Immunogenicity and Antigenicity
Learning Objectives
• Compare and contrast immunogens and antigens
• List the four major characteristics of an immunogen
• Identify the four biological factors that influence immunogenicity
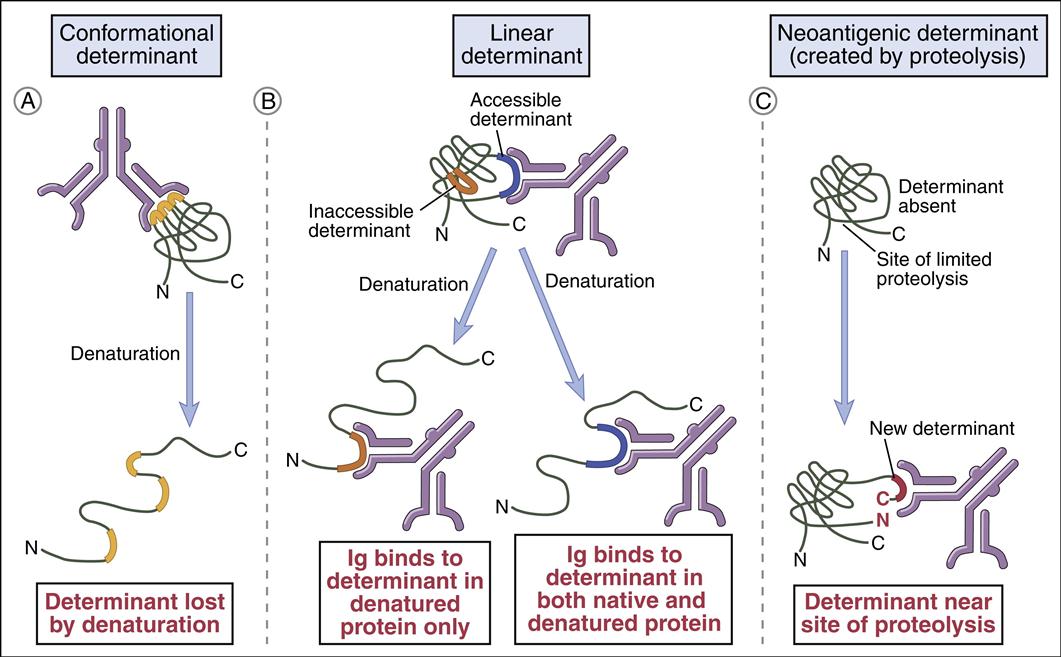
• Compare and contrast linear and conformational epitopes
• Explain the relationship between epitopes and T and B cells
• Compare and contrast isoantigens and alloantigens
• Explain the role of RhoGam in preventing an immune response to Rh-positive fetal red blood cells
• Recognize the differences between exogenous and endogenous antigens
• List the metabolites of penicillin
• Recognize the pharmaceuticals that create neoantigens on red blood cells
• Identify the drugs that cause delayed cutaneous drug reactions
Key Terms
Alloantigens
Conformational epitope
Endogenous antigen
Epitope
Exogenous antigen
Hapten
Isoantigen
Neoantigen
Linear epitope
Introduction
This chapter begins the exploration of an adaptive immune response. Unlike innate immunity, which recognizes highly conserved microbial structures, the adaptive response is designed to protect the host against microbial antigens that constantly change and evolve. As the microbe changes its tactics for infection, the immune system adapts to counter the tactic and destroy the microbe. Adaptive immunity requires the stimulation of the immune system, the proliferation of effector cells, and the synthesis of cytokines and antibodies.
The concepts of immunogenicity and antigenicity are critical to the understanding of adaptive immunity. By definition, an immunogen is a molecule that stimulates the immune system to produce a response. An antigen is the part of the immunogen that reacts with immune effector cells or soluble antibodies. The term allergen is used to denote an immunogen that elicits the production of allergic antibody.
Characteristics of an Immunogen
Foreignness
Immunogens that are considered “self” do not evoke an immune response; thus, foreignness is a critical attribute of immunogens. Phylogenetic differences between the host and the immunogen determine foreignness. For example, a vigorous response is generated when human serum albumin is injected into a mouse. In contrast, when mouse albumin is injected into a rat, only a minimal immune response is observed.
Internal Rigidity and Tertiary Structure
Most immunogens are proteins, which are complex rigid structures with a conformation defined by primary, secondary, and tertiary structures. Carbohydrates are composed of linear, repeating carbohydrate units with minimal structural rigidity. Therefore, carbohydrates are generally poor immunogens. The immunogenicity of deoxyribonucleic acid (DNA) depends on molecular weight and the extent of methylation. High-molecular-weight hypomethylated DNA is immunogenic. Other forms of DNA do not evoke an immune response.
Lipids, which are linear carbon chains with no defined tertiary structure, are rarely immunogenic because they lack structural rigidity. The exception is cardiolipin which is used as an antigen in the Wassermann test for syphilis.
Size
Size is an important determinant for antigenicity. Molecular structures less than 3000 MW (molecular weight) do not elicit an immune response, whereas maximum stimulation of the immune system is achieved with large antigens (e.g., 100,000 MW). Large macromolecules are better immunogens because they are insoluble and more easily ingested and processed by macrophages for presentation to lymphocytes.
Degradability
Immunogens must be degradable by macrophages to stimulate an immune response, antigen presenting cells must process the immunogen to yield small polypeptides between 7 and 30 amino acids. The small polypeptides are presented to lymphocytes.
Biologic Factors Influencing Immunogenicity
Genetics
Genetics influences a person’s ability to respond to specific immunogens. Genetic nonresponsiveness is the result of two defects. Some individuals lack a lymphocyte clone with a T cell receptor (TCR) directed at the antigen. Other individuals have a defect in antigen processing and cannot present the antigen to T cells.
Age
The immune response is influenced by a person’s age. Infants are born with a still-developing immune system and cannot mount an immune response to some antigens. Infants are protected by maternal antibodies that cross the placenta before birth and by antibodies in breast milk after birth. An infant’s immune system becomes fully functional between 6 and 12 months of age.
On the other end of the spectrum, the functional capacity of the immune system wanes with age, and older adults have a reduced ability to mount an effective immune response.
Ancillary Factors Influencing Immunogenicity
Concentration
The immune response is a reflection of immunogen concentration and follows a bell-shaped curve, referred to as gaussian distribution. Small amounts of antigen fail to stimulate the immune system and induce an irreversible tolerance to the antigen. Excessively large concentrations paralyze the antigen-presenting cells. Only optimal concentrations on the bell-shaped curve generate an immune response.
Route of Administration
The magnitude and the nature of the immune response are determined by the route of administration. Subcutaneous or intramuscular administration stimulates the systemic immune system and protects the host from dying of the disease. Slow release of the antigen from the subcutaneous depot stimulates the immune system maximally. When the antigen is administered by the mucosal route, the host is protected from infection and mortality from the infection. Oral administration is less effective because of rapid clearance of the antigen from the circulation or inactivation of the antigen by gastric fluids.
Epitopes
The molecular fragment of an antigen that interacts with effector cells or antibodies is called an epitope, or antigenic determinant. Proteins are usually large, complex structures that have multiple and different epitopes. One epitope, however, is dominant in the elicitation of an immune response.
Epitopes are either linear or conformational. Proteins have both linear and conformational epitopes. Linear epitopes comprise six to eight contiguous amino acids in the primary amino acid sequence of a polypeptide (Figure 3-1). Lymphocyte receptors or antibodies recognize linear epitopes in the native, fragmented, or extended conformations of the polypeptide. In contrast, conformational epitopes are created when protein segments are folded into a tertiary structure. The immune system recognizes the native conformational epitopes or the isolated fragments that retain the appropriate conformational tertiary structure.
T Cell and B Cell Recognition of Epitopes
Linear epitopes are recognized by T cells. Often, these epitopes are internal hydrophobic amino acid sequences processed by macrophages and presented to T cells in the context of human leukocyte antigen (HLA) class I and class II molecules. Processed epitopes containing 7 to 17 amino acids are presented to T lymphocytes by antigen-presenting cells. B cell epitopes from globular proteins, which range from 5 to 30 amino acids, are usually conformational. The length and flexibility of the epitope ensures high-affinity bonding to B cell receptors or circulating antibodies.
Types of Antigens
White Blood Cell Alloantigens
White blood cells express alloantigens, which are part of the body’s self-recognition system. Alloantigens are found in some, but not all, members of a species. In mice, the genes for white cell alloantigens are localized in the MHC on chromosome 17. Humans have a similar locus called the human leukocyte antigen (HLA) complex located on chromosome 6. These glycoproteins are subdivided into class I and class II antigens. These antigens are involved in the presentation of antigen (see Chapter 4), the rejection or acceptance of grafts (see Chapter 17) between members of the same species (e.g., allografts), or both. Class I HLA antigens are constitutive and are expressed on all nucleated cells. Class II antigens are inducible and are only expressed on macrophages and monocytes. Multi-parous women and transplant recipients often develop antibodies directed at alloantigens.
Transfusion-Related Acute Lung Injury
Transfusion-related acute lung injury (TRALI) is caused by the transfusion of blood products containing anti-HLA antibodies. The antibodies react with HLA molecules on circulating neutrophils, which pool in the lung capillaries and move into extravascular spaces. Antibody-coated neutrophils release free oxygen radicals, enzymes, and arachidonic acid metabolites, which damage the alveolar epithelium. Leakage of fluid into alveoli from capillaries causes pulmonary edema. Patients present with shortness of breath, hypoxia, and fever.
Red Blood Cell Antigens
The best-studied antigens in humans are found on red blood cells. Red blood cell antigens are water-soluble glycopeptides consisting of heterosaccharides attached by a glycosidic linkage at the reducing ends. The common core structure consists of a galactose and N-acetyl glucosamine attached to a glycoprotein core and is called the H-antigen. Most individuals have a fucosyltransferase enzyme that attaches a fucose to the terminal end of the H antigen. Two variants of a glycosyltransferase enzyme add additional sugars to the H antigen. The type A antigen is created when N-acetylgalactosamine is added to the terminal galactose. When an additional galactose is added to the terminal sugar, it produces a type B red cell antigen. In individuals who are heterozygous for blood group antigens, a galactosamine and N-acetylglucosamine are added to the core H antigen, which creates the AB blood type (Figure 3-2).
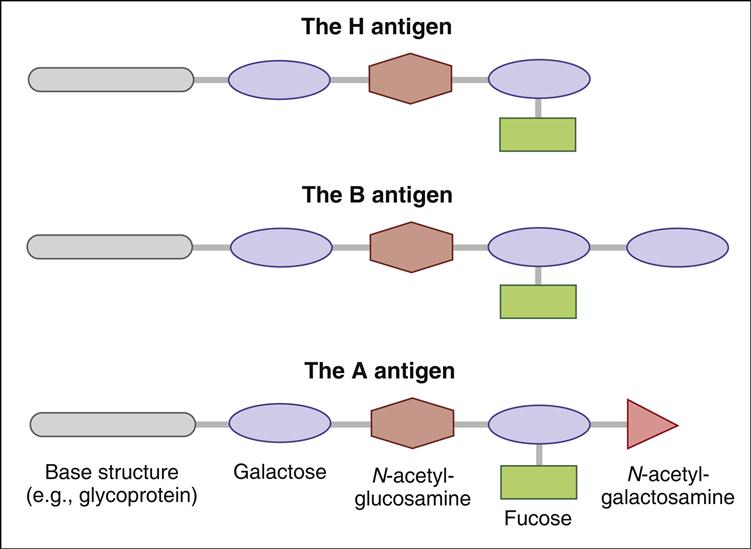
Using the ABO isoantigens, it is possible to classify red blood cell types into populations of universal donors or universal recipients. Since the O type contains an epitope that is common to all red blood cells (H antigen) and is non-immunogenic, individuals are universal donors. Conversely, persons with type AB blood are considered universal recipients because they express both A and B antigens. When transfused with type O, A, or B blood, persons with type AB blood do not mount an immune response because neither the A antigen nor the B antigen is foreign.
Antibodies directed at non-self blood group antigens are often present in serum. These natural antibodies are formed because A and B antigens are found in a wide variety of unrelated plant and animal tissues. Ingestion of these heterologous antigens stimulates the production of antibodies directed at non-self red cell antigens. For example, antibodies directed at blood group A are present in the serum of people with type B red blood cells. Conversely, persons with blood group A have anti-B antibodies in the serum. Antibodies directed at ABO blood group antigens can also be generated as a consequence of previous pregnancies, transfusions, or organ transplantations.
Hemolytic Transfusion Reactions
Hemolytic transfusion reactions occur when a patient receives red blood cells with major or minor antigens to which they have antibodies. An ABO mismatch typically occurs when a patient with group O blood type is transfused with group A, B, or AB blood cells. Antibodies in the recipient’s blood react with red cell isoantigens and activate complement that lyses the recipient’s red blood cells. As a result, hemoglobin is found in blood and urine, and microthrombi are formed. These small thrombi localize in the capillaries of the hands and feet. Obstruction of blood flow in the capillaries causes tissue necrosis and gangrene. Renal failure and cardiovascular collapse are other dangerous sequelae in these patients.
Treatment of Hemolytic Transfusion Reactions
Patients undergoing hemolytic transfusion reactions may experience mild or severe reactions. Mild symptoms include rashes, fever, and back pain. Acute kidney failure is a significant and severe problem in some patients. Treatment is directed at reducing the mild symptoms, increasing renal blood flow, and preserving urinary output (Table 3-1).
Table 3-1
Agents Used to Treat Patients Experiencing Mild or Severe Hemolytic Transfusion Reactions
| Agent | Reaction |
| Furosemide | Increases renal blood flow and preserves urinary output |
| Epinephrine | Increases bronchodilatation and peripheral vascular resistance |
| Antihistamines | Decreases the histamine response in nerve endings and blood vessels |
| Methylprednisolone | Decreases inflammation |
Rhesus Factor Isoantigens
Rhesus factor (Rh or RhD), or Rhesus antigen, is another isoantigen found on red blood cells. The name is derived from the fact that the blood antigen was first described in the Rhesus monkey. Rh antigens are nonglycosylated, hydrophobic cell membrane antigens expressed in 85% of the human population (Rh positive, or Rh+). Individuals with alterations or a deletion of the Rh protein are considered Rh negative (Rh–). If individuals with Rh– blood are exposed to Rh+ antigens, a vigorous antibody response is evoked. An Rh mismatch during transfusion causes a unique extravascular hemolytic anemia. Antibodies directed at the Rh factor do not activate complement. Hence, no intravascular hemolysis occurs. Rather, red cells are coated with antibody and removed by splenic macrophages.
Rh antigens play a significant role in transfusion reactions; however, Rh compatibility takes on an even more significant and crucial role in pregnancy. Serious problems arise when the Rh– mother is exposed to Rh+ cells. Exposure can occur as a consequence of normal delivery, trauma, or blood transfusions. Within 30 days of exposure, the mother will develop anti-Rh antibodies. However, these large (900,000 MW) IgM antibodies cannot cross the placenta. Therefore, the first child will be unaffected. If the mother is exposed to Rh T cells during a second pregnancy, small (150,000 MW) IgG antibodies are produced. These antibodies can cross the placenta and attack the fetal red blood cells, causing an autoimmune hemolytic anemia, called erythroblastosis fetalis, with severe consequences. The lysis of red cells liberates hemoglobin, which is converted to bilirubin. Accumulation of bilirubin damages the central nervous system, and the infants develop hypotonia, hearing loss, and intellectual disabilities.
Severe forms of erythroblastosis fetalis are characterized by cardiac failure, pericardial effusions, and edema (hydrops fetalis).
Treatment of Rh Incompatibility
Pooled human anti-D immune globulin (Rh IgG or RhoGAM) treatment is indicated at 28 weeks and within 72 hours after delivery if the baby is Rh+. It is also indicated following spontaneous or induced termination and any event that could lead to transplacental hemorrhage. A single dose (50 micrograms [μg]), which is administered following first-trimester pregnancy termination, is enough to neutralize 2.5 mL of fetal blood. A 300-μg dose, which can neutralize 15 mL of fetal blood, is administered for all other indications. When properly administered, the incidence of adverse effects is less than 0.5%.
Exogenous and Endogenous Antigens
Exogenous Antigens
Exogenous antigens enter the body via the oral, respiratory, and parenteral routes. In general, exogenous antigens are immunogenic structures expressed on extracellular bacteria, fungi, viruses, and pollens. Exogenous antigens are ingested by macrophages, and epitopes are presented in the context of class II molecules to Th2 cells. In some cases, exogenous antigens may be secretory products of bacteria or liberated on the death of the bacterium. For example, secreted protein exotoxins are both immunogenic and toxic to mammalian tissue. Lipopolysaccharide endotoxins are an integral part of the gram-negative cell wall and are released into the circulation following bacterial death.
Endogenous Antigens
Endogenous antigens are generated by cells infected by viruses, intracellular parasites, or tumor cells. These antigens are produced internally, processed in the cytosol, and loaded onto HLA class I molecules for presentation to CD8 cells. Antigen-specific CD8 cells then destroy the tumor cells.
Autoantigens
Autoantigens are the result of mutation, neoantigen formation, or exposure of previously hidden self-antigens. Genes producing self-proteins can mutate and create a new immunogenic protein called neoantigen. Viral infections and drugs can create neoantigens that stimulate an immune response. In some cases, auto-reactive proteins found in organs that develop late in gestation (e.g., eyes, testes) are not present when lymphocytes undergo positive and negative selection in the thymus. Therefore, auto-reactive T cells are not selected for destruction in the thymus and enter peripheral blood. Under normal circumstances, autoantigens are protected from the immune system by anatomic barriers (e.g., testes), a lack of blood vessels, or cellular structures that force immunocompetent cells to undergo apoptosis (e.g., eyes). Trauma or infection can expose autoantigens, and the resulting immune response damages the tissue.
Haptens
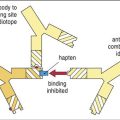
Haptens are small-molecular-weight compounds that evoke an immune response only when they are attached to carrier proteins. In vivo, haptens readily bind to serum proteins such as albumin. The combined molecular weights of albumin and the hapten need to exceed 3000 MW to stimulate the immune system. The immune response is directed at both the hapten and the carrier protein. The carrier protein has a different and unique antigenic structure after binding to the hapten.
The concept of haptens was introduced by Landsteiner. Subsequently, experiments with murine models demonstrated that immunization with m-aminobenzene sulfonate failed to elicit an immune response (Figure 3-3).
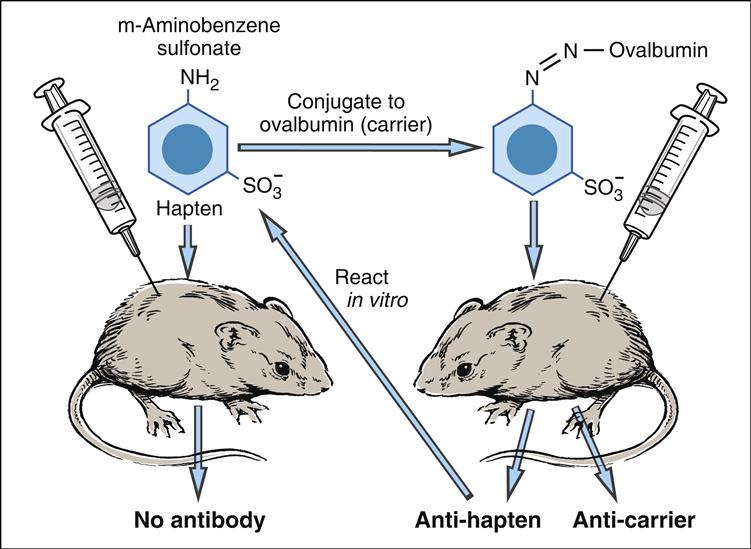
However, a vigorous antibody response was observed when m-aminobenzene sulfonate was linked to carrier protein (ovalbumin). Antibodies were directed to both the aminobenzene and the carrier protein. The phenomenon was termed haptenic response, from the Greek haptein, which means “to fasten.”
Pharmaceuticals as Haptens
Most pharmaceuticals and antibiotics are small (at or less than 3000 MW) compounds. The native drug or metabolites are often haptens that bind to serum proteins or molecules expressed on cells and elicit either an antibody response or a cellular response. An immune response to the hapten carrier complex can result in skin eruptions, asthma, anaphylaxis, and autoimmune reactions. Antibiotics and anesthetics are common biologically active haptens.
Antibiotics as Haptens
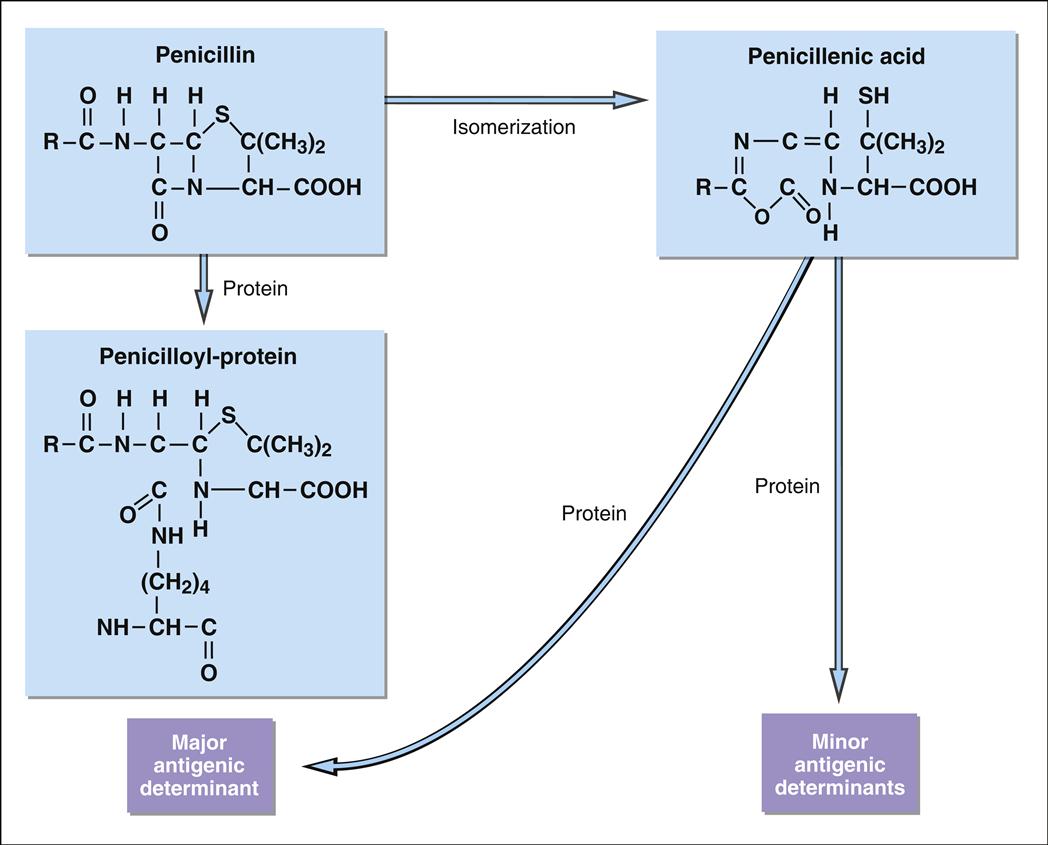
Penicillin is the leading cause of immunologically mediated adverse health effects. Approximately 2% of patients receiving penicillin therapy develop urticaria, asthma, or angioedema. Anaphylaxis, which is a serious and often fatal reaction to penicillin, results in 500 to 1000 deaths each year in the United States. Penicillin is composed of an acyl side chain linked to a β-lactam ring combined with a thiazolidine ring. Natural penicillin (PenG), penicillinase-resistant penicillin (methicillin), extended-spectrum penicillin (amoxicillin), and broad-spectrum penicillin (carbenicillin) all have the same core β-lactam ring, which is essential for antimicrobial activity. Bacteria secrete a β-lactamase that breaks the lactam ring, rendering the antibiotic ineffective and creating highly charged metabolites (Figure 3-4). The major antigenic determinant (90%–95% of the breakdown products) is a benzylpenicilloyl derivative (BPO).
Minor metabolites include parental penicillin, penicilloate, and penicilloylamine. Major and minor metabolites combine with proteins to become immunogenic.
Cephalosporins have a structure that is similar to penicillin and are reactive haptens. For reasons that are unclear, third (ceftriaxone) and fourth (cefepime) generations of cephalosporins are more involved in immunologically mediated adverse health effects. The haptenic determinants of cephalosporin are not fully delineated. It has been suggested that serologic reactivity is directed at both the acyl side chains and the β-lactam ring linked to the carriers.
Anesthetics as Haptens
Exposure to halothane may also induce an autoimmune reaction. Introduced in 1951 as a potent, nonflammable anesthetic agent, halothane is metabolized in oxidative pathways to trifluoroacetyl chloride (TFAC). In a subsequent chemical reaction, TFAC acetylates liver proteins to form a neoantigen. An immune response to the neoantigen causes halothane hepatitis. Inflammation of the liver abates when the drug is discontinued.
Pharmaceuticals and Neoantigens
Highly reactive haptens readily bind to red blood cell membranes to create immunogenic neoantigens (Box 3-1). For example, methyldopa binds to red blood cell membrane, thus creating a neoantigen. A hemolytic anemia results when antibodies react with the neoantigen and lyse the red cells.
Haptens and Cell-Mediated Reactions
Some responses to pharmaceuticals are mediated by inflammatory cells rather than by antibodies. Skin lesions, which are characterized by redness, induration, or blistering, occur 24 to 48 hours after exposure and are mediated by CD4Th1 cells and inflammatory macrophages. Inflammatory responses in the liver and kidney have also been reported. Clinical symptoms may persist after discontinuation of the drug.
Delayed hypersensitivity reactions in the skin or cutaneous drug reactions (CDRs) occur following systemic administration of a wide variety of drugs. Antimicrobial agents (sulfonamides), anticonvulsants (carbamazepine), anesthetics (lidocaine), anti-psychotics (clozapine), cardiovascular agents (procainamide, hydrazaline) and nonsteroidal anti-inflammatory drugs (diclofenac) are metabolized by the liver with the creation of reactive haptens, which bind to skin cells. An inflammatory response in the skin is characterized by widespread rashes and eruptions. Reactions in the skin usually occur 7 to 10 days after drug administration.
Summary