure, the technique permits continued drainage of the gland.
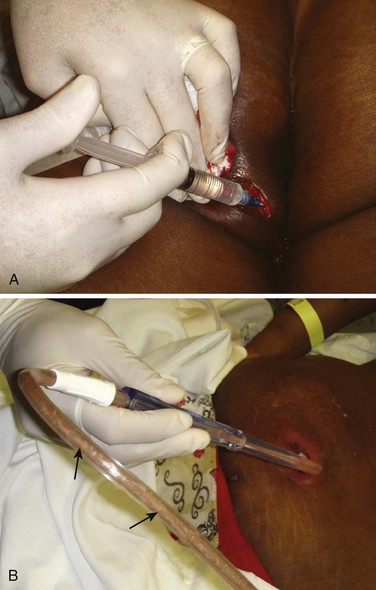
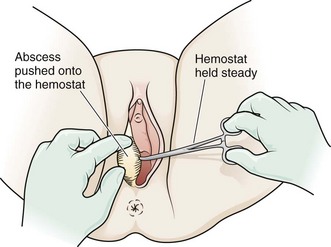
In preparation for insertion of the catheter, place the patient in the standard dorsal lithotomy position and drape the perineum (Fig. 37-20, step 1). Cleanse the area with povidone-iodine solution. If an anesthetic is deemed necessary for patient comfort and cooperation, infiltrate with lidocaine just external to the hymenal ring, where the distal duct opening should be located (at the 5- or 7-o’clock position) (see Fig. 37-20, step 2).119 Test the balloon of the Word catheter by filling it with 3 mL of water via a 25-gauge needle. Aspirate the water back and keep the needle on the catheter to serve as a handle for insertion. Make a small incision in the mucosa, not the cutaneous surface (see Fig. 37-20, step 3). Use a scalpel or a hemostat to puncture the abscess cavity (see Fig. 37-20, step 4). It can be difficult to enter this deep abscess cavity. Failure to obtain frank pus or to appreciate a “pop” when entering the abscess usually prognosticates failure of the procedure. Stabilize the abscess with the thumb and forefinger, hold the hemostat in place, and skewer the abscess onto the hemostat (Fig. 37-21). Pushing the hemostat into the immobilized abscess is technically more difficult. Make a stab incision large enough to accommodate the catheter but small enough to prohibit the inflated balloon from being extruded. Once the abscess has been entered (signaled by a palpable pop or the free flow of pus), place the deflated balloon in the abscess cavity (see Fig. 37-20, step 5). Inflate the balloon with 2 to 4 mL of water (not air). Persistent pain indicates that too much fluid has been used. Most abscesses drain for a few days, and the Word catheter often falls out within a week. Ideally, the device is left in place for 2 to 4 weeks to allow fistula formation, so follow-up is required. If the catheter falls out prematurely, it should be replaced quickly to allow fistulization. Some clinicians do not reinsert the catheter if healing has progressed significantly after the first drainage procedure.
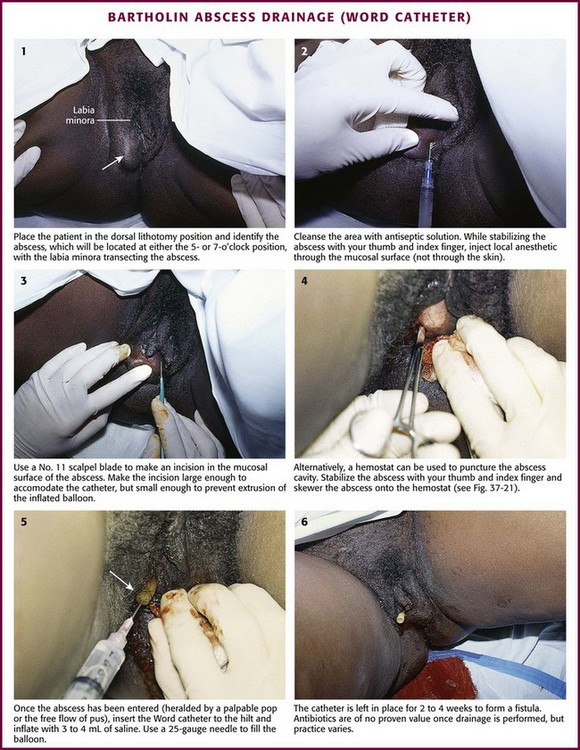
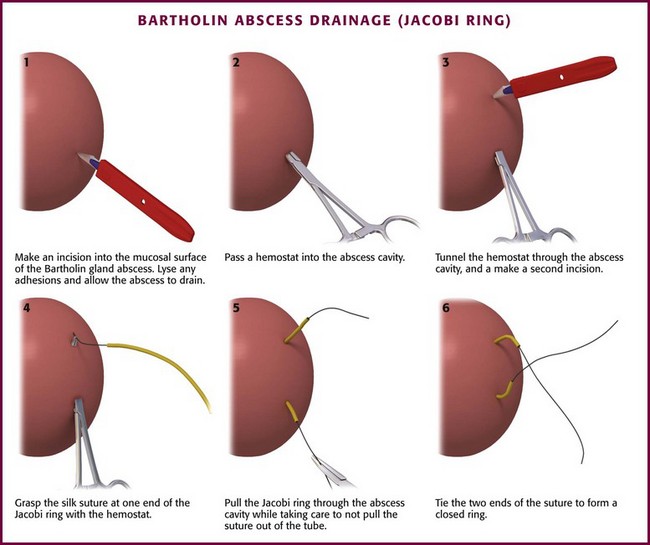
Figure 37-20 Drainage of a Bartholin abscess (Word catheter insertion.) Loop drainage (Fig. 37-13) or the Jacoby ring (Fig. 37-23) are alternative techniques.
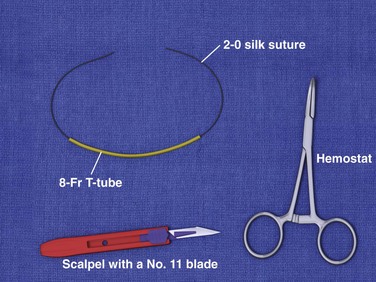
Alternative treatment strategies have also been explored. Gennis and coworkers120 designed a rubber ring catheter called the Jacobi ring from an 8-Fr T-tube threaded with 2-0 silk suture material (Fig. 37-22). The catheter enters and exits the abscess through separate incisions, and a closed ring is formed when the ends of the suture are tied (Fig. 37-23). The device was tested in a randomized study involving 38 women, 25 of whom received a ring catheter and 13 received a Word catheter. The catheters were rated similarly in terms of successful placement, abscess resolution (all resolved within 3 weeks), and recurrence rates (no recurrences at 6 months and two [one in each treatment group] at 1 year). However, the ring catheter outperformed the Word catheter in patient satisfaction.
A loop device similar to the Jacobi ring can be created from butterfly Vacutainer tubing and 2-0 suture. The availability of such equipment in most institutions makes this an attractive option. Jacobi rings or similar loop devices have the advantage of a lower risk for dislodgment than with the Word catheter. Disadvantages include the creation of two drainage tracts in comparison to one. Additionally, loop devices require pelvic rest, whereas the patient may resume intercourse with the Word cathether.121
Standard I&D can give the patient immediate relief, but this procedure is not recommended because the abscess recurrence rate is so high.122 However, if neither a Word catheter nor equipment for a loop device are available, I&D can be performed, with the caveat that the clinician and the patient must appreciate the likelihood of an unfavorable long-term outcome. Make the incision over the medial surface of the introitus (on the mucosa, not on the skin) in a line parallel to the posterior margin of the hymenal ring. The abscess cavity is slightly deeper than most cutaneous abscesses, so the clinician must be certain to enter the actual abscess cavity to achieve complete drainage. This is most easily accomplished by inserting a hemostat through the mucosal incision and spreading the tips of the instrument in the deeper soft tissue. After the contents have drained, pack the abscess for 24 to 48 hours, and start sitz baths thereafter.
CO2 laser vaporization is another technique described whereby laser technology is used to create an opening in the area of the duct’s orifice. The abscess is then evacuated and either excised, vaporized, or left intact.123 Lack of available equipment, however, precludes using this method in the ED. Silver nitrate sticks have also been used to ablate the cyst after I&D. Complications of this method include postoperative vulvar burning, labial edema, and cauterization of the surrounding mucosa.124
Empirical antibiotics are indicated only for patients with a frank abscess and local cellulitis. More than 80% of cultures from Bartholin’s cysts show no growth, and the same is true for a third of cultures from abscesses.125 Cultures that are positive typically show polymicrobial growth, usually anaerobes and especially Bacteroides species and other colonic bacteria. Much less often, N. gonorrhoeae or Chlamydia trachomatis is cultured from the abscess cavity. Chronic low-grade inflammation from a gonococcal infection has been implicated as a causative factor in cyst formation and occasionally in the development of an abscess; therefore, choose an antibiotic that will provide coverage for N. gonorrhoeae. It is reasonable to culture cervical and anal specimens for gonorrhea and chlamydia from women with a Bartholin gland abscess because of the association of these infections with sexually transmitted disease.126 However, empirical treatment of gonorrhea or chlamydia is not necessary unless the clinician has a high suspicion that a sexually transmitted organism is the infectious cause. At the current time, CA-MRSA infections of the Bartholin glands are uncommon.
Particular care must be taken when treating a pregnant woman with a Bartholin gland abscess because she is at high risk for complications. Sepsis in a pregnant woman following marsupialization of an abscess has been reported.127 Postmenopausal women with what appears to be a Bartholin gland abscess should be referred to a gynecologist to rule out malignancy. A vulvar abscess in an HIV-positive woman should raise suspicion for Kaposi’s sarcoma.128
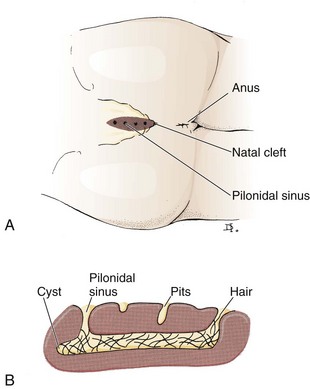
Pilonidal Abscess
Pilonidal sinuses are common malformations that occur in the sacrococcygeal area (Fig. 37-24). The cause of the sinus formation is unclear, and the malformation may occur during embryogenesis. Pilonidal cyst formation is thought to be secondary to blockage of a pilonidal sinus. The result of this obstruction is repeated soft tissue infection, followed by drainage and partial resolution, with eventual reaccumulation. The blockage is most commonly the result of hair in the region, and the lesion may in part be a foreign body (hair) granuloma. Although pilonidal sinuses are present from birth, they are not usually manifested clinically until adolescence or the early adult years. Pilonidal abscess formation most frequently affects young (often white) adult males. The sinuses and cysts are lined with stratified squamous epithelium and, after excision, may be found to contain wads of hair and debris. When cultured, pilonidal abscesses generally yield mixed fecal flora with a preponderance of anaerobes.129 Poor hygiene and repeated trauma (called “jeep bottom” in World War II) may precipitate acute infection. It is postulated that as a patient sits, microtrauma in the intergluteal cleft acts as a suction that draws hair into the superior gluteal folds. Subsequently, hairs become ingrown, bacteria invade, and an abscess developsin the sacrococcygeal region. At the current time, CA-MRSA infections are not common.
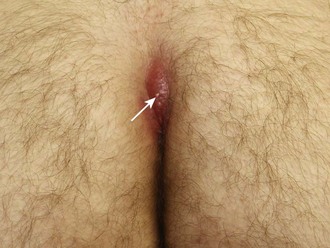
Patients with a pilonidal abscess will seek care for back pain and local tenderness. On physical examination the area is found to be indurated. Frank abscess formation may not be appreciated in this deep abscess. One will usually see barely perceptible dimples or tiny openings at the rostral end of the gluteal crease (Fig. 37-25). A hair or slight discharge may be noticed at the opening. One may find a more caudal cyst or abscess, possibly with a palpable sinus tract connecting the two. The sinus and cyst may be draining chronically, or they may become infected as the size increases and blockage occurs.130
Treatment of an acutely infected cyst is the same as previously discussed for any fluctuant abscess: all hair and pus should be removed, and the lesion should be packed (see Figs. 37-6 and 37-13). The abscess cavity may be quite large and thus necessitate a lengthy incision to ensure complete drainage. There is some evidence to support lateral, off-midline incisions. A retrospective study looked at 48 patients who had undergone lateral incisions and matched them with 48 patients who had undergone midline incisions. Those who had undergone midline incisions took approximately 3 weeks longer to heal than did those drained with a lateral incision.131 In general, reported median healing times after I&D vary between 12 and 63 days.132,133 The area may be repacked at 2- to 4-day intervals as an outpatient procedure, although some prefer to discontinue packing after the first week. Antibiotic therapy is not generally required.
Simple I&D is not usually curative, with recurrence rates of 5% to 13% reported134,135; therefore, the patient should be referred to a surgeon for removal of the cyst and sinus after the inflammatory process has resolved. Small abscesses may be incised and drained as an outpatient procedure performed under local anesthesia, but the disease process is often extensive, and general anesthesia may be required to achieve complete drainage. The extent of the cyst cavity and the volume of pus encountered on initial incision can be surprising.
Closure strategies for these potentially large wounds vary. Gencosmanoglu and Inceoglu133 concluded that a modified lay-open technique is superior to excision with primary closure for chronic pilonidal sinuses with regard to morbidity and recurrence rates. Other closure options after extensive resection of complex or recurrent sinus tracts are Z-plasty, an advancement flap, or a rotational flap. When primary closure is chosen, administration of a broad-spectrum regimen of antibiotics (e.g., preoperative infusion of cefuroxime and metronidazole followed by 5 days of oral co-amoxiclav136) offers protection against wound infections.
Perirectal Abscesses
Most anorectal infections originate in the cryptoglandular area located in the anal canal at the level of the dentate line. Abscesses within these glands can penetrate the surrounding sphincter and track in a variety of directions, with larger abscesses occurring within the perianal, intersphincteric, ischiorectal, and supralevator spaces. A small number of anorectal abscesses have a noncryptoglandular cause such as Crohn’s disease, atypical infection (e.g., tuberculosis, lymphogranuloma venereum), malignancy, or trauma.137
Perirectal infections can range from minor irritation to fatal illness. Surprisingly, clandestine infections often occur in diabetics. Successful management depends on early recognition of the disease process and adequate surgical therapy. Small abscesses can initially be managed on an outpatient basis with simple I&D, described previously. Because of the morbidity and mortality associated with inadequate treatment of these conditions, patients with large and deep abscesses should be promptly admitted to the hospital for evaluation and treatment under general or spinal anesthesia (Fig. 37-26).
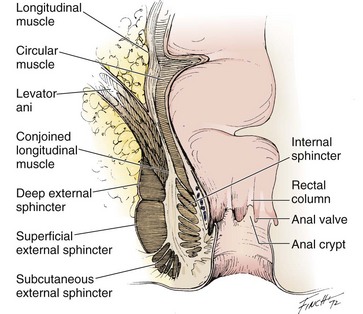
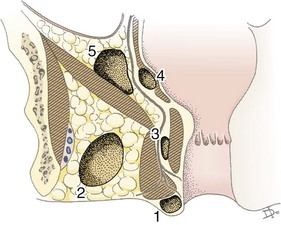
It is important to understand the anatomy of the anal canal and the rectum to appreciate the pathophysiology of these abscesses and their treatment (Fig. 37-27). The mucosa of the anal canal is loosely attached to the muscle wall. At the dentate line, where columnar epithelium gives way to squamous epithelium, there are vertical folds of tissue called the rectal columns of Morgagni that are connected at their lower ends by small semilunar folds called anal valves. Under these valves are invaginations termed anal crypts. Within these crypts are collections of ducts from the anal glands. These glands are believed to be responsible for the genesis of most, if not all perirectal abscesses. These glands often pass through the internal sphincter but do not penetrate the external sphincter.
The muscular anatomy divides the perirectal area into compartments that may house an abscess, depending on the direction of spread of the foci of the infection (Fig. 37-28).136,138
Pathophysiology
As described previously, the anal glands are mucus-secreting structures that terminate in the area between the internal and external sphincters. It is believed that most perirectal infections begin in the intersphincteric space secondary to blockage and subsequent infection of the anal glands. Normal host defense mechanisms are overwhelmed, and this results in invasion and overgrowth by bowel flora.139
If the infection spreads across the external sphincter laterally, an ischiorectal abscess is formed. If the infection dissects rostrally, it may continue between the internal and external sphincters and give rise to a high intramuscular abscess. The infection may also dissect through the external sphincter over the levator ani to form a pelvirectal abscess.140
When infection of an anal crypt extends by way of the perianal lymphatics and continues between the mucous membrane and the anal muscles, a perianal abscess forms at the anal orifice. A perianal abscess is the most common variety of perirectal infection. A perianal abscess lies immediately beneath the skin in the perianal region at the lowermost part of the anal canal. It is separated from the ischiorectal space by a fascial septum that extends from the external sphincter and is continuous with the subcutaneous tissues of the buttocks. The infection may be small and localized or it may be very large, with a wall of necrotic tissue and a surrounding zone of cellulitis.2 Perianal abscesses may be associated with a fistula in ano. A fistula in ano is an inflammatory tract with an external opening in the skin of the perianal area and an internal opening in the mucosa of the anal canal. A fistula in ano is usually formed after partial resolution of a perianal abscess, and its presence is suggested by recurrence of these abscesses with intermittent drainage. The external opening of the fissure is usually a red elevated piece of granulation tissue that may exhibit purulent or serosanguineous drainage on compression. In many cases the tract can be palpated as a cord. Patients with anal fistulas should be referred for definitive surgical excision.140
Ischiorectal abscesses are fairly common. They are bounded superiorly by the levator ani, inferiorly by the fascia over the perianal space, medially by the anal sphincter muscles, and laterally by the obturator internus muscle. These abscesses may commonly be bilateral, and if so, the two cavities communicate by way of a deep postanal space to form a “horseshoe” abscess.2
Causes of perirectal abscesses other than the cryptoglandular process have been documented but are fairly rare. It is believed that hemorrhoids, anorectal surgery, episiotomy, or local trauma may cause abscess formation by altering the local anatomy and thus destroying natural tissue barriers to infection. 141,142 Perirectal abscesses may serve as a portal of entry for organisms responsible for necrotic soft tissue infections such as Fournier’s gangrene.143
Physical and Laboratory Findings
A perianal abscess is not generally difficult to diagnose. The throbbing pain in the perianal region is acute and aggravated by sitting, coughing, sneezing, and straining. There is swelling, induration, tenderness, and a small area of cellulitis in proximity to the anus. Rectal examination of a patient with a perianal abscess reveals that most of the tenderness and induration are located below the level of the anal ring. Deeper abscesses may be difficult to localize and identify. Computed tomography is often the first imaging study given its ready availability. The sensitivity of computed tomography for anorectal abscesses, however, is only 77%.144 Therefore, when clinical suspicion is high, magnetic resonance imaging should be considered.
Patients with intersphincteric abscesses usually have dull, aching pain in the rectum rather than in the perianal region. No external aberrations of the perianal tissues are noted, but tenderness may be present. On digital examination one frequently palpates a soft, tender, sausage-shaped mass above the anorectal ring; if the mass has already ruptured, the patient may give a history of passage of purulent material during defecation.142,145
Laboratory findings do not always aid in the diagnosis. Kovalcik and colleagues139 found that less than 50% of their patients had a white blood cell count greater than 10.0 × 109/L. Cultures of perirectal abscesses usually show mixed infections involving anaerobic bacteria, most commonly B. fragilis and gram-negative enteric bacilli. MRSA, though less common, should also be considered in endemic areas.140
Treatment
Successful management of perirectal abscesses depends on adequate surgical drainage. Complications from these infections may necessitate multiple surgical procedures, prolong hospital stays, and result in sepsis and death. Bevans and associates141 retrospectively studied the charts of 184 patients who were treated surgically over a 10-year period. These patients were evaluated primarily to identify factors that contributed to morbidity and mortality. Initial drainage was performed under local anesthesia in 38% of the patients and under spinal or general anesthesia in 62%. The authors identified three key factors in those with excessive morbidity and mortality: (1) delay in diagnosis and treatment, (2) inadequate initial examination or treatment, and (3) associated systemic disease. They believed that the only way to effectively examine and adequately drain all but superficial well-localized perirectal abscesses was under spinal or general anesthesia. This assessment was supported by evidence of an increased incidence of recurrence, sepsis, and death in patients treated under local anesthesia. Drainage of deep abscesses under local anesthesia generally does not allow drainage of all hidden loculations. In addition, local anesthesia is not adequate for the treatment of associated pathologic conditions.
Use of de Pezzer catheters in anorectal abscesses has been described as an alternative to traditional incision and packing. In a series of 91 patients treated in this manner, Kyle and Isbister142 found equivalent rates of subsequent fistula surgery, less need for general anesthesia, and a shorter postoperative hospital stay than in patients treated with traditional incision and packing. Beck and coworkers146 reported successful use of catheter drainage in 55 patients with an ischiorectal abscess. Because of the complexity of ischiorectal abscesses, this technique is probably best left to the surgeon who is providing ongoing care.
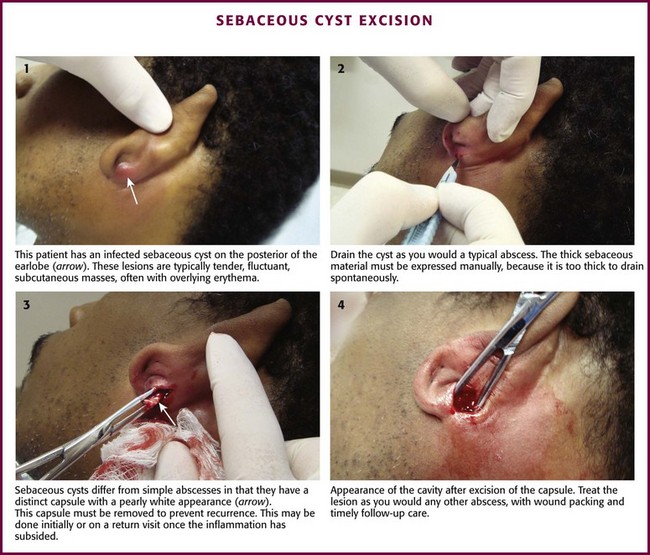
Infected Sebaceous Cyst
The initial treatment of an infected sebaceous cyst is simple I&D (Fig. 37-29). The loop drainage technique may not be suited for this abscess. The thick sebaceous material must be expressed because it is too thick to drain spontaneously. An important difference exists between infected sebaceous cysts and other abscesses. A sebaceous cyst has a definite pearly white capsule that must be excised to prevent recurrence. Traditionally, in the presence of significant inflammation it is preferable to drain the infection initially and remove the shiny capsule on the first follow-up visit or at a later visit, when it can be more easily identified. Alternatively, the entire cyst can be removed during the initial incision. At the time of capsule removal, the edges are grasped with clamps or hemostats, and the entire capsule is removed by sharp dissection with a scalpel or scissors. After excision of the capsule, the area is treated in the same manner as a healing abscess cavity. Simple drainage without excision of the capsule often leads to recurrence.
Kitamura and colleagues147 reported a randomized study of 71 patients treated by either traditional I&D or primary resection of the cyst, followed by irrigation and wound closure. In this study the patients treated by primary resection had faster healing, fewer days of pain, and less scarring.
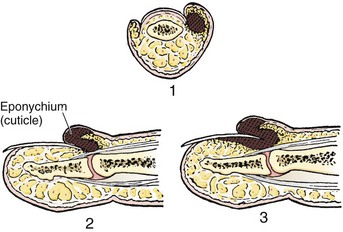
Paronychia
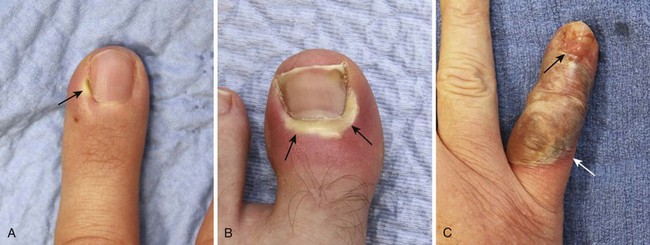
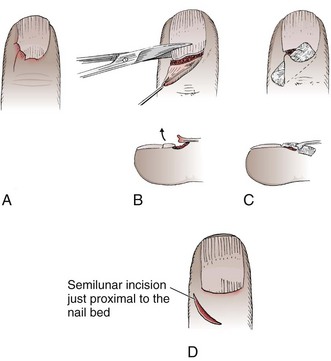
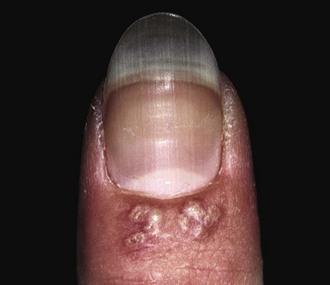
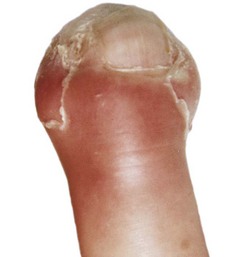
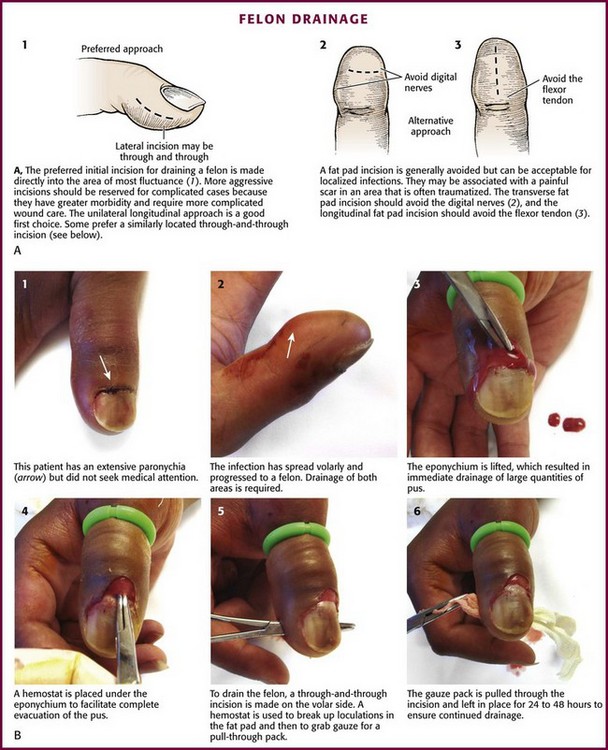
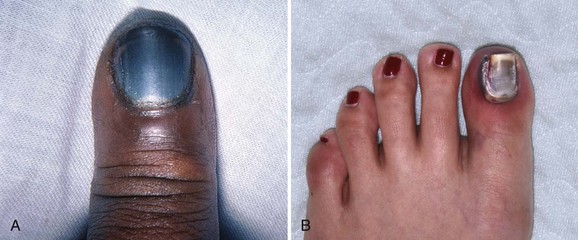
Paronychia is an infection localized to the area around the nail root (Fig. 37-30). It is a common infection probably caused by frequent trauma to the delicate skin around the fingernail and the cuticle. These type of infections have also been linked to “insults” induced by nail cosmetics (mechanical trauma, irritant reactions, and allergic reactions)148 and to a number of occupations (e.g., hair cutting and meat handling).149 When a minor infection begins, the nail itself may act like a foreign body. Usually, the infectious process is limited to the area above the nail base and underneath the eponychium (cuticle), but occasionally, it may spread to include tissue under the nail as well and form a subungual abscess. Lymphadenitis and lymphadenopathy are not usually seen. Generally, a paronychia is a mixed bacterial infection. Staphylococcus is commonly cultured from these lesions; however, anaerobes and numerous gram-negative organisms may be isolated.150 Paronychia in children is often caused by anaerobes, and it is believed that this is the result of finger sucking and nail biting. Occasionally, a group A β-hemolytic streptococcal infection will develop in a paronychia in a child with streptococcal pharyngitis who engages in thumb sucking.151,152 Paronychias involving CA-MRSA have been reported.153,154
A paronychia appears as a swelling and tenderness of the soft tissue along the base or the side of a fingernail (Fig. 37-31). Pain, often around a hangnail, usually prompts a visit to the ED. The infection begins as a cellulitis and may form a frank abscess. If the nail bed is mobile, the infectious process has extended under the nail, and a more extensive drainage procedure should be performed. Although no comparative trials of surgical management versus oral antibiotics have been performed,155 some general guidelines for the management of paronychias have become established in clinical practice. If soft tissue swelling is present without fluctuance, remission may be achieved with frequent hot soaks (six to eight times a day) and a short course of oral antibiotics (3 to 4 days).156 Incision will be of little value at this early cellulitic phase. Antibiotic treatment of localized infection is summarized in Table 37-2. If significant cellulitis is present, a broad-spectrum antistaphylococcal antibiotic (cephalosporin or semisynthetic penicillin) may be tried. Splint and elevate the digit.156,157
TABLE 37-2
Common Hand Infections, Usual Offending Organisms, and Appropriate Therapeutic Regimens
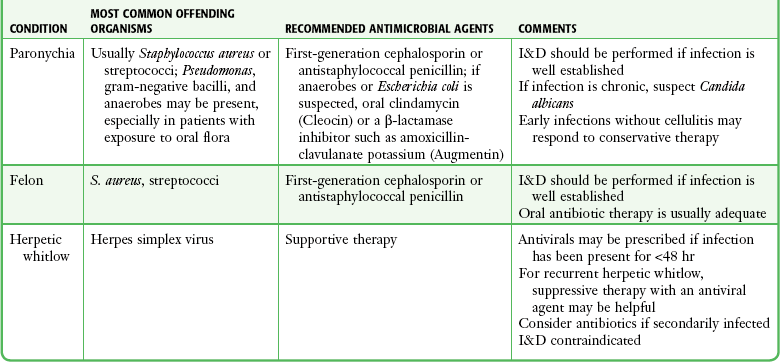
Adapted from Clark DC. Common acute hand infections. Am Fam Physician. 2003;68:167.
When a definite abscess has formed, drainage is usually curative quickly. A number of invasive operative approaches have been suggested. Actual skin incision or removal of the nail is rarely required, and neither procedure should be the initial form of treatment. One can invariably obtain adequate drainage by simply lifting the eponychial fold away from the nail matrix to allow the pus to drain. This is usually curative because a paronychia is not a cutaneous abscess per se, but rather a collection of pus in the potential space between the cuticle and the proximal end of the fingernail. Drainage may be accomplished without anesthesia in selected patients,158 but a digital nerve block is frequently required. After softening the eponychium by soaking, advance a No. 11 scalpel blade, scissors, or a 21- to 23-gauge needle parallel to the nail and under the eponychium at the site of maximal swelling (Fig. 37-32).157,159,160 Pus escapes rapidly, with immediate relief of pain. A tourniquet placed at the base of the finger may limit bleeding and aid the clinician in determining the exact extent of the infection during the drainage procedure.
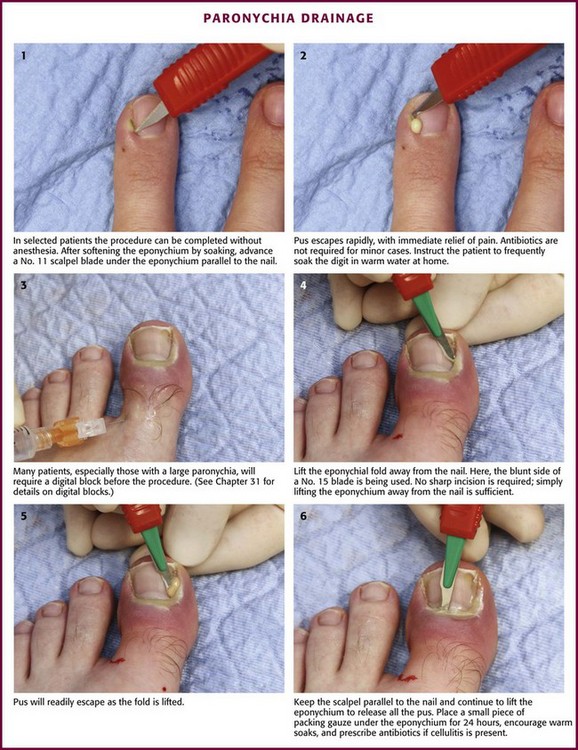
Figure 37-32 Paronychia drainage.
If more than a tiny pocket of pus is present, fan the knife tip or needle or spread the scissors under the eponychium while keeping the instrument parallel to the plane of the fingernail (see Fig. 37-32, steps 5 and 6). When a large amount of pus is drained, slip a small piece of packing gauze under the eponychium for 24 hours to provide continual drainage. Cultures are generally not indicated. Antibiotics are frequently prescribed, although they are not essential if drainage is complete or the surrounding area of cellulitis is minimal. An alternative to systemic antibiotics is to keep the operative site bathed in antibiotic ointment. After the anesthesia has worn off, start the patient on frequent soaks in warm tap water at home. In most cases the patient may easily remove the packing. At 24 to 36 hours, soak the finger in hot water and pull the gauze out; a repeated visit to a clinician is not required if healing is progressing, but follow-up examinations should always be encouraged. Once the packing is removed, cover the area with a dry, absorbent dressing. Apply an antibiotic ointment to the site for a few days. The benefit of antibiotic ointments in reducing infection is unproven, but instruct the patient on detailed use of the ointment. The ointment helps keep the bandage from sticking.
An alternative technique called the Swiss roll technique was described by Pabari and colleagues for severe acute paronychia with a run-around or contiguous infection of both nail folds. With a No. 15 scalpel blade pointing away from the nail bed, make an incision on both sides of the nail fold. The nail fold, after thorough irrigation with saline, is then rolled proximally over nonadherent dressing, like a swiss roll, and sutured to the skin with a nonabsorbable dressing. Apply a finger dressing. Remove the sutures in 48 hours and unfold the nail fold back to its original position to allow it to heal by secondary intention.161 The authors argue that this technique spares the nail plate and allows rapid healing, although no controlled studies have compared this technique with simple incision followed by packing gauze described above.
If the infection has produced purulence beneath the nail (subungual abscess), remove a portion of the nail or trephinate the nail to ensure complete drainage. As an alternative to nail removal, place a hole in the proximal part of the nail with a hot paper clip or a portable electrocautery device. Make a large opening or multiple holes to ensure continued drainage. The proximal portion of the nail is involved in most cases. Treat this by bluntly elevating the eponychium to expose the proximal edge of the nail. Elevate the proximal third of the nail from the nail bed and resect it with scissors. Leave the distal two thirds of the nail in place to act as a physiologic dressing and to decrease postoperative pain (Fig. 37-33). If purulence is found below the lateral edge of the nail, gently elevate the affected part and excise it longitudinally.162 Exercise care during this procedure to avoid damage to the nail matrix. Place a wick of gauze beneath the eponychium for 48 hours to ensure continued drainage.
Patients occasionally come to the ED complaining of a chronic, indolent paronychial infection. These seldom respond to ED intervention. Frank purulence is rarely present, and conservative treatments are often unsatisfactory. Many causes of this frustrating condition have been described, including fungal, bacterial, viral, and psoriatic conditions. Screen patients for malignancy if they have chronic paronychia that is unresponsive to therapy.159 Treatment modalities are varied, and controlled studies evaluating the various techniques are lacking. Meticulous hand care, oral and topical antimicrobial medications, and occasionally aggressive surgical intervention have been suggested.160 Refer these patients to a dermatologist or hand surgeon because of the prolonged treatment required.
Herpetic Whitlow
Herpetic whitlow is an extremely contagious infection of the distal phalanx caused by herpes simplex virus (type 1 or 2) (Fig. 37-34). In children, herpetic whitlow tends to be associated with gingivostomatosis caused by herpes simplex 1, whereas adults most commonly harbor herpes simplex 2. Inoculation occurs through a discontinuity in the skin.163 Health care providers exposed to oral secretions (e.g., dental hygienists and respiratory therapists) and patients with other herpes infections are most commonly infected.164,165 After a 2-day to 2-week incubation period, an infected individual may experience prodromal signs and symptoms such as fever, malaise, lymphadenitis, and axillary lymphadenopathy. In the affected finger the infection is characterized by tenderness followed by throbbing pain (out of proportion to the findings on physical examination), edema, and erythema. Vesicles containing clear, bloody, or cloudy fluid form and mark the most infectious stage of the process. Viral vesicles typically involve the digits but can also involve other areas of the hand.166 The lesions are usually quite painful but are self-limited and resolve in 2 to 3 weeks. I&D of herpetic whitlow is contraindicated because it may induce a secondary bacterial infection and delay healing.164,167 Treatment is symptomatic and consists of splinting, elevation, and analgesia as needed. Oral antiviral agents effective against herpes infections (acyclovir, famciclovir, or valacyclovir) can shorten the course of the disease if given early (see Box 37-3). After an accidental needlestick in health care workers, oral famciclovir may be used as a preventive.168 Infection recurs in 30% to 50% of cases, but the initial infection is usually the most severe. Acyclovir, 200 mg taken orally three to four times a day, can decrease recurrence rates.165
Consideration must be given to preventing spread of the infection. An occlusive dressing decreases the chance of viral transmission, but health care providers with herpetic whitlow should limit and perhaps even refrain from patient contact, especially until all lesions have crusted over and viral shedding has stopped.169,170 Compliance with universal precautions will decrease the likelihood of patient-to-provider transmission.
Felon
A felon is an infection of the pulp of the distal end of the finger (Fig. 37-35). The usual cause is trauma with secondary invasion by bacteria. A felon may develop in the presence of a foreign body, such as a thorn or a splinter, but often a precipitating trauma cannot be identified. An important anatomic characteristic of this area is that many fibrous septa extend from the volar skin of the fat pad to the periosteum of the phalanx; these septa subdivide and compartmentalize the pulp area. When an infection occurs in the pulp, these structures make it a closed-space infection. The septa limit swelling, delay pointing of the abscess, and inhibit drainage after incomplete surgical decompression. Pressure may increase in the closed space and initiate an ischemic process that compounds the infection. The infection can progress to osteomyelitis of the distal phalanx, septic arthritis, and flexor tenosynovitis. Although the septa may facilitate an infection in the pulp, they also provide a barrier that protects the joint space and tendon sheath by limiting proximal spread of the infection.
In most cases the offending organism is S. aureus, but mixed infections and gram-negative infection may also occur. A study looking at the bacteriology of hand infections over an 11-year period found that of 159 hand infections, 48 (30%) harbored CA-MRSA, the incidence of CA-MRSA increased by 41% per year, and IV drug use and felon infections in particular were risk factors for CA-MRSA.162 These changing patterns, along with the often protracted course of felons and concern for osteomyelitis, make this one of the few soft tissue infections in which culture may be helpful.
During the early stages of cellulitis, a felon may be controlled by treatment consisting of elevation, oral antibiotics (see Box 37-3), and warm water or saline soaks. For more developed felons, proper treatment consists of early and complete I&D.164 Antibiotics alone are not curative once suppuration has occurred. Delaying surgery may result in permanent disability and deformity.
A minor felon can usually be drained on an outpatient basis with a digital nerve block (Fig. 37-36). A long-acting anesthetic (bupivacaine) will prolong the anesthesia. A tourniquet (1.25-cm Penrose drain) can be used to allow an incision into a bloodless field. Surgical drainage must be performed carefully to avoid injury to nerves, vessels, and flexor tendons. Most felons can be managed with a limited procedure, but many surgical options have been advocated, none of which has been proved to be superior for all circumstances.164 Traditional incisions (“hockey stick” or “fish mouth”) have a propensity for complications such as sloughing of tissue and postoperative fat pad anesthesia or instability and are rarely used. The preferred initial treatment is a simple longitudinal incision over the area of greatest fluctuance,171,172 3 to 5 mm distal to the distal interphalangeal joint. The incision may be made laterally or along the volar surface (see Fig. 37-36A), although injury to the sensory nerve or digital artery is more of a concern with the lateral incision given its proximity to these structures. Frank pus may be encountered during incision, but usually only a few drops are expressed. One more often drains a combination of necrotic tissue and interstitial fluid. A foreign body should be sought even if the history is not known. A potential drawback to an incision in the middle of the fat pad is the production of a scar in a very sensitive and commonly traumatized area. The incision must not extend to the distal interphalangeal crease because of the danger of injuring the flexor tendon. The subcutaneous tissue is bluntly dissected with a hemostat to provide adequate drainage. A gauze pack may be placed in the wound for 24 to 48 hours to ensure continued drainage. Recurrent or more severe infections may require a more aggressive approach by a hand specialist. Follow-up recommendations for patients with a diagnosis of felon should include referral to a hand surgeon.
No matter which incision is made, it must not be carried proximal to the closed pulp space because of the danger of entrance into the tendon sheath or the joint capsule. A snug dressing, splinting, elevation, and adequate opioid analgesics are prerequisites for a successful outcome. Most felons are treated empirically with antibiotics for at least 5 days. A broad-spectrum cephalosporin is a reasonable choice pending cultures (if done). Antibiotics effective against CA-MRSA should also be considered given its increasing incidence. MRSA infections can be treated with vancomycin, 1 g twice a day, or linezolid and may require weeks until resolution.173 Oral linezolid may allow outpatient treatment.
Seroma and Hematoma Drainage
Although most I&D procedures are performed for decompression of purulent collections, drainage of sterile hematomas or seromas may be required in the ED. In general, the same principles used for formal drainage of pus in the soft tissues apply to drainage of a sterile fluid collection, and hence one can directly apply the principles of this chapter to the drainage of sterile fluids. In addition, when a sterile fluid collection is drained, the operator has the option of primarily closing the incision site after wound irrigation (see Chapters 34 and 35 for wound management techniques). Drainage of a soft tissue hematoma is generally best postponed for several days after the initial injury to permit hemostasis and to minimize the risk for reaccumulation of the hematoma after drainage. The procedure is generally reserved for soft tissue hematomas that are large and painful (secondary to tissue distention), and they are expected to either resolve slowly or result in soft tissue deformity if not drained. Seromas and hematomas rarely become infected if the overlying skin remains intact. It is best to avoid needle aspiration because this procedure rarely drains the collection completely and it has the potential to introduce infection into a good culture medium in a closed space. If a hematoma becomes infected, treat it as a cutaneous abscess.
Subungual Hematoma
Subungual hematomas are typically caused by hitting a fingertip with a hammer, slamming a finger in a door (Fig. 37-37), or dropping a weight on a foot. The patient’s main concern is obtaining relief from the terrible throbbing pain that increases with the pressure under the nail.
When describing the injury, the clinician should estimate the percentage of nail bed that is affected by the hematoma and discuss associated trauma to the nail margins or surrounding tissue. The examination should include tests of the extensor and flexor tendons, of circulation by capillary refill, and of the sensitivity of the area.174
If fracture of the distal phalanx is suspected (e.g., if the fingertip is unstable or the mechanism of injury suggests a fracture), obtain anteroposterior and lateral x-ray films. Radiographs differentiate tendinous from bony mallet-type injuries. Crush injuries are associated with three types of distal phalanx fractures: longitudinal, transverse, and comminuted. If the fracture is angulated, displaced, unstable, or intraarticular or involves a third or more of the articular surface, refer the patient to a hand surgeon.175 Because trephination in patients with a distal phalanx fracture converts a closed fracture into an open one, there is concern about infectious and cosmetic complications. In the two studies that examined this possible association,176,177 with fracture subsets totaling 26, no infections occurred. The presence of an underlying fracture does not contraindicate nail trephination for fear of converting a closed fracture into an open one.
Nail removal is unnecessary, even if there is an underlying distal phalanx fracture, as long as the nail margins and nail are intact.178,179 If the nail is split, avulsed or disrupted or a nail bed laceration extends to the skin, the nail should be removed and the underlying nail bed repaired (see further discussion in Chapter 35). Simple trephination of an uncomplicated subungual hematoma, even if it involves the entire subungual area, results in a good outcome functionally and cosmetically.180 Trephination should also be considered if the hematoma covers more than half the intact nail or if it is smaller but painful.
Prophylactic antibiotics are not necessary for patients with uncomplicated subungual hematomas that have been trephinated. For more serious injuries, a slight risk for infection exists, but in most cases, rigorous wound cleaning and careful soft tissue repair constitute adequate treatment.181,182
Methods of Trephination
All methods of trephination require aseptic technique. Clean the nail thoroughly and place the digit in a sterile field. Follow universal precautions because the fluid under the nail is under pressure and can spurt.183 Ensure that adequate analgesia has been achieved. Anesthesia is not always required, but a digital block can be used to calm an anxious patient and is commonly performed.
Hot cautery is the most common form of trephination and is usually done with a paper clip that has been straightened and heated in a flame (Figs. 37-38 and 37-39). Place the hot end on the nail above the center of the hematoma and apply gentle pressure until the nail is breached and the hematoma expressed. A “give” is felt as the instrument passes through the nail. Stop the pressure at this point to avoid damage to the nail bed. Blood exits rapidly, and the blackened nail regains its normal color (see Fig. 37-39, step 3). The blood usually remains fluid for 24 to 36 hours and can easily be expressed with slight pressure. Multiple holes may be needed for continued drainage. Do not use hot cautery trephination on artificial nails because they are flammable.178 Hot cautery with a paper clip is easy to perform, but hematomas treated in this way tend to reform. A cold lancet has the benefits of reducing the pain immediately, as well as reducing the likelihood of infection.179 A portable hot-wire electrocautery unit can be used, but it is difficult to obtain an adequate drainage hole without adapting the instrument (see Fig. 37-39). It can be modified to burn a larger hole by “fattening” the end of the wire loop and rotating the device slowly as the nail is penetrated or by removing a small rectangle of nail. In addition to being convenient, the cautery device is desirable because the wire stays hotter longer, thereby enhancing nail penetration.
Figure 37-38 A straightened paper clip, held with a hemostat and heated with a flame, can be used for hot cautery trephination.
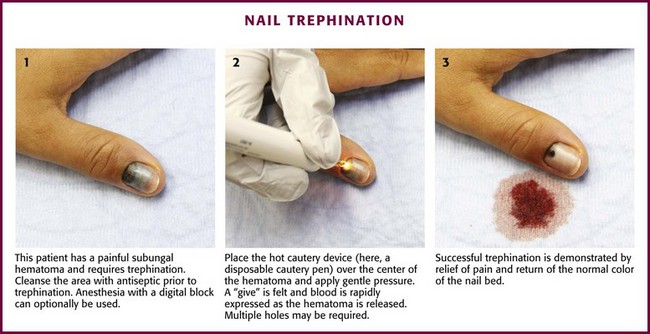
Figure 37-39 Nail trephination.
The PathFormer (Path Scientific, Carlisle, MA) is a new device that allows controlled nail trephination (“mesoscission”) and therefore minimizes patient discomfort during the procedure.183 It uses electrical resistance in the nail bed as feedback to stop and retract the drill when it penetrates the nail plate. The nail bed, with its blood supply and nerve endings, is not disturbed.
An 18- or 21-gauge needle can be rotated between the thumb and the index finger while gentle pressure is applied so that the sharp end of the needle corkscrews through the nail.183 When using a needle, the give is harder to feel, so the clinician should proceed slowly until blood is drawn. Because the hole made with a needle is small, a second hole may be required.
An alternative needle-based approach is being used by a group of Turkish dermatologists. Kaya and associates184 use extra fine, 29-gauge insulin syringes for evacuation of hematomas. After the nail is trimmed, the needle is inserted parallel to the nail plate and advanced to the distal edge of the hematoma. This technique is especially effective for small hematomas of the second, third, and fourth toenails, which are hard to trephine.
Conditions with a Similar Appearance
One condition that may be mistaken for a simple subungual hematoma is closed avulsion of the base of a fingernail occurring in conjunction with a subungual hematoma from a nail bed laceration. A common mechanism is slamming a finger in a car door, which causes sudden flexion of the distal phalanx in conjunction with a crush injury of the nail bed. The nail itself is usually stable, so generally no repair of the nail bed appears to be required. However, if the subungual hematoma extends past the confines of the nail bed, such as in the paronychial space (under the skin of the cuticle), there must be a communication between the nail bed and this space. This produces a paronychial hematoma, with blood occupying the space where pus would be located in an infectious paronychia (Fig. 37-40). When this condition is present, the avulsed proximal portion of the fingernail overlies the nail fold of the cuticle but is appreciated only after the overlying skin is opened. Open reduction (replacement) of the nail must be performed. The replaced nail often grows normally, but a lost or deformed nail is possible. Repair of the nail bed laceration is optional at this juncture but may not be required if the nail is stable. Once the injury is anatomically aligned, splinting and soaking follow as for a simple subungual hematoma. These injuries rarely become infected, and there is no evidence that the prophylactic use of antibiotics is necessary.
Mucocele
Sometimes mistaken for infection, a mucocele of the mucous membranes of the mouth is a nontender pearl-colored small lump that is felt by the patient’s tongue. It is a plugged saliva gland, and it becomes evident when it fills up with a gel-like substance. Occasionally, the patient will squeeze it to express the gel, but the mucocele may return unless excised. Under local anesthesia, the entire mass is removed by sharp dissection with a scalpel. The ulcer is left open and will heal quickly (Fig. 37-41).
References
1. McCaig LF, Nawar EW. National Hospital Ambulatory Medical Care Survey: 2004 emergency department summary. Advance Data No. 372, June 23, 2006.
2. Moreillon, P, Que, Y-A, Glauser, M. Staphylococcus aureus (including staphylococcal type shock). In: Mandell G, Bennett J, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:2321.
3. Taira, BR, Singer, AJ, Thode, HC, Jr., Lee, CC. National epidemiology of cutaneous abscesses: 1996 to 2005. Am J Emerg Med. 2009;27(3):289–292.
4. Ratnam, S, Hogan, K, March, SB, et al. Whirlpool-associated folliculitis caused by Pseudomonas aeruginosa: report of an outbreak and review. J Clin Microbiol. 1986;23:655–659.
5. Kasper, DL, Cohen-Poradosa, R. Infections due to mixed anaerobic organisms. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York: McGraw-Hill; 2008:999.
6. Simmons, RL. Life-threatening soft tissue infections. In: Peterson PK, Sabath LD, Calderón E, et al, eds. The Management of Infectious Diseases in Clinical Practice. New York: Academic Press; 1982:211.
7. Meltzer, DK. Complications of body piercing. Am Fam Physician. 2005;72:2029.
8. Ebright, JR, Pieper, B. Skin and soft tissue infections in injection drug users. Infect Dis Clin North Am. 2002;16:697.
9. Bergstein, JM, Baker, EJ, 4th., Aprahamian, C, et al. Soft tissue abscesses associated with parenteral drug abuse: presentation, microbiology, and treatment. Am Surg. 1995;61:1105.
10. Merhar, GL, Colley, DP, Clark, RA, et al. Computed tomographic demonstration of cervical abscess and jugular vein thrombosis. Arch Otolaryngol Head Neck Surg. 1981;107:313.
11. Murphy, EL, DeVita, D, Liu, H, et al. Risk factors for skin and soft-tissue abscess among injection drug users: a case-control study. Clin Infect Dis. 2001;33:35.
12. Brook, I. Microbiology of polymicrobial abscess and implications for therapy. J Antimicrob Chemother. 2002;50:805.
13. Brook, I, Finegold, SM. Aerobic and anaerobic bacteriology of cutaneous abscesses in children. Pediatrics. 1981;67:891.
14. Webb, D, Thadepalli, H. Skin and soft tissue polymicrobial infections from intravenous abuse of drugs. West J Med. 1979;130:200.
15. Schnall, SB, Holton, PD, Lilley, JC. Abscesses secondary to parenteral abuse of drugs. J Bone Joint Surg Am. 1994;76:1526.
16. Podzamczer, D, Ribera, M, Gudiol, F. Skin abscesses caused by Candida albicans in heroin abusers [letter]. J Am Acad Dermatol. 1987;16:386.
17. Boudreau, S, Hines, HC, Hood, AF. Dermal abscesses with Staphylococcus aureus, cytomegalovirus and acid-fast bacilli in a patient with acquired immunodeficiency syndrome (AIDS). J Cutan Pathol. 1988;15:53.
18. Floyd, JL, Goodman, EL. Soft-tissue abscesses in a diabetic patient. JAMA. 1981;246:675.
19. Lewis, JW, Groux, N, Elliot, JP, et al. Complications of attempted central venous injections performed by drug abusers. Chest. 1980;79:613.
20. Blumstein, H, Roberts, J. Retained needle fragments and digital dissection [letter]. N Engl J Med. 1993;328:1426.
21. Cohen, PR, Grossman, ME. Management of cutaneous lesions associated with an emergency epidemic: community-acquired methicillin-resistant Staphylococcus aureus skin infections. J Am Acad Dermatol. 2004;51:132.
22. Roberts, JR, McCawley, L, Laxton, M, et al. Genital community-associated methicillin resistant Staphylococcus aureus infection can be a sexually transmitted disease. Ann Emerg Med. 2007;50:93.
23. Ito, T, Katayama, Y, Asada, K, et al. Structural comparison of the three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1323–1336.
24. Shorr, AF. Epidemiology of staphylococcal resistance. Clin Infect Dis. 2007;45(suppl 3):S171.
25. Enright, MC, Robinson, DA, Randle, G, et al. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci U S A. 2002;99:7687.
26. Archer, GL, Niemeyer, DM, Thanassi, JA, et al. Dissemination among staphylococci of DNA sequences associated with methicillin resistance. Antimicrob Agents Chemother. 1994;38:447.
27. Fridkin, SK, Hageman, JC, Morrison, M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436.
28. Begier, EM, Frenette, K, Barrett, NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39:1446.
29. Kazakova, SV, Hageman, JC, Matava, M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352:468.
30. Herold, BC, Immergluck, LC, Maranan, MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593.
31. Adcock, PM, Pastor, P, Medley, F, et al. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis. 1998;178:577.
32. Campbell, KM, Vaughn, AF, Russell, KL, et al. Risk factors for community-associated methicillin-resistant Staphylococcus aureus infections in an outbreak of disease among military trainees in San Diego, California, in 2002. J Clin Microbiol. 2004;42:4050.
33. Aiello, AE, Lowy, FD, Wright, LN, et al. Methicillin-resistant Staphylococcus aureus among US prisoners and military personnel: review and recommendations for future studies. Lancet Infect Dis. 2006;6:335.
34. Public health dispatch: outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. JAMA. 2003;289:1377.
35. Centers for Disease Control and Prevention (CDC). Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919–922.
36. Sing, A, Tuschak, C, Hörmansdorfer, S. Methicillin-resistant Staphylococcus aureus in a family and its pet cat. N Engl J Med. 2008;358:1200.
37. Miller, LG, Perdreau-Remington, F, Bayer, AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis. 2007;44:471.
38. Frazee, BW, Lynn, J, Charlebois, ED, et al. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med. 2005;45:311.
39. Fry, DE, Barie, PS. The changing face of Staphylococcus aureus: a continuing surgical challenge. Surg Infect (Larchmt). 2011;12:191–203.
40. Naimi, DJ, LeDell, KH, Como-Sabetti, K, et al. Comparison of community- and health care–associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984.
41. Diep, BA, Chambers, HF, Graber, CJ, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148:249.
42. Diep, BA, Gill, SR, Chang, RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet. 2006;367:731.
43. Liu, C, Graber, CJ, Karr, M, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004-2005. Clin Infect Dis. 2008;46:1637.
44. Huang, SS, Platt, R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36:281.
45. Davis, SL, Perri, MB, Donabedian, SM, et al. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2007;45:1705.
46. Moran, GJ, Krishnadasan, A, Gorwitz, RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. for the EMERGEncy ID Net Study Group. N Engl J Med. 2006;355:666–674.
47. Crum, NF, Lee, RU, Thornton, SA, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943.
48. Hernandez, MB, Gofman, Y, Termotto, G, et al. Acquisition of MRSA through oral sex and treatment of carrier status in a 22 year old male: a case report. Internet. J Infect Dis. 8(2), 2010.
49. Squire, BT, Fox, JC, Anderson, C. ABSCESS: applied bedside sonography for convenient evaluation of superficial soft tissue infections. Acad Emerg Med. 2005;12:601.
50. Marantz, PR, Linzer, M, Feiner, CJ, et al. Inability to predict diagnosis in febrile intravenous drug abusers. Ann Intern Med. 1987;106:823.
51. Talan, DA, Summanen, PH, Finegold, SM. Ampicillin/sulbactam and cefoxitin in the treatment of cutaneous and other soft-tissue abscesses in patients with or without histories of injection drug abuse. Clin Infect Dis. 2000;31:464.
52. Llera, JL, Levy, RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15.
53. Hepburn, MJ, Dooley, DP, Skidmore, PJ, et al. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Intern Med. 2004;164:1669–1674.
54. Rutherford, WH, Calderwood, JW, Hart, D, et al. Antibiotics in surgical treatment of septic lesions. Lancet. 1970;1:1077.
55. Macfie, J, Harvey, J. The treatment of acute superficial abscesses: a prospective clinical trial. Br J Surg. 1977;64:264.
56. Blick, PWH, Flowers, MW, Marsden, AK, et al. Antibiotics in surgical treatment of acute abscesses. BMJ. 1980;281:111.
57. Miro, JM, Anguera, I, Cabell, CH, et al. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis. 2005;41:507–514.
58. Fowler, VG, Jr., Miro, JM, Hoen, B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–3021.
59. Wilson, W, Taubert, KA, Gewitz, M, et al. Prevention of infective endocarditis. Circulation. 2007;116:1736.
60. Infective Endocarditis Prophylaxis Expert Group. Prevention of endocarditis. 2008 update from Therapeutic guidelines: antibiotic version 13, and Therapeutic guidelines: oral and dental version 1. Melbourne: Therapeutic Guidelines Limited; 2008.
61. Friedland, GH, Klein, RS. Transmission of the human immunodeficiency virus. N Engl J Med. 1987;317:1125.
62. Warnes, CA, Williams, RG, Bashore, TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). J Am Coll Cardiol. 2008;52:e1–e121.
63. Kaye, D. Prophylaxis for infective endocarditis: an update. Ann Intern Med. 1986;104:419.
64. Fine, BC, Sheckman, PR, Bartlett, JC. Incision and drainage of soft-tissue abscesses and bacteremia [letter]. Ann Intern Med. 1985;103:645.
65. Bobrow, BJ, Pollack, CV, Jr., Gamble, S, et al. Incision and drainage of cutaneous abscesses is not associated with bacteremia in afebrile adults. Ann Emerg Med. 1997;29:404.
66. Robert-Guroff, M, Weiss, SH, Giron, JA, et al. Prevalence of antibodies to HTLV-I, -II, and -III in intravenous drug abusers from an AIDS endemic region. JAMA. 1986;255:3133.
67. Levy, N, Carlson, JR, Henrichs, S, et al. The prevalence of HTLV-III/LAV antibodies among intravenous drug users attending treatment programs in California. N Engl J Med. 1986;314:446.
68. Tsoraides, SS, Pearl, RH, Stanfill, AB, et al. Incision and loop drainage: a minimally invasive technique for subcutaneous abscess management in children. J Pediatr Surg. 2010;45:606–609.
69. Norden, C, Ruben, F. Staphylococcal infections. In: Wherle P, Top F, eds. Communicable and Infectious Diseases. 9th ed. St. Louis: Mosby; 1981:589.
70. Stevens, DL, Bisno, AL, Chambers, HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406.
71. Sandri, AM, Dalarosa, MG, Ruschel de Alcantara, L, et al. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol. 2006;27:185–187.
72. Thomas, R, Barnhill, D, Bibro, M, et al. Hidradenitis suppurativa: a case presentation and review of the literature. Obstet Gynecol. 1985;66:592–595.
73. Paletta, C, Jurkiewicz, MJ. Hidradenitis suppurativa. Clin Plast Surg. 1987;14:383–390.
74. Brown, TJ, Rosen, T, Orengo, IF. Hidradenitis suppurativa. South Med J. 1998;91:1107–1114.
75. Rose, RF, Goodfield, MJ, Clark, SM. Treatment of recalcitrant hidradenitis suppurativa with oral ciclosporin. Clin Exp Dermatol. 2006;31:154–155.
76. Slade, DE, Powell, BW, Mortimer, PS. Hidradenitis suppurativa: pathogenesis and management. Br J Plast Surg. 2003;56:451–461.
77. Fitzsimmons, JS, Guilbert, PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367–373.
78. Alikhan, A, Lynch, PJ, Eisen, DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539–561. [quiz 562-563].
79. Yazdanyar, S, Jemec, GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118–123.
80. König, A, Lehmann, C, Rompel, R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261–264.
81. Morgan, WP, Leicester, G. The role of depilation and deodorants in hidradenitis suppurativa. Arch Dermatol. 1982;118:101–102.
82. Lapins, J, Jarstrand, C, Emtestam, L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br J Dermatol. 1999;140:90–95.
83. Shah, N. Hidradenitis suppurativa: a treatment challenge. Am Fam Physician. 2005;72:1547–1552.
84. Highet, AS, Warren, RE, Weekes, AJ. Bacteriology and antibiotic treatment of perineal suppurative hidradenitis. Arch Dermatol. 1988;124:1047–1051.
85. Van der Zee, HH, Boer, J, Prens, EP, et al. The effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativa. Dermatology. 2009;219:143–147.
86. Gener, G, Canoui-Poitrine, F, Revuz, JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148–154.
87. Clemmensen, OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325–328.
88. Jemec, GB, Wendelboe, P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971–974.
89. Mortimer, PS, Dawber, RP, Gales, MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263–268.
90. Dalrymple, JC, Monaghan, JM. Treatment of hidradenitis suppurativa with the carbon dioxide laser. Br J Surg. 1987;74:420.
91. Thielen, AM, Barde, C, Saurat, JH. Long-term infliximab for severe hidradenitis suppurativa. Br J Dermatol. 2006;155:1105–1107.
92. Moul, DK, Korman, NJ. The cutting edge. Severe hidradenitis suppurativa treated with adalimumab. Arch Dermatol. 2006;142:1110–1112.
93. Ritz, JP, Runkel, N, Haier, J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164–168.
94. Mandal, A, Watson, J. Experience with different treatment modules in hidradenitis suppurativa: a study of 106 cases. Surgeon. 2005;3:23–26.
95. Kagan, RJ, Yakuboff, KP, Warner, P, et al. Surgical treatment of hidradenitis suppurativa: a 10-year experience. Surgery. 2005;138:734–740. [discussion 740-741].
96. Bohn, J, Svensson, H. Surgical treatment of hidradenitis suppurativa. Scand J Plast Reconstr Surg Hand Surg. 2001;35:305–309.
97. Bharat, A, Gao, F, Aft, RL, et al. Predictors of primary breast abscesses and recurrence. World J Surg. 2009;33:2582–2586.
98. Kvist, LJ, Rydhstroem, H. Factors related to breast abscess after delivery: a population-based study. BJOG. 2005;112:1070–1074.
99. Dixon, JM, Khan, LR. Treatment of breast infection. BMJ. 2011;342:d396.
100. Moazzez, A, Kelso, RL, Towfigh, S, et al. Breast abscess bacteriologic features in the era of community-acquired methicillin-resistant Staphylococcus aureus epidemics. Arch Surg. 2007;142:881–884.
101. Dabbas, N, Chand, M, Pallett, A, et al. Have the organisms that cause breast abscess changed with time?—Implications for appropriate antibiotic usage in primary and secondary care. Breast J. 2010;16:412–415.
102. Ulitzsch, D, Nyman, MK, Carlson, RA. Breast abscess in lactating women: US-guided treatment. Radiology. 2004;232:904–909.
103. Memish, ZA, Alazzawi, M, Bannatyne, R. Unusual complication of breast implants: Brucella infection. Infection. 2001;29:291–292.
104. Fox, LP, Geyer, AS, Husain, S, et al. Mycobacterium abscessus cellulitis and multifocal abscesses of the breasts in a transsexual from illicit intramammary injections of silicone. J Am Acad Dermatol. 2004;50:450–454.
105. Drifmeyer, E, Batts, K, Breast abscess after nipple piercing. Consultant, 2007;47(5). Available from:, http://www.consultantlive.com. [Cited 6/12/2007].
106. Eryilmaz, R, Sahin, M, Hakan Tekelioglu, M, et al. Management of lactational breast abscesses. Breast. 2005;14:375–379.
107. Schwarz, RJ, Shrestha, R. Needle aspiration of breast abscesses. Am J Surg. 2001;182:117–119.
108. Christensen, AF, Al-Suliman, N, Nielsen, KR, et al. Ultrasound-guided drainage of breast abscesses: results in 151 patients. Br J Radiol. 2005;78:186–188.
109. Berna-Serna, JD, Madrigal, M. Percutaneous management of breast abscesses. An experience of 39 cases. Ultrasound Med Biol. 2004;30:1–6.
110. Watt-Boolsen, S, Rasmussen, NR, Blichert-Toft, M. Primary periareolar abscess in the nonlactating breast: risk of recurrence. Am J Surg. 1987;153:571–573.
111. Versluijs-Ossewaarde, FN, Roumen, RM, Goris, RJ. Subareolar breast abscesses: characteristics and results of surgical treatment. Breast J. 2005;11:179–182.
112. Gupta, C, Malani, AK. Abscess as initial presentation of pure primary squamous cell carcinoma of the breast. Clin Breast Cancer. 2006;7:180.
113. Scott, BG, Silberfein, EJ, Pham, HQ, et al. Rate of malignancies in breast abscesses and argument for ultrasound drainage. Am J Surg. 2006;192:869–872.
114. Tan, YM, Yeo, A, Chia, KH, et al. Breast abscess as the initial presentation of squamous cell carcinoma of the breast. Eur J Surg Oncol. 2002;28:91–103.
115. Marzano, DA, Haefner, HK. The Bartholin gland cyst: past, present, and future. J Low Genit Tract Dis. 2004;8:195–204.
116. Brook, I. Aerobic and anaerobic microbiology of Bartholin’s abscess. Surg Gynecol Obstet. 1989;169:32–34.
117. Word, B. Office treatment of cyst and abscess of Bartholin’s gland duct. South Med J. 1968;61:514–518.
118. Omole, F, Simmons, BJ, Hacker, Y. Management of Bartholin’s duct cyst and gland abscess. Am Fam Physician. 2003;68:135–140.
119. Scott, PM. Draining a cyst or abscess in a Bartholin’s gland with a Word catheter. JAAPA. 2003;16(12):51–52.
120. Gennis, P, Li, SF, Provataris, J, et al. Jacobi ring catheter treatment of Bartholin’s abscesses. Am J Emerg Med. 2005;23:414–415.
121. Kushnir, VA, Mosquera, C. Novel technique for management of Bartholin gland cysts and abscesses. J Emerg Med. 2009;36:388–390.
122. Mathews, D. Marsupialization in the treatment of Bartholin’s cysts and abscesses. J Obstet Gynaecol Br Commonw. 1966;73:1010–1012.
123. Wechter, ME, Wu, JM, Marzano, D, et al. Management of Bartholin duct cysts and abscesses: a systematic review. Obstet Gynecol Surv. 2009;64:395–404.
124. Ergeneli, MH. Silver nitrate for Bartholin gland cysts. Eur J Obstet Gynecol Reprod Biol. 1999;82:231–232.
125. Stenchever, M, Droegemueller, W, Herbst, A, et al. Infections of the lower genital tract. In: Stenchever M, Droegemueller W, Herbst A, et al, eds. Comprehensive Gynecology. 4th ed. St. Louis: Mosby; 2002:641.
126. Bleker, OP, Smalbraak, DJ, Schutte, MF. Bartholin’s abscess: the role of Chlamydia trachomatis. Genitourin Med. 1990;66:24–25.
127. Miller, NR, Garry, DJ, Klapper, AS, et al. Sepsis after Bartholin’s duct abscess marsupialization in a gravida. J Reprod Med. 2001;46:913–915.
128. Laartz, BW, Cooper, C, Degryse, A, et al. Wolf in sheep’s clothing: advanced Kaposi sarcoma mimicking vulvar abscess. South Med J. 2005;98:475–477.
129. Brook, I, Anderson, KD, Controni, G, et al. Aerobic and anaerobic bacteriology of pilonidal cyst abscess in children. Am J Dis Child. 1980;134:679–680.
130. Arko, FR. Anorectal disorders. Am Fam Physician. 1980;22:121–126.
131. Webb, PM, Wysocki, AP. Does pilonidal abscess heal quicker with off-midline incision and drainage? Tech Coloproctol. 2011;15:179–183.
132. Courtney, SP, Merlin, MJ. The use of fusidic acid gel in pilonidal abscess treatment: cure, recurrence and failure rates. Ann R Coll Surg Engl. 1986;68:170–171.
133. Gencosmanoglu, R, Inceoglu, R. Modified lay-open (incision, curettage, partial lateral wall excision and marsupialization) versus total excision with primary closure in the treatment of chronic sacrococcygeal pilonidal sinus: a prospective, randomized clinical trial with a complete two-year follow-up. Int J Colorectal Dis. 2005;20:415–422.
134. Spivak, H, Brooks, VL, Nussbaum, M, et al. Treatment of chronic pilonidal disease. Dis Colon Rectum. 1996;39:1136–1139.
135. Søndenaa, K, Nesvik, I, Andersen, E, et al. Recurrent pilonidal sinus after excision with closed or open treatment: final result of a randomised trial. Eur J Surg. 1996;162:237–240.
136. Chaudhuri, A, Bekdash, BA, Taylor, AL. Single-dose metronidazole vs 5-day multi-drug antibiotic regimen in excision of pilonidal sinuses with primary closure: a prospective, randomized, double-blinded pilot study. Int J Colorectal Dis. 2006;21:688–692.
137. Patient Care Committee of the Society for Surgery of the Alimentary Tract (SSAT). Treatment of perineal suppurative processes. J Gastrointest Surg. 2005;9:457–459.
138. Kodner, I, Fry, R, Fleshman, JW, et al. Colon, rectum, and anus. In Schwartz S, Shires G, Spencer F, et al, eds.: Principles of Surgery, 7th ed, New York: McGraw-Hill, 1999.
139. Kovalcik, PJ, Peniston, RL, Cross, GH. Anorectal abscess. Surg Gynecol Obstet. 1979;149:884–886.
140. Brown, SR, Horton, JD, Davis, KG. Perirectal abscess infections related to MRSA: a prevalent and underrecognized pathogen. J Surg Educ. 2009;66:264–266.
141. Bevans, DW, Westbrook, KC, Thompson, BW, et al. Perirectal abscess: a potentially fatal illness. Am J Surg. 1973;126:765–768.
142. Kyle, S, Isbister, WH. Management of anorectal abscesses: comparison between traditional incision and packing and de Pezzer catheter drainage. Aust N Z J Surg. 1990;60:129–131.
143. Papachristodoulou, AJ, Zografos, GN, Papastratis, G, et al. Fournier’s gangrene: still highly lethal. Langenbecks Arch Chir. 1997;382:15–18.
144. Caliste, X, Nazir, S, Goode, T, et al. Sensitivity of computed tomography in detection of perirectal abscess. Am Surg. 2011;77:166–168.
145. Read, D, Abcarian, H. A prospective study of 404 patients with anorectal abscess. Dis Colon Rectum. 1979;22:566.
146. Beck, DE, Fazio, VW, Lavery, IC, et al. Catheter drainage of ischiorectal abscesses. South Med J. 1988;81:444–446.
147. Kitamura, K, Takahashi, T, Yamaguchi, T, et al. Primary resection of infectious epidermal cyst. J Am Coll Surg. 1994;179:607.
148. Dahdah, MJ, Scher, RK. Nail diseases related to nail cosmetics. Dermatol Clin. 2006;24:233–239. [vii].
149. Gaar, E. Occupational hand infections. Clin Occup Environ Med. 2006;5:369–380. [viii].
150. Whitehead, SM, Eykyn, SJ, Phillips, I. Anaerobic paronychia. Br J Surg. 1981;68:420–422.
151. Brook, I. Bacteriologic study of paronychia in children. Am J Surg. 1981;141:703–705.
152. Neviaser, R. Infections. In Green DP, ed.: Operative Hand Surgery, 2nd ed, New York: Churchill Livingstone, 1988.
153. Geng, W, Yang, Y, Wang, C, et al. Skin and soft tissue infections caused by community-associated methicillin-resistant Staphylococcus aureus among children in China. Acta Paediatr. 2010;99:575–580.
154. Imahara, SD, Friedrich, JB. Community-acquired methicillin-resistant Staphylococcus aureus in surgically treated hand infections. J Hand Surg [Am]. 2010;35:97–103.
155. Shaw, J, Body, R. Best evidence topic report. Incision and drainage preferable to oral antibiotics in acute paronychial nail infection? Emerg Med J. 2005;22:813–814.
156. Daniel, CR. Paronychia. Dermatol Clin. 1985;3:461–464.
157. Lee, TC. The office treatment of simple paronychias and ganglions. Med Times. 1981;109(9):49–51. [54-55].
158. Ogunlusi, JD, Oginni, LM, Ogunlusi, OO. DAREJD simple technique of draining acute paronychia. Tech Hand Up Extrem Surg. 2005;9:120–121.
159. Rockwell, PG. Acute and chronic paronychia. Am Fam Physician. 2001;63:1113–1116.
160. Baran, R, Bureau, H. Surgical treatment of recalcitrant chronic paronychias of the fingers. J Dermatol Surg Oncol. 1981;7:106–107.
161. Pabari, A, Iyer, S, Khoo, CT. Swiss roll technique for treatment of paronychia. Tech Hand Up Extrem Surg. 2011;15:75–77.
162. Halvorson, GD, Halvorson, JE, Iserson, KV. Abscess incision and drainage in the emergency department. J Emerg Med. 1985;3:295–305.
163. Polayes, IM, Arons, MS. The treatment of herpetic whitlow—a new surgical concept. Plast Reconstr Surg. 1980;65:811–817.
164. Avitzur, Y, Amir, J. Herpetic whitlow infection in a general pediatrician—an occupational hazard. Infection. 2002;30:234–236.
165. Laskin, OL. Acyclovir and suppression of frequently recurring herpetic whitlow. Ann Intern Med. 1985;102:494–495.
166. Gill, MJ, Arlette, J, Buchan, K. Herpes simplex virus infection of the hand. A profile of 79 cases. Am J Med. 1988;84:89–93.
167. Feder, HM, Long, SS. Herpetic whitlow. Epidemiology, clinical characteristics, diagnosis, and treatment. Am J Dis Child. 1983;137:861–863.
168. Manian, FA. Potential role of famciclovir for prevention of herpetic whitlow in the health care setting. Clin Infect Dis. 2000;31:E18.
169. Palenik, CJ, Miller, CH. Occupational herpetic whitlow. J Indiana Dent Assoc. 1982;61(6):25–27.
170. Rosato, FE, Rosato, EF, Plotkin, SA. Herpetic paronychia—an occupational hazard of medical personnel. N Engl J Med. 1970;283:804–805.
171. Clark, DC. Common acute hand infections. Am Fam Physician. 2003;68:2167–2176.
172. Kilgore, ES, Brown, LG, Newmeyer, WL, et al. Treatment of felons. Am J Surg. 1975;130:194–198.
173. Connolly, B, Johnstone, F, Gerlinger, T, et al. Methicillin-resistant Staphylococcus aureus in a finger felon. J Hand Surg [Am]. 2000;25:173–175.
174. Gamston, J. Subungual haematomas. Emerg Nurse. 2006;14(7):26–34.
175. Uttaravoli, P, Stair, T, Subungual hematoma. Common Simple Emergencies. Longwood Information: Washington, DC, 2007. Available at, http://www.ncemi.org/cse/cse1007.htm.
176. Seaberg, DC, Angelos, WJ, Paris, PM. Treatment of subungual hematomas with nail trephination: a prospective study. Am J Emerg Med. 1991;9:209–210.
177. Roser, SE, Gellman, H. Comparison of nail bed repair versus nail trephination for subungual hematomas in children. J Hand Surg [Am]. 1999;24:1166–1170.
178. Salazard, B, Launay, F, Desouches, C, et al. [Fingertip injuries in children: 81 cases with at least one year follow-up.]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90:621–627.
179. Salter, SA, Ciocon, DH, Gowrishankar, TR, et al. Controlled nail trephination for subungual hematoma. Am J Emerg Med. 2006;24:875–877.
180. Batrick, N, Hashemi, K, Freij, R. Treatment of uncomplicated subungual haematoma. Emerg Med J. 2003;20:65.
181. Suprock, MD, Hood, JM, Lubahn, JD. Role of antibiotics in open fractures of the finger. J Hand Surg [Am]. 1990;15:761–764.
182. Stevenson, J, McNaughton, G, Riley, J. The use of prophylactic flucloxacillin in treatment of open fractures of the distal phalanx within an accident and emergency department: a double-blind randomized placebo-controlled trial. J Hand Surg [Br]. 2003;28:388–394.
183. Skinner, PB. Management of traumatic subungual hematoma. Am Fam Physician. 2005;71:856.
184. Kaya, TI, Tursen, U, Baz, K, et al. Extra-fine insulin syringe needle: an excellent instrument for the evacuation of subungual hematoma. Dermatol Surg. 2003;29:1141–1143.