Figure 30.1 Intracellular events following interaction of the APC with the naive T cell. The antigenic peptide fragment is loaded on the MHC II complex on the APC. Interaction with the CD3 receptor on the T cell results in signal 1. Interactions between CD28 on the T cell and CD80/86 on the APC result in signal 2, which is a verification step for T cell activation. The whole process then activates intracellular kinases, resulting in activation of calcineurin. Calcineurin activates a transcription factor NFAT, which upregulates expression of IL-2. The IL-2 binds to the CD25 molecule, triggering a cascade of intracellular signalling pathways, resulting in cell proliferation. The various immunosuppressants are shown in the diagram acting on their respective targets. Mab, monoclonal antibody.
30.3.5.4 ROLE OF CALCINEURIN
Calcineurin is a Ca2+-dependent protein phosphatase, which has an important role in the control of intracellular Ca2+ signalling. Both signal 1 and signal 2 are necessary for T cell activation. When an APC interacts with a TCR on a T cell, there is an increase in the intracytoplasmic calcium which activates calcineurin. Calcineurin activates (dephosphorylates) a transcription factor NFAT (nuclear factor of activated T cells), which is then translocated into the nucleus, where it upregulates the expression of IL-2. IL-2 is associated with T cell activation and proliferation, and it is believed that the amount of IL-2 produced by the helper T cells influences the extent of immune response significantly. Calcineurin is the target of calcineurin inhibitor (CNI) drugs such as cyclosporin and tacrolimus [9].
30.3.6 Recognition of graft antigens by the recipient’s T cells
Interaction of donor antigens with the recipient T cells will provoke a fierce rejection response. Three nonmutually exclusive pathways have been described.
30.3.6.1 DIRECT PATHWAY
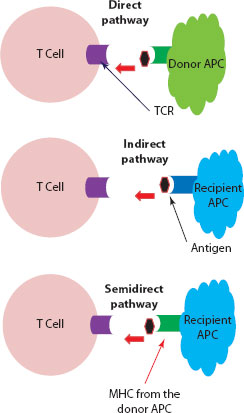
The donor organ will carry a variable number of passenger dendritic cells with a high density of allo-MHC molecules. These passenger APCs are capable of directly stimulating the recipient’s T cells. In the direct pathway, recipient T cells will recognise MHC molecules on the surface of donor APCs. The degree of MHC polymorphism between individuals will largely affect peptide binding, and consequently the immune response.
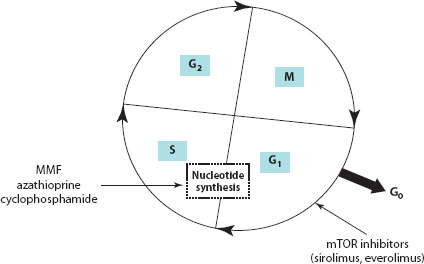
Figure 30.2 Cell cycle showing the various phases. MMF, azathioprine and cyclophosphamide inhibit nucleotide synthesis and thereby cell replication. SRL and everolimus inhibit the progression of T cell proliferation from the G1 to the S phase of the cell cycle. S, synthesis phase; M, mitosis phase; G, gap phase.
30.3.6.2 INDIRECT PATHWAY
With time, the donor APCs in the graft will be replaced by recipient APCs, which will then process and present donor antigenic peptides using their own MHC platform.
30.3.6.3 SEMIDIRECT PATHWAY
In this mechanism, it has been proposed that recipient APCs will acquire intact MHC molecules from donor cells and then display this foreign package on their own cell surface for interaction with the host T cell [7,10] (Figure 30.3).
30.3.7 Events that follow naive T cell activation
Newly transplanted organs are subject to severe inflammation due to several nonimmunological factors, such as injury during organ procurement, cold preservation in nonphysiological fluids, surgical trauma and reperfusion injury. This will lead to a hostile cytokine milieu with release of inflammatory mediators. The characteristics of the inflammatory environment in which donor-reactive CD4+ T cells recognise donor antigens determine the immune phenotype of these cells. When CD4+ T cells are activated in the presence of IL-12 (usually produced by activated, mature dendritic cells), they become tissue-destructive, interferon (IFN)-γ–producing T helper (Th1 response). In contrast, CD4+ T cells that are activated in the presence of IL-4 differentiate into Th2 cells, which produce IL-4 and IL-5. In the absence of pro-inflammatory cytokines, transforming growth factor (TGF)-β induces expression of Foxp3 and differentiation of CD4+ T cells into Tregs. In contrast, expression of TGF-β with IL-6 or IL-21 prevents development of the transplant-protective Tregs; instead, the antigen-reactive CD4+ T cells become IL-17-producing T cells (Th17), which are highly cytopathic. The overall outcome is determined by an unpredictable balance between the hostile rejection-promoting helpers (Th1, Th2, Th17 and possibly more) and the tolerogenic Tregs [10].
Figure 30.3 Recognition of graft antigens by the recipient’s T cells. Direct pathway: Dendritic cell (APC) from the donor tissue presents the antigen to the recipient’s T cell (TCR) through its own MHC. Indirect pathway: Dendritic cell from the recipient (host) will process the foreign antigen from the allograft and load it onto its MHC platform for engagement with the TCR on the recipient’s T cell. Semidirect pathway: Here, the recipient’s dendritic cells will acquire intact MHC molecules from the donor cells and use this foreign MHC platform to present the antigen to the recipient T cell.
Several postulated mechanisms of donor cell injury have been described. Cytokine secretion from CD4+ T cells will recruit and activate CD8+ T cells, B cells, NK cells and macrophages, all of which have the potential to mount an immune response. Furthermore, certain cytokines, such as IFN-γ, upregulate expression of MHC on the target donor cell thereby, making it susceptible to the weaponry of CD8+ T cells. The final ‘lethal hit’ for the cytolytic processes is triggering of apoptosis in the target cell [7].
30.4 IMMUNOLOGICAL TOLERANCE IN THE LIVER
The liver tends to be a rather tolerogenic microenvironment for a variety of reasons. The portal venous system drains the gastrointestinal tract and therefore is an inevitable thoroughfare for microbial antigens, microbe-associated molecular patterns (MAMPs), and food-derived antigens. There is a constant traffic of MAMPs, such as lipopolysaccharide (LPS) and other endotoxins. The reactivity of the liver cells towards MAMPs is attenuated by the development of a functional state of refractoriness following repeated exposure and is termed ‘endotoxin tolerance’. The prevalence of MAMPs in the gut and liver is physiological and not usually associated with infection. Therefore, it is important to maintain immune tolerance under these conditions to avoid autoimmunity. Liver has high levels of IL-10 (anti-inflammatory cytokine) and PD-L1 (programmed death-1 ligand-1). Also, the threshold for T cell activation is set rather high, as a result of which a state of immune tolerance prevails and tends to dampen T cell immune response. This is achieved by the cooperation of various tolerogenic liver-resident APC populations and immune regulatory mediators such as IL-10, prostanoids and TGF-β, which are released locally within the liver. These mechanisms are likely to be responsible for the spontaneous acceptance of liver allografts observed in animal models and selected patients. Nevertheless, immunosuppression is a key element of posttransplant care to maintain and enhance survival of allografts [10,11].
30.5 CURRENT STATUS OF IMMUNOSUPPRESSIVE AGENTS IN CHILDREN
Early liver transplantation was complicated by frequent episodes of rejection resulting in graft loss. Posttransplant outcomes have improved significantly with the advent of CNIs, rescue management strategies with more robust immunosuppressive agents and advances in surgical techniques. The wide array of drugs offers the option to use combinations with nonoverlapping toxicity profiles so that doses of individual drugs can be kept at a minimum to avoid toxicity. However, in paediatrics there are only a few clinical studies with robust data to rationalise the use of some useful drugs, leading to frequent off-licence usage in the paediatric age group. Table 30.1 lists the commonly used and newer agents in post–liver transplant immunosuppression. Table 30.2 summarises the mode of action, pharmacokinetics and common side effects of some of the common agents used.
30.5.1 Calcineurin inhibitors
CNIs (cyclosporin* and tacrolimus†) have been used widely and recommended as part of initial immunosuppressive therapy following a liver transplant. The use of cyclosporin (Box 30.2) in liver transplantation has largely been superseded by tacrolimus given its superior efficacy [12]. Tacrolimus is a macrolide immunosuppressant first isolated in 1985 from an actinomycete Streptomyces tsukubaensis, obtained from soil samples from Mount Tsukuba in Japan. It was first used for renal transplantation in Pittsburgh in 1989, and then subsequently was approved for liver transplantation in 1994 in the United States. Like cyclosporin, tacrolimus acts primarily by interfering with T cell activation. Cyclosporin binds to its specific cytoplasmic immunophilin, cyclophilin, and tacrolimus binds to a corresponding protein, FK506 binding protein 12 (FKBP12). These cyclosporin–or tacrolimus–immunophilin complexes inhibit the activity of calcineurin, a calcium-dependent phosphatase. Inhibition of calcineurin impedes calcium-dependent transduction and inactivates transcription factors, particularly NFAT. NFAT is believed to initiate gene transcription for the formation of lymphokines such as interleukin-2 and γ-interferon. The clinical result of this NFAT inhibition is immunosuppression [9,13].
Tacrolimus inhibits lymphocyte activation in vitro 10–100 times more potently than cyclosporin. Its oral bio-availability ranges between 5% and 67%, with the rate and extent of absorption greatest under fasting conditions. Drug concentration monitoring is crucial because tacrolimus has high inter- and intraindividual variability in pharmacokinetics, and a narrow therapeutic index [9].
Tacrolimus has become a common choice for immuno-suppression following liver, kidney and heart transplantation. It is licensed for use in the prophylaxis of transplant rejection in children. Its safety profile and efficacy is well established through robust paediatric studies [14,15]. A meta-analysis of 16 randomised trials comparing cyclosporin with tacrolimus for liver transplantation showed that tacrolimus is superior to cyclosporin in preventing AR [12].
Table 30.2 Pharmacological profile and adverse effects
|
Drug |
Pharmacological action |
Pharmacokinetics and dynamics |
Adverse effects |
|
Tacrolimus |
Binds to FK506 binding protein 12 and inhibits calcineurin; calcineurin inhibition consequently interrupts T cell activation and proliferation |
• Variable oral bioavailability • Peak blood levels after 1–2 h • Half-life of 8–24 h • Highly bound to albumin and a-1 acid glycoprotein • Metabolised by cytochrome p450 isoenzymes |
Nephrotoxicity, neurotoxicity, lymphomas and lymphoproliferative disease, cardiomyopathy, anaemia, chronic diarrhoea, onset of diabetes mellitus, electrolyte disturbances |
|
Sirolimus |
Binds to mTOR and inhibits cytokine-driven T cell proliferation |
• Hydrophobic drug with low solubility in aqueous solutions • Long half-life • Metabolised by cytochrome p450 system |
Hyperlipidaemia, proteinuria, myelosuppression, poor wound healing, pneumonitis, hypersensitivity reactions |
|
Mycophenolate mofetil |
Blocks purine nucleotide synthesis by inhibiting type 2 IMPDH |
• Bioavailability is 90%–94% or oral preparations • Has substantial enterohepatic circulation, contributing to its GI toxicity |
Gastrointestinal side effects, neutropenia, opportunistic infections |
|
IL-2 receptor antibodies |
Bind to the a chain of the IL-2 receptor, resulting in inhibition of clonal proliferation of the T cells |
• Half-life of 7.2 days • Suppressor effects last for 3–4 weeks |
Hypersensitivity reactions |
|
Campath-1H |
Monoclonal antibody directed against the CD52 surface antigen, resulting in lymphocyte depletion from the circulation |
• Plasma elimination half-life of approximately 12 days |
Hypersensitivity reactions |
Cyclosporine A can be credited with the successful rejuvenation of an entire chapter in medicine – organ transplantation. Its discovery can be said to start with a collection of soil samples in Norway in 1969 by employees of the Swiss drug company Sandoz searching for rare examples of fungi. From this was isolated the previously unknown Tolypocladium inflatum, whose metabolites were exhibited by antifungal and immunosuppressive properties via selective action on lymphocytes. By 1975, the now isolated drug was being tested in animals, particularly in the laboratory of Roy Calne in Cambridge, United Kingdom. The first human trials in renal transplants [23] and later in other organs, including two liver transplants, were reported in 1979 [24].
The controversy? Jean-Francoise Borel has been widely credited with its discovery as the head of the immunology department in Sandoz, but Hartman Stähelin, who made key discoveries in its bioassay and was actually in charge of the larger pharmacology department and supervised Borel, disputed this interpretation of history.
30.5.1.1 LONG-ACTING TACROLIMUS
Once-a-day tacrolimus preparation is now available and could be used in children and young people who can swallow a pill. Once-a-day use of tacrolimus in this dosage form has been shown to be safe and effective. It could also have an added advantage of improving adherence to the medication [16,17].
30.5.2 m-TOR inhibitors (sirolimus and everolimus)
SRL (also called rapamycin) is a macrolide compound derived from Actinomyces hygroscopicus. It was discovered in soil samples brought from Easter Island (Rapa Nui) by the Canadian Medical Research Expedition between December 1964 and February 1965. SRL has a long history dating from the 1970s, at the same time when cyclosporin was discovered, and was considered to have antifungal properties. Because of its immunosuppressive effects, it was not further developed for clinical use as an antibiotic. The advent of tacrolimus and the recognition of its structural similarities with SRL led to further research. SRL was launched for clinical use in organ transplantation in 1999 [8,13]. Everolimus, on the other hand, is a newer addition, essentially a derivative of SRL sharing its mechanism of action and a similar profile overall.
SRL binds to the same intracellular immunophilin, FKBP12 in T cells, and is similar to tacrolimus in this respect. However, the two drugs act synergistically rather than competitively and also differ in their mechanism of action. Tacrolimus (which inhibits calcineurin after binding to FKBP12) inhibits early events in the T cell activation, particularly the expression of IL-2 in the G0–G1 stage of the cell cycle. SRL, on the other hand, does not bind to calcineurin, but instead binds to target molecules with kinase activity called mammalian target of rapamycin (mTOR), also known as FRAP and RAFT. The inhibition of mTOR suppresses cytokine-driven T cell proliferation, resulting in inhibition of the progression from the G1 to the S phase of the cell cycle.
In paediatric liver transplantation, SRL has been used in combination with steroids, MMF or low-dose CNIs. It is not a favoured drug in the early posttransplant period due to its known complications of hepatic artery thrombosis and poor wound healing. SRL does not have the nephrotoxicity profile shown by CNIs and does not reduce glomerular filtration rate (GFR). SRL appears to be a safe alternative to CNIs, especially when used in patients with CNI-induced toxicities. Also, it has an effective role in chronic rejection or steroid-resistant rejection. However, further studies are needed to evaluate SRL in the paediatric population. Although widely used, its current usage in paediatric liver transplant is outside the product licence [4,9].
30.5.3 Mycophenolate mofetil
MMF is an ester pro-drug that is hydrolysed to the active compound mycophenolic acid (MPA). In 1969, MMF was discovered to prevent de novo purine nucleotide synthesis by inhibiting type 2 inosine monophosphate dehydrogenase (IMPDH) and the production of guanosine nucleotides such as guanosine monophosphate (GMP). Depletion of de novo guanosine causes a lack of deoxyguanosine triphosphate, suppressing DNA synthesis. Therefore, cells cannot replicate unless they are able to maintain GMP levels through the purine salvage pathway. Because B and T cells lack a key enzyme for this salvage pathway, hypoxanthine guanine phosphoribosyl transferase, they rely on de novo synthesis entirely and are unable to replicate in the presence of MMF, staying in the S phase of the cell cycle [8,18]. In liver transplantation, MMF has been used as an alternative immunosuppressive agent in patients with chronic rejection or severe CNI toxicity. In paediatric liver transplantation, it has been widely used as a CNI-sparing agent; however, there are no suitable randomised controlled trials to rationalise its role in the paediatric population [9]. MMF is teratogenic; hence, use in the adolescent age group should be considered carefully.
30.5.4 Azathioprine
Azathioprine is a derivative of 6-mercatopurine and acts as an antimetabolite of DNA and RNA synthesis. Azathioprine is more myelotoxic and hepatotoxic than MMF. Since 2000, many centres have substituted azathioprine with MMF. Generally, its use is considered in light of MMF intolerance or where teratogenicity should be considered, as in an adolescent patient [4].
30.5.5 Interleukin-2 receptor antibodies (basiliximab)
Although a combination of high-dose steroids and CNIs remains the mainstay of immunosuppression in the immediate posttransplant period, some centres use induction agents such as basiliximab as an initial hard hit to suppress immune response. Basiliximab is a chimeric monoclonal antibody which selectively inhibits the IL-2 receptor, thereby blocking T cell proliferation. The IL-2 receptor is composed of three noncovalently bound chains – the α, β and γ chains. The α chain (also referred to as CD25 or T cell activation antigen) is upregulated in activated T lymphocytes, resulting in internalisation of the TCR. The monoclonal antibody directly binds to the α subunit with high affinity, preventing IL-2 from binding to its receptor. This therefore leads to a halt in T cell proliferation in response to circulating IL-2 [19].
IL-2 receptor antibodies provide a safe and effective induction immunosuppression in paediatric liver transplant recipients and are associated with a lower incidence of AR episodes. The safety data is, however, only short term, and the conclusion is based on nonrandomised studies in children and from adult trials. At our centre, basiliximab is considered for induction in children with renal dysfunction, transplant after recurrent graft loss due to recurrent AR or chronic rejectionand sometimes autoimmune liver disease. Basiliximab is also considered in children where allograft rejection continues despite optimal levels of tacrolimus and other adjuvant immunosuppressants. Also, another common scenario is when steroids fail to reverse allograft rejection, although data on its use in steroid-resistant rejection is very limited. In the past, potent antilymphocyte preparations such as antithymocyte globulin and OKT3 have been used in our centre, but with the advent of basiliximab, their use has largely been superseded. Although widely used nowadays, basiliximab is not licensed for use in paediatric liver transplant. Clear recommendations on use in children cannot be established until adequately powered randomised controlled trials are performed.
30.5.6 Alemtuzumab (Campath-1H™)
Campath-1H* is a humanised, monoclonal antibody directed against CD52 determinants on the surface of human B cells, T cells and monocytes. CD52 is the most prevalent cell surface antigen on lymphocytes. Campath-1H results in a profound and prolonged depletion of T cells from the peripheral circulation after systemic administration, although its plasma elimination half-life is approximately 12 days. B cells, NK cells and monocytes are also depleted by alemtuzumab to a lesser extent. Its effect on lymph node lymphocyte depletion takes 3–5 days, compared with less than 1 hour in peripheral lymphocytes. It has been tested extensively in lymphoid malignancies, autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and organ transplantation.
Although its use in children has been limited to date, so far it appears to be well tolerated in children [20,21]. The University of Miami reported their experience in 10 children with liver transplants where they used induction with Campath-1H. Through their preliminary experience, Campath-1H was found to be well tolerated and rejection-free survival was prolonged among recipients with AIH despite a low level of maintenance immunosuppression [22]. Thus, alemtuzumab appears to be an attractive future option for induction in organ transplantation. However, there is a need for more robust studies with long-term follow-up before its role can be established, particularly with respect to safety and use in children. Our centre’s experience is limited to a few patients who developed persistent rejection under a setting where other agents, such as IL-2 antibodies and antithymocyte globulin, were ineffective. Due to the profound immunosuppressive effects, patients commenced on Campath-1H should be given prophylaxis therapy against opportunistic infections.
30.5.7 Novel emerging therapeutic agents
Several new biologics are currently under investigation as potential transplant therapeutic agents, targeted to a defined antigen ligand with the goal of inhibiting a single pathway or cell type involved in the rejection process. The specificity of the mode of action of such agents is aimed at minimising the adverse effects observed with the currently available drugs. FK778, JAK3 inhibitors, FTY720 and so forth are examples of some novel agents that have been developed recently and investigated for their potential benefit. Another example is belatacept (LEA29Y), which is a humanised monoclonal antibody, which acts by causing a costimulatory blockade during activation of a naive T cell. This drug has been approved for adult renal transplantation by the Food and Drug Administration (FDA), but not for liver transplantation [9].
There are compelling reasons to improve the currently available regimens and immunosuppressive agents in spite of the advances and progress made. Important goals using the various regimens include reducing renal toxicity from CNIs while preserving graft function, and lowering the incidence of opportunistic infections, post-transplant lymphoproliferative disease (PTLD) and other malignancies. However, all the available agents have shown little effect on the prevention of chronic allograft dysfunction. Future research should therefore look beyond the realms of short-term efficacy of the immunosuppressants and focus on the long-term preservation of graft function with optimal immunosuppression and minimal toxicities.
An ideal transplant would be an autologous graft that is engineered entirely from the stem cells of the recipient. Given the pace at which health research and technology are progressing, we can certainly be hopeful of the potential to grow stem cells in order to engineer tissues, including a whole organ such as the liver. This would circumvent the problem of allograft rejection and totally eliminate the need for immunosuppression. Use of Tregs to induce tolerance in liver transplant recipients has been a subject of recent clinical studies, and the outcome of these trials is eagerly awaited.
1. Terminology for hepatic allograft rejection. International Working Party. Hepatology 1995; 22: 648–654.
2. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology 1997; 25: 658–663.
3. Muiesan P, Vergani D, Mieli-Vergani G. Liver transplantation in children. Journal of Hepatology 2007; 46: 340–348.
4. Thangarajah D, O’Meara M, Dhawan A. Management of acute rejection in paediatric liver transplantation. Paediatric Drugs 2013 15: 459–471.
5. Neil DA, Hubscher SG. Current views on rejection pathology in liver transplantation. Transplant International 2010; 23: 971–983.
6. Chinen J, Buckley RH. Transplantation immunology: solid organ and bone marrow. Journal of Allergy and Clinical Immunology 2010; 125 (2 Suppl 2): S324–S335.
7. Delves PJ. Roitt’s Essential Immunology. 12th ed. Wiley-Blackwell, Singapore, 2011.
8. Mukherjee S, Mukherjee U, A comprehensive review of immunosuppression used for liver transplantation. Journal of Transplantation 2009; 2009: 701464. doi:10.1155/2009/701464.
9. Coelho T, Tredger JM, Dhawan A. Current status of immunosuppressive agents for solid organ transplantation in children. Pediatric Transplantation 2012; 16: 106–122.
10. Sanchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology 2011; 140: 51–64.
11. Kern M, Popov A, Kurts C, et al. Taking off the brakes: T cell immunity in the liver. Trends in Immunology 2010; 31: 311–317.
12. McAlister VC, Haddad E, Renouf E, et al. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. American Journal of Transplantation 2006; 6: 1578–1585.
13. Gummert JF, Ikonen T, Morris RE. Newer immunosuppressive drugs: a review. Journal of the American Society of Nephrology 1999; 10: 1366–1380.
14. Jain A, Mazariegos G, Kashyap R, et al. Comparative long-term evaluation of tacrolimus and cyclosporine in pediatric liver transplantation. Transplantation 2000; 70: 617–625.
15. Jain A, Singhal A, Fontes P, et al. One thousand consecutive primary liver transplants under tacrolimus immunosuppression: a 17-to 20-year longitudinal follow-up. Transplantation 2011; 91: 1025–1030.
16. Chisholm MA, Middleton MD, Modified-release tacrolimus. Annals of Pharmacotherapy 2006; 40: 270–275.
17. Kramer BK, Charpentier B, Bäckman L, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. American Journal of Transplantation 2010; 10: 2632–2643.
18. Hood KA, Zarembski DG. Mycophenolate mofetil: a unique immunosuppressive agent. American Journal of Health System Pharmacology 1997; 54: 285–294.
19. Church AC. Clinical advances in therapies targeting the interleukin-2 receptor. Quarterly Journal of Medicine 2003; 96: 91–102.
20. Knechtle SJ. Present experience with Campath-1H in organ transplantation and its potential use in pediatric recipients. Pediatric Transplantation 2004; 8: 106–112.
21. Morris PJ, Russell NK. Alemtuzumab (Campath-1H): a systematic review in organ transplantation. Transplantation 2006; 81: 1361–1367.
22. Kato T, Selvaggi G, Panagiotis T, et al. Pediatric liver transplant with Campath 1H induction – preliminary report. Transplant Proceedings 2006; 38: 3609–3611.
23. Calne RY, White DJ, Thiru S, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 1979; ii (8104–8105): 1323–1327.
24. Calne RY, White DJ, Thiru S, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 1979; ii: 1327.
* Cyclosporin(e) A (original name) – isolated in 1969 from a soil fungus Tolypocladium inflatum (Beauveria nivea). Its immunosuppressive properties were identified by J. F. Borel (Swiss scientist working for Sandoz).
† Tacrolimus (originally FK506) – the name is derived from ‘Tsukuba macrolide immunosuppressant’, as it was isolated from soil bacteria Streptomyces tsukubaensis isolated from Mount Tsukuba, Japan.
* Campath – the name derives from a series of antibodies developed by the University of Cambridge pathology department.