Chapter 43 Hysteroscopic Management of Intrauterine Adhesions and Uterine Septa
INTRAUTERINE ADHESIONS
Etiology
Synechiae refers to adhesions that join together body parts, and this term is commonly used as a synonym for adhesion within the uterine cavity. In 1948, Asherman was the first to describe the frequency of uterine synechiae and the etiologic symptoms associated with the condition.1 Since then, the presence of intrauterine adhesions has also commonly become known as Asherman’s syndrome.1,2 Genital tuberculosis was first described by Netter in 1956.3 The classic observations were chronic inflammation of the endometrium resulting in severe intrauterine adhesions and often complete destruction of the endometrium, also known as Netter’s syndrome. It is quite evident that the development of adhesions requires at least one, if not all, aspects of uterine trauma and local infection to occur.
The main predisposing factor for its development is overzealous curettage postpartum or postabortion. Therefore, a mainstay in the prevention of adhesions is the avoidance of unnecessary curettage. Early detection of intrauterine synechiae is also a key preventive feature after intrauterine surgery, curettage, or spontaneous abortion to identify early adhesions that are filmy, thin, and easily resected with prompt adhesiolysis.4–6
Prevalence
The exact incidence of uterine adhesions in women of reproductive age is not known, but it is an infrequent cause of secondary amenorrhea, infertility, and recurrent pregnancy loss. The reported incidence of intrauterine adhesions with diagnostic hysteroscopy is only 1%, and the overall prevalence may be in the range of 1.5%.6,7 However, the prevalence of intrauterine adhesions seen in women undergoing hysterosalpingography (HSG) is reported to be 2.7%; in women with subfertility, adhesions are seen in 4% of cases.8,9
Dicker and colleagues4 also reported that previous abortion does not predispose to intrauterine adhesions in a review of 144 women; hence, the prevalence with an uncomplicated curettage is 2.1%. The incidence of Asherman’s syndrome in a select group of women especially after curettage for missed or incomplete abortion is reported in the range of 17%, but rates as high as 30% are reported in the literature, the majority of which are mild in severity.10–13 Furthermore, in at-risk women, such as those who have undergone postpartum or incomplete curettage, the rate is speculated to be even higher.14,15
The prevalence of intrauterine adhesions in women undergoing postpartum curettage or repeat curettage for a previously incomplete curettage for missed abortion or medical abortion was reported in a prospective study of 50 women and found to be present in 40%, of whom 75% had grade II to IV intrauterine adhesions as classified by the European Society of Hysteroscopy.21 In women with menstrual disorders, a statistically significant 12-fold increase in moderate to severe Asherman’s syndrome was noted. A history of prior abortion or infection during the initial surgery was associated with a mildly but not statistically significant increase in the risk of Asherman’s syndrome.
Risk Factors
Risk factors that should raise the index of suspicion for the presence of intrauterine adhesions include postpartum or postabortion curettage causing menstrual disturbance.11 Friedler and colleagues11 reported on the incidence of intrauterine adhesions with one or multiple abortions. The incidence of intrauterine adhesions after one, two, or three or more abortions was 16.3%, 14%, and 32%, respectively. The severity of adhesions was higher if at least two or more abortions were documented. There was no association between the number of previous intrauterine procedures and the presence of adhesions in asymptomatic women, but significant correlation was seen in women with menstrual disorders. The presence of infection at the initial procedure increased the presence of adhesions in this study, although it failed to reach statistical significance.
Pathophysiology
Any intervention that destroys the endometrium may generate adhesions of the myometrium in the opposing uterine walls. The key predictive factor to intrauterine adhesions is the gravid uterus. The gestational changes noted with a gravid uterus soften the uterine wall, resulting in greater denudation of the basalis layer with surgical intervention. The basalis layer is the regenerative layer of the endometrium.16
The most common contributing factor is an overzealous postpartum or postabortion curettage. The critical timing of the development of adhesions after uterine trauma in a gravid uterus is the first 4 weeks of the puerperium. The mechanism is probably secondary to an excessive form of wound healing. Pregnancy was found to be the predisposing factor in 91% of cases, of which 66.7% were postabortion curettage, 21.5% postpartum curettage, 2% cesarean section, and only 0.6% evacuation of a molar pregnancy.16 The contribution of infection at the time of the initial intervention in the pathophysiology of this syndrome is unknown and remains controversial13,17–19 Other reported causes include genital tuberculosis and previous uterine surgery, such as myomectomy.20
The gross pathologic changes seen in the uterus can range from marginal adhesions to complete obliteration of the cavity. The adhesions can vary from markedly dense to filmy. Adjacent to intrauterine adhesions are areas of endometrial sclerosis, which is more pronounced with dense adhesions.21
A key prognostic factor in the regeneration of endometrial tissue after adhesiolysis is the presence of the endometrial basalis, which serves as the anchoring point for the formation of new endometrium. Hence, muscular adhesions carry the worst prognosis because they are devoid or deficient in the regenerative basalis layer.22 The source of intrauterine adhesions can be endometrium, myometrium, or connective tissue, each of which has distinct characteristics.
The presence of adhesions within the uterine cavity can limit uterine muscle activity and endometrial perfusion, resulting in atrophy and consequently diminishing the amount of receptive endometrium available for normal implantation.23 Impairment of normal growth and development of an implanted embryo is due to the limited space available and the impaired blood supply to the embryo.22 The postulated mechanism for poor reproductive outcome or recurrent pregnancy loss in women with intrauterine adhesions includes implantation failure secondary to endometrial atrophy with defective vascularization.
Scanty endometrium with a damaged blood supply results in a lack of response to estrogenic stimuli associated with the extent of the adhesions present.23,24 Adhesions can also exist near the tubal ostia and cause tubal ostia obliteration or occlusion, further hindering implantation.
Classification
The classification systems in existence for intrauterine adhesions include those that classify according to pathologic localization or according to hysteroscopic findings that define the location and extent of uterine adhesions. In addition, a few prognostic classification systems based on menstrual pattern exist.6 The most commonly reported classification systems used include those by Valle and Sciarra,25 Donnez and Nisolle,26 the European Society for Hysteroscopy,27 March and coworkers,28 the American Society for Reproductive Medicine (ASRM; formerly the American Fertility Society),29 and Nasr and coworkers.30
The only two classification systems that include menstrual pattern as a prognostic factor are the ASRM and Nasr classification systems.29,30 The potential value of including in the classification system the presence or absence of menstrual abnormalities may be indicative of the amount of endometrium available for regeneration after adhesiolysis and may be of prognostic significance.
Intrauterine adhesions at the time of hysteroscopy have been classified by March and colleagues28 into three categories as mild, moderate, and severe (Table 43-1). Briefly, adhesions can be characterized either by multiple avascular strands of fibrous tissue between the anterior and posterior uterine walls, vascularized and dense adhesions that contain inactive endometrium or myometrium or muscular adhesions where there is no notable endometrial basalis.
Table 43-1 March Intrauterine Adhesion Classification System
| Grade | Finding |
|---|---|
| Minimal |
From March CM, Israel R, March AD: Hysteroscopic management of intrauterine adhesions. Am J Obstet Gynecol 130:653–657, 1978.
March Classification
Intrauterine adhesions can be further characterized based on the extent of uterine involvement.28 Minimal adhesions are said to be present if less than one fourth of the uterine cavity is involved, the adhesions are thin and filmy, and the fundal and ostia areas are minimally involved or devoid of any adhesions. Moderate adhesions involve one fourth to three fourths of the uterine cavity; no agglutination of the uterine wall is seen, only adhesions are present, and the tubal ostia and fundus are only partially occluded. Severe adhesions involve more than three fourths of the uterine cavity, with agglutination of the uterine walls or thick bands with occlusions of the tubal ostia and the upper uterine cavity. The March classification system is simple and easy to apply, but it is not prognostic.28 The most accurate classification system used that describes the adhesion site and density is the European Society for Hysteroscopy system, but it is very difficult to apply or utilize clinically.27
American Society for Reproductive Medicine Classification
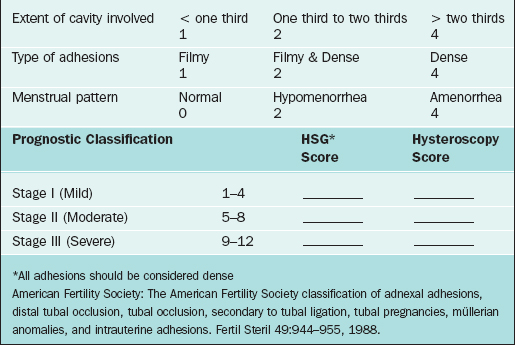
According to the 1988 ASRM classification system, synechiae are classified in three stages, with stage III being complete obliteration of the uterine cavity (Table 43-2).11 The ASRM classification system provides both an indirect and direct grading of intrauterine adhesions with HSG and hysteroscopy, respectively. The location of the adhesions is presumed to be prognostic for reproductive outcome given that most implantation occurs in the top fundal portion of the uterine cavity, and cornual adhesions may cause tubal obstruction.
Clinical Manifestations
The most common single presentation is infertility, representing 43% of reported cases; second is amenorrhea, representing 37% of cases.31 The rate of abnormal placentation, although elevated in women with intrauterine adhesions, is the least common presentation reported in women with intrauterine adhesions.
Diagnosis
Radiologic Imaging
Transvaginal sonography has replaced transabdominal sonography as an imaging tool for detection of uterine pathology.32 Transvaginal ultrasonography (TVUS) was assessed in 200 patients being investigated for infertility. The overall sensitivity in the detection of endometrial pathology was 98.9%, with a positive predictive value of 94.3% and false-positive rate of 5.5%.32 The overall specificity of TVUS for normal findings was 31.3% and the negative predictive value was 71.4%; however, these data were limited given the small number of patients with normal findings reported. In this study the positive predictive value for the detection of intrauterine adhesions was 98.5%.32 The sonogram was performed in the periovulatory phase of the menstrual cycle.
Another report assessed the predictive value of TVUS in 74 infertile women undergoing diagnostic hysteroscopy. The sensitivity, specificity, and positive and negative predictive value for the specific diagnosis of intrauterine adhesions were 80%, 100%, 100%, and 97%, respectively.33 However, in a separate series TVUS was unable to detect any of the cases of intrauterine adhesions, with three false-positive diagnoses,34 resulting in a sensitivity and positive predictive value of 0%. These conflicting reports could be due to technique, especially variation in the time in the cycle when the scan was performed.
The poor result of TVUS in some centers has been improved with the introduction of saline infusion sonohysterography, also referred to as sonohysterography. Sonohysterography is performed with TVUS and can further enhance the detection of intrauterine adhesions. Saline solution serves as a homogeneous, echo-free contrast medium, enabling better visualization of the uterine cavity than TVUS alone. Alborzi and colleagues34 published the largest series to date to evaluate the diagnostic accuracy of HSG and sonohysterography compared to laparoscopy and hysteroscopy as the gold standard. The prospective study reviewed 86 women with infertility. In this study sonohysterography had a high diagnostic accuracy for the detection of Asherman’s syndrome, greater than that for HSG, with a sensitivity of 76.8%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 97.7%.
In a separate study both HSG and sonohysterography had similar diagnostic accuracy.36 Sensitivity of both studies was 75%; HSG had a positive predictive value of 43% and sonohysterography had a positive predictive value of 50%. The negative predictive value of both studies was close to 100%.35 The use of magnetic resonance imaging (MRI) in the diagnosis of intrauterine adhesions remains to be elucidated.36
Technique
Transvaginal ultrasound should be performed in the late follicular or early luteal phase of the cycle (cycle day 7, 14, or 21) using a real-time sector transvaginal probe with imaging frequencies of 5 and 7.5 MHz. In fact, the periovulatory phase is the best time to evaluate the uterine cavity contours because the endometrium is thick enough to appear more echogenic than the myometrium and not too thick to obscure the midline echo. The classic appearance of the three-layer endometrium enables better imaging of uterine defects than the postmenstrual endometrium, which is thin, less than 3 mm.
The contour of the endometrial cavity is inspected for both irregularities and echo pattern of the myometrium–endometrium interphase in the sagittal (long axis) and transverse plane. The diagnostic criteria used for the diagnosis of intrauterine adhesions is interruption in the midline echo in the periovulatory phase of the cycle. The typical appearance is focal, hyperechoic, irregular, often cordlike structures seen within the echo-free space between the basalis layers, which interrupt the continuity of the endometrial cavity. These structures can vary in size from 2 to 6 mm or in location within the cavity.33
Surgical Treatment
Historical and Uncommonly Used Techniques
The classic blind dilation and curettage has gradually been abandoned in favor of the hysteroscopic approach for intrauterine adhesiolysis.16,22,25,37,38 Hysteroscopy can be performed in an outpatient setting and allows both the determination of the extent of adhesions under direct visualization and prompt treatment concurrently. A number of studies report resection of intrauterine adhesions by hysterotomy. In a review of 31 cases in 12 published studies, only 16 patients (52%) conceived, of which 8 resulted in live births, and 4 patients required a cesarean hysterectomy secondary to placenta accreta.16
Operative hysteroscopy has replaced dilation and curettage or blind dissection in the treatment of intrauterine adhesions and is the treatment of choice.16,22,25,37,38
Hysteroscopic Surgery
The mainstays of treatment for intrauterine adhesions are early detection and prompt surgical treatment to minimize further complications. Hysteroscopy has become not only an accurate tool for the diagnosis of adhesions, but also the main method for their treatment. Hysteroscopic lysis of adhesions is indicated when the extent of adhesions is moderate to severe or access to tubal ostia is blocked. The significance of mild adhesions is still controversial, yet surgical treatment may be considered if all other causes of infertility or recurrent pregnancy loss have been excluded or successfully corrected with persistent reproductive failure. It also appears prudent to restore the uterine cavity to normal before assisted reproductive technology (ART) treatment, yet evidence remains sparse.4
An additional use, albeit with limited data, is the treatment of intrauterine adhesions secondary to genital tuberculosis prior to in vitro fertilization–embryo transfer. However, the reformation rate is reported at 100%.37
Technique
Operative hysteroscopy is performed classically under general anesthesia using either an operative hysteroscope or resectoscope. Hysteroscopic adhesiolysis can be performed under local anesthesia in an outpatient setting with or without analgesics.16 The uterine cavity is distended using nonconductive hypo-osmolar solution under manometric control if electrosurgery is used. The most common solutions used are glycine (1.5%), sorbitol (2.7%), and mannitol (0.54%). The basic technique involves resection of the intrauterine adhesions by sharp or blunt dissection. Successful hysteroscopic resection can be accomplished by the use of sharp dissection using semirigid scissors, electrosurgery, or fiberoptic laser.
The adhesions are incised with a high-frequency resectoscopic manual electrode needle using an angled electrode, 90-degree loop. The theoretical concern with the use of electrocautery is the negative effect of thermal damage on the endometrium and myometrium with potential risk of uterine rupture with a subsequent pregnancy. If the extent of adhesiolysis is severe, the use of laparoscopy has been promoted to reduce the risk of uterine perforation.16,25,28 Reports also exist of the use of intraoperative fluoroscopic division of adhesions, but often adhesiolysis is incomplete.39 However, there is no clear advantage for the use of electrosurgery or laser over sharp adhesiolysis using hysteroscopic scissors through the operative channel of the hysteroscope. Recent reports also include the use of the coaxial bipolar electrode system in a limited number of cases for intrauterine adhesiolysis. Its efficacy and safety appear to be similar to the conventional techniques used, but further studies need to be conducted.40,41
A case report introduced the concept of hysteroscopic adhesiolysis with guidance of a laparoscopic ultrasound in a patient with infertility and intrauterine synechiae.42 A 7.5-MHz laparoscopic ultrasound transducer was introduced though a 10-mm port; placing the scanning surface of the probe firmly on the uterine serosa yielded a high-quality real-time image and safe resection of the synechiae. The potential benefit over conventional transabdominal techniques includes proper localization of the hysteroscopic instruments and greater definitions of the uterine walls to minimize uterine perforation. The technique holds promise, but further studies need to be conducted before it replaces other procedures.42–44
Adhesiolysis begins inferiorly and is carried out cephalad until a panoramic view of the endometrial cavity can be obtained and the tubal ostia are seen. The initiation of the adhesiolysis is from the internal os. Filmy and central adhesions should be cut first; marginal and dense adhesions should be approached last.39 The maintenance of adequate distension is key to the successful resection of intrauterine adhesions. Valle and Sciarra25 described the use of methylene blue injection through the inferior channel of the hysteroscope when it is difficult to ascertain adhesions from a normal endometrial lining. This technique may help delineate a plane of cleavage for safe adhesiolysis. Operating time for hysteroscopic adhesiolysis varies depending on the severity of adhesions present and often may require a second intervention for adequate resection. The average operating time is 30 minutes, with a range of 10 to 60 minutes.
The published studies that describe the successful use of a coaxial bipolar electrode surgical system report similar results to conventional techniques.40,41,45–47 In a series of 50 patients, of which 12 were identified with uterine adhesions and the other 12 with uterine septum, septoplasty, and intrauterine adhesiolysis was successfully completed without any major complications reported.40 The use of the bipolar vaporization system was well-tolerated, safe, and likely an effective alternative to the conventional hysteroscopic techniques in the treatment of intrauterine adhesions. A key advantage appears to be the small diameter of the electrodes used, which can easily be inserted through a #5 French working channel of a 5.5-mm hysteroscope, as well as the use of normal saline solution, which is associated with reduced incidence of hyponatremia.
Women with severe intrauterine adhesions and complete obliteration of the uterine cavity are the most challenging surgical cases. The key to the success of treatment is in defining the correct plane of dissection without causing a uterine perforation.28 McComb and Wagner48 have described a simplified technique for women with severe Asherman’s syndrome. In a series of six women successful adhesiolysis was accomplished using a Pratt cervical dilator first. This allowed some space to start the hysteroscopy. The curved tip points laterally toward the uterine cornua aligned with the plane of the uterine corpus. The technique is performed bilaterally, thus converting the obliterated cavity in the configuration of a uterine septum.
Similar techniques have been used with the use of fluoroscopic guidance with a 16-gauge, 80-mm Tuohy needle that is introduced alongside a 5-mm hysteroscope with similar results.39 The goal is to create a passageway using either the dilator or needle with subsequent division of the adhesions under direct vision with the hysteroscopic scissors. These specialized techniques often require multiple procedures to achieve adequate adhesiolysis.39,49,50
Radiologic Techniques
Blunt disruption of adhesions using either insemination or HSG balloon catheters has also been described.51,52 Fluoroscopic-guided lysis of intrauterine adhesions was originally reported by Ikeda and colleagues51 and further developed by Karande and colleagues.52 Both procedures can be performed at the time of diagnostic HSG. Karande described a small series of patients in whom in-office lysis of intrauterine adhesions under gynecoradiologic control using balloon tip HSG catheters or hysteroscopic scissors inserted through the main port of the catheter was performed. Successful lysis of adhesions occurred in 81.2% of the patients, but in women with moderate or severe adhesions the procedure was abandoned secondary to significant discomfort, only leading to partial resection of the adhesions.
Recent reports have also described a therapeutic role of sonohysterography for the treatment of uterine adhesions. Coccia and coworkers53 described a newly developed treatment technique for intrauterine adhesions based on sonohysterography. The technique uses saline infusion under pressure lavage of the uterine cavity to mechanically disrupt adhesions. Saline solution is initially injected slowly (5 to 10 mL) and then under increasing pressure until pain is experienced by the patient, at which point the infusion is stopped. The same procedure is repeated several times until complete uterine distension is visualized with no residual interruption of the endometrial contour nor filling defects noted.
Prophylactic antibiotics and an estrogen/progesterone preparation were used after the procedure. This procedure was named the PLUG method (pressure lavage under ultrasound guidance; see Chapter 30); however, generalization of the results is limited given the small sample size (7 patients) reported. The procedure was well-tolerated, not requiring local or general anesthesia; the mean duration of the procedure was 17 minutes. The technique was more efficacious for the lysis of mild adhesions. A high rate of recurrence was noted in women with moderate to severe adhesions, due to a higher rate of incomplete adhesiolysis. Menstrual cyclicity was restored in all patients, but limited data on fertility were available. Only one of the three infertile women treated had a live birth.
Postoperative Care
Despite advances in the development of techniques for adhesiolysis, the two basic problems associated with poor outcome with these procedures still exist: the inability to treat extensive or severe adhesions and the lack of methods to prevent recurrence of the adhesions postoperatively. The use of intrauterine devices, Foley catheters, antibiotics, steroids, and high-dose estrogen postoperatively to prevent recurrence of adhesions is still widely debated, and no consensus exists.16
Intrauterine Device
An IUD was traditionally used. However, the modern IUD, such as the copper-bearing IUD and the Progestasert, may have too small a surface area or cause too much of an inflammatory reaction to be of any benefit. The reported preference was to use the Lippes Loop IUD.16,54 The value of the IUD is to keep the raw, freshly dissected surfaces separated during the initial phase of healing in hopes of reducing the chance of adhesion reformation.
Most surgeons now use a Foley catheter with an inflatable balloon to keep the freshly dissected uterine surfaces separated. The Foley catheter is inserted into the uterus posthysteroscopy and remains in situ for approximately 1 week in most circumstances. Those that do not recommend its use suggest that it may impede endometrial regeneration by the simple pressure on the uterine wall from the inflated balloon.16 A uterine balloon stent (Cook OB/GYN, Spencer, Ind.) has been developed with a balloon that is heart-shaped and flat to conform better to the uterine cavity, although no comparative data is available for this device.
Schenker16 reported on 642 patients with menstrual disorders treated with an IUD; 92% regained normal menses and 8% were hypomenorrheic. Among the 405 infertile women, 55% conceived, of whom 61% had a term delivery, 25% aborted, 7% had preterm labor, and 7% had abnormal placentation (placenta accreta). In 167 women who had insertion of a Foley balloon after adhesiolysis, 47% conceived, of whom 68% had a term delivery, 8% had preterm labor, 18% aborted, and 5% had placenta accreta. Of note, in 292 women with mild intrauterine adhesions without treatment, 133 (45%) conceived, of which 30% were term, 23% preterm, 40% aborted, and 13% had placenta accreta.
Prevention
The use of adhesion prevention barriers has been also reported to prevent intrauterine adhesions after curettage. A recent randomized, controlled, prospective trial describes the use of a bioresorbable membrane postoperatively within the endometrial cavity in 150 women with incomplete or missed abortion with or without a history of at least one or more prior curettage. In women with no prior history of curettage who used the membrane, all were able to conceive within 8 months after adhesiolysis compared to women who did not use the membrane, in whom only 54% conceived. In women who did not conceive with a history of prior curettage and the use of the membrane, 90% were found to be adhesion-free compared to only 50% in the untreated group. Further studies are needed before its routine use is advocated.55
Complications
The complications associated with hysteroscopic adhesiolysis can be viewed in two major categories: those intrinsic to operative hysteroscopy and those related to the technique of adhesiolysis itself. The complications related to operative hysteroscopy are reviewed in detail in Chapter 45. In summary, the reported complications seen with operative hysteroscopy include the possibility of fluid overload, perforation, and pelvic organ injury that includes thermal damage. The overall complication rate associated with hysteroscopy is reported to be 2.7%.45,56 Furthermore, the potential risk seen with the use of the electrosurgical system includes genital burns.57,58
The rate of uterine perforation during hysteroscopic surgery is less than 2% (1.6%), of which most are recognized (97%) during the procedure. The perforation risk is highest with intrauterine adhesiolysis or during hysteroscopic adhesiolysis.16,59 The risk of postoperative infectious complications is 1.42%, but the risk of early-onset endometritis is highest after lysis of synechiae compared to other hysteroscopic procedures, including uterine septa.59
Vaginal delivery is still recommended in women after hysteroscopic adhesiolysis unless extensive damage has occurred through thermal injury or a fundal perforation has occurred. Reports exist of potential catastrophic events, albeit rare, of uterine perforation with subsequent pregnancy or labor with or without uterine perforation. The risk of abnormal placentation is in the range of 8% and is speculated to be secondary to defective lamina basalis seen after adhesiolysis.60 It is recommended that pregnancy surveillance be vigilantly pursued to detect or rule out abnormal placentation as its lack of detection can lead to catastrophic events to both the mother and fetus.
Surgical Results
A number of series have been published reporting the outcome of hysteroscopic treatment of intrauterine adhesions. However, randomized clinical trials are lacking. There are also few reports about women with untreated intrauterine adhesions. Schenker16 reported on 292 women with mild intrauterine adhesions without treatment, of whom 133 (45%) conceived, of which 30% were term, 23% preterm, 40% aborted, and 13% had placenta accreta.
A report of 40 consecutive women with recurrent pregnancy loss (24 women) or infertility (16 women) resulting from intrauterine adhesions showed excellent surgical results with mild or moderate disease.38 Of the 40 women, 10 had mild adhesions, 20 had moderate adhesions, and 10 had severe adhesions according to the March classification system.28 Hysteroscopic adhesiolysis was performed with hysteroscopic scissors or monopolar electrosurgery. Prophylactic antibiotics were used; postoperatively, a pediatric Foley catheter was introduced and estrogen was administered. In women with hypomenorrhea as the presenting complaint, 67% reported normal menstrual cycles after adhesiolysis. All women with recurrent pregnancy loss conceived after adhesiolysis; 71% of pregnancies were term or preterm with a viable pregnancy. Among the women with infertility, 62% conceived resulting in a 37.5% live birth rate. Adhesion reformation was absent or rare in women with mild or moderate adhesions, reported as 0 to 10%. However, adhesion reformation was seen in 60% of women with severe intrauterine adhesions; none of the patients with severe adhesions conceived. Only one perforation was reported in a patient with severe adhesions.
Valle and Sciarra25 reviewed 81 infertile women and reported a term pregnancy rate of 81%, 66%, and 15%, respectively, in women with mild, moderate, and severe disease. Among these women with recurrent pregnancy loss the term pregnancy rate was 94%, 89%, and 65% in those with mild, moderate, and severe adhesions, respectively. The literature is unified and quite clear in that women with severe intrauterine adhesions, the reproductive outcome remains poor even after hysteroscopic adhesiolysis.25,38 In Valle and Sciarra’s series, of the 30 women with infertility and severe adhesions, 43% of women conceived, resulting in only a 10% live birth rate. Adhesion reformation rate is also strongly correlated with the extent of severity of the initial degree of adhesions, as is the reproductive outcome after hysteroscopic adhesiolysis. The recurrence rate of severe adhesions was 48.9% and decreased to 35% after repeat adhesiolysis.25
Zikopoulos and coworkers41 reported on 46 women who underwent hysteroscopic adhesiolysis either with the resectoscope or the coaxial bipolar electrical system. It is the largest series in which the coaxial bipolar system was used. Three women required a second attempt because of the presence of adhesions postoperatively and one patient required a third procedure. Restoration of menses in women with oligomenorrhea or amenorrhea was noted in 93% of the women.
The pregnancy complication rate noted was a preterm rate of 50% and hysterectomy for abnormal placentation (placenta accreta) in 2 of the 20 patients (10%). In addition, Zikopoulos and colleagues41 reviewed the literature of existing studies; they identified seven published studies in the past decade that examined delivery rates in women undergoing hysteroscopic adhesiolysis using an array of techniques. The overall delivery rate was 38.1% (48/126) among all the studies analyzed.
Pabuccu and colleagues38 reported the highest success rate among women with recurrent abortion with a delivery rate of 70.8% versus women with infertility, with a 37.5% delivery rate. The overall delivery rate was similar to that reported by Siegler and Valle60 in 1988 after reviewing a series of published studies that reported on a total of 775 women, of which 302 (38.9%) achieved a term delivery.
Women with adhesions secondary to genital tuberculosis carry the worse prognosis even after hysteroscopic adhesiolysis.37 In a series of 12 women undergoing 15 attempts at hysteroscopic lysis of uterine adhesions despite adequate restoration of the uterine cavity postoperatively, the reformation rate for severe adhesions was 100%, with a 25% uterine perforation rate. Women with severe intrauterine adhesions secondary to tuberculosis carry a poor prognosis; these authors recommend alternative fertility options such as surrogacy if initial attempts at surgical adhesiolysis have failed.
UTERINE SEPTA
Embryology of the Genital Ducts
Congenital uterine anomalies can arise from essentially three general categories of müllerian developmental defects that can either be partial or complete. The three categories include major disturbances of development, formation or fusion of the müllerian ducts, resulting in either arrest of development (agenesis) of the uterovaginal primordium, failure of canalization, failure of fusion, or failure of resorption. A septate uterus arises from the partial or complete failure of resorption of the midline septum between the two müllerian ducts. The embryology of the female genital tract is reviewed in detail in Chapter 12.
Briefly, both the Wolffian (mesonephric) and müllerian (paramesonephric) pairs of ducts coexist from 5 to 8 weeks’ gestation during the indifferent stage of development. In the absence of müllerian-inhibiting substance produced by the Sertoli cells in the developing testis, the müllerian ducts develop into the uterus, fallopian tubes, and upper vaginal fornices. The Wolffian ducts regress with the absence of androgens. Fusion of the caudal portions of the mullerian ducts form a Y shaped structure called a uterovaginal primordium. The caudal tip of the uterovaginal primordium inserts into the dorsal wall of the urogenital sinus. Fusion with subsequent canalization forms the entire reproductive tract up to the level of the hymen, a process that is initiated at 9 weeks of gestation and completed by 20 weeks.61
The classic unidirectional theory of fusion of the müllerian ducts, caudad to cephalad, has been challenged given the existence of müllerian defects that cannot be explained by the classical unidirectional theory. These types of anomalies are incompatible with the theory of linear caudad to cephalad müllerian fusion, as described by Crosby and Hill in 1962.62 The basis of the theory was that uterine development results from fusion of the müllerian ducts during the 11th to 13th week of embryonic life, beginning at the caudal-most aspect, known as the müllerian tubercle, and proceeding in a cranial direction. Septal resorption was thought to follow shortly thereafter, beginning at any point of fusion and moving in either or both directions.
An alternative theory of progression was proposed by Musset and colleagues63 and Muller and colleagues,64 in which the medial aspect of the müllerian ducts begin to fuse in the middle and proceed in both the cephalad and caudal directions simultaneously. It is then followed by rapid cellular proliferation between the ducts, forming the uterine corpus and cervix, and septal resorption, all of which occur in both directions simultaneously. The medial portion of the müllerian ducts fuses between the urogenital sinus and the isthmus of the uterus approximately in the 10th week of embryologic life and proceeds simultaneously both in the cephalad and caudad direction; a thick septum forms at the uterine fundus by the 13th week of gestation. Septal resorption, which begins in the isthmic portion and proceeds simultaneously in both directions, takes place between 13 and 20 weeks’ gestation. Failure of fusion of the müllerian ducts occurs by 10 weeks’ gestation and failure of resorption by 20 weeks.
Etiology
The etiology of a septate uterus remains to be elucidated. Sporadic case reports on family pedigrees suggest familial aggregation exists, but no clear genetic cause has been linked to the development of a septate uterus.65–67 The few studies that exist report not only affected siblings, but also vertical transmission with affected mothers and daughters with similar müllerian defects. A number of genes and their interaction with environmental factors likely play a role in the development of a septate uterus, yet the true etiology is unknown.68
In general, 92% of women with congenital uterine anomalies have a normal karyotype, 46,XX; approximately 8% of women have an abnormal karyotype.69 In rare cases, early in utero exposure to radiation, infection such as rubella, and teratogens (diethylstilbestrol, thalidomide) has been implicated as the causal factor of the uterine anomaly. Furthermore, müllerian anomalies can coexist with anomalies in other organ systems, particularly in the genitourinary tract, but these are infrequently seen with a septate uterus. Hence, a complete diagnostic workup of all organ systems possibly involved in müllerian anomalies is prudent, based on the type of uterine abnormality present.
Classification
A number of classification systems have been reported for müllerian anomalies. A single classification system needs to be uniformly adopted to allow the proper classification of patients and the ability to compare data from both national and international publications using the same system. Buttram and Gibbons70 devised a classification system of müllerian anomalies based on six groups of abnormalities. The ASRM in 1988 incorporated and revised Buttram’s classification system based on the major uterine anatomic types in existence (Table 43-3).29 The classification system proposed by the ASRM in 1988 is most commonly used to describe or define müllerian defects.
The classification system organizes the anomalies into six major uterine anatomic types or categories. Two basic categories of uterine anomalies exist secondary to partial or complete failure of resorption of the müllerian ducts, classified as type V or VI. A septate uterus is among the vertical fusion defects described by the ASRM classification system. It is classified as type V; type Va is a complete septate uterus and type Vb is a partial septate uterus (see Fig. 12-3 in this book).
Type VI is an arcuate uterus in the ASRM classification (see Fig. 12-3).29 The ASRM classifies an arcuate uterus into a separate category, but an arcuate uterus could be classified as a form of partial septate uterus, given that it is externally unified and has the same embryologic origin as a septate uterus (i.e., failure of resorption of the midline septum). The basic rationale of the ASRM for placing the arcuate uterus in a separate category was the hypothesis that an arcuate uterus behaves benignly and surgical treatment is often not indicated.29 Hence, an arcuate uterus can often be classified as a septate uterus with a small septum and minimal fundal indentation (dimple).
The advent of MRI has further characterized and confirmed the presence of a complete uterine septum, dual cervices, and longitudinal vaginal septum; these anomalies do not fit the classification system used by Buttram and Gibbons.70 Case reports document the presence of a double cervix and vagina with a septate uterus.71–73 This anomaly is classified by Toaff and coworkers72 as a type 1A; this further supports Musset’s theory of müllerian development,63 which is further advocated by McBean and Brumsted.73
Incidence
The true incidence of congenital uterine anomalies in the general population remains unknown, with variations in the reported incidence from study to study. The difficulty in trying to ascertain the true incidence of uterine anomalies lies primarily with the asymptomatic nature of most anomalies. However, recent improvements in uterine imaging techniques have increased their detection.
The reported incidence of müllerian or uterine anomalies is between 0.5% and 6% of reproductive-age women and is highest among women with poor reproductive outcome. The estimated prevalence of uterine anomalies in all women is 2% to 3%.74–76 A review of a series of 679 women who underwent surgical sterilization by laparoscopy or laparotomy with a history of normal reproductive outcome demonstrated an incidence of uterine anomalies of 3.2%, of which 90% were septate uteri, 5% bicornuate, and 5% didelphic.75
In another review of a similar group of 323 women with normal reproductive outcome undergoing hysteroscopic sterilization, the reported incidence was 6%.76 The uterine anomalies were primarily septate or bicornuate uterus. Similar results have been reported by others.77 In a prospective study of 336 patients undergoing hysteroscopy for the evaluation of infertility, müllerian defects were noted in 5.4%, a rate similar to that reported in the general population. In contrast, a series of 228 women with uterine anomalies noted a 9.1% frequency of infertility that could be explained by other causes.78
The most common uterine abnormality seen in women with poor reproductive outcome is either a septate or bicornuate uterus, with an equal distribution in prevalence of both types of abnormalities. The overall incidence of mullerian defects reported in a series by Acien74 was 5% among women with normal reproductive history, 3% among infertile women, 5% to 10% among women with first-trimester recurrent miscarriage, and greater than 25% in women with late first- or early second-trimester loss or preterm delivery. Patients with an arcuate uterus were excluded in this incidence report. Combining a large group of infertile and fertile women from several studies, the most frequent to least frequent anomaly is bicornuate uterus, arcuate uterus, incomplete uterine septum, uterus didelphys, complete uterine septum, and unicornuate uterus.74 In this combined series of women, a bicornuate uterus and a complete or partial septum, which included an arcuate uterus, represented 74% of the uterine anomalies, equally divided between the two groups.
In a summary of five major studies available in the literature that reported on 2992 cases with uterine anomalies, the overall mean incidence of uterine malformation in the general population of fertile women was 4.3%.79 After excluding earlier prevalence studies in which a septate uterus was often misdiagnosed as bicornuate due to the limitations of the imaging techniques, the frequency distribution in 1392 cases was arcuate uterus, 18.3%; septate uterus, 34.9%; and bicornuate uterus, 26%; with unicornuate uterus, didelphys uterus, and müllerian agenesis representing the rest. Hence, approximately one third of the cases were a septate uterus, which can be successfully treated hysteroscopically.
This report further subdivided the incidence of müllerian anomalies in women with infertility and recurrent pregnancy loss.79 In women with infertility, depending on the study reviewed, the incidence varies between 1% and 26%. In reviewing a total of 3640 cases from existing studies, the overall mean incidence of mullerian defects in infertility patients was 3.4% which is similar to that noted in the general population (4.3%), suggesting that the septate uterus has no impact on fertility.
The key presentation in women with a septate uterus is difficulty in maintaining a pregnancy and not a decreased ability to conceive (infertility). In women with recurrent pregnancy loss, this same review reported a mean overall incidence of 12.6%, a rate higher than reported for the general population and infertile women.79 A higher incidence was seen in women with late abortion and preterm delivery than in women with earlier abortions.
In women with recurrent pregnancy loss, the relative frequency of a septate versus bicornuate uterus is less clear. This lack of clarity is often attributed to old surgical data that often did not definitely differentiate a septate from a bicornuate uterus. In one of the largest studies of patients with recurrent pregnancy loss evaluated with either laparoscopy or sonohysterography, the septate uterus was more prevalent in women with recurrent pregnancy loss than the controls.80 Several case series have demonstrated that the septate uterus is more prevalent than bicornuate uterus in women with recurrent pregnancy loss.81,82
Pathophysiology
The presence of a septate uterus is thought to impair normal reproductive performance by increasing the risk or incidence of early and late abortion, preterm delivery, and the rate of obstetric complications. In a case series of women with uncorrected uterine anomalies, it was observed that 48% with septate uterus had a term pregnancy and 15% had preterm labor compared to 39% and 21%, respectively, in women with bicornuate uterus.83 The fetal survival rate was high in both groups (50% to 60%). In a separate report, first-trimester spontaneous abortions were associated with a septate uterus but not a bicornuate uterus. Definitive evidence is lacking linking infertility with uterine anomalies. The ability to conceive does not appear to be impaired in women with a septate uterus.
The data does support the concept that a uterine septum imparts an elevated risk of recurrent pregnancy loss. Women with uncorrected uterine septa may experience an elevated risk of spontaneous abortion in the first or second trimester, and less commonly premature birth and abnormal fetal presentation.83,84 Yet, the association between a uterine septa and pregnancy loss is not clear and data are contradictory.
In a case series of women with unexplained infertility, the spontaneous abortion rate was 22% in women with septate uterus, compared to approximately 35% in women with bicornuate or unicornuate uterus.85 The risk of pregnancy loss in women in whom the uterine septum is only an incidental finding during an evaluation for infertility is unclear.86,87
The septum is primarily composed of avascular, fibromuscular tissue. Hence, it has been proposed that the endometrium lining the septum responds poorly to estrogen, resulting in irregular differentiation and estrogenic maturation.23,88–90 Implantation on this poorly vascularized, fibrous septum leads to abnormal implantation, defective embryonic development, and subsequent abortion.87,91,92 The diminished size of the uterine cavity may have also have a role in the pathogenesis of pregnancy loss.
There appears to be a higher risk of poor obstetrical outcome with longer septa. Smaller septa that involve less than half of the uterine cavity are less likely to cause recurrent pregnancy loss. A smaller septum, defined as a septal length less than half of the length of the uterus, was less likely to cause recurrent pregnancy loss than larger septa; however, smaller septa are still associated with recurrent pregnancy loss.93
Diagnosis
Radiologic Imaging
Hysterosalpingography is the most commonly used diagnostic radiologic technique to determine congenital or acquired uterine defects and tubal patency. HSG is performed between the 5th and 12th day of the menstrual cycle. The diagnosis of a septate uterus is generally suspected by an HSG but often misdiagnosed.94 HSG is a simple, safe, relatively noninvasive radiologic procedure performed under fluoroscopic guidance that enables visualization of the uterine cavity, but it is limited in its ability to differentiate between a septate and bicornuate uterus (see Chapter 29). Consequently, the limitations of the HSG require additional evaluation of the external uterine contour or configuration with either surgical intervention or more recently with ultrasonography or MRI for a definitive diagnosis.
Hysterosalpingography is a useful screening test and should be the first step in the evaluation of the uterine cavity. The gold standard for the accurate and proper classification for the diagnosis of a uterine anomaly has been laparoscopy and hysteroscopy, especially in women in whom the fundal contour of the uterus needs to be confirmed. Prophylactic antibiotics before the procedure are recommended in high-risk patients (e.g., those with a history of pelvic inflammatory disease or cardiac disease). The most commonly used antibiotic is doxycycline. Sedation is not routinely required because the procedure is associated with mild discomfort that is relieved by an anti-inflammatory agent given 30 to 60 minutes before the procedure. Details of this procedure are given in Chapter 29.
In one study, 26 of 336 infertile women who underwent a workup for infertility with both an HSG and hysteroscopy had a diagnosis of a müllerian anomaly. Eighteen of the 26 had a confirmation of the müllerian defect seen on HSG.95 Other studies have demonstrated similar findings; however, the sensitivity and positive predictive value is highly dependent on the type of uterine malformation in question.96,97
The presence of a uterine septum is best diagnosed with a TVUS in the transverse plane. The classic findings include myometrial echoes dividing the fundal endometrial image (Fig. 43-1). The added technique of both three- and four-dimensional ultrasound may prove to be more efficacious in the diagnosis of uterine septum, but currently no clear benefit over standard TVUS has been demonstrated.
A sonohysterogram has some advantages over the traditional TVUS for the diagnosis of uterine defects. This procedure is performed using a conventional transvaginal ultrasound transducer with a frequency of 5 MHz, a uterine injector with or without a balloon tip, and physiologic saline solution. The saline solution is slowly infused to achieve adequate uterine distension and the cavity evaluated in both the transverse and longitudinal plane for any defects. The procedure takes on average 5 to 10 minutes to perform in experienced hands. Neither analgesia nor sedation is routinely required because the procedure is associated with minimal discomfort. The risk of major complications after sonohysterography is similar to that seen with an HSG and is reported in the range of 1% to 2%, primarily secondary to pelvic inflammatory disease.97–99 Details of this procedure are given in Chapter 30.
In one study the correlation between preoperative TVUS and hysteroscopic findings in 200 infertile women was reported.32 The overall positive predictive value of TVUS in detecting endometrial pathology was 94.3%, the sensitivity was 98.9% and the false-positive rate was 5.5%. The positive predictive value for the detection of a uterine abnormality with the use of TVUS was highest for intrauterine septa, at 100%.
In a series of 65 infertile women, the diagnostic accuracy for all imaging techniques was compared to hysteroscopy.97 Both TVUS and sonohysterography had a 0% false-positive rate with a specificity and positive predictive value of 100%. In contrast HSG had a 96.4% specificity and a 66.7% positive predictive value. The sensitivity of sonohysterography was 77.8% compared to 44.4% for both HSG and TVUS. These data suggest that sonohysterography can replace an HSG as the primary method for the detection of uterine septum.
In another, larger case series of 86 women, the diagnostic accuracy of HSG and sonohysterography was compared to laparoscopy and hysteroscopy.34 The use of sonohysterography for uterine anomalies had a sensitivity of 94%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 96.2%. These data are similar to those of the previous study,97 but they had less favorable results; the major limitation of sonohysterography was with the detection of an arcuate uterus.
Magnetic resonance imaging can accurately predict the presence of uterine anomalies and has become the imaging method of choice to confirm inconclusive results from other methods. A number of recent reports describe the effective use of MRI in the evaluation of uterine anomalies.71,100–103 MRI is both sensitive and specific in differentiating between the types of uterine anomalies present, including the arcuate uterus.
The clear advantages of an MRI include the ability to distinguish between myometrial and endometrial tissue, image the uterus in several planes, and define uterine contour.100,104–106 The MRI can accurately detect the fundal contour to include measuring the intercornual diameter, which is required to differentiate a bicornuate from a septate uterus. A septate uterus is defined by a smooth fundal contour with an intercornual diameter of less than 4 cm. In addition, Fedele and coworkers100 remarked that a fundal indentation of less than 10 mm and an angle between the medial margins of the hemicavities of less than 60 degrees seen on MRI differentiates a septate from a bicornuate uterus. Similar findings were reported by others71 (see Chapter 31).
Furthermore, the uterine septum can be further characterized by the presence or absence of myometrial tissue and vascularization of the septum versus only a fibrous consistency throughout its entire length seen with a septum. The positive predictive value for MRI for the detection of uterine septum has been reported as high as 100%.106 Several studies have reported correct diagnosis of uterine anomalies in 100% of cases when using MRI as an adjunct to surgery.100,101 In a separate review of 23 cases of müllerian anomalies, the correct diagnosis was made in 96% of cases with MRI, compared to 85% for TVUS.102
Another advantage of MRI is its ability to detect the associated anomalies in other organ systems typically seen with müllerian anomalies, such as renal or urinary tract anomalies.106 The major disadvantages include the lack of portability and higher costs compared with other imaging modalities.
Laparoscopy and hysteroscopy together have been traditionally used to detect and characterize uterine septums. In a prospective, randomized study the authors106 found no significant difference between hysteroscopy, saline infusion hysterosonography, and HSG in the evaluation of uterine cavity in infertile women. The main limitation of hysteroscopy is the inability to determine the contour of the uterus and therefore definitively differentiate between uterine anomalies such as a bicornuate or septate uterus.
Surgical Treatment
Historical Perspective
In 1907, Strassman described the abdominal metroplasty. It was the surgical technique of choice for unification of the uterine horns in bicornuate and didelphic uteri. Jones and Jones108 and Tompkins109 in 1953 and 1962, respectively, made further modifications to the technique of abdominal metroplasty. Despite the achievement of excellent outcomes with abdominal metroplasty, there were sufficient major disadvantages associated with the technique that have rendered these procedures obsolete.110,111 The disadvantages include risk of hemorrhage, significant pelvic adhesions, prolonged postoperative recovery with associated high morbidity, and the need for operative delivery (cesarean section) if pregnancy is achieved.108,109
Women can also attempt pregnancy 2 months after resection versus 3 to 6 months with abdominal metroplasty. Operative hysteroscopic metroplasty is now the accepted surgical technique for the treatment of uterine septum.112,113
Indications
Limited data support prophylactic treatment for the prevention of poor reproductive outcome in subsequent pregnancies even if the first pregnancy is normal. Acien evaluated the pregnancy outcome in 176 patients with untreated müllerian defects.74 He reported on the pregnancy outcome with a first or subsequent pregnancy. The abortion rates were the same with the first pregnancy as with all subsequent pregnancies. However, the pregnancy outcome was worse with subsequent pregnancies than with a first pregnancy, with a lower term delivery rate and higher preterm rate. In the subgroup of 31 patients with a septate uterus, a total of 89 pregnancies were achieved. The pregnancy outcome for the first versus all subsequent pregnancies was 21% versus 23% for spontaneous abortions, 8% versus 23% for preterm delivery, and 71% versus 54% for term delivery. Hence, women with septate uterus had worse reproductive outcomes with subsequent pregnancies than with the first one. The normal uterus was associated with an 8% abortion rate, 6% preterm delivery rate, and 85% term delivery rate.
Hysteroscopic Technique
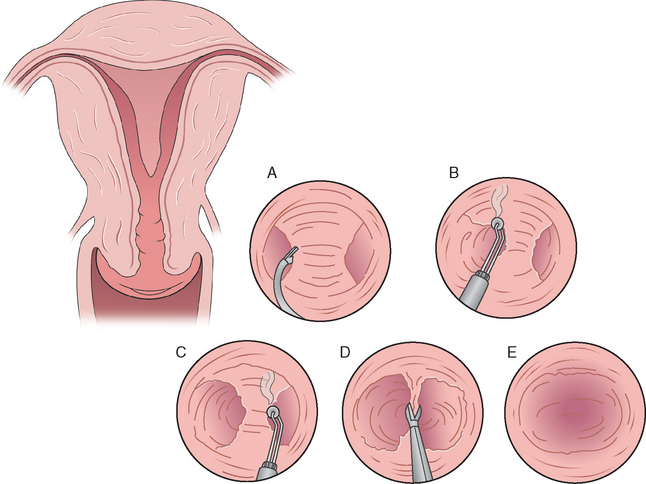
Most of the large published studies on hysteroscopic metroplasty used microscissors as the method of choice for surgical resection of the septum.112–114 Two of the most common instruments used to accomplish either incision or resection of the septum are the rigid and semirigid flexible scissors (Fig. 43-2). These are #7 French instruments passed through the operative channel of the hysteroscope. Alternatively, a wire loop can be introduced through a #21 French sheath of an operative resectoscope. There are bipolar systems that use a typical operative hysteroscope; instruments are introduced through the working channel that has electrocautery capabilities for incision. The limitations with the use of scissors include more difficulty in dissecting or cutting a broad-based septum. The theoretical concern with the use of electrocautery is the negative effect of thermal damage on the endometrium and myometrium with potential risk of uterine rupture with a subsequent pregnancy.
The use of the laser for hysteroscopic metroplasty has been reported with equivalent postoperative results. However, its superiority over the other techniques is unproven.115,116 A number of studies exist confirming the efficiency and safety of the use of Nd:YAG laser for hysteroscopic metroplasty.117 Similar pregnancy outcomes have been reported with conventional methods. The clear advantage of the Nd:YAG laser is the bloodless nature of the operation and rapid incision of the septum. The use of a flexible fibroscope and the absence of need for cervical dilation are additional advantages. However, its expense makes it prohibitive.
Resection of the septum with the use of a coaxial bipolar electrode surgical system is effective in normal saline solution. The bipolar instrument is passed through a #5 French operating channel of a 5.5-mm hysteroscope.45,118–120 The technique involves either vaporization or excision of the septum. The two basic modes include the vapor cut mode and the desiccation mode, with a range of power settings available (1 to 200 watts), the former being more advantageous for women with infertility. To date limited experience exists with respect to hysteroscopic metroplasty using this form of energy source, but this technique likely holds promise given the theoretical lower risk of fluid overload with normal saline solution as the distension media used and the minimized risk of electrosurgical genital burns.45,118–120 Fernandez and colleagues120 reported on 12 women undergoing septoplasty with the coaxial bipolar system with similar results to traditional surgical techniques. The coaxial bipolar electrode surgical system appears safe and well-tolerated and is likely an effective alternative to conventional hysteroscopic techniques, but further studies need to be completed.
Concurrent laparoscopy and hysteroscopy has been the gold standard for resection of the septum, yet more recent data suggest that intraoperative transabdominal ultrasound can be used both safely and adequately. However, few studies exist, with limited sample size.121,122 The added advantage to the use of intraoperative transabdominal sonography during septum resection is the ability for ultrasound to determine the thickness of the uterine wall and minimize resection of the myometrium. However, there are no data that show that the use of the laparoscope or intraoperative ultrasound has better outcomes than operative hysteroscopy alone.
The surgical technique used for a complete uterine septum includes placement of either a plastic uterine dilator or balloon HSG catheter or Foley balloon through the contralateral cervix to indent the septum wall, as well as prevent the loss of distension medium through the second cervical opening.123 The hysteroscope is then inserted in the opposite cervix and resection is initiated over the indented septum wall, enabling a safe resection. It is important to identify the point above the cervix in which the resection can be initiated. Once the passage is created, resection is then completed while sparing the cervix tissue. Limited studies exist, but the recommendation is to spare the cervical portion and preserve the septum below the internal os to minimize the risk of cervical incompetence in subsequent pregnancies.123–125
Radiologic Techniques
Resection of a uterine septum using radiology techniques has been described.126,127 Karande and Gleicher127 reported on 14 patients who underwent in-office resection of a uterine septum under fluoroscopic control. Resections were carried out with the technique described for intrauterine adhesions, using either hysteroscopic scissors in combination with a specially designed balloon catheter or in select cases using 2-mm microlaparoscopic scissors. Successful resection was reported in all patients with minimal complaints of major discomfort. The procedure was completed within 20 to 30 minutes, with an associated radiation exposure time of less than 7 minutes. However, no pregnancy outcomes were reported in the study. Some potential advantages appear to be the lack of need for anesthesia, the performance in an office setting, the depth perception provided with fluoroscopic control, and abolished risk of fluid overload. No direct comparative trials exist comparing radiologic resection and hysteroscopic metroplasty. Although the technique holds promise, further studies are needed and hysteroscopic metroplasty still remains the standard of care for the resection of uterine septa.
Postoperative Care
A number of adhesion prevention techniques have been described after hysteroscopic metroplasty. Postoperative estrogens or intrauterine contraceptive devices have been used. However, randomized studies in women undergoing hysteroscopic resection of the uterine septum have noted no difference in the postoperative intrauterine adhesion rates with follow-up imaging either by HSG or hysteroscopy with the use of either agent.114,128,129 Furthermore, endometrial preparation with gonadotropin-releasing hormone agonists, gestogens, or danazol are not efficacious nor required because the endometrium is thin enough in the follicular phase of the cycle. In addition, the use of postoperative antibiotics is not required. Postoperative formation of intrauterine adhesions is rare, as are postoperative infections. New endometrium develops within 2 months after hysteroscopic metroplasty simply by endogenous estrogen that stimulates endometrial re-epithelization, as reported by Candiani and coworkers.130
Complications
Hysteroscopic metroplasty is the accepted treatment for the septate uterus.112 Although it is a relatively safe procedure with documented improvement in reproductive outcome in women with recurrent pregnancy loss, it is not free of complications. The operative complications of hysteroscopy have been reported to occur in 2.7% of hysteroscopies and include uterine perforation, excessive hemorrhage, air embolus, pulmonary edema, excessive glycine absorption, and infection.87 After hysteroscopic metroplasty the uterine walls are considered strong enough to support labor. However, among the most catastrophic events are those that occur during labor, such as uterine rupture.131 Uterine rupture in labor has been reported after uncomplicated operative hysteroscopy without rupture as well as after uterine perforation during hysteroscopic metroplasty.131–137
The complications associated with hysteroscopic metroplasty can be viewed in two major categories: those intrinsic to operative hysteroscopy and those related to the technique and instruments used for septum resection. The complications related to operative hysteroscopy are reviewed in detail in Chapter 45. In summary, the reported complications seen with operative hysteroscopy include the possibility of fluid overload, perforation, and pelvic organ injury, including thermal damage if electrode surgical systems are used. The overall complication rate associated with hysteroscopy is reported to be 2.7%.87 The distension media usually used with the monopolar electrosurgical system are sorbitol or glycine. The septum resection hence needs to be carried out in a timely fashion to minimize excessive fluid overload and the development of hyponatremia, cerebral edema, and possible death. The use of normal saline solution with either the operative hysteroscope or the bipolar electrosurgical system is associated with reduced risk of electrolyte changes and hyponatremia with excessive fluid absorption, but the risk is not abolished. Report exists of fluid overload with the use of saline solution, pulmonary edema, and brain edema.45 Furthermore, the risk potential seen with the use of the electrosurgical system includes electrosurgical genital burns, seen mostly with the use of the monopolar systems.57,58
The major concern with the use of the electrosurgical systems is uterine rupture at the actual site of the septum with a later pregnancy secondary to weakening of the uterine wall from thermal damage. The rate of uterine perforation is lower with resection of a uterine septum than it is for intrauterine adhesiolysis and is reported to be less than 1%.59 Vaginal delivery is still recommended in women after hysteroscopic metroplasty unless extensive damage has occurred through thermal injury or a fundal perforation has occurred. Reports exist of potential catastrophic events, albeit rare, including uterine perforation with subsequent pregnancy or labor. Uterine rupture has been reported with both uncomplicated hysteroscopic metroplasty, as well as after uterine perforation during hysteroscopic metroplasty. The rupture, most often fundal in location, can extend from cornua to cornua and likely is secondary to either a weakened myometrium from extensive resection of the septum into the myometrium, thermal damage from the use of the electrical system, or an undiagnosed uterine perforation.131–138 However, metroplasty may weaken the uterine wall regardless of the method used and despite the absence of perforation. Angell and colleagues136 reported a review of the literature on uterine rupture at term with or without uterine perforation. It is possible that the septate uterus is congenitally weakened; hence an inherent risk exists before metroplasty. Once uterine rupture occurs, successful outcomes by cesarean section with a subsequent pregnancy are possible.139
Outcome
Many studies exist that report both presurgical and postsurgical outcome in women with hysteroscopic metroplasty. However, to date there are no published randomized clinical trials that compare pregnancy outcome in a treated and untreated group of symptomatic women. Hence, surgical outcomes after treatment of septate uterus are based on retrospective studies evaluating the reproductive outcome of women, often using patients as their own control. The overall reported rate of successful pregnancy after hysteroscopic metroplasty is 85% to 90%.78,108,113,116 In a small retrospective series of 40 women, Hickok90 reported a miscarriage rate of 77.4%, delivery rate of 22.6%, and uncomplicated delivery rate of 6.5% preoperatively compared to a miscarriage rate of 18.2%, delivery rate of 81.8%, and uncomplicated delivery rate of 77.3% postoperatively.
Venturoli and coworkers140 reviewed a large series of 141 women who underwent a hysteroscopic metroplasty with a partial septate uterus (class Vb, ASRM). Two groups of women were identified: Group I (69 patients) presented with primary unexplained infertility of at least 2 years’ duration and group II (72 patients) with two or more recurrent losses within 3 years and no prior deliveries. The mean age of the women was similar in both groups, and only women who wanted to conceive were included in the study. The mean postoperative follow-up period was 36 months (±19.5). The pregnancy rate postseptum resection for group I and group II was 52.1% and 52.7%, respectively; the spontaneous abortion rate was 20% and 25%, respectively. The preterm and term delivery rates were similar in groups I and II, 10% and 15.5%, respectively, for term delivery and 52.5% and 46.2%, respectively, for preterm.
Kupesic141 reviewed the reproductive outcome from 13 studies in women with untreated septate uterus and reported on 1304 pregnancies with a miscarriage rate of 81.9% and a preterm delivery rate of 9.6%, but the author cautions that the group of women reviewed may represent a biased group; women may have been excluded who had a septate uterus and normal reproductive outcome. Kupesic also reported a review of the existing literature with regard to reproductive outcome before and after hysteroscopic metroplasty for septate uterus. In 388 patients, 1059 pregnancies were achieved before metroplasty and 362 pregnancies after surgery. The miscarriage rate, preterm delivery rate, and term delivery rate were 87.8%, 9.0%, and 3.2%, respectively, before metroplasty versus 14.6%, 5.2%, and 80.1%, respectively, after metroplasty.
However, there still remains some debate whether metroplasty improves the outcome in women with recurrent pregnancy loss. In a series of 146 women reported by Kirk and colleagues,142 no increase in the number of living children was found after metroplasty even in women with uterine septum.
Hysteroscopic metroplasty in women with recurrent pregnancy loss does not impair their fertility potential. Multiple studies report that patients with recurrent pregnancy loss and normal fertility were able to conceive at least once after hysteroscopic metroplasty. Normal monthly fecundity exists for such women.79,115,143 The chance of pregnancy was not affected by maternal age, number of previous pregnancy losses, or method of septal resection (microscissors, resectoscope, or laser) nor by the type of septum present, partial or complete.91,92
In contrast, women with infertility and septal resection appear to have lower cumulative pregnancy rates and live birth rates. This is probably related to the presence of additional infertility factors compromising the chance of pregnancy, or the possibility that presence of the septum was not the cause of the infertility.115 In women with primary infertility and a septate uterus, the data are limited as to whether there is an improvement in the pregnancy rate.
The possible adverse effect of the presence of a septate uterus on the outcome of ART is still debated. The existing studies do not demonstrate any impairment in ovarian response to stimulation nor implantation rates in the presence of müllerian anomalies, including septate uterus. However, the studies do report a higher rate of abortion and preterm delivery if the septum is uncorrected.79,144,145 Although hysteroscopic metroplasty is not intended to enhance fertility, it may be indicated for the improvement of pregnancy outcome, especially after multiple failed assisted reproductive cycles.
1 Asherman JG. Amenorrhea traumatica. J Obstet Gynaecol Br Emp. 1948;55:23-30.
2 Asherman JG. Traumatic intrauterine adhesions. BJOG. 1950;57:892-896.
3 Netter AP, Musset R, Lambert A, Salomon Y. Traumatic uterine synechiae: A common cause of menstrual insufficiency, sterility and abortion. Am J Obstet Gynecol. 1956;71:368-375.
4 Dicker D, Ashkenazi J, Dekel A, et al. The value of hysteroscopic evaluation in patients with preclinical in vitro fertilization abortions. Hum Reprod. 1996;11:730-731.
5 Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7:351-354.
6 Al-Inany H. Intrauterine adhesions. An update. Acta Obstet Gynecol Scand. 2001;80:986-993.
7 Fedorkow D, Pattinson HA, Taylor PJ. Is diagnostic hysteroscopy adhesiogenic? Int J Fertil. 1991;36:21-22.
8 Sweeney WJ. Intrauterine synechiae. Obstet Gynecol. 1966;27:284-289.
9 Sirbu P, Coman A, Vexler E. Gynecol Obstet (Paris). 1957;56:521-528.
10 Adoni A, Palti Z, Milwidsky A. The incidence of intrauterine adhesions following spontaneous abortions. Int J Fertil. 1982;27:117-178.
11 Friedler S, Margalioth EJ, Kafka I, Yaffe H. Incidence of post-abortion intra-uterine adhesions evaluated by hysteroscopy—a prospective study. Hum Reprod. 1993;8:442-444.
12 Golan A, Schneider D, Avrech O. Hysteroscopic findings after missed abortion. Fertil Steril. 1992;58:508-510.
13 Romer T. Postabortion hysteroscopy—a method for early diagnosis of congenital and acquired intrauterine causes of abortions. Eur J Obstet Gynecol Reprod Biol. 1994;57:171-173.
14 Lurie S, Appelman Z, Katz Z. Curettage after midtrimester termination of pregnancy—is it necessary? J Reprod Med. 1991;36:786-788.
15 Westendorp ICD, Ankum WM, Mol BWJ, Vonk J. Prevalence of Asherman’s syndrome after secondary removal of placental remnants or a repeat curettage for incomplete abortion. Hum Reprod. 1998;13:3347-3350.
16 Schenker JG. Etiology and therapeutic approach to synechia uteri. Eur J Obstet Gynecol Reprod Biol. 1996;65:109-113.
17 Jensen PA, Stromme WB. Amenorrhea secondary to puerperal curettage (Asherman’s syndrome). Am J Obstet Gynecol. 1972;113:150-157.
18 Taylor PJ, Cumming DC, Hill PJ. Significance of intrauterine adhesions detected hysteroscopically in eumenorrheic infertile women and the role of antecedent curettage in their formation. Am J Obstet Gynecol. 1981;139:239-242.
19 Polishuk WZ, Schenker JG. Induction of intrauterine adhesions in the rabbit with autogenous fibroblast implants. Am J Obstet Gynecol. 1973;115:789-794.
20 Yaffe H, Ron M, Polishuk WZ. Amenorrhea, hypomenorrhea and uterine fibroids. Am J Obstet Gynecol. 1978;130:599-601.
21 Sugimoto O. Diagnostic and therapeutic hysteroscopy for traumatic intrauterine adhesions. Am J Obstet Gynecol. 1978;131:539-547.
22 March CM. Update: Intrauterine adhesions. Fertil News. XVIV, 1996.
23 Sparac V, Kupesic S, Ilijas M, et al. Histologic architecture and vascularization of hysteroscopically excised intrauterine septa. J Am Assoc Gynecol Laparosc. 2001;8:111-116.
24 Polishuk WZ, Siew FP, Gordon R, et al. Vascular changes in traumatic amenorrhea and hypomenorrhea. Int J Fertil. 1977;22:189-192.
25 Valle RF, Sciarra JJ. Intrauterine adhesions: Hysteroscopic diagnosis, classification, treatment and reproductive outcome. Am J Obstet Gynecol. 1988;158:1459-1470.
26 Donnez J, Nisolle M. Hysteroscopic lysis of intrauterine adhesions (Asherman’s syndrome). In: Donnez J, editor. Atlas of Laser Operative Laparoscopy and Hysteroscopy. New York: Press-Parthenon Publishers; 1994:305-322.
27 Wamsteker K, DeBlok SJ. Diagnostic hysteroscopy: Technique and documentation. In: Sutton C, Diamond M, editors. Endoscopic Surgery for the Gynecologist. New York: Lippincott Williams and Wilkins; 1995:263-276.
28 March CM, Israel R, March AD. Hysteroscopic management of intrauterine adhesions. Am J Obstet Gynecol. 1978;130:653-657.
29 American Fertility Society. The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, Müllerian anomalies, and intrauterine adhesions. Fertil Steril. 1988;49:944-955.
30 Nasr AL, Al-Inany HG, Thabet SM, Aboulghar M. A clinicohysteroscopic scoring system of intrauterine adhesions. Gynecol Obstet Invest. 2000;50:178-181.
31 Schenker JG, Margalioth EJ. Intrauterine adhesions. An updated appraisal. Fertil Steril. 1982;37:593-610.
32 Narayan R, Gosway RK. Transvaginal sonography of the uterine cavity with hysteroscopic correlation in the investigation of infertility. Ultrasound Obstet Gynecol. 1993;3:129-133.
33 Shalev J, Meizner I, Bar-Hava I, et al. Predictive value of transvaginal sonography performed before routine diagnostic hysteroscopy for evaluation of infertility. Fertil Steril. 2000;73:412-417.
34 Alborzi S, Dehbashi S, Khodaee R. Sonohysterosalpingographic screening for infertile patients. Int J Gynecol Obstet. 2003;82:57-62.
35 Fedele L, Bianchi S, Dorta M, Vignali M. Intrauterine adhesions: Detection with transvaginal ultrasound. Radiology. 1996;199:757-759.
36 Bacelar AC, Wilcock D, Powell M, Worthington BS. The value of MRI in the assessment of traumatic intra-uterine adhesions (Asherman’s syndrome). Clin Radiol. 1995;50:80-83.
37 Bukulmez O, Yarali H, Gurgan T. Total corporal synechiae due to tuberculosis carry a very poor prognosis following hysteroscopic synechialysis. Hum Reprod. 1999;14:1960-1961.
38 Pabuccu R, Atay V, Orhon E, et al. Hysteroscopic treatment of intrauterine adhesions is safe and effective in the restoration of normal menstruation and fertility. Fertil Steril. 1997;68:1141-1143.
39 Broome JD, Vancaillie TG. Fluoroscopically guided hysteroscopic division of adhesions in severe Asherman syndrome. Obstet Gynecol. 1999;93:1041-1043.
40 Fernandez H, Gervaise A, de Tayrac R. Operative hysteroscopy for infertility using normal saline solution and a coaxial bipolar electrode: A pilot study. Hum Reprod. 2000;15:1773-1775.
41 Zikopoulos KA, Kolibianakis AM, Plateau P, et al. Live delivery rates in sub fertile women with Asherman’s syndrome after hysteroscopic adhesiolysis using resectoscope or the Versapoint system. RBM Online. 2004;8:720-725.
42 Bulent Tiras M, Oktem M, Noyan V. Laparoscopic intracorporeal ultrasound guidance during hysteroscopic adhesiolysis. Eur J Obstet Gynecol Reprod Biol. 2003;108:80-84.
43 Goldberg BB, Liu JB, Kuhlman K, et al. Endoluminal gynecologic ultrasound: Preliminary results. J Ultrasound Med. 1991;10:583-590.
44 Goldberg BB, Liu JB, Merton DA, et al. Sonographically guided laparoscopy and mediastinoscopy using miniature catheter-based transducers. J Ultrasound Med. 1993;12:49-54.
45 Vilos GA. Intrauterine surgery using a new coaxial bipolar electrode in normal saline solution (Versa point*): A pilot study. Fertil Steril. 1999;72:740-743.
46 Lindheim SR, Kavic S, Shulman SV, Sauer MV. Operative hysteroscopy in the office setting. J Am Assoc Gynecol Laparosc. 2000;7:65-69.
47 Marwah V, Bhandari SK. Diagnostic and interventional microhysteroscopy with the use of the coaxial bipolar electrode system. Fertil Steril. 2003;79:413-417.
48 McComb PF, Wagner BL. Simplified therapy for Asherman’s syndrome. Fertil Steril. 1997;68:1047-1050.
49 Lancet M, Mass N. Concomitant hysteroscopy and hysterography in Asherman’s syndrome. Int J Fertil. 1981;26:267-272.
50 Neuwirth RS, Hussein AR, Schiffman BM, Amin HK. Hysteroscopic resection of intrauterine scars using a new technique. Obstet Gynaecol. 1982;60:111-113.
51 Ikeda T, Morita A, Imamura A, Mori I. The separation procedure for intrauterine adhesions (synechia uteri) under roentgengraphic view. Fertil Steril. 1981;36:333-338.
52 Karande V, Levrant S, Hoxsey R, et al. Lysis of intrauterine adhesions using gynaecoradiological techniques. Fertil Steril. 1997;68:658-662.
53 Coccia ME, Becattini C, Bracco GL, et al. Pressure lavage under ultrasound guidance: A new approach for outpatient treatment of intrauterine adhesion. Fertil Steril. 2001;75:601-606.
54 Massouras HG. Intrauterine adhesions: A syndrome of the past, with the use of the Massouras duck’s Foot No.2 intrauterine contraceptive device. Am J Obstet Gynecol. 1973;116:576-578.
55 Tsapanos VS, Stathopoulou LP, Papathanassopoulou VS, Tzingounis VA. The role of Seprafilm bioresorbable membrane in the prevention and therapy of endometrial synechiae. J Biomed Matern Res. 2002;63:10-14.
56 Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: Predicting patients at risk. Obstet Gynecol. 2000;96:517-520.
57 Vilos GA, D’Souza I, Huband D. Genital tract burns during roller ball endometrial coagulation. J Am Assoc Gynecol Laparosc. 1997;4:273-276.
58 Vilos GA, Brown G, Graham G, et al. Genital tract electrical burns during hysteroscopic endometrial ablation: A report of 13 cases in United States and Canada. J Am Assoc Gynecol Laparosc. 2000;7:141-144.
59 Agostini A, Cravello L, Shojai R, et al. Postoperative infection and surgical hysteroscopy. Fertil Steril. 2002;77:766-768.
60 Siegler AM, Valle RF. Therapeutic hysteroscopic procedures. Fertil Steril. 1988;50:685-701.
61 Lin PC, Bhatnagar KP, Nettleton GS, Nakajima ST. Female genital anomalies affecting reproduction. Fertil Steril. 2002;78:899-915.
62 Crosby WM, Hill EC. Embryology of the müllerian duct system. Obstet Gynecol. 1962;20:507-515.
63 Musset R, Muller P, Netter A, et al. Etat du haut appareil urinaire chez les porteuses de malformations uterines, Etude de 133 observations. Presse Med. 1967;75:1227-1232.
64 Muller PP, Musset R, Netter A, et al. Etat du haut appareil urinaire chez les porteuses de malformations uterines, Etude de 133 observations. Presse Med. 1967;75:1331-1336.
65 Ergun A, Pabuccu R, Atay V, et al. Three sisters with septate uteri: Another reference to bi-directional theory. Hum Reprod. 1997;12:140-142.
66 Elias S, Simpson JL, Carson SA, et al. Genetic studies in complete mullerian fusion. Obstet Gynecol. 1984;63:276-281.
67 Verp MS, Simpson JL, Elias S, et al. Heritable aspects of uterine anomalies. I. Three families aggregates with Müllerian fusion anomalies. Fertil Steril. 1983;40:80-86.
68 Simpson JL. Genes and chromosomes that cause female infertility. Fertil Steril. 1985;44:725-739.
69 Harger JH, Archer DF, Marchese SG, et al. Etiology of recurrent pregnancy loss and outcome of subsequent pregnancies. Obstet Gynecol. 1983;62:574-581.
70 Buttram VC, Gibbons WE. Müllerian anomalies: A proposed classification (an analysis of 144 cases). Fertil Steril. 1979;32:140-146.
71 Hundley AF, Fielding JR, Hoyte L. Double cervix and vagina with septate uterus: An uncommon Müllerian malformation. Obstet Gynecol. 2001;98:982-985.
72 Toaff ME, Lev-Toaff AS, Toaff R. Communicating uteri: Review and classification with introduction of two previously unreported cases. Fertil Steril. 1984;41:661-679.
73 McBean JH, Brumsted JR. Septate uterus with cervical duplication: A rare malformation. Fertil Steril. 1994;62:415-417.
74 Acien P. Incidence of Müllerian defects in fertile and infertile women. Hum Reprod. 1997;12:1372-1376.
75 Simon C, Martinez L, Pardo F, et al. Müllerian defects in women with normal reproductive outcome. Fertil Steril. 1991;56:1192-1193.
76 Cooper JM, Houck RM, Rigberg HS. The incidence of intrauterine abnormalities found at hysteroscopy in patients undergoing elective hysteroscopic sterilization. J Reprod Med. 1983;28:659.
77 Ashton D, Amin HK, Richart RM, Neuwirth RS. The incidence of asymptomatic uterine anomalies in women undergoing transcervical tubal sterilization. Obstet Gynecol. 1988;72:28-30.
78 Heinonen PK, Pystynen PP. Primary infertility and uterine anomalies. Fertil Steril. 1983;40:311-316.
79 Grimbizis GF, Camus M, Tarlatzis BC, et al. Clinical implications of uterine malformations and hysteroscopic treatment results. Hum Reprod. 2001;7:161-174.
80 Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66:24-29.
81 Raga F, Bauset C, Remohi J, et al. Reproductive impact of congenital Müllerian anomalies. Hum Reprod. 1997;12:2277-2281.
82 Raga F, Bonilla-Musoles F, Blanes J, Osborne NG. Congenital Müllerian anomalies: Diagnostic accuracy of three-dimensional ultrasound. Fertil Steril. 1996;65:523-528.
83 Ludmir J, Samuels P, Brooks S, Mennuti MT. Pregnancy outcome of patients with uncorrected uterine anomalies managed in a high risk-obstetric setting. Obstet Gynecol. 1990;75:906-910.
84 Ben-Rafael Z, Seidman DS, Recabi K, Bider D. Uterine anomalies. A retrospective, matched-control study. J Reprod Med. 1991;36:723-727.
85 Jewelewicz R, Husarni N, Wallach EE. When uterine factors cause infertility. Contemp Obstet Gynecol. 1980;16:95.
86 DeCherney AH, Russell JB, Graebe RA, Polan ML. Resectoscopic management of mullerian fusion defects. Fertil Steril. 1986;45:726-728.
87 Propst AM, Hill JA. Anatomic factors associated with recurrent pregnancy loss. Semin Reprod Med. 2000;18:341-350.
88 Pellerito J, McCarthy S, Doyle M, et al. Diagnosis of uterine anomalies: Relative accuracy of MR imaging, endovaginal sonography, and hysterosalpingography. Radiology. 1992;183:795-800.
89 Dabirashrafi H, Bahadori M, Mohammad K, et al. Septate uterus: New idea on the histologic features of the septum in this abnormal uterus. Am J Obstet Gynecol. 1995;172:105-107.
90 Hickok LR. Hysteroscopic treatment of the uterine septum: A clinician’s experience. Am J Obstet Gynecol. 2000;182:1414-1420.
91 Fedele L, Dorta M, Brioschi D, et al. Pregnancies in septate uteri: Outcome in relation site of uterine implantation as determined by sonography. Am J Radiol. 1989;152:781-784.
92 Fedele L, Bianchi S, Marchini M, et al. Ultrastructural aspects of endometrium in infertile women with septate uterus. Fertil Steril. 1996;65:750-752.
93 Proctor JA, Haney AF. Recurrent first trimester pregnancy loss is associated with uterine septum but not with bicornuate. Fertil Steril. 2003;80:1212-1215.
94 Sheth SS, Sonkawde R. Uterine septum misdiagnosed on hysterosalpingogram. Int J Gynecol Obstet. 2000;69:261-263.
95 Preutthipan S, Linasmita V. A prospective comparative study between hysterosalpingography and hysteroscopy in the detection of intrauterine pathology in patients with infertility. J Obstet Gynaecol Res. 2003;29:33-37.
96 Fayez JA, Mutie G, Schneider PJ. The diagnostic value of hysterosalpingography and hysteroscopy in infertility investigation. Am J Obstet Gynecol. 1987;156:558-560.
97 Soares SR, dos Reis MMBB, Camargos AF. Diagnostic accuracy of sonohysterography, transvaginal sonography, and hysterosalpingography in patients with uterine diseases. Fertil Steril. 2000;73:406-411.
98 Soules MR, Spadoni LR. Oil versus aqueous media for hysterosalpingography: A continuing debate based on many options and few facts. Fertil Steril. 1982;38:1-11.
99 Keltz MD, Olive DL, Kim AH, Arici A. Sonohysterography for screening in recurrent pregnancy loss. Fertil Steril. 1997;67:670-674.
100 Fedele L, Dorta M, Brioschi D, et al. Magnetic resonance imaging of double uteri. Obstet Gynecol. 1989;74:844-847.
101 Carrington BM, Hriacak H, Nuruddin RN, et al. Müllerian duct anomalies: MR imaging and detection. Radiology. 1990;176:715-720.
102 Doyle MB. Magnetic resonance imaging in müllerian fusion defects. J Reprod Med. 1992;37:33-38.
103 Saleem SN. MR imaging diagnosis of uterovaginal anomalies: Current state of the art. Radiographics. 2003;23:e13.
104 Mintz M, Thickman D, Gussman D, Kressel H. MR evaluation of uterine anomalies. Am J Roentgenol. 1987;148:287-290.
105 Pellerito JS, McCarthy SM, Doyle MB, et al. Relative accuracy of MR imaging, endovaginal sonography, and hysterososalpingography. Radiology. 1992;183:795-800.
106 Takagi H, Matsunami K, Noda K, et al. Magnetic resonance imaging in the evaluation of double uterus and associated urinary tract anomalies: A report of five cases. J Obstet Gynaecol. 2003;23:525-527.
107 Brown SE, Coddington CC, Schnorr J, et al. Evaluation of out patient hysteroscopy, saline infusion hysterosonography, and hysterosalpingography in infertile women: A prospective randomized study. Fertil Steril. 2000;74:1029-1034.
108 Jones HW, Jones GES. Double uterus as an etiological factor of repeated abortion, indication and surgical repair. Am J Obstet Gynecol. 1953;65:325-331.
109 Tompkins P. Comments on the bicornuate uterus and twinning. Surg Clin North Am. 1962;42:1049-1055.
110 Ayhan A, Yucel I, Tuncer ZS. Reproductive performance after conventional metroplasty: An evaluation of 102 cases. Fertil Steril. 1992;57:1194-1196.
111 Candiani GB, Fefele L, Parazzini F, Zamberletti D. Reproductive prognosis after abdominal metroplasty in bicornuate or septate: A life table analysis. BJOG. 1990;97:613-617.
112 Israel R, March CM. Hysteroscopic incisions of the septate uterus. Am J Obstet Gynecol. 1984;149:66-73.
113 March CM, Israel R. Hysteroscopic management of recurrent abortion caused by septate uterus. Am J Obstet Gynecol. 1987;156:834-842.
114 Vercellini P, Fedele L, Arcaini L, et al. Value of intrauterine device insertion and estrogen administration after hysteroscopic metroplasty. J Reprod Med. 1989;34:447-450.
115 Fedele L, Arcaini L, Parazzini F, et al. Reproductive prognosis after hysteroscopic metroplasty in 102 women: Life table analysis. Fertil Steril. 1993;59:768-772.
116 Choe JK, Baggish MS. Hysteroscopic treatment of septate uterus with neodymium-YAG laser. Fertil Steril. 1992;57:81-84.
117 Jourdain O, Dabysing F, Harle T, et al. Management of septate uterus by flexible hysteroscopy and Nd:YAG laser. Int J Gynecol Obstet. 1998;63:159-162.
118 Kung RC, Vilos GA, Thomas B, Riddick DH. A new bipolar system for performing operative hysteroscopy in normal saline. J Am Assoc Gynecol Laparosc. 1999;6:331-336.
119 Lindheim SR, Kavic S, Shulman SV, Sauer MV. Operative hysteroscopy in the office setting. J Am Assoc Gynecol Laparosc. 2000;7:65-69.
120 Fernandez H, Gervaise A, Tayrac R. Operative hysteroscopy for infertility using normal saline solution and a coaxial bipolar electrode: A pilot study. Hum Reprod. 2000;15:1773-1775.
121 Dabirashrafi H, Mohammed K, Moghadami-Tabbriz N. Three-contrasts method hysteroscopy: The use of real-time ultrasonography for monitoring intrauterine operations. Fertil Steril. 1992;57:450-452.
122 Letterie GS, Kramer DJ. Intraoperative ultrasound guidance for intrauterine endoscopic surgery. Fertil Steril. 1994;62:654-656.
123 Romer T, Lober R. Hysteroscopic correction of a complete septate uterus using a balloon technique. Hum Reprod. 1997;12:478-479.
124 Daly DC, Tohan N, Walters C, et al. Hysteroscopic resection of the uterine septum in the presence of a septate cervix. Fertil Steril. 1983;39:560-563.
125 Rock JA, Murphy AA, Cooper WHIV. Resectoscopic techniques for the lysis of a class V complete uterine septum. Fertil Steril. 1987;48:495-496.
126 Gleicher N, Pratt D, Levrant S, et al. Gynaecoradiological uterine resection. Hum Reprod. 1995;10:1801-1803.
127 Karande VC, Gleicher N. Resection of uterine septum using gynaecoradiological techniques. Hum Reprod. 1999;14:1226-1229.
128 Assaf A, Serour G, Elkady A, El Agizy H. Endoscopic management of the intrauterine septum. Int J Gynaecol Obstet. 1990;32:43-51.
129 Dabirashrafi H, Mohammad-Tabrizi M. Is estrogen necessary after hysteroscopic incision of the uterine septum? J Am Assoc Gynecol Laparosc. 1996;3:623-625.
130 Candiani GB, Vercellini P, Fedele L, et al. Repair of the uterine cavity after hysteroscopic septal incision. Fertil Steril. 1990;54:991-994.
131 Kerimis P, Zolti M, Sinwany G, et al. Uterine rupture after hysteroscopic resection of uterine septum. Fertil Steril. 2002;77:618-620.
132 Lobaugh ML, Bammel BM, Duke D, Webster BW. Uterine rupture during pregnancy in a patient with a history of hysteroscopic metroplasty. Obstet Gynecol. 1994;83:838-840.
133 Tannous W, Hamou J, Henry-Suchet J, et al. Uterine rupture during labor following hysteroscopy. Presse Med. 1996;25:159-161.
134 Gabriele A, Zanetta G, Pasta F, Colombo M. Uterine rupture after hysteroscopy metroplasty and labor induction. A case report. J Reprod Med. 1999;44:642-644.
135 Halvorson LM, Aserkoff RD, Oskowitz SP. Spontaneous uterine rupture after hysteroscopic metroplasty with uterine perforation. A case report. J Reprod Med. 1993;38:236-238.
136 Angell NF, Domingo JT, Siddiqi N. Uterine rupture at term after uncomplicated hysteroscopic metroplasty. Obstet Gynecol. 2002;100:1098-1099.
137 Howe RS. Third-trimester uterine rupture following hysteroscopic uterine perforation. Obstet Gynecol. 1993;81:827-829.
138 Creinin M, Chen M. Uterine defects in a twin pregnancy with a history of hysteroscopic fundal perforation. Obstet Gynecol. 1992;79:879-880.
139 Al Sakka M, Dauleh W, AL Hassani S. Case series of uterine rupture and subsequent pregnancy outcome. Int J Fertil Womens Med. 1999;44:297-300.
140 Venturoli S, Colombo FM, Vianello F, et al. A study of hysteroscopic metroplasty in 141 women with a septate uterus. Arch Gynecol Obstet. 2002;266:157-159.
141 Kupesic S. Clinical implications of sonographic detection of uterine anomalies for reproductive outcome. Ultrasound Obstet Gynecol. 2001;18:387-400.
142 Kirk EP, Chuong CJ, Coulam CB, Williams TJ. Pregnancy after metroplasty for uterine anomalies. Fertil Steril. 1993;59:1164-1168.
143 Homer HA, Li TC, Cooke ID. The septate uterus: A review of management and reproductive outcome. Fertil Steril. 2000;73:1-14.
144 Marcus S, Al-Shawaf T, Brinsden P. The obstetric outcome of in vitro fertilization and embryo transfer in women with congenital uterine malformation. Am J Obstet Gynecol. 1996;175:85-89.
145 Guirgis RR, Shrivastav P. Gamete intrafallopian transfer (GIFT) in women with bicornuate uteri. J In Vitro Fertil Embryo Transfer. 1990;7:283-284.