Hypothyroidism and Myxedema Coma
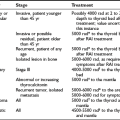
The first step in the spontaneous development of primary hypothyroidism is a slight decrease in thyroid secretion of thyroxine (T4), which causes increased release of TSH. The decreased T4 secretion results in a modest decrease in the serum concentration of free thyroxine (FT4), which still remains within the normal reference range, but serum TSH increases to values above the upper normal limit because of the exquisite sensitivity of the pituitary thyrotroph for circulating thyroid hormone (giving rise to the log-linear relationship between serum TSH and FT4). The condition is known as subclinical hypothyroidism. The increase in TSH induces preferential thyroid secretion of triiodothyronine (T3) by stimulating the synthesis of T3 more than T4 and by increasing thyroidal 5′-monodeiodination of T4 into T3.1,2 The fractional conversion rate of T4 to T3 in extrathyroidal tissues (notably the brain) increases. These mechanisms result in a relative overproduction of T3 compared with T4 and serve—in view of the greater biological potency of T3 than T4—to restrict the impact of thyroid hormone deficiency in peripheral tissues. This preferential T3 production explains why in subclinical hypothyroidism the serum concentration of T3 sometimes exceeds the upper normal limit. Progression of thyroid disease causes a greater decline in thyroidal secretion of T4 and results in serum FT4 levels below the normal reference range and a further rise in serum TSH; serum T3 remains within normal limits because of maintenance of T3 production. Finally, when serum T4 has decreased even further, serum T3 values fall into the subnormal range. Hypothyroidism is a graded phenomenon (Fig. 14-1) that ranges from subclinical hypothyroidism to myxedema coma, the most severe manifestation of the syndrome.
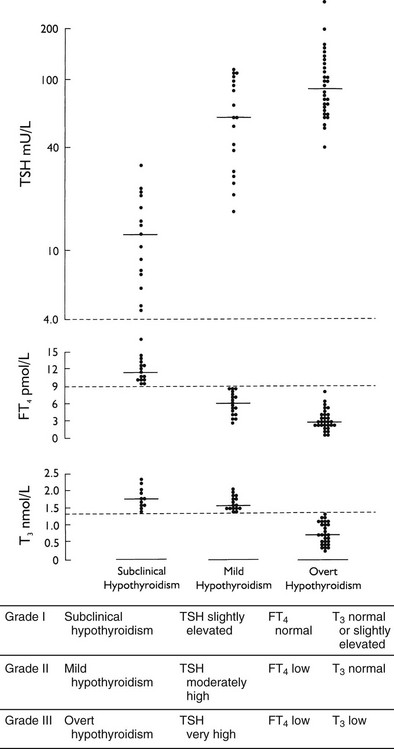
FIGURE 14-1 Individual and median values of thyroid function tests in various grades of primary hypothyroidism. Interrupted horizontal lines indicate upper (thyroid-stimulating hormone [TSH]) and lower (free thyroxine [FT4] and triiodothyronine [T3]) limits of the normal reference range. Progression from grade I to III can be observed in the transition from the euthyroid to the severely hypothyroid state and vice versa on treatment of hypothyroidism.
History
Hypothyroidism as a clinical syndrome was described in 1874 by Gull under the name of myxedema in view of the swollen skin (edema) and its excessive content of mucin (myx-). In 1883, Semon noted striking similarities between patients with myxedema and patients who had undergone total thyroidectomy. The Clinical Society of London nominated a committee to investigate this matter. In 1888, the committee reported in what has become a classic paper3 that cretinism, myxedema, and postthyroidectomy changes all were due to loss of thyroid function. In 1891, Murray reported cure of myxedema by hypodermic injections of sheep thyroid extract. Simply eating ground or dried animal thyroid tissue proved equally effective. The active principle of thyroid extract was isolated by Kendall on Christmas Day, 1914, and was named thyroxine. Harrington elucidated the precise constitution of thyroxine in 1926 and was able to synthesize it. Desiccated thyroid remained the usual treatment for hypothyroidism, however, because thyroxine was more expensive and less efficacious owing to poor absorption of the free acid. As of the 1960s, levothyroxine sodium surplanted gradually desiccated thyroid as the preferred treatment modality for hypothyroidism.
Epidemiology
Primary hypothyroidism is a common disease worldwide, especially in iodine-deficient areas. It also is a prevalent disease in iodine-replete regions. The most extensive epidemiologic data have been obtained from a population-based study of subjects 18 years old and older in Whickham County in northeast England (Table 14-1).4,5 The initial survey was done between 1972 and 1974, with a follow-up 20 years later. The data seem representative of other countries inasmuch as similar figures have been reported from Sweden, Japan, and the United States.6 Most striking are the high prevalence (especially of subclinical hypothyroidism), the marked female preponderance, and the increasing occurrence with advancing age. The mean age at diagnosis of hypothyroidism in women is 60 years. Most cases are due to chronic autoimmune thyroiditis (incidence of 3.5 per 1000 women per year), followed by destructive treatment for thyrotoxicosis (incidence of 0.6 per 1000 women per year). The probability of spontaneous hypothyroidism developing in women at a particular time increases with age: from 1.4 per 1000 per year at ages 20 to 25, to 14 per 1000 per year at 75 to 80 years. Risk factors for progression to overt hypothyroidism include the presence of thyroid autoantibodies and an already elevated TSH (Table 14-2). The risk correlates directly with the serum concentration of thyroid peroxidase autoantibodies and with the extent of the TSH increase. The probability that hypothyroidism will develop increases even at TSH levels in the high-normal range of 2 to 5 mU/L, independent of age or antibody status.5,7
Table 14-1
Prevalence and Incidence of Primary Hypothyroidism in Adults as Established in the Whickham Survey
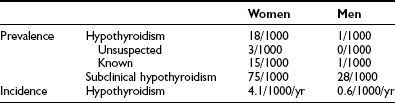
Table 14-6
Characteristics and Treatment of Myxedema Coma
| Hypothyroxinemia | Large doses of intravenous levothyroxine |
| Hypothermia | Blankets, no active rewarming |
| Hypoventilation | Mechanical ventilation |
| Hypotension | Cautious volume expansion with crystalloid or whole blood |
| Hyponatremia | Mild fluid restoration |
| Hypoglycemia | Glucose administration |
| Hypocortisolemia | Glucocorticoid administration |
| Precipitating event | Identification and elimination by specific treatment |
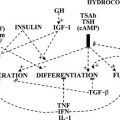
Pathogenesis
The various causes of hypothyroidism can be classified according to their site of interference (in the hypothalamus-pituitary, in the thyroid gland, or in the peripheral target tissues) and their nature (organic lesions resulting in loss of functional tissue, or functional disturbances resulting in deficient hormone biosynthesis and release) (Table 14-3). Most cases of hypothyroidism are acquired and permanent; congenital hypothyroidism and transient forms of hypothyroidism are in the minority.
Table 14-2
Percentage of Women Acquiring Spontaneous Primary Hypothyroidism During 20 Years of Follow-up in the Whickham Survey

Central Hypothyroidism
Reduced T4 secretion in central hypothyroidism is due to insufficient stimulation of the thyroid gland by TSH, which is caused by lesions in the pituitary (secondary hypothyroidism) or the hypothalamus (tertiary hypothyroidism resulting from deficient thyrotropin-releasing hormone [TRH] release). The term central hypothyroidism is preferred because lesions sometimes involve both sites, which prevents clear-cut distinction. Although an absent TSH response to exogenous TRH would suggest a pituitary cause, and a delayed response would suggest a hypothalamic cause,8 the TSH profiles after TRH are not well correlated to the anatomic site of the lesion. Basal serum TSH values in central hypothyroidism can be low, normal, or even slightly elevated (up to 10 mU/L).9,10 The apparent paradox of central hypothyroidism in the presence of a normal or increased serum TSH concentration is explained by the reduced biological activity of TSH in these patients related to abnormal sialylation of TSH. Central hypothyroidism also is associated with a decreased nocturnal TSH surge (because of loss of the usual nocturnal increase in TSH pulse amplitude, but not TSH pulse frequency), which might hamper further maintenance of normal thyroid function.11,12
The prevalence of central hypothyroidism in the general population is unknown; a rough estimate is 0.005%. The sex distribution is about equal, and central hypothyroidism occurs with peaks in childhood and in adults 30 to 60 years old.13 Congenital cases are due to pituitary hypoplasia, midline defects such as septo-optic dysplasia (TSH deficiency in 20%), Rathke’s pouch cysts, or rare loss-of-function mutations in genes encoding for TRH receptors, the TSH-β subunit, or pituitary transcription factors. Childhood cases are caused most often by craniopharyngioma (TSH deficiency in 53%) or cranial irradiation for brain tumors (TSH deficiency in 6%).14 Adult cases most frequently are due to pituitary macroadenomas (hypothyroidism in 10% to 25%) and pituitary surgery or irradiation. TSH deficiency caused by loss of functional tissue usually becomes manifest after the development of growth hormone and gonadotropin deficiency.13 TSH deficiency sometimes may disappear after selective adenomectomy.15 Cranial radiotherapy for brain tumors causes hypothyroidism in 65%, depending on the radiation dose; the onset varies between 1 and 26 years after irradiation.16
Radiotherapy for pituitary tumors is followed by hypothyroidism in at least 15% (55% when combined with surgery).17 Less common causes include traumatic brain injury and subarachnoid hemorrhage,18 ischemic necrosis from postpartum hemorrhage (Sheehan’s syndrome) and severe shock, pituitary apoplexy (hemorrhage in a pituitary adenoma), infiltrative diseases, and lymphocytic hypophysitis.19 Lymphocytic hypophysitis most likely is an autoimmune disease that occurs predominantly in women during pregnancy and the postpartum period and is characterized by a pituitary mass and hypopituitarism.19 Despite the many known causes of central hypothyroidism, idiopathic cases are still encountered.
In critically ill patients receiving dopamine, serum TSH and the T4 production rate decrease by 60% and 56%, respectively, as a result of direct inhibition of pituitary TSH.20 Transient functional inhibition of TSH release is observed after withdrawal of long-term levothyroxine suppressive therapy, which may last 6 weeks.21 Glucocorticoid excess dampens pulsatile TSH release, which rarely results in decreased serum FT4.22 Octreotide therapy does not cause hypothyroidism despite its inhibition of TSH secretion. High doses of bexarotene, a specific retinoid X receptor agonist used in the treatment of cutaneous T cell lymphoma, cause central hypothyroidism by strongly inhibiting TSH secretion.23
Chronic Autoimmune Thyroiditis
Hypothyroidism secondary to chronic autoimmune thyroiditis is caused mainly by destruction of thyrocytes. The goitrous variant (hypothyroid Hashimoto’s goiter) is characterized by massive lymphocytic infiltration of the thyroid with the formation of germinal centers, oxyphilic changes in thyrocytes called Hürthle or Askanazy cells, and some fibrosis. In the atrophic variant (atrophic myxedema), fibrosis is the predominant feature, along with lymphocytic infiltration. The less common goitrous variant is characterized by a diffuse goiter of firm “rubbery” consistency; the histology remained essentially unaltered after 20 years, and the goiter did not regress despite T4 treatment in 43% of cases.24 Many patients with chronic autoimmune thyroiditis are euthyroid, and a few have an initial transient hyperthyroid stage (labeled as Hashitoxicosis). Hashimoto’s disease is used by many authors as an umbrella term to indicate autoimmune-mediated destruction of thyrocytes, frequently but not always resulting in hypothyroidism, as opposed to Graves’ disease, in which TSH receptor–stimulating antibodies usually result in hyperthyroidism. The two disease entities overlap and can be viewed as opposite ends of a continuous spectrum of thyroid autoimmunity. Destruction of thyrocytes and development of hypothyroidism in Hashimoto’s disease are mediated by cytotoxic T cells and cytokines (especially interferon-γ and tumor necrosis factor) released by infiltrating T cells and macrophages. Humoral immunity appears less important in this respect, but (a subset of) thyroid peroxidase (TPO) antibodies may contribute via antibody-dependent, cell-mediated cytotoxicity, complement-mediated cytotoxicity, and inhibition of TPO enzymatic activity. TSH receptor–blocking antibodies enhance thyroid atrophy and hypothyroidism, possibly also by inducing apoptosis; their prevalence is low except in Japanese patients.25
Genetic and environmental factors enhance the susceptibility of individuals to develop the disease and may determine the direction of the evolving autoimmune reaction. Autoimmune thyroid disease runs in families (80% of patients have a positive family history) and is four to ten times more common in women. Autoimmune hypothyroidism in whites is weakly associated with HLA-DR and CTLA4 polymorphisms; other, still unidentified genes probably are involved. Iodine intake has been identified as an environmental factor because the prevalence of autoimmune hypothyroidism is higher in iodine-replete than in iodine-deficient areas,26 and the incidence increases after supplemental iodine is introduced. Smoking decreases the risk for developing TPO antibodies and hypothyroidism.27
Reversible Autoimmune Hypothyroidism
Chronic Autoimmune Thyroiditis
Autoimmune hypothyroidism may revert spontaneously into euthyroidism in connection with the disappearance of TSH receptor–blocking antibodies.28 The presence of a goiter and high thyroidal radioiodine uptake increase the likelihood of spontaneous recovery.29 The incidence of spontaneous recovery is about 5%,30 but in Japan—in the face of a high ambient iodine intake—iodide restriction alone restores euthyroidism in one third of patients.29 Autoimmune hypothyroidism, however, is permanent in most patients. Peculiar cases of alternating hypothyroidism and hyperthyroidism are explained by changes in coexisting TSH receptor–blocking and TSH receptor–stimulating antibodies.31
Silent and Postpartum Thyroiditis
Silent or painless thyroiditis and postpartum thyroiditis are variant forms of chronic autoimmune thyroiditis. Thyroid histology shows lymphocytic infiltration with no germinal centers or fibrosis. The autoimmune attack is intense (resulting mainly in T cell–mediated destructive thyroiditis) but transient, which explains the characteristic pattern of transient thyrotoxicosis followed by transient hypothyroidism in the recovery stage. Each stage lasts 2 to 8 weeks. Most patients remain asymptomatic and revert spontaneously to euthyroidism. Occurrence is common in the first year after delivery: The incidence of postpartum thyroiditis is 4% to 6% and 25% in patients with type 1 diabetes mellitus.32–34 Several patterns are recognized: Thyrotoxicosis alone occurs in 38%, thyrotoxicosis followed by hypothyroidism occurs in 26%, and hypothyroidism alone occurs in 36%. TPO antibodies in serum of 100 kU/L or greater at 12 weeks’ gestation predict to a certain extent postpartum thyroiditis (positive predictive value 0.50, negative predictive value 0.98).33 Thyroid antibody titers decrease in the second and third trimesters and increase postpartum. Women with postpartum thyroiditis are at risk for recurrent postpartum thyroiditis after delivery (about 40%) and for permanent hypothyroidism (20% to 30% after 5 years) related to higher antibody titers and absence of a thyrotoxic phase.
Cytokine-Induced Thyroiditis
Treatment for malignant tumors or for hepatitis C or B with interleukin-2 or interferon-α is causally related to the de novo occurrence of TPO antibodies and the development of thyroid dysfunction.35,36 Typical features are similar to features of silent and postpartum thyroiditis and include sudden onset, biphasic pattern of thyrotoxicosis followed by hypothyroidism (although hypothyroidism alone is most frequent), and spontaneous resolution after discontinuation of treatment. The incidence is about 6%; risk factors include female sex and preexisting TPO antibodies.36
Postoperative and Postirradiation Hypothyroidism
Total thyroidectomy results in overt hypothyroidism within 1 month. Subtotal thyroidectomy for Graves’ hyperthyroidism is followed by hypothyroidism in 40% after 10 years37; risk factors include a small thyroid remnant, lymphocytic infiltration, and subsequent exposure to iodine. Most patients become hypothyroid in the first year after surgery; thereafter, the cumulative incidence of hypothyroidism increases by only 1% to 2% per year. Immediate postoperative hypothyroidism does not always indicate permanent hypothyroidism; it may resolve spontaneously by 6 months. Subtotal thyroidectomy for (toxic) nodular goiter carries a much lower risk (about 15%) for postoperative hypothyroidism.
Radioactive Iodine
Radioactive iodine (131I) treatment for Graves’ hyperthyroidism results in a cumulative incidence of hypothyroidism of 70% after 10 years,37 depending on the dose of 131I administered. Most cases occur in the first year (spontaneous return to euthyroidism is observed in some patients); thereafter, the annual incidence of hypothyroidism is 0.5% to 2%, also related to persisting chronic autoimmune thyroiditis. Hypothyroidism after 131I treatment for toxic nodular goiter is less common (6% to 13%).38 131I treatment for nontoxic goiter to reduce goiter size carries a cumulative risk of 58% for the development of hypothyroidism in 8 years, the risk being related to the (relatively high) dose of 131I and the presence of TPO antibodies.39 Hypothyroidism caused by ionizing radiation has been reported in subjects exposed to atomic or hydrogen bomb explosions.
External Irradiation
External radiotherapy of the neck for Hodgkin’s or non-Hodgkin’s lymphoma causes hypothyroidism in 25% to 50% of patients; the risk is related to the radiation dose, the use of iodine-containing contrast agents before radiotherapy, and the duration of follow-up.40 The risk is decreased when the thyroid is shielded during mantle field irradiation. External radiotherapy for head and neck cancer has an actuarial risk of 40% for the development of subclinical hypothyroidism and 15% for overt hypothyroidism 3 years after treatment.41 Another study with a median follow-up of 4.4 years reports a 5-year incidence rate of 48%, with a median time of 1.4 years (range, 0.3 to 7.2 years) to the onset of elevated TSH values.42 Total body irradiation with subsequent bone marrow transplantation for acute leukemia or aplastic anemia is associated with (mainly subclinical) hypothyroidism in about 25% and usually occurs after 1 year; it is transient in half of patients.43
Infiltrative and Infectious Diseases
A rare cause of hypothyroidism is thyroidal infiltration by systemic disease.44 Hypothyroidism is observed in the course of invasive fibrous thyroiditis of Riedel’s (30% to 40%), cystinosis (86% in adults), progressive systemic sclerosis, and amyloidosis. Infections of the thyroid gland are rare and are associated with preexisting thyroid disease and immunocompromising conditions. Occasionally, damage to the thyroid causes hypothyroidism. In contrast, hypothyroidism in the recovery phase of subacute thyroiditis of de Quervain (related to previous viral infections) is a common event.
Iodine Deficiency and Iodine Excess
Hypothyroidism can be caused by iodine deficiency or iodine excess. Inorganic iodide in excess of daily doses of 500 to 1000 µg inhibits organification of iodide, known as the Wolff-Chaikoff effect. Usually, the thyroid gland escapes the Wolff-Chaikoff effect after several weeks because autoregulatory mechanisms inhibit thyroid iodide transport, and the intrathyroidal iodine concentration consequently falls below the level required for inhibition of organification. Failure to escape results in hypothyroidism, which occurs in the presence of underlying thyroid disease, such as chronic autoimmune thyroiditis, previous subacute or postpartum thyroiditis, and 131I or surgical therapy.45 Iodide-induced hypothyroidism may be due to inorganic iodide or organic iodine compounds that are deiodinated in vivo. Sources of iodine excess include an iodine-rich diet (e.g., in Japan with high consumption of seaweed29) and iodine-containing medications, such as potassium iodide, vitamins, kelp, topical antiseptics, radiographic contrast agents, and amiodarone.45 The incidence of amiodarone-induced hypothyroidism in areas with high environmental iodine intake is higher than in areas with low iodine intake (22% and 5%)46; cases occur predominantly in the first 18 months of amiodarone treatment, especially in women with preexisting thyroid antibodies.47
Drug-Induced Hypothyroidism
Drugs that cause hypothyroidism through interference with thyroid hormone production or release in the thyroid gland48 include thiouracils and imidazoles (used as treatment for thyrotoxicosis), lithium, cytokines (see Reversible Autoimmune Hypothyroidism), iodine (see Iodine Deficiency and Iodine Excess), and a variety of environmental and industrial goitrogenic chemicals. Examples of the latter group include naturally occurring goitrogens, such as flavonoids and resorcinol (present in watersheds of the coal-rich and shale-rich regions of Colombia and Kentucky), and industrial pollution with polychlorinated biphenyls. Lithium inhibits thyroidal iodide transport and release of T4 and T3. Long-term lithium treatment results in goiter in 50%, subclinical hypothyroidism in about 20%, and hypothyroidism in about 20%; goiter and hypothyroidism usually occur in the first 2 years of treatment, especially in patients with preexisting thyroid antibodies. Tyrosine kinase inhibitors like sunitinib induce hypothyroidism in about 50%; the responsible mechanism is incompletely understood.49,50
Consumptive Hypothyroidism
Hepatic and cutaneous hemangiomas often express high levels of type 3 iodothyronine-5-deiodinase (D3), which catalyzes the conversion of T4 and T3 into biologically inactive rT3 and 3,3′-T2. In infants with large hemangiomas, hypothyroidism can occur as the result of D3-induced degradation of thyroid hormone at rates that exceed the synthetic capacity of the thyroid gland.51,52 Removal of the tumor restores euthyroidism.
Clinical Features
Systemic manifestations vary considerably, depending on the cause, duration, and severity of the hypothyroid state. The characteristic clinical finding is slowing of physical and mental activity and many organ functions. The characteristic pathologic finding is accumulation of hyaluronic acid and other glycosaminoglycans in interstitial tissue, which is related to loss of the inhibitory effects of thyroid hormone on the synthesis of hyaluronate, fibronectin, and collagen by fibroblasts.53 The hydrophilic properties of glycosaminoglycans lead to a peculiar mucinous nonpitting edema (myxedema) that is most obvious in the dermis but can be present in many organs.
Energy and Nutrient Metabolism
Serum leptin in some but not all studies is slightly low, returning to normal levels after treatment.54,55 Whether thyroid hormone regulates leptin secretion independent of body mass index and body fat remains controversial.56,57 The other adipocytokines have normal (adiponectin) or slightly low (resistin) serum concentrations.55 Hypothyroidism delays glucose absorption from the intestine. Insulin secretion in response to oral glucose is appropriate for the slightly flattened oral glucose tolerance curve. Hepatic gluconeogenesis and glucose use usually remain normal, and blood glucose levels are maintained within normal limits. The occurrence of hypoglycemia in hypothyroid patients should alert the physician to concomitant diseases (e.g., hypopituitarism). The development of hypothyroidism in patients with insulin-dependent diabetes mellitus may require lowering of the insulin dose to counteract the decreased rate of insulin degradation.
Synthesis and degradation of proteins are reduced in hypothyroidism; one of the obvious consequences during childhood is impaired growth. Biosynthesis of fatty acids and lipolysis also are reduced. An increase in total cholesterol in serum occurs, largely as the result of an increase in low-density lipoprotein (LDL)-cholesterol (explained by decreased expression of the T3-responsive liver LDL receptor, which is involved in LDL clearance), combined with an increase in apolipoprotein B, lipoprotein (a), and possible triglycerides.58 An increase in the oxidizability of LDL particles occurs,59 along with a decrease in the metabolism of serum remnant-like particles reflecting chylomicrons and very low-density lipoprotein (VLDL) remnants.60 HDL2 but not HDL3 is increased modestly with higher apoprotein AI but not AII. The changes in serum lipids result in an atherogenic lipid profile that is reversible upon treatment.
Skin and Appendages
Skin changes are prevalent among hypothyroid patients. The skin is dry, pale, thick, and rough with scales, and it feels cold. Dryness is related to decreased function of sebaceous and sweat glands. Pallor is related to decreased skin blood flow and anemia. Yellowish discoloration of the skin may be present, especially on the palms and soles, because of the deposition of carotene, which is converted to a lesser extent to vitamin A. The thick rough skin with scales is caused by mucinous swelling of the dermis and hyperkeratosis of the stratum corneum in the epidermis. The nonpitting swelling is most marked in the extremities and the face and gives rise to the so-called myxedema face (Fig. 14-2). This classic appearance of primary hypothyroidism is seen less often nowadays, probably because of earlier diagnosis achieved by widespread use of the TSH assay. The hair becomes dull, coarse, and brittle. Hair loss occurs in 50%; it usually is diffuse and involves the scalp, beard, and genital hair and less often the eyebrows. Nail deformities also are common: The nails become thin and brittle, have grooves, and grow more slowly.
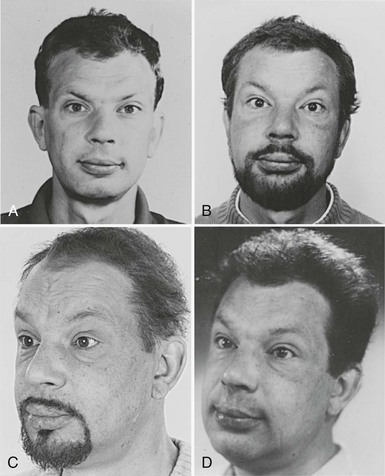
FIGURE 14-2 Appearance of a 47-year-old man 12 years (A), 5 years (B), and 3 years (C) before hypothyroidism secondary to atrophic myxedema (D) was diagnosed. Note the typical myxedema face characterized by puffy nonpitting swelling of the skin and coarse facial features. (Reproduced with permission of the patient.)
Nervous System
Thyroid hormones are essential for normal brain development; congenital hypothyroidism, if left untreated, results in mental retardation and neurologic abnormalities. In adult hypothyroid patients, a generalized decrease in regional cerebral blood flow and in cerebral glucose metabolism has been shown.61 Studies using phosphorus 32 nuclear magnetic resonance spectroscopy of the frontal lobe of adult hypothyroid patients reported reversible alterations in phosphate metabolism.62 The low-voltage electroencephalogram, prolonged central motor conduction time, and reduced visual and somatosensory-evoked potential amplitude with longer latency in adult hypothyroid patients are reversible with T4 treatment. These findings indicate that the adult human brain is a thyroid hormone–responsive organ and provide a biological basis for the prevalent neurobehavioral symptoms and cognitive impairment associated with adult hypothyroidism.61,63,64
Typically, a hypothyroid patient is slow in movement and thought, is less alert, and is less able to concentrate and memorize. Speech becomes slow and often hoarse. Hearing can be impaired. The patient sleeps longer and may fall asleep during the daytime. Hypothyroidism is listed as one of the rare but treatable causes of dementia.65 Patients may accept the limitations in physical and mental activity as part of the unavoidable aging process, but many become anxious or depressed. Rarely, severe anxiety and agitation occur, a condition known as myxedematous madness. Depression develops in more than 40%, most likely related to reduced synthesis and turnover of brain 5-hydroxytryptamine; central 5-hydroxytryptamine activity is reduced in hypothyroid patients.66
Thyroid hormone deficiency may give rise to several reversible neurologic syndromes. Cerebellar ataxia may occur, especially in elderly people, and is associated with an unsteady gait and intention tremor. More common (30%) is the carpal tunnel syndrome, which is linked to entrapment of the median nerve by thickening of the connective tissue of tendon sheaths.67,68 Complaints of paresthesias occur in 64%, and signs of sensorimotor axonal neuropathy are observed in 42%.67 Hashimoto’s encephalopathy is a vaguely defined condition in which otherwise unexplained clinical manifestations of central nervous system dysfunction are linked to the presence of TPO antibodies; serum TSH can be normal or slightly elevated.69 The condition responds to glucocorticoids, but the relationship to thyroid autoimmunity is currently uncertain.
Musculoskeletal System
Muscle symptoms are prevalent in hypothyroid patients and include myalgia, weakness, stiffness, cramps, and easy fatigability.67,68,70 The biochemical substrate of these complaints is provided in part by an increase in the inorganic phosphate-to-adenosine triphosphate (ATP) ratio in resting muscle and by an important decrease in phosphocreatine in working hypothyroid muscle with a greater decrease in intracellular pH than in controls.71 Impairment of mitochondrial oxidative metabolism also has been shown in subclinical hypothyroidism.72 Transition from white fast type II to red slow type I muscle fibers is involved in the change in muscle bioenergetics, which is probably multifactorial. The histopathology varies; most common is type II fiber atrophy, but fiber hypertrophy may be present along with interstitial edema and sarcoplasmic degeneration.70 Rarely, chronic hypothyroid myopathy results in increased volume of muscles (notably in the tongue and extremities), which may cause entrapment syndromes.73 Serum creatine kinase (MM fraction derived from skeletal muscle) is often elevated and correlates with the severity of hypothyroidism. The decreased rate of muscle contractility in hypothyroidism is evident from slow deep tendon reflexes. The half-relaxation time of the Achilles reflex is prolonged in many hypothyroid patients, but substantial overlap is seen in euthyroid subjects.
Cardiovascular System
Changes in cardiovascular dynamics in hypothyroidism include an increase (of 50% to 60%) in peripheral vascular resistance and a decrease (of 30% to 50%) in cardiac output.74,75 Aortic stiffness is increased.76 As a result, mean blood pressure is largely unaltered, although systolic pressure may decrease and diastolic pressure may increase. The increase in systemic vascular resistance is due to endothelial dysfunction and impaired vascular smooth muscle relaxation. The decrease in cardiac output is due to a decrease in stroke volume and heart rate. The pre-ejection time and isovolumetric contraction time are prolonged, and the ventricular relaxation rate during diastole is slower.75 The mechanism of reduced cardiac contractility with subnormal systolic and diastolic performance is multifactorial. Changes in T3-dependent myocardial gene expression are involved, especially in genes that code for calcium regulatory proteins.77 Blood volume is decreased. Edema may develop through albumin extravasation as a result of increased capillary permeability; it may give rise to pericardial, pleural, or peritoneal effusions.
Cardiovascular symptoms of hypothyroid patients include dyspnea and decreased exercise tolerance; the hemodynamic response to exercise usually is preserved. Physical examination may reveal a slow pulse rate, diastolic hypertension (in 20%), weak heart sounds, occasionally cardiac enlargement (caused by pericardial effusion or, rarely, by T4-reversible cardiomyopathy77), and peripheral nonpitting or pitting edema (rarely caused by heart failure except when cardiac disease is preexisting). The electrocardiogram may show bradycardia, low-voltage conduction disturbances, and nonspecific ST-T changes. Symptomatic ischemic heart disease with anginal complaints occurs in about 3%; the reduced need for oxygen in view of the hypometabolic state might give some protection.78 The atherogenic profile of serum lipids and the hyperhomocysteinemia79 in hypothyroidism suggest a greater prevalence of coronary atherosclerosis in these patients.80
Respiratory System
Respiratory symptoms of hypothyroidism include shortness of breath and sleep apnea. Shortness of breath can be caused by the cardiac effects of thyroid hormone deficiency, by weakness of respiratory muscles, by pleural effusion, or by impaired pulmonary function. In most nonobese hypothyroid patients, pulmonary function is nearly normal. Reduced ventilatory drive is observed in 34%; the depressed response to hypercapnia or hypoxia usually is restored rapidly on T4 treatment.81 Severe obstructive sleep apnea occurs in 7.7%,82 in part as a result of increased size of the tongue and pharyngeal muscles with a slow and sustained muscle contraction; the contribution of reduced ventilatory drive to sleep apnea is less marked but can be substantial in obese patients.
Urogenital System
Renal plasma flow and glomerular filtration rate are reduced in hypothyroidism in accordance with the changes in cardiovascular hemodynamics. Serum creatinine is increased by 10% to 20%, and hyponatremia sometimes occurs.79,83 Hyponatremia is associated with increased total body water and sodium content in hypothyroidism, which is a result of the increased vascular permeability and extravascular accumulation of hydrophilic glycosaminoglycans. Free water clearance in hypothyroidism is diminished, regardless of the presence of hyponatremia. Plasma arginine vasopressin frequently is increased in hypothyroid patients; arginine vasopressin levels increase normally in response to hypertonic saline, but they are not suppressed normally after water ingestion. The syndrome of inappropriate antidiuresis in hypothyroidism is not fully understood,84 but a purely renal vasopressin-independent mechanism seems to be involved,85 presumably related to increased expression of aquaporin water channels in the kidney.86 The significance of low serum atrial natriuretic peptide concentrations in hypothyroidism is unclear.72
Reproductive System
Juvenile hypothyroidism results in delayed sexual maturation; it seldom results in precocious puberty (explained by spillover of the action of TRH on gonadotropes and the action of TSH on follicle-stimulating hormone receptors87,88). In adult hypothyroid men, semen analysis is usually normal; erectile dysfunction is common but fully reversible.89 Serum sex hormone–binding globulin, free testosterone, follicle-stimulating hormone, and luteinizing hormone levels most often are normal. In adult hypothyroid women, pulsatile gonadotropin release in the follicular phase is normal,90 but the ovulatory surge may not occur. Irregular—often anovulatory—cycles occur in 23% (three times as often as in the general population); oligomenorrhea and menorrhagia are most common.91 Some patients are seen initially with the galactorrhea-amenorrhea syndrome, which is due to hyperprolactinemia induced by thyroid hormone deficiency. Despite restricted fertility, conception may occur with a successful pregnancy outcome. Pregnancy-induced hypertension is two to three times more common in hypothyroid women. The prevalence of an elevated TSH among pregnant women is about 2%.92 The IQs of children born to affected mothers are 7 points lower than the IQs of controls.93 The spontaneous abortion rate is higher in hypothyroid mothers who do not receive adequate levothyroxine treatment than in mothers who do.94
Gastrointestinal System
Hypothyroidism causes a decrease in electrical and motor activity of the esophagus, stomach, small intestine, and colon. Gastric emptying and intestinal transit times are prolonged.95 The decreased motility explains the common complaint of constipation, which may range from mild to severe (rarely with paralytic ileus and intestinal pseudo-obstruction). Small intestinal bacterial overgrowth is common,96 but intestinal absorption is mostly normal. Malabsorption may be due to pernicious anemia or celiac disease, both of which frequently are associated with autoimmune hypothyroidism. About 25% of patients with chronic autoimmune thyroiditis have parietal cell antibodies; some patients have achlorhydria and vitamin B12 malabsorption. Myxedematous ascites is rare.
Slightly abnormal liver function test results are common97 but usually fully reversible (except when caused by associated autoimmune liver disease). Hypotonia of the gallbladder may occur.
Hematopoietic System
Hemostasis
Hypothyroid patients may have bleeding symptoms, such as easy bruising, menorrhagia, or prolonged bleeding after tooth extraction. Hypothyroidism is associated with a hypocoagulable state: Bleeding time in vivo and clotting time in vitro are prolonged. Coagulation tests reveal low or normal factor VIII activity and decreased von Willebrand factor antigen and activity.98,99 Desmopressin rapidly reduces these abnormalities100 and may be valuable for the acute treatment of bleeding or as cover for surgery. Usually the clinical relevance of these abnormalities is limited, as illustrated by no excess blood loss or bleeding complications during and after surgery in a large series of hypothyroid patients.101
Endocrine System
The decrease in growth hormone secretion in hypothyroidism is related to an increase in hypothalamic somatostatinergic tone102 and results in low insulin-like growth factor (IGF-1) serum concentrations. It may cause dramatic growth retardation in hypothyroid children. Serum IGF-2, IGF-binding protein-1 (IGFBP-1), and IGFBP-3 are also decreased, whereas IGFBP-2 is increased; these changes are reversed with T4 treatment.103
Moderate hyperprolactinemia occurs in 8% of hypothyroid patients, especially in young women, but is not related to the severity of thyroid hormone deficiency.104 It may cause galactorrhea and amenorrhea, particularly in long-standing hypothyroidism. Increased expression of hypothalamic TRH due to diminished negative feedback exerted by T3 might explain the (reversible) hyperprolactinemia in hypothyroidism.
Hypothyroidism in the presence of a pituitary mass does not always indicate central hypothyroidism. The hypersecretion of TSH in primary hypothyroidism is accompanied by hyperplasia and hypertrophy of pituitary thyrotrophs. Rarely, this alteration may cause a distinct pituitary macroadenoma in severely hypothyroid patients with high TSH levels (even with impaired vision) that shrinks after thyroid hormone therapy.105
Adrenal Cortex
Metabolic clearance and, to a lesser extent, production of cortisol are decreased in hypothyroidism106; serum cortisol and 24-hour urinary cortisol remain within normal limits. The adrenal response to exogenous adrenocorticotropic hormone and the pituitary response to hypoglycemia or metyrapone usually are maintained or slightly decreased. Some patients with chronic autoimmune thyroiditis have associated autoimmune adrenalitis. Hypocortisolemia by itself may cause slightly elevated TSH levels that return to normal with glucocorticoid replacement, illustrating the negative feedback of cortisol on TSH secretion.107
Hypothyroidism decreases angiotensinogen production in the liver and serum angiotensin-converting enzyme and plasma renin activity. Serum aldosterone remains normal: The decrease in clearance is neutralized by a decrease in secretion.74 The effects of these changes in the renin-angiotensin-aldosterone system are minimal and are not responsible for the hypertension in hypothyroid patients.
Sympathoadrenal System
Serum norepinephrine concentrations are increased in hypothyroid patients because of an increased production rate; epinephrine production is not affected. The increased central sympathetic output seems to be compensatory for the reduced response to catecholamines in target tissues such as the heart.108 Mechanisms involved include a reduced number of β-adrenergic receptors and postreceptor defects, which contributes to impaired lipolysis, glycogenolysis, and gluconeogenesis.
Diagnosis
Syndromal Diagnosis
Statistical methods based on the frequency of symptoms and signs in patients and controls have been applied to the clinical diagnosis of hypothyroidism. The Billewicz score consists of points given in a weighted manner for the presence or absence of 17 symptoms and signs.109 Application of this score to patients suspected of having hypothyroidism increases the pretest probability of hypothyroidism by 15% to 19%.110 A simpler score is derived by awarding 1 point each for the presence of 12 symptoms and signs (Table 14-4)111; because of a high frequency in euthyroid controls, cold intolerance and pulse rate had predictive values less than 70% and were excluded from the score. The score was higher in older than in younger control women; correction for age is done by adding 1 point for women younger than age 55. The positive predictive value for hypothyroidism is 96.9% with a score of 6 or more points; the negative predictive value for exclusion of hypothyroidism is 94.2% with a score of 2 points or less. Sixty-two percent of all overt hypothyroid and 24% of subclinical hypothyroid patients are classified as clinically hypothyroid by this new score; the corresponding figures with the Billewicz score are 42% and 6%. Receiver operating curves of both scores, however, are similar.
Table 14-3
Central (Hypothalamic/Pituitary) Hypothyroidism
Tumors (pituitary adenoma, craniopharyngioma, meningioma, dysgerminoma, glioma, metastases)
Trauma (surgery, irradiation, head injury)
Vascular (ischemic necrosis, hemorrhage, stalk interruption, aneurysm of internal carotid artery)
Infections (abscess, tuberculosis, syphilis, toxoplasmosis)
Infiltrative (sarcoidosis, histiocytosis, hemochromatosis)
Chronic lymphocytic hypophysitis
Congenital (pituitary hypoplasia, septo-optic dysplasia, basal encephalocele)
Primary (Thyroidal) Hypothyroidism
Loss of functional thyroid tissue
Chronic autoimmune thyroiditis
Reversible autoimmune hypothyroidism (silent and postpartum thyroiditis, cytokine-induced thyroiditis)
Surgery and irradiation (131I or external irradiation)
Functional defects in thyroid hormone biosynthesis and release
Peripheral (Extrathyroidal) Hypothyroidism
The clinical diagnosis of hypothyroidism can be easy but also difficult because of the nonspecific nature of the symptoms and signs and the marked diversity of findings.112 It is incompletely understood why the clinical manifestations of thyroid hormone deficiency vary considerably among patients. Chilliness, paresthesias, weight gain, and muscle cramps occur less frequently in elderly patients, who also have fewer clinical signs than younger patients.113 Smokers have more severe manifestations of hypothyroidism.114
Biochemical Assessment
The ideal diagnostic test for hypothyroidism would be one that accurately measures the effect of thyroid hormone deficiency on target tissues. Peripheral tissue function tests, such as serum cholesterol and creatine kinase, lack sufficient sensitivity and specificity to be of much use. Serum TSH is the best assay for detection of hypothyroidism. By using the flow diagram presented in Fig. 14-3, the following results can be obtained:
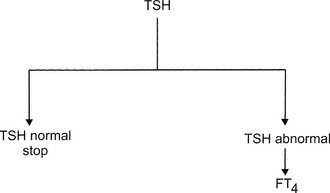
FIGURE 14-3 Flow diagram for the biochemical diagnosis of hypothyroidism. FT4, Free thyroxine; TSH, thyroid-stimulating hormone.
1. TSH normal. Euthyroidism is almost certain, and no additional tests are necessary. The only exception is central hypothyroidism; usually, clinical examination offers sufficient clues to suspect hypothalamic/pituitary disease because isolated TSH deficiency is rare.
2. TSH elevated, FT4 decreased. Primary hypothyroidism is almost always present. In a few cases, TSH values of 5 to 15 mU/L are associated with central hypothyroidism.
3. TSH elevated, FT4 normal. The results indicate subclinical hypothyroidism and sometimes nonthyroidal illness.
4. TSH elevated, FT4 increased. This peculiar combination of test results indicates one of the rare patients with thyroid hormone resistance or thyrotoxicosis caused by a TSH-producing adenoma.
5. TSH decreased, FT4 decreased. These results are compatible with central hypothyroidism, hypothyroidism after recent therapy for thyrotoxicosis, or nonthyroidal illness.
6. TSH decreased, FT4 increased or normal. Hypothyroidism is excluded. The results indicate overt thyrotoxicosis, subclinical hyperthyroidism, or rarely, nonthyroidal illness.
Nosologic Diagnosis
Clues for potentially reversible hypothyroidism can be obtained from the history (recent delivery? exposure to iodine excess? use of antithyroid drugs? recent thyroid surgery or 131I therapy?). In patients with chronic autoimmune thyroiditis, the presence of a goiter, preserved thyroidal radioiodine uptake, and homogeneous distribution of the tracer increase the likelihood of reversible hypothyroidism.29
Treatment
Replacement with Thyroxine
T4 is prescribed as levothyroxine sodium, which comes in tablets of different strength. The sodium salt increases the gastrointestinal absorption of levothyroxine, which is greater in the fasting (80%) than in the fed state (60%).115 Absorption of T4 seems to be greater in the evening at 22.00 hours than in the morning at 06.30 hours before breakfast.116 Generic and brand name levothyroxine preparations are most often bioequivalent,117 but altered bioavailability from changes in the formulation of preparations has been reported.118 About 25% of the exogenous T4 is converted into T3 and provides 80% of the circulating T3 pool.115 The half-life of serum T4 is approximately 7 days, which allows a single daily dose of levothyroxine sodium. Omission of an occasional tablet has little or no clinical relevance.
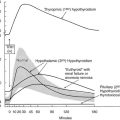
The initial daily dose of levothyroxine sodium depends on the severity and the duration of the hypothyroid state, the age of the patient, and the coexistence of cardiac disease. In the case of mild hypothyroidism, short duration of hypothyroidism, young age, and no heart disease, one may opt to start with a full replacement dose (on average 1.6 µg/kg/d, but with large interindividual variation).119 In the case of more severe or long-standing hypothyroidism, older age, and especially the presence of ischemic heart disease, it is prudent to start with a low dose (25 to 50 µg daily). Too high a starting dose under these circumstances may be poorly tolerated by the patient, who is accustomed to a low metabolic rate, which is now reversed. The patient may experience agitation, palpitations, and worsening or development of anginal complaints because of the increased need for oxygen. Individualization of the initial dose is recommended, and the same holds true for the rate at which the initial dose is increased until the full replacement dose has been reached. In high-risk cases, the daily levothyroxine sodium dose can be increased by 25 to 50 µg every 4 weeks, and it takes 3 to 6 months before the euthyroid state is restored. The mean replacement dose of levothyroxine sodium is 125 µg daily, in line with the daily production rate of 100 µg of thyroxine in normal subjects; it varies, however, between 50 and 200 µg (Fig. 14-4). The final dose required is a function of body weight (especially lean body mass) and initial TSH value,120 but it is not always predictable. The dose should be titrated against serum TSH and FT4 concentrations. These assays should be done no earlier than 4 to 6 weeks after a change in T4 dose, when a new steady state has been established. One aims for TSH values in the low normal range, which results in FT4 values that are significantly higher than values in controls (see Fig. 14-4) and often slightly above the upper normal limit.115 The high T4 levels under these circumstances serve to maintain serum T3 (predominantly derived from 5′-deiodination of T4) in the midnormal range. In patients with central hypothyroidism, one should rely primarily on normalization of serum FT4, which frequently suppresses serum TSH to less than 0.1 mU/L.121
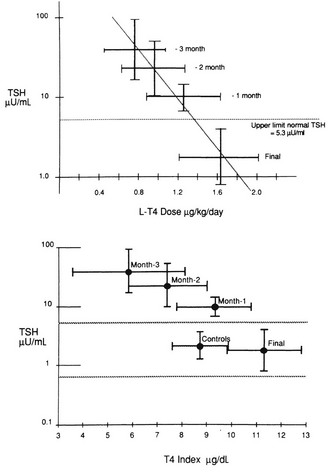
FIGURE 14-4 Dosage titration of levothyroxine (L-T4) (top panel) and the free thyroxine (T4) index (bottom panel) as a function of serum thyroid-stimulating hormone (TSH). Values are expressed by month, counting backward from the final dose. The bars represent 1 standard deviation from the mean. (Reprinted from Fish LH, Schwartz HL, Cavanaugh J, et al: Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism: role of triiodothyronine in pituitary feedback in humans. N Engl J Med 316:764–770, 1987. Copyright © 1987, Massachusetts Medical Society. All rights reserved.)
Some patients feel better with a slightly higher dose of levothyroxine sodium and consequently suppressed TSH. This dose can be accepted as long as serum T3 is still within the normal range and serum TSH is (arbitrarily) not lower than 0.2 mU/L. TSH values of 0.1 mU/L or less carry a risk for atrial fibrillation122 and bone loss123 (especially in postmenopausal women). Long-term levothyroxine therapy at TSH-suppressive doses increases the risk for ischemic heart disease in patients younger than age 65 years.124 No or just a slight excess of fractures has been observed in patients maintained with levothyroxine even if TSH is suppressed.125,126
Factors Requiring Dose Adjustment
When euthyroidism has been restored by the full replacement dose of levothyroxine, this usually suffices to check the patient’s thyroid state once a year. The main reason for the annual follow-up visit is to enhance compliance with lifelong levothyroxine sodium treatment. Some patients need adjustment of the levothyroxine sodium dose for reasons outlined in Table 14-5.
Table 14-4
Accuracy of 12 Symptoms and Signs in the Diagnosis of Primary Hypothyroidism
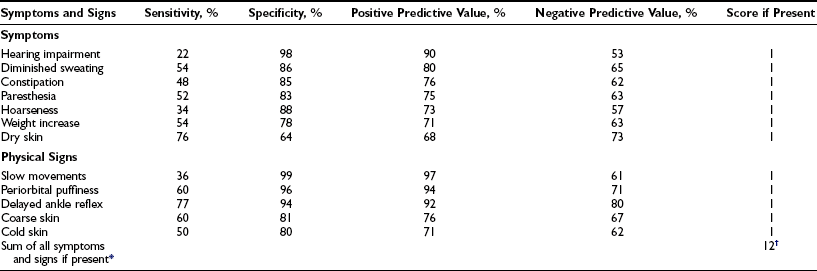
*Add 1 point for women younger than 55 years.
†Hypothyroid, ≥6 points; intermediate, 3 to 5 points; euthyroid, ≤2 points.
From Zulewski H, Müller B, Exer P, et al: Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab 82:771–776, 1997. Copyright © 1997, The Endocrine Society.
Increased Dose Requirement
T4 is absorbed mainly from the small intestine, which explains the higher dose requirements in malabsorption and short-bowel syndromes.127 Nonspecific absorption of T4 by dietary fibers decreases the bioavailability of T4 and necessitates a higher dose in patients with high intake of dietary fiber (whole-wheat bread, granola, bran).128 T4 and T3 conjugates are excreted in bile and partially deconjugated in the intestine, with the release of small amounts of T4 and T3 for reabsorption. Interference with this enterohepatic circulation of thyroid hormone by bile acid–sequestering agents may cause a slight increase in TSH in levothyroxine-treated patients, but not in normal subjects.129 Other drugs, such as sucralfate,130 aluminum hydroxide,131 ferrous sulfate,132 raloxifene,133 and calcium carbonate,134 also decrease the absorption of T4. Serum T4 is often above normal in many levothyroxine-treated patients with normal TSH. The effect of these drug interactions is modest and largely can be avoided by taking levothyroxine sodium and the other drug several hours apart.
Considerable weight gain may increase the need for T4. Estrogen therapy135 and pregnancy136 also require additional thyroid hormone in most hypothyroid patients, probably because of increased serum concentrations of T4-binding globulin. Levothyroxine requirements increase by about 50% in most pregnant women during the first half of pregnancy and plateau by week 16; median onset of TSH increase occurs at 8 weeks’ gestation.136 It is prudent to anticipate these events, and assessment of thyroid function in each trimester is recommended. After delivery, the dose used before pregnancy can be reinstituted.
Several antiepileptic and tuberculostatic drugs increase the clearance of T4 by stimulating the mixed-function oxygenases responsible for hepatic drug oxidation.137 Serum TSH increases in levothyroxine-treated hypothyroid patients when amiodarone is administered,138 possibly as a result of the inhibition of T4 conversion into T3. The mechanism by which other drugs, such as sertraline139 and chloroquine,140 increase T4 requirements is unknown. The most common reason for persistently elevated TSH values despite apparently adequate replacement doses is poor compliance of the patient with intake of levothyroxine tablets. Noncompliance is a challenge to the treating physician who seeks to solve this difficult management problem; in the process, it is important to not lose the patient’s confidence. One option is to administer levothyroxine under supervision once weekly.141 A slightly larger dose than seven times the normal daily dose may be required; a single weekly dose of 1000 µg of levothyroxine sodium given orally seems to be effective and well tolerated.
Decreased Dose Requirement
Considerable weight loss may decrease the need for levothyroxine. In women receiving long-term levothyroxine replacement therapy, administration of androgens for breast cancer may result—via a decrease in serum T4-binding globulin—in thyrotoxicosis within 4 weeks; the levothyroxine dose has to be reduced by 25% to 50%.142 Production and metabolic clearance rates of T4 are slightly decreased in old age; the net result is no change in the serum FT4 concentration. The levothyroxine replacement dose decreases in elderly people by about 25% in association with the decrease in lean body mass with age.143
Other Thyroid Hormone Preparations
Combination of Levothyroxine and Liothyronine
Synthetic levothyroxine and liothyronine have been combined in a single tablet in a ratio of 4 : 1. New interest in this kind of formula has been inspired by animal studies showing that the euthyroid state of thyroidectomized rats could be restored in all tissues only by the combination of levothyroxine and liothyronine and not by levothyroxine alone,144 and likewise by human studies reporting impaired psychological well-being and neurocognitive functioning of hypothyroid patients despite adequate treatment with thyroxine.145,146 However, a meta-analysis of 11 randomized clinical trials found no differences in the effectiveness of T4 and T3 combination therapy versus T4 monotherapy in terms of bodily pain, depression, anxiety, fatigue, and quality of life.147
Interference with Coexistent Conditions
Ischemic Heart Disease
Levothyroxine increases the need for oxygen in the myocardium. Worsening or de novo development of anginal complaints should call for tempering of the levothyroxine dose or institution of antianginal drugs. Alternatively, coronary artery bypass surgery or angioplasty is a safe procedure, even when euthyroidism has not yet been restored.101,148
Surgery
Surgery in hypothyroid patients is associated with increased risk for several minor perioperative complications.101 A higher incidence of heart failure and gastrointestinal and neuropsychiatric complications has been reported. Patients have fever less frequently with infection.
Drugs
The metabolism of many drugs is slowed in hypothyroidism, which results in higher sensitivity to a loading dose and a lower maintenance dose. Hypothyroid patients can experience marked respiratory depression after a single small dose of morphine. Restoration of the euthyroid state may require dose adjustments, for example, an increase in the dose of digoxin or insulin. Treatment for adult growth hormone deficiency with recombinant human growth hormone decreases serum FT4, sometimes into the hypothyroid range.149
Myxedema Coma
Myxedema coma is a rare, life-threatening clinical condition in patients with long-standing, severe untreated hypothyroidism. The term is largely a misnomer because most patients are not comatose. Rather, the entity represents a form of decompensated hypothyroidism in which a precipitating event leads to functional disorders of the cardiovascular and central nervous systems, which, if not recognized and reversed, frequently have a fatal outcome.150
Diagnosis
The three key diagnostic features of myxedema coma are as follows:
1. Altered mental status: from disorientation and lethargy to psychosis and coma
2. Defective thermoregulation: hypothermia or the absence of fever despite infectious disease
3. Precipitating event: cold exposure, infection, drugs (diuretics, sedatives, tranquilizers), trauma, stroke, heart failure, gastrointestinal bleeding
Treatment
Rapid institution of thyroid hormone replacement therapy and supportive measures (Table 14-6) is essential for a successful outcome; the prognosis remains poor, however, with a mortality of at least 20%. Patients should be monitored closely for vital signs.
Table 14-5
Conditions Requiring Adjustment of the Replacement Dose of Thyroxine for Hypothyroidism
Increased Dose Requirement
Decreased intestinal absorption of T4
Malabsorption (e.g., celiac disease) and short-bowel syndrome127
Drugs: bile acid sequestering agents (colestipol, cholestyramine129), α-sucralfate,130 aluminum hydroxide,131 ferrous sulfate,132 raloxifene,133 calcium carbonate134
Amiodarone,138 sertraline,139 chloroquine140
Decreased Dose Requirement
Subclinical Hypothyroidism and Screening
Subclinical hypothyroidism is defined as an elevated serum TSH concentration in the presence of normal serum FT4 and T3. It is a prevalent condition (see Table 14-1) that occurs most often in women and elderly people.4 The causes can be endogenous (chronic autoimmune thyroiditis, subacute thyroiditis, postpartum thyroiditis) or exogenous (131I therapy, thyroidectomy, antithyroid drugs).
The natural course of subclinical hypothyroidism secondary to chronic autoimmune thyroiditis is reasonably well known.5 Spontaneous return to normal TSH values occurs in 5% to 6%. Progression to overt hypothyroidism is a common event, especially if thyroid antibodies are present (see Table 14-2); the annual incidence is about 5%.
Systemic Manifestations
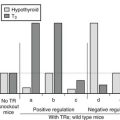
The term subclinical hypothyroidism suggests the absence of symptoms and signs of thyroid hormone deficiency, but clinical experience tells otherwise. Nonspecific complaints (fatigue and weight gain), depressive feelings, and mild cognitive disturbances (poor ability to concentrate, poor memory) can be present. Subjects score higher on a clinical scale for hypothyroidism (Fig. 14-5).111 Peripheral tissue function tests frequently indicate a limited degree of thyroid hormone deficiency; examples include prolongation of the Achilles tendon reflex relaxation time, prolongation of systolic time intervals, a decrease in cardiac contractility, impairment of muscle energy metabolism, and an increase in LDL cholesterol.151
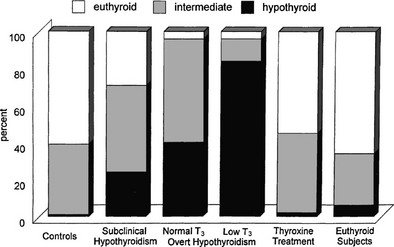
FIGURE 14-5 Assessment of hypothyroidism by a clinical score in 50 patients with overt hypothyroidism, 80 age-matched controls, 93 patients with subclinical hypothyroidism, 67 hypothyroid patients treated with thyroxine, and an additional 109 euthyroid subjects. T3, triiodothyronine. (From Zulewski H, Müller B, Exer P, et al: Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab 82:771–776, 1997. Copyright © 1997, The Endocrine Society.)
Treatment
Treatment for subclinical hypothyroidism is still debated. A Cochrane analysis of randomized clinical trials found that levothyroxine therapy had no effect on survival, cardiovascular morbidity, symptoms, or quality of life, although lipid profiles and left ventricular function improved.152 Nevertheless, meta-analyses of population-based studies demonstrate a higher prevalence and incidence of ischemic heart disease and mortality, at least in women and subjects younger than 65 years.153,154 In view of this, thyroxine therapy might be considered in subjects younger than 65 years if TSH is >10 mU/L and/or TPO antibodies are present. In case TSH is <10 mU/L and TPO antibodies are absent, thyroxine therapy still might be warranted in individuals with a high background for cardiovascular risk, symptoms, pregnancy, and infertility.151 When in doubt because of nonspecific complaints, a trial of T4 treatment for at least 3 months can be considered.
Set Point
Subclinical hypothyroidism seems to be a misnomer, but how some subjects with subclinical thyroid dysfunction experience clinical symptoms and signs, whereas other subjects are really asymptomatic despite similar serum FT4 concentrations, remains an unanswered question. Repeated measurements of thyroid function in the same individual over time show a narrow fluctuation around the mean value. Apparently, each individual is characterized by a fixed relationship between serum TSH and FT4 concentrations; this point can be considered the working point of the pituitary-thyroid axis of that individual. From longitudinal observations in subjects in whom abnormal thyroid function develops, it can be deduced that an intraindividual log-linear relationship exists between TSH and FT4, as depicted by a straight line115; the working point moves along this line, upward in the case of hypothyroidism (Fig. 14-6). The position of the working point of an individual determines the changes in thyroid function test results that are maximally allowed within the conventional reference range before they are labeled abnormal. In the example depicted in Fig. 14-6, the subject with subclinical hypothyroidism and a serum FT4 of 12 mol/L might have an original working point at 18 mol/L (indicating a decrease in serum FT4 by 33%) or at 15 mol/L (indicating a decrease in serum FT4 by 20%). It is conceivable that symptoms and signs are present in the former but not in the latter situation.
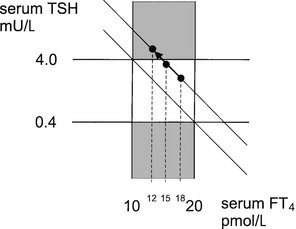
FIGURE 14-6 The log-linear relationship between thyroid-stimulating hormone (TSH) and free thyroxine (FT4) of a particular individual is depicted by a straight line; the working point of that individual moves upward along this line if hypothyroidism develops. Variation between individuals is given by the different location of the working point on the same or parallel lines. The upper hatched area represents subclinical hypothyroidism, and the central area encompasses the normal range of TSH and FT4. For further explanation, see the text.
Screening
In view of the high prevalence of thyroid disease in the general population, the question arises of whether a screening program for adults is justified.6 A simple, inexpensive, and accurate screening test is available: the sensitive TSH assay. The disease to be screened (hypothyroidism and thyrotoxicosis) has a high prevalence and can be treated effectively. The burden of disease is limited, however, and it has not been proved that clinical outcome is improved by early diagnosis and treatment in the asymptomatic stage. Nevertheless, a computer-derived decision model concludes that it is cost effective to screen persons in the general community for mild hypothyroidism with a serum TSH combined with a serum cholesterol every 5 years after the age of 35 years.155 Screening of elderly women is especially cost effective. For the time being, case finding is a suitable alternative, that is, determination of serum TSH in patients (especially pregnant women and older women) who consult the physician because of unrelated problems. A high degree of suspicion of thyroid function disorder is warranted in patients with nonspecific complaints.
References
1. Ishii, H, Inada, M, Tanaka, K, et al. Induction of outer and inner ring monodeiodinases in human thyroid gland by thyrotropin. J Clin Endocrinol Metab. 1983;57:500–505.
2. Lum, SM, Nicoloff, JT, Spencer, CA, et al. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest. 1984;73:570–575.
3. Ord, WM. Report of a committee of the Clinical Society of London nominated December 14, 1883 to investigate the subject of myxoedema. Trans Clin Soc Lond. 1888;8:15.
4. Tunbridge, WMG, Evered, DC, Hall, R, et al. The spectrum of thyroid disease in the community: the Whickham survey. Clin Endocrinol. 1977;7:481–493.
5. Vanderpump, MPJ, Tunbridge, WMG, French, JM, et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham survey. Clin Endocrinol. 1995;43:55–68.
6. Wang, C, Crapo, LM. The epidemiology of thyroid disease and implication for screening. Endocrinol Metab Clin North Am. 1997;26:189–218.
7. Strieder, TGA, Tijssen, JGP, Wenzel, BE, et al. Prediction of progression to overt hypo- or hyperthyroidism in female relatives of patients with autoimmune thyroid disease using the Thyroid Events Amsterdam (THEA) score. Arch Int Med. 2008;168:1–7.
8. Faglia, G. The clinical impact of the thyrotropin-releasing hormone test. Thyroid. 1998;8:903–908.
9. Horimoto, M, Nishikawa, M, Ishihara, T, et al. Bioactivity of thyrotropin (TSH) in patients with central hypothyroidism: comparison between in vivo 3,5,3′-triiodothyronine response to TSH and in vitro bioactivity of TSH. J Clin Endocrinol Metab. 1995;80:1124–1128.
10. Persani, L, Ferretti, E, Borgato, S, et al. Circulating thyrotropin bioactivity in sporadic central hypothyroidism. J Clin Endocrinol Metab. 2000;85:3631–3635.
11. Samuels, MH, Lillehei, K, Kleinschmidt-Demasters, BK, et al. Patterns of pulsatile glycoprotein secretion in central hypothyroidism and hypogonadism. J Clin Endocrinol Metab. 1990;70:391–395.
12. Adriaanse, R, Brabant, G, Endert, E, et al. Pulsatile TSH release in patients with untreated pituitary disease. J Clin Endocrinol Metab. 1993;77:205–209.
13. Vance, ML. Hypopituitarism. N Engl J Med. 1994;330:1651–1662.
14. Schmiegelow, M, Feldt-Rasmussen, U, Rasmussen, AK, et al. A population-based study of thyroid function after radiotherapy and chemotherapy for a childhood brain tumor. J Clin Endocrinol Metab. 2003;88:136–140.
15. Arafah, BM. Reversible hypopituitarism in patients with large non-functioning pituitary adenomas. J Clin Endocrinol Metab. 1986;62:1173–1179.
16. Constine, LS, Woolf, PD, Cann, D, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328:87–94.
17. Snijder, PJ, Fowble, BF, Schatz, NJ, et al. Hypopituitarism following radiation therapy of pituitary adenomas. Am J Med. 1986;81:457–462.
18. Schneider, HJ, Kreitschmann-Andermahr, I, Ghigo, E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429–1438.
19. Bellastella, A, Bizzarro, A, Coronella, C, et al. Lymphocytic hypophysitis: a rare or underestimated disease? Eur J Endocrinol. 2003;149:363–376.
20. Kaptein, EM, Spencer, CA, Kamile, MB, et al. Prolonged dopamine administration and thyroid hormone economy in normal and critically ill subjects. J Clin Endocrinol Metab. 1980;51:387–393.
21. Vagenakis, AG, Braverman, LE, Azizi, F, et al. Recovery of pituitary thyrotropic function after withdrawal of prolonged thyroid suppression therapy. N Engl J Med. 1975;293:681–684.
22. Adriaanse, R, Brabant, G, Endert, E, et al. Pulsatile thyrotropin secretion in patients with Cushing’s syndrome. Metabolism. 1994;43:782–786.
23. Sherman, SI, Gopal, J, Haugen, BR, et al. Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med. 1999;340:1075–1079.
24. Hayashi, Y, Tamai, H, Fukata, S, et al. A long-term clinical, immunological, and histological follow-up study of patients with goitrous chronic lymphocytic thyroiditis. J Clin Endocrinol Metab. 1985;61:1172–1178.
25. Arikawa, K, Ichikawa, Y, Yoshida, T, et al. Blocking type antithyrotropin receptor antibody in patients with nongoitrous hypothyroidism: its incidence and characteristics of action. J Clin Endocrinol Metab. 1985;60:953–959.
26. Laurberg, P, Pedersen, KM, Hreidarsson, A, et al. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab. 1998;83:765–769.
27. Asvold, BO,  , T, Nilsen, TI, et al. Tobacco smoking and thyroid function: a population-based study. Arch Int Med. 2007;167:1428–1432.
, T, Nilsen, TI, et al. Tobacco smoking and thyroid function: a population-based study. Arch Int Med. 2007;167:1428–1432.
28. Takasu, N, Yamada, T, Takasu, M, et al. Disappearance of thyrotropin-blocking antibodies and spontaneous recovery from hypothyroidism in autoimmune thyroiditis. N Engl J Med. 1992;326:513–518.
29. Kasagi, K, Iwata, M, Misaki, T, et al. Effect of iodine restriction on thyroid function in patients with primary hypothyroidism. Thyroid. 2003;13:561–567.
30. Nikolai, TF. Recovery of thyroid function in primary hypothyroidism. Am J Med Sci. 1989;297:18–21.
31. Kraiem, Z, Baron, E, Kahana, L, et al. Changes in stimulating and blocking TSH receptor antibodies in a patient undergoing three cycles of transition from hypo- to hyperthyroidism and back to hypothyroidism. Clin Endocrinol. 1992;36:211–216.
32. Gerstein, HC. How common is postpartum thyroiditis? A methodologic overview of the literature. Arch Intern Med. 1990;150:1397–1400.
33. Kuypens, JL, Pop, VJ, Vader, HL, et al. Prediction of postpartum thyroid dysfunction: can it be improved? Eur J Endocrinol. 1998;139:36–43.
34. Alvarez-Marfany, M, Roman, SH, Drexler, AJ, et al. Long-term prospective study of postpartum thyroid function in women with insulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1994;79:10–16.
35. Vialettes, B, Guillerand, MA, Viens, P, et al. Incidence rate and risk factors for thyroid dysfunction during recombinant interleukin-2 therapy in advanced malignancies. Acta Endocrinol. 1993;129:31–38.
36. Prummel, MF, Laurberg, P. Interferon-α and autoimmune thyroid disease. Thyroid. 2003;13:547–551.
37. Nofal, MN, Beierwaltes, WH, Patno, ME. Treatment of hyperthyroidism with sodium iodide I-131, a 16-year experience. JAMA. 1966;197:605–610.
38. Huysmans, DA, Corstens, FH, Kloppenborg, PW. Long-term follow-up in toxic solitary autonomous thyroid nodules treated with radioactive iodine. J Nucl Med. 1991;32:27–30.
39. Le Moli, R, Wesche, MFT, Tiel-van Buul, MMC, et al. Determinants of long-term outcome of radioiodine therapy of sporadic non-toxic goitre. Clin Endocrinol. 1999;50:783–789.
40. Smith, RE, Adler, RA, Clark, P, et al. Thyroid function after mantle irradiation in Hodgkin’s disease. JAMA. 1981;245:46–49.
41. Tell, R, Sjödin, H, Lundell, G, et al. Hypothyroidism after external radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 1997;39:303–308.
42. Mercado, G, Adelstein, DJ, Saxton, JP, et al. Hypothyroidism: A frequent event after radiotherapy and after radiotherapy with chemotherapy for patients with head and neck carcinoma. Cancer. 2001;92:2892–2897.
43. Katsanis, E, Shapiro, RS, Robison, LL, et al. Thyroid dysfunction following bone marrow transplantation: long-term follow-up of 80 pediatric patients. Bone Marrow Transplant. 1990;5:335–340.
44. Pearce, EN, Farwell, AP, Braverman, LE. Thyroiditis. New Engl J Med. 2003;348:2646–2655.
45. Braverman, LE. Iodine and the thyroid: 33 years of study. Thyroid. 1994;4:351–356.
46. Martino, E, Safran, M, Aghini-Lombardi, F, et al. Environmental iodine intake and thyroid dysfunction during chronic amiodarone therapy. Ann Intern Med. 1984;101:28–34.
47. Trip, MD, Wiersinga, WM, Plomp, TA. Incidence, predictability, and pathogenesis of amiodarone-induced thyrotoxicosis and hypothyroidism. Am J Med. 1991;91:507–511.
48. Surks, MI, Sievert, R. Drugs and thyroid function. N Engl J Med. 1995;333:1688–1694.
49. Desai, J, Yassa, L, Marqusee, E, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumours. Ann Int Med. 2006;145:660–664.
50. Mannavola, D, Coco, P, Vannucchi, G, et al. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab. 2007;92:3531–3534.
51. Huang, SA, Tu, HM, Harney, JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–189.
52. Huang, SA, Fish, SA, Dorfman, DM, et al. A 21-year-old woman with consumptive hypothyroidism due to a vascular tumor expressing type 3 iodothyronine deiodinase. J Clin Endocrinol Metab. 2002;87:4457–4461.
53. Smith, TJ, Bahn, RS, Gorman, CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989;10:366–391.
54. Diekman, MJ, Romijn, JA, Endert, E, et al. Thyroid hormones modulate serum leptin levels: observations in thyrotoxic and hypothyroid women. Thyroid. 1998;8:1081–1086.
55. Iglesias, P, Alvarez Fidalgo, P, Codoceo, R, et al. Serum concentrations of adipocytokines in patients with hyperthyroidism and hypothyroidism before and after control of thyroid function. Clin Endocrinol. 2003;59:621–629.
56. Hsieh, CJ, Wang, PW, Wong, ST, et al. Serum leptin concentrations of patients with sequential thyroid function changes. Clin Endocrinol. 2002;57:29–34.
57. Brackhik, M, Marcisz, C, Giebel, S, et al. Serum leptin and ghrelin levels in premenopausal women with stable body mass index during treatment of thyroid dysfunction. Thyroid. 2008;18:545–550.
58. Pearce, EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep. 2004;6:451–456.
59. Diekman, T, Demacker, PNM, Kastelein, JJP, et al. Increased oxidizability of low-density lipoproteins in hypothyroidism. J Clin Endocrinol Metab. 1998;83:1752–1755.
60. Ito, M, Takamutsu, J, Matsuo, T, et al. Serum concentrations of remnant-like particles in hypothyroid patients before and after thyroxine replacement. Clin Endocrinol. 2003;58:621–626.
61. Constant, EL, De Volder, AG, Ivanocin, A, et al. Cerebral blood flow and glucose metabolism in hypothyroidism: a positron emission tomography study. J Clin Endocrinol Metab. 2001;86:3864–3870.
62. Smith, CD, Ain, KB. Brain metabolism in hypothyroidism studied with 31P magnetic-resonance spectroscopy. Lancet. 1995;345:619–620.
63. Dugbartey, AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413–1418.
64. Burmeister, LA, Ganguli, M, Dodge, HH, et al. Hypothyroidism and cognition: Preliminary evidence for a specific defect in memory. Thyroid. 2001;11:1177–1185.
65. Knopman, DS, Petersen, RC, Cha, RH. Incidence and causes of nondegenerative nonvascular dementia: a population-based study. Arch Neurol. 2006;63:218–221.
66. Cleare, AJ, McGregor, A, O’Keane, V. Neuroendocrine evidence for an association between hypothyroidism, reduced central 5-HT activity and depression. Clin Endocrinol. 1995;43:713–719.
67. Duyff, RF, Van den Bosch, J, Laman, DM, et al. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry. 2000;68:750–755.
68. Cakir, M, Samanci, N, Balci, N, et al. Musculoskeletal manifestations in patients with thyroid disease. Clin Endocrinol. 2003;59:162–167.
69. Chong, JY, Rowland, LP, Utiger, RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol. 2003;60:164–171.
70. Madariaga, M. Polymyositis-like syndrome in hypothyroidism: review of cases reported over the past twenty-five years. Thyroid. 2002;12:331–336.
71. Kaminsky, P, Robin-Lherbier, B, Brunotte, F, et al. Energetic metabolism in hypothyroid skeletal muscle, as studied by phosphorus magnetic resonance spectroscopy. J Clin Endocrinol Metab. 1992;74:124–129.
72. Monzani, F, Caraccio, N, Siciliano, G, et al. Clinical and biochemical features of muscle dysfunction in subclinical hypothyroidism. J Clin Endocrinol Metab. 1997;82:3315–3318.
73. Hsu, I-H, Thadhani, RI, Daniels, GH. Acute compartment syndrome in a hypothyroid patient. Thyroid. 1995;5:305–308.
74. Diekman, MJM, Harms, MPM, Endert, E, et al. Endocrine factors related to changes in total peripheral vascular resistance after treatment of thyrotoxic and hypothyroid patients. Eur J Endocrinol. 2001;144:339–346.
75. Klein, I, Danzi, S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735.
76. Obuobie, K, Smith, J, Evans, LM, et al. Increased central arterial stiffness in hypothyroidism. J Clin Endocrinol Metab. 2002;87:4662–4666.
77. Ladenson, PW, Sherman, SI, Baughman, KL, et al. Reversible alterations in myocardial gene expression in a young man with dilated cardiomyopathy and hypothyroidism. Proc Natl Acad Sci U S A. 1992;89:5251–5255.
78. Bengel, FM, Nekolla, SG, Ibrahim, T, et al. Effect of thyroid hormones on cardiac function, geometry, and oxidative metabolism assessed noninvasively by positron emission tomography and magnetic resonance imaging. J Clin Endocrinol Metab. 2000;85:1822–1827.
79. Diekman, MJM, van der Put, NM, Blom, HJ, et al. Determinants of changes in plasma homocysteine in hyperthyroidism and hypothyroidism. Clin Endocrinol. 2001;54:197–204.
80. Cappola, AR, Ladenson, PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88:2438–2444.
81. Ladenson, PW, Goldenheim, PD, Ridgway, EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84:877–883.
82. Pelttari, L, Rauhala, E, Polo, O, et al. Upper airway obstruction in hypothyroidism. J Intern Med. 1994;236:177–181.
83. Hollander, JG den, Wulkan, RW, Mantel, MJ, et al. Correlation between severity of thyroid dysfunction and renal function. Clin Endocrinol. 2005;62:423–427.
84. Hanna, FWF, Scanlon, MF. Hyponatraemia, hypothyroidism and role of arginine-vasopressin. Lancet. 1997;350:755–756.
85. Sahun, M, Villabona, C, Rosel, P, et al. Hypothyroidism is associated with plasma hypo-osmolality and impaired water excretion that is vasopressin-independent. J Endocrinol. 2001;168:435–445.
86. Yeum, CH, Kim, SW, Kim, NH, et al. Increased expression of aquaporin water channels in hypothyroid rat kidney. Pharmacol Res. 2002;46:85–88.
87. Bruder, JM, Samuels, MH, Bremner, WJ, et al. Hypothyroidism-induced macroorchidism: Use of a gonadotropin-releasing hormone agonist to understand its mechanism and augment adult stature. J Clin Endocrinol Metab. 1995;80:11–16.
88. Anasti, JN, Flack, MR, Froehlich, J, et al. A potential novel mechanism for precocious puberty in juvenile hypothyroidism. J Clin Endocrinol Metab. 1995;80:276–279.
89. Krassas, GE, Tziomalos, K, Papadopoulou, F, et al. Erectile dysfunction in patients with hyper- and hypothyroidism: how common and should we treat? J Clin Endocrinol Metab. 2008;93:1815–1819.
90. Samuels, MH, Veldhuis, JD, Henry, P, et al. Pathophysiology of pulsatile and copulsatile release of thyroid-stimulating hormone, luteinizing hormone, follicle-stimulating hormone, and alpha-subunit. J Clin Endocrinol Metab. 1990;71:425–432.
91. Krassas, GE, Pontikides, N, Kaltsas, FL, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol. 1999;50:655–659.
92. Smallridge, RC, Ladenson, PW. Hypothyroidism in pregnancy: consequences to neonatal health. J Clin Endocrinol Metab. 2001;86:2349–2353.
93. Haddow, JE, Palomaki, GE, Allan, WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555.
94. Abalovich, M, Gutierrez, S, Alcaraz, G. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12:63–68.
95. Rahman, Q, Haboubi, NY, Hudson, PR, et al. The effect of thyroxine on small intestinal motility in the elderly. Clin Endocrinol. 1991;35:443–446.
96. Lauritano, EC, Bilotta, AL, Gabrielli, M, et al. Association between hypothyroidism and small intestinal bacterial overgrowth. J Clin Endocrinol Metab. 2007;92:4180–4184.
97. Saha, B, Maity, C. Alterations of serum enzymes in primary hypothyroidism. Clin Chem Lab Med. 2002;40:609–611.
98. Squizzato, A, Romualdi, Büller, HR, et al. Clinical review: thyroid dysfunction and effects on coagulation and fibrinolysis: a systematic review. J Clin Endocrinol Metab. 2007;92:2415–2420.
99. Manfredi, E, Zaane, B van, Gerdes, VE. Hypothyroidism and acquired von Willebrand’s syndrome: a systematic review. Haemophilia. 2008;14:423–433.
100. Erfurth, EM, Ericsson, U-BC, Egervalh, K, et al. Effect of acute desmopressin and of long-term thyroxine replacement on haemostasis in hypothyroidism. Clin Endocrinol. 1995;42:373–378.
101. Ladenson, PW, Levin, AA, Ridgway, EC, et al. Complications of surgery in hypothyroid patients. Am J Med. 1984;77:261–266.
102. Valcavi, R, Valente, F, Dieguez, C, et al. Evidence against depletion of the growth hormone (GH)-releasable pool in human primary hypothyroidism: studies with GH-releasing hormone, pyridostigmine, and arginine. J Clin Endocrinol Metab. 1993;77:616–620.
103. Miell, JP, Zini, M, Quin, JD, et al. Reversible effects of cessation and recommencement of thyroxine treatment on insulin-like growth factors (IGFs) and IGF-binding proteins in patients with total thyroidectomy. J Clin Endocrinol Metab. 1994;79:1507–1512.
104. Raber, W, Gesol, A, Nowotny, P, et al. Hyperprolactinaemia in hypothyroidism: clinical significance and impact of TSH normalization. Clin Endocrinol. 2003;58:185–191.
105. Sarlis, NJ, Brucker-Davis, F, Doppman, JL, et al. MRI-demonstrable regression of a pituitary mass in a case of primary hypothyroidism after a week of acute thyroid hormone therapy. J Clin Endocrinol Metab. 1997;82:808–811.
106. Iranmanesh, A, Lizarralde, G, Johnson, ML, et al. Dynamics of 24-hour endogenous cortisol secretion and clearance in primary hypothyroidism assessed before and after partial thyroid hormone replacement. J Clin Endocrinol Metab. 1990;70:155–161.
107. Topliss, DJ, White, EL, Stockigt, JR. Significance of thyrotropin excess in untreated primary adrenal insufficiency. J Clin Endocrinol Metab. 1980;50:52–56.
108. Silva, JE. Intermediary metabolism and the sympathoadrenal system in hypothyroidism. In: Braverman LE, Utiger RD, eds. The thyroid: a Fundamental and Clinical Text. ed 9. Philadelphia: Lippincott Williams & Wilkins; 2005:817–823.
109. Billewicz, WL, Chapman, RS, Crooks, J, et al. Statistical methods applied to the diagnosis of hypothyroidism. QJM. 1969;38:255–266.
110. Seshadri, MS, Samuel, BU, Kanagasabapathy, AS, et al. Clinical scoring system for hypothyroidism: is it useful? J Gen Intern Med. 1989;4:490–492.
111. Zulewski, H, Müller, B, Exer, P, et al. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab. 1997;82:771–776.
112. Tachman, ML, Guthrie, GP. Hypothyroidism: diversity of presentation. Endocr Rev. 1984;5:456–465.
113. Doucet, J, Trivalle, C, Chassagne, P, et al. Does age play a role in clinical presentation of hypothyroidism? J Am Geriatr Soc. 1994;42:984–986.
114. Müller, B, Zulewski, H, Huber, P, et al. Impaired action of thyroid hormone associated with smoking in women with hypothyroidism. N Engl J Med. 1995;333:964–969.
115. Fish, LH, Schwartz, HL, Cavanaugh, J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism: role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764–770.
116. Bolk, N, Visser, TJ, Kalsbeek, A, et al. Effect of evening vs morning thyroxine ingestion on serum thyroid hormone profiles in hypothyroid patients. Clin Endocrinol. 2007;66:43–48.
117. Dong, BJ, Hauck, WW, Gambertoglio, JG, et al. Bioequivalence of generic and brand-name levothyroxine products in the treatment of hypothyroidism. JAMA. 1997;227:1205–1213.
118. Olveira, G, Almaraz, MC, Soriguer, F, et al. Altered bioavailability due to changes in the formulation of a commercial preparation of levothyroxine in patients with differentiated carcinoma. Clin Endocrinol. 1997;46:707–711.
119. Roos, A, Linn-Rasker, SP, Domburg, RT van, et al. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Intern Med. 2005;165:1714–1720.
120. Kabadi, UM, Jackson, T. Serum thyrotropin in primary hypothyroidism: a possible predictor of optimal daily levothyroxine dose. Arch Intern Med. 1995;155:1046–1048.
121. Shimon, I, Cohen, O, Lubetsky, A, et al. Thyrotropin suppression by thyroid hormone replacement is correlated with thyroxine level normalization in central hypothyroidism. Thyroid. 2002;12:823–827.
122. Sawin, CT, Geller, A, Wolf, PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252.
123. Uzzan, B, Campos, J, Cucherat, M, et al. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab. 1996;81:4278–4289.
124. Leese, GP, Jung, RT, Guthrie, C, et al. Morbidity in patients on l-thyroxine: a comparison of those with a normal TSH to those with a suppressed TSH. Clin Endocrinol. 1992;37:500–503.
125. Vestergaard, P, Weeke, J, Hoeck, HC, et al. Fractures in patients with primary idiopathic hypothyroidism. Thyroid. 2000;10:335–340.
126. Sheppard, MC, Holder, R, Franklyn, J. Levothyroxine treatment and occurrence of fracture of the hip. Arch Intern Med. 2002;162:338–343.
127. d’Estève-Bonetti, L, Bennet, AP, Malet, D, et al. Gluten-induced enteropathy (coeliac disease) revealed by resistance to treatment with levothyroxine and alfacalcidol in a 68-year old patient: a case report. Thyroid. 2002;12:633–636.
128. Liel, Y, Harman-Boehm, I, Shany, S. Evidence for a clinically important adverse effect of fiber-enriched diet on the availability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metab. 1996;81:857–859.
129. Harmon, SM, Seifert, CF. Levothyroxine-cholestyramine interaction reemphasized. Ann Intern Med. 1991;115:658–659.
130. Havrankova, J, Lahaie, R. Levothyroxine binding by sucralfate [erratum appears in Ann Intern Med 118:398, 1993]. Ann Intern Med. 1992;147:445–446.
131. Sperber, AD, Liel, Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminium hydroxide. Arch Intern Med. 1992;152:183–184.
132. Campbell, NRC, Hasinoff, BB, Stalts, H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010–1013.
133. Siraj, ES, Gupta, MK, Reddy, SS. Raloxifene causes malabsorption of levothyroxine. Arch Intern Med. 2003;163:1367–1370.
134. Singh, N, Singh, PN, Hershman, JM. Effects of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283:2822–2825.
135. Arafah, BM. Increased need for thyroxine in women with hypothyroidism during estrogen therapy. N Engl J Med. 2001;344:1743–1749.
136. Alexander, EK, Marqusee, E, Lawrence, J, et al. Timing and magnitude of increases in levothyroxine requirements dusring pregnancy in women with hypothyroidism. New Engl J Med. 2004;351:241–249.
137. Isley, WL. Effect of rifampin therapy on thyroid function tests in a hypothyroid patient on replacement l-thyroxine. Ann Intern Med. 1987;107:517–518.
138. Figge, J, Dluhy, RG. Amiodarone-induced elevation of thyroid stimulating hormone in patients receiving levothyroxine for primary hypothyroidism. Ann Intern Med. 1990;113:553–555.
139. McCowen, KC, Garber, JR, Spark, R. Elevated serum thyrotropin in thyroxine-treated patients with hypothyroidism given sertraline. N Engl J Med. 1997;337:1010–1011.
140. Munera, Y, Hugues, FC, Le Jeunne, C, et al. Interaction of thyroxine sodium with antimalarial drugs. Br Med J. 1997;314:1593.
141. Grebe, SKG, Cooke, RR, Ford, HC, et al. Treatment of hypothyroidism with once weekly thyroxine. J Clin Endocrinol Metab. 1997;82:870–875.
142. Arafah, BM. Decreased levothyroxine requirement in women with hypothyroidism during androgen therapy for breast cancer. Ann Intern Med. 1994;121:247–251.
143. Griffin, JE. Hypothyroidism in the elderly. Am J Med Sci. 1990;299:334–345.
144. Escobar-Morreale, HF, Escobar del Ray, F, Obregon, MJ, et al. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. 1996;137:2490–2502.
145. Saravanan, P, Chau, W-F, Roberts, N, et al. Psychological well-being in patients on ‘adequate’ doses of L-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol. 2002;57:577–585.
146. Wekking, EM, Appelhof, BC, Fliers, E, et al. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol. 2005;153:747–753.
147. Grozinsky-Glasberg, S, Fraser, A, Nahskoni, E, et al. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006;91:2592–2599.
148. Sherman, SI, Ladenson, PW. Percutaneous transluminal coronary angioplasty in hypothyroidism. Am J Med. 1991;90:367–370.
149. Porretti, S, Giavoli, C, Ronchi, C, et al. Recombinant human GH replacement therapy and thyroid function in a large group of adult GH-deficient patients: when does L-T4 therapy become mandatory? J Clin Endocrinol Metab. 2002;87:2042–2045.
150. Wartofsky, L. Myxedema coma. Endocrinol Metab Clin North Am. 2006;35:687–698.
151. Biondi, B, Cooper, DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131.
152. Villar HC, Saconato H, Valente O, et al: Thyroid hormone replacement in subclinical hypothyroidism, Cochrane Database Syst Rev 18:CD003419, 2007.
153. Ochs, N, Auer, R, Bauer, DC, et al. Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med. 2008;148:832–845.
154. Razvi, S, Shakoor, A, Vanderpump, M, et al. The influence of age on the relationship between subclinical hypothyroidism and ischaemic heart disease: a metaanalysis. J Clin Endocrinol Metab. 2008;93:2998–3007.
155. Danese, MD, Powe, NR, Sawin, CT, et al. Screening for mild thyroid failure at the periodic health examination: a decision and cost-effectiveness analysis. JAMA. 1996;276:285–292.