Chapter 93 Imaging Evaluation and Endovascular Treatment of Vasospasm
The phenomenon of cerebral vasospasm following aneurysmal subarachnoid hemorrhage (aSAH) was first described in the 1950s by Ecker and Riemenschneider,1 and substantial contributions have been made in subsequent decades to the clinical and pathophysiologic understanding of this debilitating condition. The traditional belief has been that subarachnoid blood products trigger vasospasm of the proximal, large-caliber cerebral vessels, which consequently leads to impaired cerebral perfusion and eventual infarction of the affected tissue. Modern investigations of this phenomenon, which has been termed delayed cerebral ischemia (DCI), have further implicated distal microvascular disease2 and dysregulated proliferation of smooth muscle and endothelial cells in its pathophysiology.3,4 While the complete picture of its pathogenesis remains to be fully elucidated, there is strong evidence that the presence of severe vasospasm correlates with the development of DCI and subsequent cerebral infarcts. Prompt diagnosis and treatment of vasospasm prior to the onset of permanent ischemic damage are therefore essential to improving survival and preventing secondary loss of neurologic function in the aSAH patient population.5
Historically, the most common cause of mortality following initial hemorrhage from aSAH was rebleeding. Advancements in surgical and endovascular techniques and a general trend toward more aggressive, early repair of aneurysm have led to a decrease in the incidence of this complication.6 DCI from vasospasm has thus emerged as the most common cause of secondary morbidity and death. From literature and clinical experience, this reversible narrowing of cerebral vessels typically occurs between 3 and 14 days7–9 following initial hemorrhage, with a peak incidence around day 7.10 Up to 70% of patients demonstrate angiographically visible vasospasm within this time window, but only 20% to 30% of these cases develop clinical evidence of cerebral ischemia and consequently require acute therapy.7,8,11,12 Of symptomatic patients, up to 50% suffer devastating neurologic deficits or death as a result of clinically significant vasospasm, highlighting the importance of prompt diagnosis and therapeutic management.9,13
To assess which patients are at highest risk of developing vasospasm, various groups have suggested clinical factors, including Hunt and Hess grading and characteristics of the subarachnoid clot on computed tomography (CT) (i.e., initial presence, volume, density, and duration) that associate with an increased risk of vasospasm.6,7,8 Other clinical variables, including young patient age, poor neurologic grade, greater-than-normal thickness of the subarachnoid clot, intraventricular or intracerebral hemorrhage, and history of smoking, have been associated with the development of more severe vasospasm.8,11,12 While these considerations can aid in the care of aSAH patients, no comprehensive prediction algorithm exists to determine which patients will suffer DCI. Therefore, there continues to be no substitute for vigilant clinical monitoring and careful decision making by an experienced, multidisciplinary care team to prevent or limit the neurologic injury from vasospasm.14
Diagnostic Imaging
Transcranial Doppler Ultrasonography
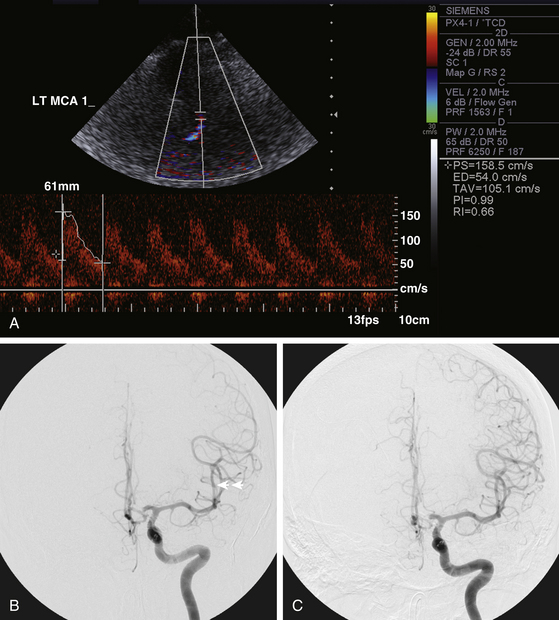
Transcranial Doppler (TCD) ultrasonography has been widely employed as the first-line modality for monitoring cerebral vasospasm in aSAH patients (Fig. 93-1). It is noninvasive, inexpensive, and easily performed at the bedside, making it particularly appropriate for daily evaluations. The sensitivity of TCD is highest for vasospasm in the proximal middle cerebral artery (MCA) but decreases in other vascular territories.15 It also varies depending on the adequacy of vessel insonation.16 The diagnostic value of TCD comes from its high specificity—detection of normal flow velocities can effectively exclude the presence of vasospasm.17 It is thus an excellent modality for initial patient triage.
Noncontrast Head CT
For patients with changes in clinical exam or TCD findings, noncontrast head CT should be performed before any subsequent interventions are considered. This modality offers a rapid survey for infarctions in the territory of suspected vasospasm and rules out other etiologies of neurologic deterioration, including hydrocephalus and rehemorrhage. The identification of developing infarcts has important implications for subsequent treatment decision making, because the restoration of flow to these areas typically provides minimal recovery of neurologic function and can potentially lead to further decline as a result of reperfusion hemorrhage.18,19
Computed Tomography Angiography and Perfusion
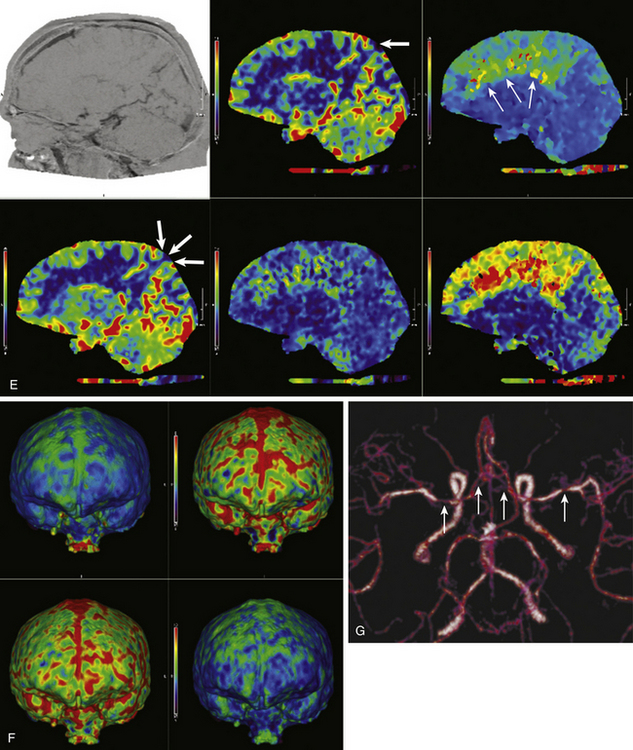
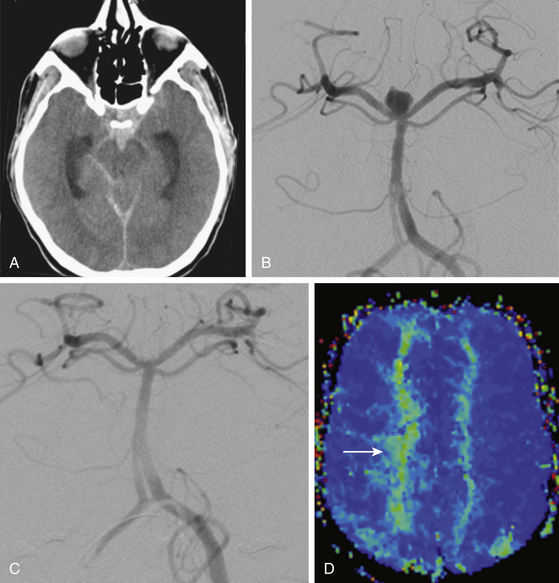
Computed tomography angiography (CTA) and computed tomography perfusion (CTP) imaging have undergone significant advancements in recent years and have emerged as effective modalities for triaging vasospasm patients toward endovascular therapy (Fig. 93-2). CTA has been shown to be highly accurate for detection of severe vasospasm (more than 50% luminal reduction) and has excellent negative predictive value.20,21 The severity of vasospasm can be overestimated in certain vascular territories, and metallic artifacts from coils or clips can hinder the assessment of nearby territories. Despite these limitations, CTA provides an informative and practical assessment of cerebral vessel caliber in patients with concerning symptoms. The recent addition of CTP scans to the vasospasm imaging armamentarium has allowed insight into the hemodynamic implications of CTA findings. Stereotypical patterns of perfusion abnormality can indicate the presence of either reversible ischemia, which should be addressed promptly to maximize penumbral recovery, or irreversible ischemia, which is a contraindication to aggressive therapy. This distinction is essential for the appropriate triage of patients toward endovascular therapy, because the treatment of infarcted territories potentially leads to further morbidity. A combined, multimodality, CT-based approach has been implemented at many institutions, allowing acquisition of conventional CT, CTA, and CTP images in one setting. This protocol is well suited for aSAH patients for whom lengthy transport or imaging studies may be unfeasible. Clinicians need to be wary of radiation exposure when ordering repeated CT studies; there have been reports of associated sequelae in the medical literature and lay press.22
Magnetic Resonance Angiography and Perfusion-Weighted Magnetic Resonance Imaging
Magnetic resonance angiography and perfusion-weighted magnetic resonance imaging (MRI) have been used for detecting vasospasm (Fig. 93-2) but have failed to achieve wider adoption for vasospasm imaging due to logistical impracticability of MRI in acutely sick patients.
Medical Therapies for Cerebral Vasospasm
Although the scope of this chapter is endovascular management for vasospasm, we must emphasize that medical therapy remains the first-line and mainstay treatment for a majority of patients. Hemodynamic therapy via induced hypervolemia, hypertension, and hemodilution (triple-H therapy) has achieved widespread use, and studies have demonstrated efficacy in improving cerebral perfusion, as well as clinical outcomes.23 Patients who do not respond to this treatment or who cannot tolerate sufficient periods of hyperdynamic therapy due to underlying medical comorbidities are candidates for endovascular intervention.
Overview of Endovascular Therapies for Cerebral Vasospasm
The goal of endovascular therapy for symptomatic cerebral vasospasm is to restore blood flow to ischemic parenchyma and salvage the penumbra region (Figs. 93-1, 93-3, and 93-4). These interventions are not the first-line treatment due to inherent procedural risks and intensive resource requirements, but when performed in appropriately selected patients, they can produce excellent angiographic and clinical outcomes.
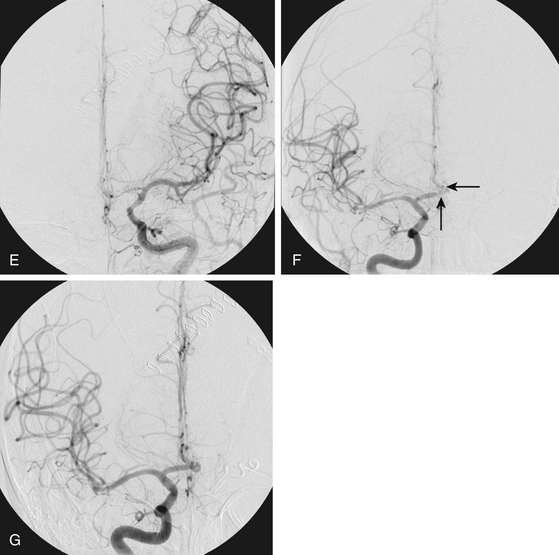
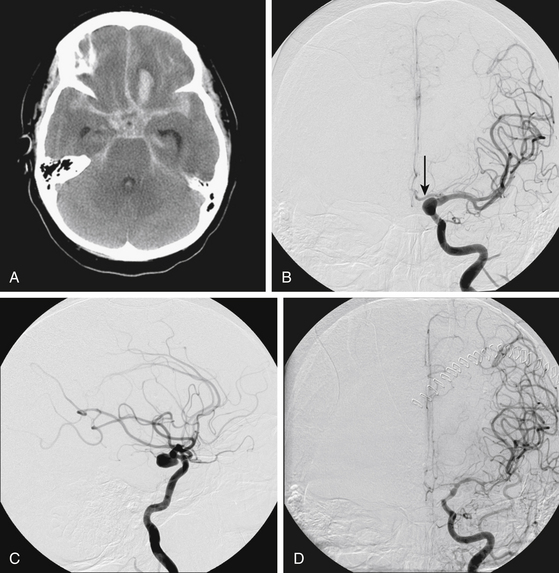
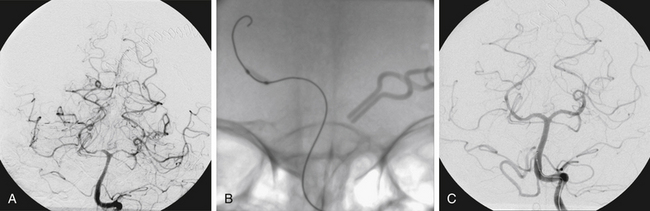
FIGURE 93-4 Combined therapy (IA verapamil and balloon angioplasty) of vasospasm. A, An initial CT scan shows diffuse SAH and a left frontal intraparenchymal hematoma. The cause of this SAH was believed to be a left-sided posterior communicating artery aneurysm, shown on anteroposterior (B) and lateral (C) projections. The aneurysm was clipped. In B, the left A1 segment is hypoplastic (arrow). D, The patient experienced symptomatic vasospasm. An angiogram reveals severe vasospasm of the left M1 segment and moderate spasm of the supraclinoid ICA.FIGURE 93-4, cont’dE, The left MCA and distal ICA were treated with angioplasty with good result. The A1 segment was intentionally not angioplastied since it was a congenitally hypoplastic vessel. Angioplasty of a hypoplastic vessel should be avoided to prevent the risk of vessel rupture. F, Restoration of anterior cerebral artery (ACA) perfusion was addressed from the contralateral, dominant right A1. This right ICA angiogram reveals vasospasm of the distal right A1 and proximal A2 segments (arrows). G, The spastic right A1 segment was treated with balloon angioplasty. A subsequent angiogram shows improved vessel caliber of the target segment and more robust filling of the distal ACA branches.
IA Vasodilator Infusion
IA vasodilator infusions have been employed widely for the pharmacologic treatment of cerebral vasospasm, but the efficacy and duration of these agents remains modest. For distal vasospasm that cannot be readily accessed by mechanical angioplasty devices, IA vasodilator infusions nonetheless provide an important means to improve blood flow and prevent permanent ischemic damage (Fig. 93-1). Anecdotal reports have also suggested the use of IA vasodilator infusion as an adjunct to angioplasty to reduce the vasomotor tone of the vessel and potentially decrease the subsequent risk of acute vessel rupture during balloon dilation (Fig. 93-4).
Papaverine
The first pharmacologic vasodilator that was employed broadly for treatment of cerebral vasospasm was papaverine, an alkaloid of the opium group with a half-life of 2 hours that acts as a nonspecific vasodilator by increasing cyclic adenosine monophosphate levels in smooth muscle cells.24,25 Studies examining the efficacy of the drug demonstrated angiographic improvement in 75% of cases, but modest clinical improvements were achieved in only 25% to 52% of patients.19,26,27 Furthermore, these improvements were often transient, and some patients required multiple treatments, which were associated with worsened complication profiles.25 Described complications include raised intracranial pressure (ICP), seizures, hypotension, transient brain stem depression, worsening vasospasm, monocular blindness if infusion is performed proximal to the ophthalmic artery origin, and gray matter injury by direct neurotoxicity.18,27,28 The combination of these risks with the papaverine’s relatively short duration of action have led to the replacement of this drug by calcium channel blockers (CCBs) for IA treatment of vasospasm in modern practice.
Calcium Channel Blockers
As a class, CCBs have been met with the greatest recent investigational interest because of their excellent safety profiles and consistent efficacy. Their implementation as IA agents was logical, because there has been extensive experience with intravenous administration of these drugs to patients before and after definitive aneurysm therapy. Mechanistically, the benefit of these drugs have traditionally been attributed to inhibition of voltage-gated calcium channels in smooth muscle cells, but evidence that patients can demonstrate a positive clinical response without corresponding improvements to angiographic vasospasm have alluded to the presence of indirect benefits, such as neuroprotective effects.29 Verapamil, nimodipine, and nicardipine have been the most studied of the CCBs, but randomized, controlled trials examining the use of these drugs for treatment of vasospasm remain to be conducted, and their use toward this application still remains off-label. As such, the optimal doses, infusion rates, and retreatment intervals remain to be definitively studied and vary substantially between groups.
Verapamil is a phenylalkylamine CCB with a half-life of approximately 7 hours. Preliminary studies have yielded mixed results, with some groups reporting angiographic and clinical improvement30 while others have been unable to demonstrate statistically significant effects.31 The ambiguity of these data may be due to the lack of quantified analysis and blinded radiographic review among some of these studies.32 The appropriate dose to achieve therapeutic effects remains uncertain. On one hand, groups have reported the doses as high as 41 mg per procedure33 but Feng et al. demonstrated increased vessel diameter in 44% of patients and neurologic improvement in 29% with only 3 mg per procedure.30 The appropriate time course of administration also remains to be elucidated—most studies to date have employed bolus infusion strategies, but it has been suggested that prolonged infusions may offer greater clinical benefit. Albanese et al. recently examined the efficacy of long-term IA infusion (average of 7.8 hours) with an average dose per vessel of 164.6 mg in 12 patients and reported improved vessel caliber in 89% of treated vessels, with favorable clinical outcome at 8 to 12 months in 73% of patients.34 As optimization of the treatment protocol improves through additional research, the potential for further improvements in angiographic and clinical outcomes is optimistic.
Nimodipine is a dihydropyridine CCB with a half-life of approximately 8 to 9 hours that works through mechanisms similar to those of verapamil and possesses a similar safety profile. It has not been available for use in the United States but has been adopted in Europe and Australasia. Biondi et al. reported clinical improvement 19 of 25 patients (76%) in the first 24 hours following IA nimodipine infusion.35 At 3 to 6 months follow-up, 18 patients (72%) had a favorable clinical outcome. Successful dilation of infused vessels, however, occurred in only 13 out of 30 (43%) of procedures, so the results raise some uncertainty as to whether improvements in vessel caliber were truly responsible for the positive outcomes. Doses of up to 3 mg per vascular territory were used (for a total of 1-5 mg per session) at a rate of 1 mg over 10 to 15 minutes to minimize hypotension. No episodes of increased ICP or other complications were noted. The use of continuous nimodipine infusion for vasospasm that is refractory to bolus treatment has been reported in a series of 9 patients by Wolf et al.36 The authors used a 2 mg per hour or 2 mg every 30 minutes for a three-times-daily infusion schedule and showed improvements in CTP patterns and positive long-term clinical outcomes in 3 patients (33%). However, another 3 patients (33%) died from refractory vasospasm, so further investigations are necessary before definitive recommendations for the use of continuous infusions can be made.
Nicardipine is a dihydropyridine CCB that is more selective for vascular smooth muscle than for cardiac smooth muscle and possesses a half-life of approximately 16 hours (Fig. 93-1). In the series by Badjatia et al., 44 vessels were treated in 18 patients at doses of 0.5 to 6 mg per vessel.37 DSA and TCD demonstrated reductions in vasospasm in all patients, and clinical improvement was observed in 42% of patients. Although the drug was generally well tolerated, 4 patients suffered transient increases in ICP and 1 had a persistent increase that necessitated termination of nicardipine infusion. In a subsequent paper, Tejada et al. reported a series of 20 treatments in 11 patients for which they attained effective angiographic responses of more than 60% increase in arterial diameter in all patients and clinical improvement in the Glasgow coma scale or resolution of focal symptoms in 10 of 11 patients (91%) following IA infusion of 10 to 40 mg of nicardipine.38 Linfante et al. showed similarly promising results, with angiographic vasospasm improvement in 95% of cases following 2 to 25 mg of IA nicardipine infusion in 22 patients with symptomatic vasospasm, 50% of whom were functionally independent at the time of discharge.39 Although retreatment is required in some patients, the current literature and experience suggest that the duration of effect of nicardipine is favorable when compared to other IA agents.
The benefits of IA nicardipine infusions may also extend to the microvascular and parenchymal levels, which may not be evidenced by angiographic examination of larger proximal vessel vasospasm.40 In terms of complications, modest reductions in blood pressure of between 17 and 23 mm Hg have been reported with the use of IA nicardipine, and vasopressor support may occasionally be needed.38,39,41
Other Pharmacologic Agents under Investigation
Aside from the previously mentioned drugs, agents including magnesium sulfate, 3-3-hydroxy-3-methylglutaryl–coenzyme A reductase inhibitors, nitric oxide donors, and endothelin-1 antagonists have been used, but their application remains limited.
Transluminal Balloon Angioplasty
Introduction and Vessel Selection
The use of TBA in cerebral vasospasm was first described in the 1980s by Zubkov et al.42 and has been refined substantially in the decades since. The precise mechanisms by which mechanical angioplasty produces durable patency and resistance to recurrent vasospasm remains to be fully delineated but may include flattening of the smooth muscle and endothelial cells, disruption of the extracellular matrix, and separation of connections between cellular basement membranes.43,44 Although the vessels seem to lose some responsiveness to pharmacologic vasodilators following angioplasty, there is no evidence that TBA causes significant structural damage to vessels when performed with appropriate safety considerations.8,43
All patients for whom the use of TBA is considered must undergo a detailed review of baseline angiographic findings. Assessment of vessel morphology and diameter is especially important as a means to differentiate congenitally hypoplastic vessels (most commonly seen in A1 segments, intradural vertebral arteries, P1 segments, and posterior communicating arteries) from vessels in acute spasm (Fig. 93-4). Attempts to angioplasty these hypoplastic segments can result in catastrophic, acute vessel rupture and must be avoided. In addition, vessels near the site of recent surgical clipping should be not be angioplastied, because fatal rupture has been reported under these circumstances.45
The segments that are traditionally considered amenable for angioplasty are the more proximal, large (more than 2 mm) vessels of the circle of Willis, including the vertebral, basilar, and supraclinoid internal carotid arteries, as well as M1 segments. The adoption of dedicated neurovascular balloons, which have improved safety profiles and trackability, have allowed farther reach into the immediate distal branches of these vessels, including the posterior communicating arteries, A1, proximal A2, M2, P1, and P2 segments (Fig. 93-3).
Balloon Technologies
Historically, the first endovascular balloons used for TBA in cerebral vasospasm were borrowed from the larger coronary interventional field. These balloons are composed of a stiff polyethylene or nylon membrane on a double-lumen shaft, which allows for balloon inflation and passage of a guidewire. Sizing decisions need to be conservative, because the rigid characteristics of these balloons can easily cause vessel rupture if the diameter of the chosen balloon is excessive—Eddleman et al. have suggested, as a general reference, 2-mm-diameter balloons for MCA spasm and 1.5-mm-diameter balloons for M2 or A1 segments.14 Shorter coronary balloons such as the Maverick (Boston Scientific) have favorable characteristics, including improved maneuverability and less vessel straightening on inflation. The Gateway balloon (Boston Scientific) was more recently developed from coronary technologies for dedicated intracranial use and can be used for treatment of intracranial vasospasm, but it is primarily designed for the treatment of intracranial atherosclerotic stenoses.
The advent of intracranial balloon catheters has substantially improved the safety of TBA procedures, because these balloon systems are made of a softer, semipermeable silicone/elastomer membrane mounted on a flexible, single-lumen catheter shaft (Fig. 93-3). The single-lumen system is much softer than its coronary counterparts but has the slight disadvantage of lacking a continuous flush option. Examples of current neurovascular balloon catheter systems include the Hyperglide (ev3 Neurovascular) and Hyperform (ev3 Neurovascular), both of which come in a number of diameter and length configurations. The Hyperform is more compliant, but the Hyperglide has more length options and is thus better suited for angioplasty of longer arterial segments. Both are designed for navigation over an atraumatic, 0.010-inch X-Pedion guidewire. In occasional situations in which steerability in tortuous arteries with acute angles becomes cumbersome, a microcatheter can be placed into the target vessel first, followed by balloon exchange over a 0.010-inch exchange length X-Celerator microwire (ev3 Neurovascular).
Balloon Inflation
We prefer to inflate the balloons with a threaded, precisely calibrated, 1-ml Cadence (ev3 Neurovascular) syringe that enables adjustment in the 0.01-ml range. To decrease the risk of acute vessel rupture, the goal of angioplasty should generally not be to achieve the projected normal caliber of the target vessel but rather to improve vessel caliber enough to augment flow to ischemic brain regions.
Efficacy
A number of series have examined the efficacy of TBA for the treatment of subarachnoid hemorrhage (SAH)–related vasospasm. A review by Hoh and Ogilvy of the overall rate of clinical improvement among recent reports, which included a total of 530 patients, found an average rate of 62% with a range of 11% to 93%.25 The studies analyzed in this review include that of Eskridge et al. (50 patients and 170 vessel segments, demonstrating 61% sustained neurologic improvement at 72 hours),46 Bejjani et al. (31 patients with 72% neurologic improvement),47 Higashida et al. (28 patients with 61% neurologic improvement),19 and Fujii et al. (19 patients with 63% neurologic improvement).48 These data were limited by the lack of matched controls, but further investigations comparing vascular territories treated with TBA to territories in the same patient that were not treated with TBA have shown decreased rates of infarction on CT following TBA (7% for treated vs. 38% for untreated).49
The timing of TBA for cerebral vasospasm is thought to be a critical component in achieving maximum clinical benefit in patients with symptomatic vasospasm, but the exact therapeutic window remains to be fully determined. Rosenwasser et al. published a retrospective study on vasospasm patients treated with TBA inside and outside of a 2-hour window and reported that the rate of angiographic improvement was not significantly different between the two groups (90% of patients treated within 2 hours vs. 88% of patients treated outside 2 hours) but that the rate of clinical improvement was significantly higher in patients treated sooner (70% of patients treated within 2 hours vs. 40% of patients treated outside 2 hours).50 Although the use of prophylactic TBA has also been suggested, a recent phase II clinical trial for the prophylactic use of TBA in Fisher grade III SAH patients failed to show improvements in clinical outcome at 3 months follow-up, so this use cannot currently be recommended.51
Complications
Complications during TBA include vessel rupture, thromboembolism, and reperfusion hemorrhage and are essential considerations for the procedure, because each of these complications can be potentially devastating.19,42,44,46,52 Vessel rupture is perhaps the most feared of these events, and reviews have cited rates between 0% and 7.7% with a mean occurrence of 1.1%.25 With the wide adoption of dedicated neurovascular balloon systems and increased technical experience, the rate of rupture in current practice is likely trending toward the lower end of that spectrum. Particular care must still be taken in cases of severe vessel spasm in which limited distal visualization can be predisposed to wire- or catheter-induced perforation of small branch vessels. Thrombus formation can occur around the balloon or within the catheter lumen and should be prevented by appropriate patient heparinization unless contraindicated.
Combination Therapy
The use of current IA vasodilator agents in conjunction with TBA may possibly provide improvements in patient outcomes, but the evidence in literature remains limited. In theory, the use of IA agents prior to TBA can offer an initial improvement in proximal vessel diameter to facilitate safer subsequent navigation of endovascular balloon catheters. Alternatively, once proximal vessel spasm has been addressed with mechanical angioplasty, pharmacologic agents may be able to more easily access and treat distal and microvascular spasm (Fig. 93-3). Given the excellent safety profiles of the CCBs that are most commonly used for IA infusions today, the addition of pharmacologic agents to mechanical angioplasty is unlikely to add significant risk to the procedure and can be used at the discretion of the care team. Future studies investigating the benefit of modern IA vasodilators as an adjunct to TBA are needed before definitive recommendations can be made for practice.
Conclusions
Perfusion scans, if available, should be interpreted carefully prior to aggressive endovascular therapy to identify evidence of significant, established, irreversible ischemia. Either CTA/CTP or MRI/magnetic resonance perfusion studies can be utilized based on institutional practices to help increase the safety and positive impact of endovascular therapeutic techniques. TBA is currently the most reliable option for durable treatment of proximal vasospasm and should be instituted for these vessel segments whenever technically possible. IA vasodilator infusions should be employed for distal vasospasm and can provide an adjunct to mechanical angioplasty. Although the safety profiles of these pharmacologic agents are favorable, patient vital signs and cerebrovascular parameters should be monitored closely to avoid additional morbidity from hypotension or elevated ICP. Because many of the technologies used for the modern diagnosis and treatment of symptomatic cerebral vasospasm remain to be fully investigated, further research will be important for clarification of the optimal role of endovascular therapies in managing patients within the post-SAH vasospasm window.
Albanese E., Russo A., Quiroga M., et al. Ultrahigh-dose intraarterial infusion of verapamil through an indwelling microcatheter for medically refractory severe vasospasm: initial experience. Clinical article. J Neurosurg. 2010;113:913-922.
Badjatia N., Topcuoglu M.A., Pryor J.C., et al. Preliminary experience with intra-arterial nicardipine as a treatment for cerebral vasospasm. AJNR Am J Neuroradiol. 2004;25:819-826.
Biondi A., Ricciardi G.K., Puybasset L., et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. AJNR Am J Neuroradiol. 2004;25:1067-1076.
Dorsch N., King M. A review of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Part I: incidence and effects. J Clinical Neuroscience. 1994:19-26.
Ecker A., Riemenschneider P.A. Arteriographic demonstration of spasm of the intracranial arteries, with special reference to saccular arterial aneurysms. J Neurosurg. 1951;8:660-667.
Eddleman C.S., Hurley M.C., Naidech A.M., et al. Endovascular options in the treatment of delayed ischemic neurological deficits due to cerebral vasospasm. Neurosurg Focus. 2009;26:E6.
Eskridge J.M., McAuliffe W., Song J.K., et al. Balloon angioplasty for the treatment of vasospasm: results of first 50 cases. Neurosurgery. 1998;42:510-516. discussion 516–517
Feng L., Fitzsimmons B.F., Young W.L., et al. Intraarterially administered verapamil as adjunct therapy for cerebral vasospasm: safety and 2-year experience. AJNR Am J Neuroradiol. 2002;23:1284-1290.
Fergusen S., Macdonald R.L. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;60:658-667. discussion 667
Firlik K.S., Kaufmann A.M., Firlik A.D., et al. Intra-arterial papaverine for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Surg Neurol. 1999;51:66-74.
Frontera J.A., Claassen J., Schmidt J.M., et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery. 2006;59:21-27. discussion 21–27
Fujii Y., Takahashi A., Yoshimoto T. Effect of balloon angioplasty on high grade symptomatic vasospasm after subarachnoid hemorrhage. Neurosurg Rev. 1995;18:7-13.
Higashida R.T., Halbach V.V., Dowd C.F., et al. Intravascular balloon dilatation therapy for intracranial arterial vasospasm: patient selection, technique, and clinical results. Neurosurg Rev. 1992;15:89-95.
Hoh B.L., Ogilvy C.S. Endovascular treatment of cerebral vasospasm: transluminal balloon angioplasty, intra-arterial papaverine, and intra-arterial nicardipine. Neurosurg Clin N Am. 2005;16:501-516. vi
Janardhan V., Biondi A., Riina H.A., et al. Vasospasm in aneurysmal subarachnoid hemorrhage: diagnosis, prevention, and management. Neuroimaging Clin N Am. 2006;16:483-496. viii-ix
Jestaedt L., Pham M., Bartsch A.J., et al. The impact of balloon angioplasty on the evolution of vasospasm-related infarction after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2008;62:610-617. discussion 610–617
Kassell N.F., Helm G., Simmons N., et al. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg. 1992;77:848-852.
Keuskamp J., Murali R., Chao K.H. High-dose intraarterial verapamil in the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;108:458-463.
Linfante I., Delgado-Mederos R., Andreone V., et al. Angiographic and hemodynamic effect of high concentration of intra-arterial nicardipine in cerebral vasospasm. Neurosurgery. 2008;63:1080-1086. discussion 1086–1087
Macdonald R.L., Pluta R.M., Zhang J.H. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256-263.
Mazumdar A., Rivet D.J., Derdeyn C.P., et al. Effect of intraarterial verapamil on the diameter of vasospastic intracranial arteries in patients with cerebral vasospasm. Neurosurg Focus. 21, 2006. E15
McGuinness B., Gandhi D. Endovascular management of cerebral vasospasm. Neurosurg Clin N Am. 2010;21:281-290.
Rosenwasser R.H., Armonda R.A., Thomas J.E., et al. Therapeutic modalities for the management of cerebral vasospasm: timing of endovascular options. Neurosurgery. 1999;44:975-979. discussion 979–980
Tejada J.G., Taylor R.A., Ugurel M.S., et al. Safety and feasibility of intra-arterial nicardipine for the treatment of subarachnoid hemorrhage-associated vasospasm: initial clinical experience with high-dose infusions. AJNR Am J Neuroradiol. 2007;28:844-848.
Wolf S., Martin H., Landscheidt J.F., et al. Continuous selective intraarterial infusion of nimodipine for therapy of refractory cerebral vasospasm. Neurocrit Care. 2010;12:346-351.
1. Ecker A., Riemenschneider P.A. Arteriographic demonstration of spasm of the intracranial arteries, with special reference to saccular arterial aneurysms. J Neurosurg. 1951;8:660-667.
2. Neil-Dwyer G., Lang D.A., Doshi B., et al. Delayed cerebral ischaemia: the pathological substrate. Acta Neurochir (Wien). 1994;131:137-145.
3. Borel C.O., McKee A., Parra A., et al. Possible role for vascular cell proliferation in cerebral vasospasm after subarachnoid hemorrhage. Stroke. 2003;34:427-433.
4. Mayberg M.R., Okada T., Bark D.H. Morphologic changes in cerebral arteries after subarachnoid hemorrhage. Neurosurg Clin N Am. 1990;1:417-432.
5. Weidauer S., Lanfermann H., Raabe A., et al. Impairment of cerebral perfusion and infarct patterns attributable to vasospasm after aneurysmal subarachnoid hemorrhage: a prospective MRI and DSA study. Stroke. 2007;38:1831-1836.
6. Haley E.C.Jr., Kassell N.F., Torner J.C. The International Cooperative Study on the Timing of Aneurysm Surgery. The North American experience. Stroke. 1992;23:205-214.
7. Janardhan V., Biondi A., Riina H.A., et al. Vasospasm in aneurysmal subarachnoid hemorrhage: diagnosis, prevention, and management. Neuroimaging Clin N Am. 2006;16:483-496. viii-ix
8. Macdonald R.L., Pluta R.M., Zhang J.H. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256-263.
9. Dorsch N., King M. A review of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Part I: incidence and effects. J Clinical Neuroscience. 1994:19-26.
10. McGuinness B., Gandhi D. Endovascular management of cerebral vasospasm. Neurosurg Clin N Am. 2010;21:281-290.
11. Fergusen S., Macdonald R.L. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;60:658-667. discussion 667
12. Frontera J.A., Claassen J., Schmidt J.M., et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery. 2006;59:21-27. discussion 21–27
13. Bleck T. Rebleeding and vasospasm after SAH: new strategies for improving outcome. J Crit Illn. 1997:572-574.
14. Eddleman C.S., Hurley M.C., Naidech A.M., et al. Endovascular options in the treatment of delayed ischemic neurological deficits due to cerebral vasospasm. Neurosurg Focus. 2009;26:E6.
15. Zubkov A.Y., Rabinstein A.A. Medical management of cerebral vasospasm: present and future. Neurol Res. 2009;31:626-631.
16. Soustiel J.F., Bruk B., Shik B., et al. Transcranial Doppler in vertebrobasilar vasospasm after subarachnoid hemorrhage. Neurosurgery. 1998;43:282-291. discussion 291–283
17. Lysakowski C., Walder B., Costanza M.C., et al. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke. 2001;32:2292-2298.
18. Firlik K.S., Kaufmann A.M., Firlik A.D., et al. Intra-arterial papaverine for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Surg Neurol. 1999;51:66-74.
19. Higashida R.T., Halbach V.V., Dowd C.F., et al. Intravascular balloon dilatation therapy for intracranial arterial vasospasm: patient selection, technique, and clinical results. Neurosurg Rev. 1992;15:89-95.
20. Binaghi S., Colleoni M.L., Maeder P., et al. CT angiography and perfusion CT in cerebral vasospasm after subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2007;28:750-758.
21. Wintermark M., Ko N.U., Smith W.S., et al. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol. 2006;27:26-34.
22. Imanishi Y., Fukui A., Niimi H., et al. Radiation-induced temporary hair loss as a radiation damage only occurring in patients who had the combination of MDCT and DSA. Eur Radiol. 2005;15:41-46.
23. Lennihan L., Mayer S.A., Fink M.E., et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2000;31:383-391.
24. Liu J.K., Couldwell W.T. Intra-arterial papaverine infusions for the treatment of cerebral vasospasm induced by aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2005;2:124-132.
25. Hoh B.L., Ogilvy C.S. Endovascular treatment of cerebral vasospasm: transluminal balloon angioplasty, intra-arterial papaverine, and intra-arterial nicardipine. Neurosurg Clin N Am. 2005;16:501-516. vi
26. Kassell N.F., Helm G., Simmons N., et al. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg. 1992;77:848-852.
27. McAuliffe W., Townsend M., Eskridge J.M., et al. Intracranial pressure changes induced during papaverine infusion for treatment of vasospasm. J Neurosurg. 1995;83:430-434.
28. Clouston J.E., Numaguchi Y., Zoarski G.H., et al. Intraarterial papaverine infusion for cerebral vasospasm after subarachnoid hemorrhage. AJNR Am J Neuroradiol. 1995;16:27-38.
29. Laskowitz D.T., Kolls B.J. Neuroprotection in subarachnoid hemorrhage. Stroke. 2010;41:S79-S84.
30. Feng L., Fitzsimmons B.F., Young W.L., et al. Intraarterially administered verapamil as adjunct therapy for cerebral vasospasm: safety and 2-year experience. AJNR Am J Neuroradiol. 2002;23:1284-1290.
31. Mazumdar A., Rivet D.J., Derdeyn C.P., et al. Effect of intraarterial verapamil on the diameter of vasospastic intracranial arteries in patients with cerebral vasospasm. Neurosurg Focus. 2006;21:E15.
32. Sehy J.V., Holloway W.E., Lin S.P., et al. Improvement in angiographic cerebral vasospasm after intra-arterial verapamil administration. AJNR Am J Neuroradiol. 2010;31(10):1923-1928.
33. Keuskamp J., Murali R., Chao K.H. High-dose intraarterial verapamil in the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;108:458-463.
34. Albanese E., Russo A., Quiroga M., et al. Ultrahigh-dose intraarterial infusion of verapamil through an indwelling microcatheter for medically refractory severe vasospasm: initial experience. Clinical article. J Neurosurg. 2010;113:913-922.
35. Biondi A., Ricciardi G.K., Puybasset L., et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. AJNR Am J Neuroradiol. 2004;25:1067-1076.
36. Wolf S., Martin H., Landscheidt J.F., et al. Continuous selective intraarterial infusion of nimodipine for therapy of refractory cerebral vasospasm. Neurocrit Care. 2010;12:346-351.
37. Badjatia N., Topcuoglu M.A., Pryor J.C., et al. Preliminary experience with intra-arterial nicardipine as a treatment for cerebral vasospasm. AJNR Am J Neuroradiol. 2004;25:819-826.
38. Tejada J.G., Taylor R.A., Ugurel M.S., et al. Safety and feasibility of intra-arterial nicardipine for the treatment of subarachnoid hemorrhage-associated vasospasm: initial clinical experience with high-dose infusions. AJNR Am J Neuroradiol. 2007;28:844-848.
39. Linfante I., Delgado-Mederos R., Andreone V., et al. Angiographic and hemodynamic effect of high concentration of intra-arterial nicardipine in cerebral vasospasm. Neurosurgery. 2008;63:1080-1086. discussion 1086–1087
40. Nogueira R.G., Lev M.H., Roccatagliata L., et al. Intra-arterial nicardipine infusion improves CT perfusion-measured cerebral blood flow in patients with subarachnoid hemorrhage-induced vasospasm. AJNR Am J Neuroradiol. 2009;30:160-164.
41. Avitsian R., Fiorella D., Soliman M.M., et al. Anesthetic considerations of selective intra-arterial nicardipine injection for intracranial vasospasm: a case series. J Neurosurg Anesthesiol. 2007;19:125-129.
42. Zubkov Y.N., Nikiforov B.M., Shustin V.A. Balloon catheter technique for dilatation of constricted cerebral arteries after aneurysmal SAH. Acta Neurochir (Wien). 1984;70:65-79.
43. Megyesi J.F., Findlay J.M., Vollrath B., et al. In vivo angioplasty prevents the development of vasospasm in canine carotid arteries. Pharmacological and morphological analyses. Stroke. 1997;28:1216-1224.
44. Sedat J., Chau Y., Popolo M., et al. Restenosis after balloon angioplasty for cerebral vasospasm. Cardiovasc Intervent Radiol. 2009;32:337-340.
45. Linskey M.E., Horton J.A., Rao G.R., et al. Fatal rupture of the intracranial carotid artery during transluminal angioplasty for vasospasm induced by subarachnoid hemorrhage. Case report. J Neurosurg. 1991;74:985-990.
46. Eskridge J.M., McAuliffe W., Song J.K., et al. Balloon angioplasty for the treatment of vasospasm: results of first 50 cases. Neurosurgery. 1998;42:510-516. discussion 516–517
47. Bejjani G.K., Bank W.O., Olan W.J., et al. The efficacy and safety of angioplasty for cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1998;42:979-986. discussion 986–977
48. Fujii Y., Takahashi A., Yoshimoto T. Effect of balloon angioplasty on high grade symptomatic vasospasm after subarachnoid hemorrhage. Neurosurg Rev. 1995;18:7-13.
49. Jestaedt L., Pham M., Bartsch A.J., et al. The impact of balloon angioplasty on the evolution of vasospasm-related infarction after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2008;62:610-617. discussion 610–617
50. Rosenwasser R.H., Armonda R.A., Thomas J.E., et al. Therapeutic modalities for the management of cerebral vasospasm: timing of endovascular options. Neurosurgery. 1999;44:975-979. discussion 979–980
51. Zwienenberg-Lee M., Hartman J., Rudisill N., et al. Effect of prophylactic transluminal balloon angioplasty on cerebral vasospasm and outcome in patients with Fisher grade III subarachnoid hemorrhage: results of a phase II multicenter, randomized, clinical trial. Stroke. 2008;39:1759-1765.
52. Firlik A.D., Kaufmann A.M., Jungreis C.A., et al. Effect of transluminal angioplasty on cerebral blood flow in the management of symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 1997;86:830-839.