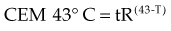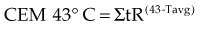Chapter 21 Hyperthermia
The Biology of Hyperthermia
The Arrhenius Relationship and Thermal Isoeffect Dose
Arrhenius found a temperature dependence of sucrose hydrolysis in the presence of various acids that was a logarithmic function of the absolute temperatures at which the reactions were conducted.1 These observations were physiologically relevant in that the rate of cellular metabolism increases as the temperature rises in cells or tissues, up to a point at which thermal damage is created. The principles discovered by Arrhenius extend to cell killing by HT as well. The temperature dependence of the rate of cell killing is referred to as the Arrhenius relationship.
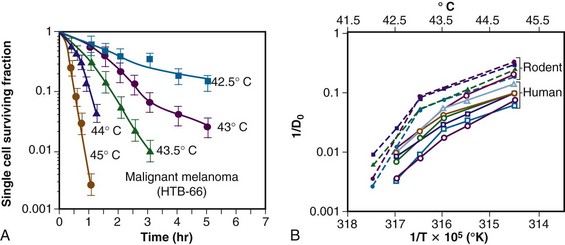
The temperature dependence of the rate of cell killing has been used as a method for thermal dosimetry. This is done by plotting temperature versus log of the slope of the survival curve (1/Do). Do is analogous to a Do in a radiation survival curve (the dose to reduce survival to 37% of a starting value on the exponential portion of the survival curve). In the case of HT, “dose” is the number of minutes at a specified temperature (Fig. 21-1). Typically, Arrhenius plots have a biphasic curve and the point at which there is a change in slope is referred to as a breakpoint. Above the breakpoint for nearly all cell types, a change in temperature of 1° C doubles the rate of cell killing. Acquired resistance to HT killing (thermotolerance) that occurs during HT is responsible for the change in slope of the heat cell survival curve at longer heating times for temperatures below 43° C (see Fig. 21-1A). Arrhenius plots for human cell and rodent lines are well described. The breakpoint for human cells is near 43.5° C, and the slope below the breakpoint is between 2 and 4. The change in slope below the breakpoint is thought to be due to thermotolerance induction at lower temperatures. The breakpoint for rodent cell lines is lower than that of humans, at about 43° C. The slopes of Arrhenius plots derived from in vivo studies are virtually identical to those derived from in vitro studies.
Sapareto and Dewey2 proposed using the Arrhenius relationship to normalize thermal data from HT treatments. The rationale came from the observations that time-temperature histories are not stable and that they vary from patient to patient, and that temperatures within tumors are almost always nonuniform. Using the Arrhenius relationship, it would be possible to convert all time-temperature data to an equivalent number of minutes at a standard temperature. The formulation takes the following form:
The CEM 43° C (thermal isoeffect dose) formulation has been used extensively and successfully in clinical trials to assess the efficacy of heating. This is in spite of the fact that the R values and breakpoints have historically been derived from studies done in rodents, which are not exactly equivalent to human cells.*,3
Mechanisms of Hyperthermic Cytotoxicity
Cellular and Tissue Responses to Hyperthermia
Protein is the primary target for hyperthermic cytotoxicity. When cells are exposed to elevated temperatures (≥41° C), damage is inflicted in multiple sites, but the predominant molecular target appears to be protein. The heat of inactivation for cell killing and thermal damage to tissues is in the range of that necessary for protein denaturation (130 to 170 kcal/mol). Additional evidence for protein being the primary target for cell killing is the importance of heat shock proteins in protecting thermotolerant cells from thermal damage. One of the primary functions of heat shock proteins is to refold other proteins that have been damaged.4
Some organelles are especially important in controlling thermal response. For example, modification of membrane lipid content or use of membrane active agents, such as alcohols, can sensitize cells to heat killing, but the sensitization is probably related to destabilization of the membrane as it relates to lipid-protein interactions.5 The cytoskeleton of cells is particularly heat sensitive.6 When it is collapsed by heat, there is disruption of cytoskeletal-dependent signal transduction pathways as well as inhibition of cell motility.7,8 Enzymes in the respiratory chain are more heat sensitive than enzymes in the glycolytic pathway.9 The heat sensitivity of the centriole leads to chromosomal aberrations following thermal injury.10 Finally, the DNA repair process is heat sensitive, and this may be one of the mechanisms that leads to heat-induced radiosensitization and chemosensitization.11
Physiologic Response to Hyperthermia
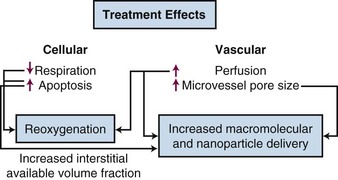
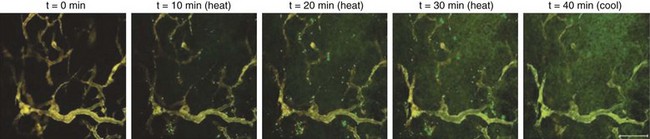
As temperatures increase, there is an increase in blood flow. The temperature threshold for this change is 41° to 41.5° C in skin.12 Changes in vascular permeability also occur, leading to edema formation in the heated volume. At higher thermal doses, vascular stasis and hemorrhage develop. The change in normal tissue perfusion upon heating is much greater (10 times) than one sees in tumors (1.5 to 2 times).13 Mechanisms underlying vascular stasis may include arteriovenous shunting, thrombus formation, and leukocyte plugging.14 Importantly, physiologic changes occur in tumors at lower temperatures that are potentially beneficial (Fig. 21-2).
Taking Advantage of the Physiologic Response to Hyperthermia
Improvement in Macromolecular and Liposomal Drug Delivery
A liposome is a small lipid vesicle (≈100 nm in diameter) that contains water or saline in the center. Drugs can be loaded into liposomes at high concentration. It has been recently shown that HT increases microvascular pore sizes preferentially in tumor microvessels, which leads to enhanced liposomal accumulation in tumor.15 The threshold for increased liposomal extravasation is 40° C, and it increases by a factor of 2 for every degree of temperature rise until vascular damage occurs.16 The effects of HT on liposomal extravasation have been studied extensively in preclinical models, but a twofold to thirteenfold enhancement in accumulation occurs in spontaneous soft tissue sarcomas of pet cats, an encouraging result that may translate to human tumors.17 HT also increases the available volume fraction (fraction of tissue not occupied by cells or stromal fibers) that may develop as cells undergo either necrosis or apoptosis following heat treatment.18 This increases the tissue space available for nanoparticles to be deposited. The increase in liposomal drug delivery achieved with HT has been shown to result in increased antitumor effect in many preclinical studies.19 HT has been used in conjunction with liposomal doxorubicin and irradiation in one small clinical series of patients with chest wall recurrences of breast cancer. Encouraging results were obtained, although drug concentrations were not measured in this series.20
For relatively small agents, such as most chemotherapeutic drugs, there is no advantage gained via increased vascular permeability to macromolecules or nanoparticles unless the drugs are protein-bound.21 For drugs with a molecular weight of less than 1000 MW, the primary mechanism that governs drug transport is diffusion,22 which is not highly temperature dependent. However, for molecules larger than 1000 MW, the primary driving force for transport is convection, which is controlled by the pressure gradient across the vessel wall. Accordingly, HT can augment the transvascular delivery of agents such as monoclonal antibodies23 and polymeric peptides that carry drugs or radioisotopes.24 Dreher and colleagues used a novel peptide polymer that undergoes an inverse-phase transition at 41° C to “pump” polymer into tumors. The inverse-phase transition refers to a physical feature of the polymer that renders it to be water soluble below 41° C; above this temperature, the polymer comes out of solution in aggregates. The aggregation occurs primarily intravascularly but is reversible. Therefore, when the tissue is cooled down, the particles rapidly disaggregate and diffuse into the tissue down their concentration gradient25 (Fig. 21-3). This same type of peptide polymer could be used for noninvasive thermometry with magnetic resonance imaging (MRI) or electron paramagnetic resonance (EPR) imaging because the interaction of the polymer with water changes when it aggregates. Proof of principle for this method was shown using EPR imaging.26
Effects of HT on Tumor Metabolism and Oxygenation.
Enzymes for aerobic metabolism are more heat sensitive than those involved in anaerobic metabolism.9 Decreases in adenosine triphosphate (ATP) concentration after HT are inversely related to the temperatures achieved during HT in preclinical models and canine sarcomas27,28 and are related to the pathologic complete response (pCR) rate in human sarcomas.27 Decreases in ATP and increases in lactate concentration occur following tumor blood flow reduction after HT.29 In human patients with soft tissue sarcomas who were treated preoperatively with HT and radiation therapy, reduction in the ratio of ATP to inorganic phosphate (Pi) (measured using phosphorus-31 [31P] magnetic resonance spectroscopy) was significantly correlated with a higher probability for pCR.27 These results are consistent with the theory that tumor respiration can decrease after HT treatment.
A shift toward anaerobic metabolism would decrease oxygen consumption rates, which could lead to improvement in tumor oxygenation. Some of the benefits of HT may therefore result from improvements in oxygenation.30 Several preclinical studies have shown that mild temperature heating (between 40° and 42° C for an hour) can lead to improvements in the oxygen partial pressure (pO2) in the tumor up to 24 hours after heating.31 However, in some models, the extent of reoxygenation is quite heterogeneous between and even within individual tumors.32 It has been reported that HT improves tumor oxygenation in canine and human soft tissue sarcomas, with reoxygenation being associated with a greater probability for pCR in human tumors.33,34 In a canine study, median temperatures of less than 44° C improved oxygenation, whereas median temperatures higher than 44° C for 60 minutes led to decreased oxygenation.33 In patients with locally advanced breast cancer who were treated in a phase II study with preoperative HT plus radiotherapy and taxol, there was a significantly increased likelihood for achieving pCR if the tumors reoxygenated 24 hours after the first HT treatment. The probability for achieving a pCR was greater when the median temperatures were below 41.5° C.35 Improvement in oxygenation does not occur in all tumors, however, and currently there is no method for predicting which tumors will show this effect.36 In summary, mild temperature elevations during HT will benefit some patients if HT induces reoxygenation.31 To take full advantage of this effect, it will be necessary to use methods to enhance the reoxygenation effect, such that it occurs in the majority of patients. Song and Griffin have suggested that the addition of hyperoxic gas breathing after HT but during radiotherapy may achieve this goal.37,38
Physiologic Approaches to Enhance Thermal Cytotoxicity
pH Modification
It is well established that acidification can enhance sensitivity to HT. Changes in intracellular pH are responsible for increased thermal sensitivity.39,40 The most widely studied method to achieve acidification has been induction of hyperglycemia. The logic is that excess glucose load will push tumors toward glycolysis and lactic acid production.41,42 However, hyperglycemia alone is insufficient to reliably achieve adequate acidification.43,44 The addition of agents that can selectively acidify tumor intracellular pH, such as glucose combined with the respiratory inhibitor meta-iodobenzylguanadine (MIBG), have the potential to further enhance hyperthermic cytotoxicity selectively in tumor tissues45,46 because the acidification appears to be selective for tumors. Quercetin, a naturally occurring flavinoid, inhibits thermotolerance induction, particularly at a low pH, and can accordingly increase the thermal sensitivity of cells.47–49 An alternative approach is to block the extrusion of hydrogen ions from cells, which is normally accomplished via membrane-bound pumps. Use of such agents, in combination with acidification of the extracellular space, can lead to enhanced hyperthermic cell killing both in vitro and in vivo.50
Hyperthermia and Metastases
HT causes abrupt changes in tumor microvascular function, as described above, which could enhance tumor cell shedding from the heated site. Preclinical studies are mixed on this issue. One report showed that local HT alone enhanced the metastatic rate of B16 melanoma.51,52 When curative doses of radiotherapy were added to HT in the B16 melanoma model, however, the incidence of lymph node and lung metastases was decreased, compared with controls.52 The combination of curative doses of radiation and HT has generally been reported in other tumor models to reduce the incidence of metastases,53,54 although in a few models, simultaneous administration of HT with irradiation has been reported to increase metastases.53,54
The question of whether local HT increases the risk for metastasis is difficult to answer in clinical trials unless the primary therapy has a high probability for local control. This is because of the problem of competing risks. In a phase III trial of canine patients with primary malignant melanomas treated with the combination of HT and irradiation or irradiation alone, no difference in the likelihood for metastasis between the two groups was seen.55 However, local recurrence was a common event, and its onset was frequently followed by the appearance of distant metastases. In a phase III randomized trial of human melanomas treated with thermoradiotherapy versus radiotherapy alone, there was significant improvement in the likelihood for survival when the local tumor was controlled. Because the use of HT in that trial resulted in improved local control, the implication is that the combination therapy reduced the probability for metastases.56,57 In a study comparing graded doses of irradiation with and without HT for the treatment of canine soft tissue sarcomas, higher normal tissue temperatures in the region of the tumor were correlated with lower likelihood for distant metastases.58 A large series of patients (n = 95) with previously untreated high-grade soft tissue sarcomas who were treated preoperatively with HT and irradiation achieved a local control rate of nearly 90%, but 50% developed distant metastases.59 This rate of metastasis is essentially identical to that seen with preoperative radiotherapy alone, however, suggesting that HT had an undetectable influence on metastases.60
Normal Tissue Damage from Hyperthermia
Thresholds for thermal damage depend on the tissue type being heated and the severity of the injury. Mild damage can merely lead to edema, whereas more severe injury can lead to massive necrosis and organ failure. This subject has been reviewed in detail.3 The ranking of tissue thermal sensitivities does not follow classical principles derived from other cytotoxic agents, such as irradiation or chemotherapy. With such agents, the most sensitive tissues are those with the highest proliferative potential or activity. For HT, the most sensitive tissue classification includes brain tissue, which is composed of cells with almost no proliferative potential, as well as testicular tissue, which has high proliferative potential. There has been speculation as to whether tumor tissues might be more sensitive to thermal damage than normal tissues. Many studies have compared tumor tissue with normal cells tissue in vitro. There is no inherent difference in the thermal sensitivity of the two types of tissue cells. However, the microenvironment of tumors, which is often acidotic and nutritionally deprived, leads to an increase in thermal sensitivity that has been reported in a clinical series.61
Radiotherapy and Hyperthermia
Rationale for Combining Hyperthermia with Radiotherapy
When irradiation is combined with HT, complementary effects occur. Cells in S phase are radioresistant but are sensitive to HT. Hypoxic cells are three times more resistant to radiation compared with aerobic cells, but there is no difference in thermal sensitivity between aerobic and hypoxic cells. As discussed above, HT can lead to reoxygenation, which will further improve radiotherapy response.31,33,34 Finally, HT inhibits the repair of both sublethal and potentially lethal damage by inactivating crucial DNA repair pathways.62–64
Factors to Consider when Combining Hyperthermia with Radiotherapy
The interaction between radiation and HT is described by the thermal enhancement ratio (TER), which is defined as the ratio of doses of radiation to achieve an isoeffect for radiotherapy alone compared with radiotherapy plus HT. TERs for local tumor control have been estimated for a number of human tumors using historical control data for radiation alone.65 In most tumor types examined, these ratios were higher than 1. Assessment of normal tissue TER has not been attempted except in a few cases. For those examples, TER values for normal tissue damage have been less than those for tumor tissue damage in the same patient population, suggesting that there is potential for therapeutic gain with the use of radiotherapy combined with HT compared with radiotherapy alone.65 Prospective randomized trials in dogs with spontaneous tumors have also shown evidence for improved local tumor control with radiotherapy plus HT compared with radiotherapy alone,58,66,67,68 with no clinically observable increase in the frequency of clinically relevant late normal tissue complications. In one canine trial, enhancement of late radiation damage (as assessed histologically) was reported, and the duration of acute radiation complications was prolonged.68 There is clinical evidence, however, that excessively high intratumoral temperatures (i.e., >45° C for 60 min) can lead to damage to surrounding normal tissues, an effect that is often caused by rapid tumor regression.66,69 Such damage is not easily repaired and can lead to chronic tissue consequences, such as fibrosis, fistula formation, and bone necrosis.
Two cases of fatal pelvic necrosis have been reported as a late complication in patients with locally advanced cervical cancer treated with thermoradiotherapy,70 and there was speculation as to whether the complication was the result of HT. More recently, however, this rare complication has been reported following chemoradiotherapy treatment of cervical cancer in two small series.71,72 Thermal doses achieved in the HT series were several degrees lower than the radiotherapy doses reported to be associated with enhancement of normal tissue complications. This finding strongly suggests that these two cases were not the result of HT but more likely resulted from the radiotherapy treatment; An additional complication may have been the heavy smoking history of both patients.70
Hyperthermia and Chemotherapy
Rationale for Using Hyperthermia with Chemotherapy
Many chemotherapeutic agents have been shown to exhibit synergism with HT, including cisplatin and related compounds, melphalan, cyclophosphamide, nitrogen mustards, anthracyclines, nitrosoureas, bleomycin, mitomycin C, and hypoxic cell sensitizers.21 The mechanisms that underlie the synergy may include (1) increased cellular uptake of drug, (2) increased oxygen radical production, (3) increased DNA damage and inhibition of repair, and (4) reversal of drug resistance mechanisms.73,74–76 Hypoxia and pH are also important in the thermochemotherapeutic response.77–81 There are some classes of drugs, such as etoposide and vinca alkaloids, that do not synergistically interact with HT.21
Rationale for Using Hyperthermia with Targeted Agents
Very little is known about whether HT affects the cytotoxicity of newer targeted agents, such as those that block epidermal growth factor receptor (EGFR), mitogen-activated protein (MAP) kinase, mammalian target of rapamycin (mTOR), or other signal transduction pathways. Identification of targets can be accomplished by examining changes in gene expression brought on by HT treatment. Such studies have been conducted in vitro and in vivo. Genomic analysis of cells from U937, a human myelomonocytic tumor, exhibited differential expression in approximately 1000 genes, following a noncytotoxic treatment of 41° C for 30 minutes.82 A higher thermal exposure of 42° C for 90 minutes led to more significant change in proapoptotic signaling pathways,83 in addition to the expected heat shock response in both cases. Genome-wide analysis has also been done on one rat tumor line in vivo, following local heating (43° C for 60 min).84,85 The effects of HT were rather pleiotropic, involving over 1200 genes and a host of cellular responses, including apoptosis regulation, cell cycle control, MAP kinase- and calcium-regulated cell signaling, and genes involved in angiogenesis and metabolism regulation. There was also substantial down-regulation of a number of genes involved in immune function at 3 hours after treatment, which recovered to baseline by 24 hours. These results suggested that HT does not adversely affect immune function. The fact that HT appears to augment proapoptotic signaling pathways suggests that it could work additively with targeted agents that promote apoptosis. To date, such strategies have not been examined, however.
It has been reported recently that HT can induce autophagy via activation of nuclear factor kappa B (NFκB) as a protective mechanism against cell death.86 Therefore it would make sense to consider combining agents that inhibit NFKB activation with HT as a means of enhancing thermal cell killing. Alternatively, the combination of HT at 43° C with increased oxidative stress can increase cell killing via inhibition of the Akt pathway and induction of irreversible autophagy.87 Pajonk and coworkers88 reported that inhibition of proteasome activity in combination with HT at 44° C for 60 minutes was particularly effective in augmenting apoptosis, and, in addition, caused substantial radiosensitization.
There has been some effort, however, related to the idea of using HER2-targeted thermosensitive liposomes to enhance specificity of drug delivery to HER2-positive tumors.89 Alternatively, magnetic nanoparticles have been coated with HER2 antibodies to enhance delivery to tumor cells, where they can induce HT when placed in a high-powered magnetic field.90,91 Targeted nanorods have also been investigated for therapeutic potential with HT.92
Factors to Consider when Combining Hyperthermia with Chemotherapy
Temperature Dependence
The degree of enhancement of cytotoxicity has been shown to be temperature dependent for many drugs.93 Combinations of camptothecins with HT have not shown consistent results in vitro. The interaction between these agents and HT is schedule and temperature dependent.94,95 In one report, temperatures up to 41.8° C increased the activity of topoisomerase II; this finding may explain the increased activity of these drugs at elevated temperatures.94
In Vitro Results May Not Predict In Vivo Activity
Tubulin-binding agents, such as taxol, show no evidence for interaction in vitro,96 but studies of these agents in combination with radiation therapy in vivo are more encouraging.97 There may be physiologic consequences of the combination of taxol and HT that make the combination work better than predicted from in vitro studies.
Sequencing
For most drugs (excluding 5-fluorouracil [5-FU] and perhaps other antimetabolites), the optimal sequence for heating and drug therapy is to administer both simultaneously or to give the drug immediately before the onset of heating. For platinum-containing drugs, the tissue extraction rate is increased with HT, further substantiating the rationale for use of this sequence.98 Most antimetabolites do not interact with HT when given concomitantly.21 However, 5-FU has been shown to interact supra-additively with HT by maintaining temperatures between 39° and 41° C. Temperatures in this range lead to enhanced conversion to active metabolites, thereby increasing drug cytotoxicity. In addition, continuous infusion protocols with this drug may lead to cell cycle block in S phase, a part of the cell cycle that is relatively sensitive to HT.99
Immunologic Effects of Hyperthermia
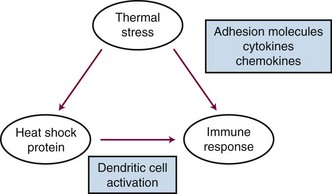
Heat shock proteins have been recently recognized for their potential role in regulating immune responses. There are several recognized functions of these proteins: (1) They are known to bind, in a noncovalent fashion, to immunogenic peptides. (2) When tumor cells are exposed to HT, heat shock protein-peptide complexes are presented on the cell surface. These complexes can be recognized by antigen-presenting cells (dendritic cells) via major histocompatibility complex (MHC) class I molecules. Once dendritic cells have received this type of stimulus, they migrate to lymph nodes, where they prime T-cell lymphocytes to be cytotoxic toward cells that express the peptide-heat shock protein complex. HT has been shown to enhance the rate of dendritic cell migration.100 (3) Heat shock proteins also induce dendritic cell maturation and proinflammatory cytokine release.101,102 (4) Cell membrane localization of heat shock proteins also activates the innate immune system by activating natural killer (NK) cells.103 Other sources of cellular stress such as viral infection, fever, hypoxia, and radiation exposure have been shown to up-regulate heat shock proteins as well. Because this process appears to occur naturally, there have been efforts to exploit the use of HT to produce tumor-derived vaccines and to augment the in vivo response to such vaccines.102 HT has also been reported to up-regulate a number of proinflammatory cytokines and adhesion molecules that facilitate immune cell trafficking across endothelial cells to gain access to the tumor interstitium.104 Additionally, shed heat shock proteins, with or without associated peptides, may act as chemokines to attract immune cells (particularly, macrophages) toward a region of tissue that has undergone heat stress.105 A summary of some of the key immunologic effects of HT is shown in Figure 21-4.
Hyperthermia Physics and Engineering
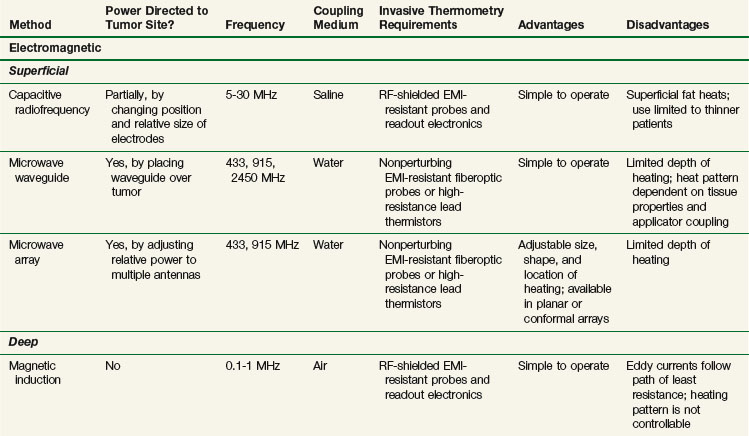
Clinical HT is usually achieved by exposing tissues to nonionizing radiation (e.g., electromagnetic [EM] or ultrasonic [US] fields) or by conducting heat into tissue from a heated source (e.g., hot pad or needle). Although these modalities deposit energy in tissue by different physical mechanisms, they have general similarities. Uniformity of heating is sensitive to the heterogeneity of tissue properties, geometry of blood flow, and practical problems of coupling the energy source into tissue. HT can be delivered noninvasively with externally applied power sources or invasively by placing heat sources either interstitially or inside natural body cavities. A brief presentation of noninvasive methods is provided below and is summarized in Table 21-1. Invasive methods have been developed extensively and include radiofrequency electrodes, microwave antennas, hot water tubes, ferromagnetic implant rods or seeds, and ultrasound transducers. Further details on all methods are available elsewhere.*
Electromagnetic Heating
Electromagnetic Heating Devices
Electromagnetic heating devices can be separated into two categories: superficial applicators with effective penetration into tissue of less than 4 cm, and deep heating devices that have effective penetration of more than 4 cm. Superficial devices include waveguides (Fig. 21-5) and microstrip or patch antennas (Fig. 21-6) operating at microwave frequencies of 433, 915, or 2450 MHz.111,112,113,114 Microwave energy is usually coupled into tissue through a temperature-controlled de-ionized water bolus to maintain the skin temperature below 44° C.
Capacitive Heating Technique
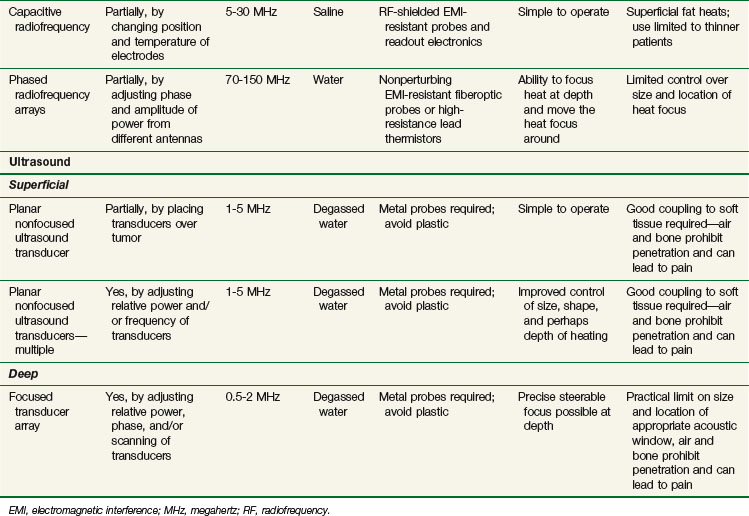
The capacitive heating technique uses radiofrequency fields of between 5 and 30 MHz to generate electric current between two or more conductive electrodes. Heating tends to be concentrated nearest the electrodes, but temperature-controlled saline boluses are used to reduce hot spots on the skin surface and help cool superficial fat.115 Using different sizes of electrodes shifts the maximum power deposition toward the smaller electrode. This technique can be used with large electrodes on the skin116,117 and one or more small intracavitary electrodes such as a balloon electrode for esophageal tumors.118 The thickness of the superficial fat layer is a significant factor for this method because of extra heating in high-resistance fat tissue relative to deeper-lying, high-conductivity soft tissues. To date, radiofrequency capacitive heating has been used most widely in Asia, where patients tend to be thinner.
Radiofrequency Phased Array
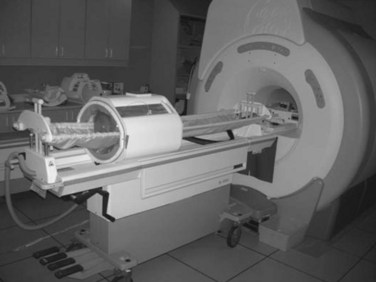
The third option for heating with electromagnetic fields is the radiofrequency phased array.119,120,121,122 These devices consist of an array of radiofrequency antennas arranged in a geometric pattern surrounding the target body region.123 Antennas are driven with multiple independently controllable power amplifiers but use a common radiofrequency source, allowing the radiofrequency fields from all antennas to add together to form a focus in the center of the array. The focus can usually be shifted off center and into the tumor by variation of the relative phase of the signals driving each amplifier. For an array with multiple antennas in phase, one can achieve better and deeper power deposition into tissue than is possible with operation of the antennas independently (i.e., driving the antennas without phase addition). By varying the phase and amplitude of multiple antennas, the phased array technique has more flexibility for controlling power deposition than the magnetic induction and capacitive techniques, which depend heavily on tissue properties and have few power control variables. Figure 21-7 shows one example of a 12-antenna annular phased array applicator mounted on the patient table of an MRI system. This MR-compatible 100-MHz radiofrequency phased array applicator fits inside the bore of a 1.5-T magnet, allowing MR imaging of a temperature rise inside the body during HT treatment.
Ultrasound Heating
Energy is coupled into tissue using temperature-controlled degassed water. Single- and multiple-transducer unfocused devices have been designed for heating superficial tumors (2 to 5 cm); these devices typically operate in the 1- to 5-MHz range.110,124–126 Deep heating with ultrasound is accomplished by using scanned focused transducers, phased arrays, or multiple scanned focused transducers, normally in the 0.5- to 2-MHz range.127,128
Measurement of Temperatures during Hyperthermia
Invasive Thermometry
Invasive thermometry, the current standard, involves physically placing thermometry probes into the tumor within implanted needles or catheters to read subsurface temperatures.129,130,131 Although the accuracy of invasive thermometers is sufficiently precise (typically, ±0.2° C) to resolve important differences in thermal dose (as described above), this type of thermometry has many disadvantages. Disadvantages include discomfort to the patient and risk of hemorrhage and/or infection, the expense of physician time required for catheter placement, and the necessity for imaging to verify placement of thermometers. In addition, the sparse nature of the data obtained makes it difficult to spatially control power deposition or to alter treatment to improve temperature distributions.132,133 The most common method for doing invasive thermometry is to insert blind-ended catheters into a tumor, using either ultrasound or computed tomography (CT) guidance.130 Alternatively, for deep-seated tumors, thermometers may be placed inside orifices that are surrounded by tumor, such as the rectum, urethra, or cervix.134,135 Thermometers are then placed inside these catheters during treatment. The probes can be multipoint sensors that remain fixed in place, or the sensors can be moved cyclically during treatment to record temperatures at many points along the catheter. There are guidelines published for how these measurements should be taken for all HT devices.130,136–138 In spite of its sparse nature, invasive thermometry can provide valuable information about the quality of a treatment. A number of clinical reports have correlated temperature-related parameters to clinical response.66,139,140,141–144
Noninvasive Thermometry
To address the shortcomings of invasive thermometry, a number of noninvasive techniques are under investigation. Infrared thermography has been available for years for monitoring surface temperature distributions.145 Although spatial resolution and accuracy are excellent, the method requires open access to visualize the surface, so it is not compatible with most heating technologies. A new approach for monitoring two-dimensional surface temperature distributions, even when the surface is buried under a water bolus-coupled applicator, involves use of high-density arrays of fiberoptic sensors mounted in a large surface-conforming thermal monitoring sheet (TMS) configuration.146,147 Other noninvasive thermometry approaches under development include electrical impedance tomography (EIT), which is sometimes called applied potential tomography,148,149 ultrasonic temperature imaging using changes in backscattered energy,150,151 electron paramagnetic resonance (EPR) imaging,26 active microwave imaging or tomography,152 microwave radiometry,153–159 and real-time multislice MR thermal imaging (MRTI).
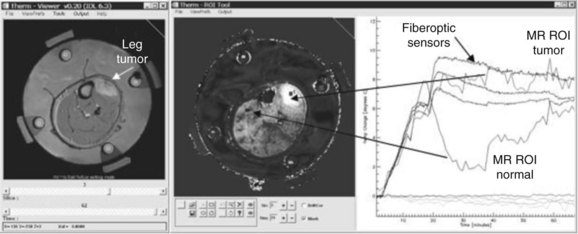
Although there are clinical applications for each of these technologies, MRTI is being studied intensively for volumetric characterization and control of deep tissue hyperthermia. Although several MR tissue parameters are sensitive to changes in temperature, attention has focused in recent years on investigation of the change in water proton resonance frequency shift (PRFS),160,161,162 even in tissues with low water content such as breast tissue.163,164 Noninvasive MR thermometry has been shown to assist in the delivery of HT treatments and can offer better characterization of the treatment efficacy by allowing calculation of the required thermal metrics from volumetric data. Figure 21-8 shows an example of noninvasive thermometry with MRTI during heat treatment of a lower leg sarcoma with a four-antenna, 140-MHz mini-annular phased array applicator. The method in human patients has yielded a temperature resolution of better than 1° C compared with calibrated invasive sensors.165 Moreover, MRI offers opportunities for validation of treatment planning systems,166–168 dynamic control of treatment delivery,169,170–172 and post-treatment assessment of tissue damage.173 The use of MRTI has been increasing rapidly in recent years for clinical applications in both moderate- and high-temperature thermal therapies such as radiofrequency, laser, and high-intensity focused ultrasound.135,174,175 The International Journal of Hyperthermia summarized the status of MR thermometry in a special issue,162,176,177,178 and additional background information can be found in review articles by Rieke and Butts179 and MacFall and Soher.161
In a recent paper by Craciunescu and colleagues,165 important steps toward rapid volumetric real-time imaging-guided HT treatments were presented. With update times of the order of minutes, the real-time treatment control claim made here is in agreement with an accepted definition of real time given for thermal therapies by Rieke and Butts,179 as “an update time that is small compared to significant changes in temperatures during treatment.” This update time is different in HT (minutes or more) versus ablation therapies (less than a second). This whole process lends credibility to the idea that future HT systems will use image feedback to control and focus heating in tumors.
Clinical Hyperthermia
General Considerations
Hyperthermia Alone
HT alone has been studied in the treatment of superficial tumors. Although an occasional response is noted, the duration is typically quite short.180,181 No long-term tumor control has been described with the use of HT as a sole treatment modality. HT alone remains widely practiced in certain alternative and complementary medicine clinics. Its use, however, is not supported by any peer-reviewed published scientific data.
Hyperthermia with Radiotherapy
A large number of reports attest to the efficacy of HT in combination with radiotherapy. The majority of publications have been from phase II trials involving patients with superficial malignant disease which is more amenable to heating) as a component of more generalized disease, such as local recurrence of breast carcinoma on the chest wall. With the addition of HT to irradiation, clinical response rates have approximately doubled, from 25% to 35% with radiation alone to 50% to 70% with radiotherapy plus HT.182 In addition to phase II studies, several phase III trials have now been conducted worldwide with approximately two-thirds published in the English-language literature. These trials generally have been positive, lending additional validity to the information obtained from phase II studies.
Hyperthermia with Chemotherapy
Published data on the efficacy of locoregional HT in conjunction with chemotherapy is much less frequent. Both animal and human data have been reviewed.182 Particularly encouraging phase II results were reported for sarcomas,183 which led to a recently completed phase III trial (see section on phase III trials). A small phase III study from Japan compared the preoperative combination of bleomycin and cisplatin with and without HT for esophageal cancer.184 The pathologic response rate was higher in the combined treatment arm compared with the CT-alone arm. A phase III trial, recently reported by Issels and associates,185 compared neoadjuvant thermochemotherapy with chemotherapy followed by surgical resection and radiotherapy for locally advanced soft tissue sarcomas (see section on phase III trials, below, for details).
Tri-modality Therapies with Hyperthermia, Radiotherapy, and Chemotherapy
The combination of HT, chemotherapy, and irradiation has been evaluated preclinically and has been shown to yield superior antitumor effects.186 Clinical studies have reported encouraging results with this approach as well. For example, pilot clinical studies have evaluated the combination of the hypoxic cell sensitizer etanidazole, irradiation, and HT for superficial tumors.187 A small randomized phase III trial involving patients with esophageal cancer showed a higher pathologic complete response (pCR) rate when HT was added to chemoradiotherapy compared with chemoradiotherapy alone.188 More recently, the combination of HT plus cisplatin plus radiotherapy has been evaluated in patients with locally advanced cervical cancer. Twelve patients were treated in this series, with local control being achieved in 10 of them. The two patients with local failure presented with local recurrence following hysterectomy.189 In a follow-up report, Westermann and associates190 combined results of this trial (with longer follow-up) with results from several other institutions that had conducted similar studies. A total of 68 patients were included in the analysis. Of these, 61 achieved a complete response. At a median follow-up of 538 days, 74% of patients were disease free. This is a favorable outcome, compared with historical controls.
The combination of 5-FU, HT, and radiation therapy yielded favorable responses in patients with locally advanced colorectal cancer in a phase II study.191 This formed the basis for a randomized phase III trial of 5-FU plus irradiation with or without HT by investigators in Germany.
Normal Tissue Damage from HT
HT does not significantly increase the early or late toxicity of radiation.58,65,67,68 The most common toxicities of HT are superficial or subcutaneous tissue burns. They are usually first- or second-degree burns and are small in volume. Such burns occur in approximately 5% of patients treated in the Duke experience; characteristically, they do not exceed 3 to 4 cm in maximum diameter. Third-degree burns are infrequently observed (<1% incidence). Complications from thermometry catheters are infrequent if the catheters are removed following each HT treatment.192 When catheters are left in place throughout the course of HT (e.g., for several weeks), the frequency of complications rises significantly, particularly of secondary infections.193
There are potential medical contraindications to deep regional HT related to the physiologic stress. Guidelines for patient selection for deep HT have been developed.138,194
Relationship between Thermal Dose and Outcome
Quality assurance for delivery and measurement of temperatures during HT is essential to identify quantifiable thermal dose parameters. Negative phase III clinical trials conducted by the Radiation Therapy Oncology Group (RTOG) in the 1980s were attributed to this issue.195–198 Quality assurance standards as well as data acquisition procedures were subsequently established by both the RTOG130,138 and the European Society of Hyperthermic Oncology (ESHO).194,199 The biologic underpinning for thermal dosimetry was discussed previously in the biology section of this chapter.
Dosimetry for HT is distinctly different from that for radiation therapy in that the amount of energy deposited does not correlate with the degree of damage. This is because damage is related to the amount of temperature rise and the time of exposure, both of which are governed by the net balance between how much energy is deposited and how much is carried away by thermal conduction and perfusion. In the case of HT, the dosimetric unit used most often has been one that was derived empirically from evaluation of the Arrhenius relationship for cell killing in vitro as well as from numerous studies of degree of tissue damage as a function of temperature and time of exposure.3 As was described in detail above, the unit most often used has been cumulative equivalent minutes at 43° C (CEM = 43° C). Application of this concept to the clinic, however, requires integration of nonuniform temperature history data with CEM43°C.
Retrospective evaluation of many phase II and III trial results have shown positive relationships between measures of thermal dose and treatment outcome, suggesting that higher thermal doses will yield better responses when HT is combined with radiotherapy.139,141–144 In all positive studies reported to date, thermal analysis was performed retrospectively. It has been more difficult to correlate variations in thermal dose administered a priori and clinical outcome. Two studies compared various numbers of heat treatments given in combination with fractionated radiotherapy,200,201 using the assumption that more treatments would yield a greater cumulative thermal dose. Neither study showed any benefit with higher numbers of HT fractions, which could in part be due to the fact that there was considerable overlap in thermal doses between the treatment arms. This is because temperature is a stronger determinant of thermal dose than time-at-temperature (see biology section, above). In a canine study, radiation therapy combined with whole body HT plus local HT was compared with local HT alone. The CEM43°CT90 was higher in the group that received whole body HT, but there was no difference in the duration of local control between the two treatment arms.202 One potential explanation for the lack of improvement in local control might have been the induction of thermotolerance during the whole body HT, which was administered prior to application of local heating.
The first attempt to prospectively control thermal dose, as prescribed by the CEM43°CT90, was a study of preoperative radiotherapy plus HT for soft tissue sarcomas. The outcome variable was the pCR rate assessed at the time of surgery, and the percentage of patients predicted to achieve a pCR rate as a result of achieving a CEM43T90 of more than 10 minutes was predicted to be 75%, based on prior phase II studies.142 The pCR rate in this study was 54%, which was below the 95% confidence level for the projected rate.59 A number of explanations are possible for the failure to achieve the projected pCR rate, including the sparseness of the temperature information gained. Recently, it was reported that other mitigating physiologic factors may have confounded the results of this study.203
Two additional phase III trials have been completed by the same group—one in patients with superficial tumors and the other in pet dogs with soft tissue sarcomas. In both of these trials, there was a clear separation in thermal doses between the two treatment arms.140,204 Most importantly, an improved response and duration of local control was observed in the groups that prospectively received the higher thermal dose. These are the first two and only trials conducted in which prospective control of thermal dose has yielded demonstrated improvement in the local tumor response rate and/or the control rate. They clearly indicate that better prospective control of thermal dose may ultimately lead to methods to truly optimize HT treatment.
Overview of Phase III Clinical Trials of Hyperthermia Plus Radiotherapy
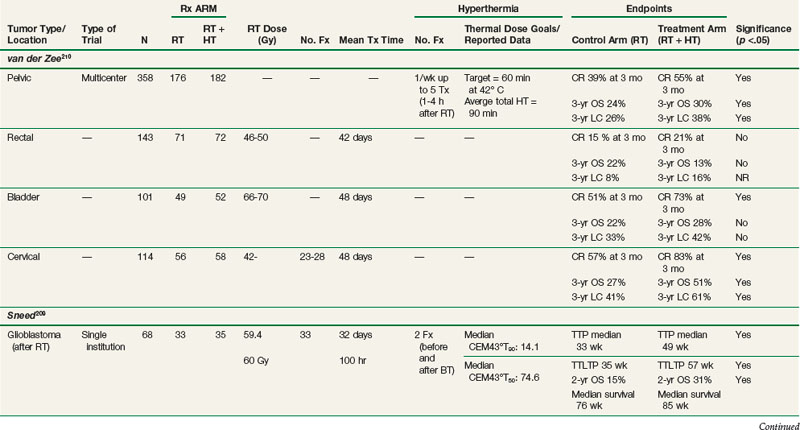
Phase III trials have involved a wide variety of diseases, including superficially and deeply situated malignant tumors. Trials have been conducted with either palliative or curative intent. A summary of the most important of these trials is presented in Table 21-2.
Breast Cancer Trials
Chest wall recurrences of breast cancer are difficult to control with conventional approaches, and many patients develop such recurrences despite prior adjuvant radiotherapy or systemic therapy. Because breast tumors are superficial, they have been amenable to HT trials. Five separate phase III trials were combined for analysis in an international collaborative study by Vernon and colleagues.205 Patients were randomized to either radiotherapy alone or radiotherapy with HT. Treatment was prescribed according to ESHO and RTOG guidelines. HT techniques differed somewhat between institutions but are well documented, as is information concerning temperature distributions and thermal dosimetry.144 Overall, the five trials demonstrated a significant improvement in the complete response rate for patients receiving HT plus radiotherapy (59%) compared with radiotherapy alone (41%), with an odds ratio of 2.3 (95% confidence interval, 1.4 to 3.8) (see Table 21-2). The greatest effect was observed in patients with recurrent lesions in previously irradiated areas where further irradiation was of necessity limited to low doses. No survival advantage was seen.
Trials of Other Superficial Malignant Tumors
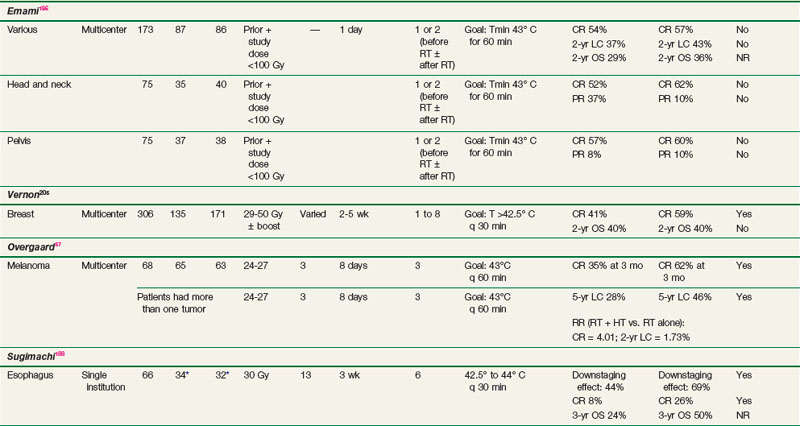
A study was conducted by the RTOG in the 1980s comparing radiotherapy alone with radiotherapy plus HT in superficial measurable tumors (RTOG 8104).197,198 Of the 307 patients included in the study, approximately half had head and neck tumors, one-third had breast carcinomas (with chest wall recurrences), and the remainder had a variety of superficial malignant tumors. Patients treated with radiotherapy plus HT had a complete response rate of 32% compared with 30% for those receiving radiotherapy alone. Subgroup analysis revealed significant improvements in the duration of local control only in patients with tumors less than 3 cm and in those with breast and/or chest wall recurrences. It was postulated that the better outcome in smaller tumors was a consequence of better heating. This trial was plagued by highly variable heating techniques and crude thermal dosimetry. These problems led to the development of subsequent RTOG guidelines for performing HT.130
Head and Neck Cancer Trials
There are two randomized series demonstrating an advantage to HT combined with radiotherapy (see Table 21-2). The first study by Datta and associates206 randomized 65 patients to radiation alone versus radiotherapy and HT. Radiotherapy doses consisted of 50 Gy in 5 weeks to the primary site and regional lymphatics followed by 10 to 15 Gy given to sites of gross disease in daily fractions of 2 Gy. HT was given twice a week, with 72 hours between each session. The HT plus radiotherapy arm showed significant improvement in response in patients with stage III and IV disease. For example, patients with stage III disease receiving combined treatment had a 58% complete response rate compared with 20% in the radiotherapy-alone group. There was no benefit for patients with stage I and II disease, with more than 90% of these patients achieving a complete response with either treatment. This trial from India evaluated both the primary site as well as neck nodes, despite the recognized difficulties of effectively delivering heat to most primary head and neck tumors.
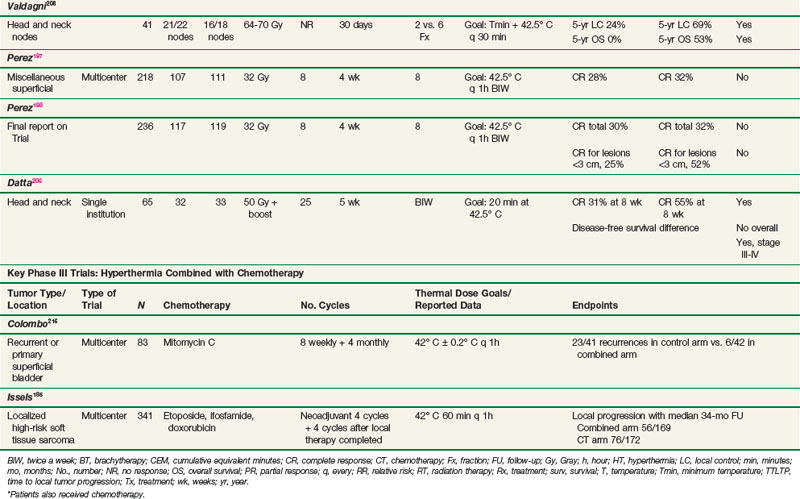
A second trial from Italy by Valdagni and colleagues207,208 restricted treatment and evaluation to metastatic cervical lymph nodes. This study randomized 41 patients with advanced locoregional squamous cell carcinoma of the head and neck to treatment with either radiotherapy alone or radiotherapy combined with HT. Long-term follow-up allowed for analysis of response, duration of local control, and survival times. The 5-year actuarial local control rate in the neck with radiotherapy alone versus radiotherapy plus HT was 24% versus 69%, and the 5-year overall survival rate was 0% versus 53%; all of these differences were statistically significant. There was no clear enhancement of toxicity or any clear relationship between thermal dose received and outcome. Nonetheless, this well-executed, well-described phase III trial, despite the relatively small number of patients, is an important component of the evidence suggesting the value of HT.
A trial of interstitial HT was carried out primarily in head and neck patients by the RTOG.196 This trial studied 173 patients with persistent or recurrent tumors after prior radiotherapy and/or surgery that were amenable to interstitial radiotherapy. The lesion site was the head and neck in approximately 45% of patients and the pelvis in approximately 40%, with miscellaneous sites accounting for the rest. The patients were randomized to receive interstitial radiotherapy alone with or without interstitial HT. Overall, there was no difference in complete response rates or 2-year survival rates. There were major quality assurance issues with this trial. Only one patient in the entire group was considered to have had adequate HT. This trial, as well as the previously mentioned RTOG trial, provided impetus for the subsequent development of HT guidelines by the RTOG.137
Esophageal Cancer Trials
Two randomized studies demonstrated an advantage to the addition of HT to chemoradiotherapy or chemotherapy alone in the neoadjuvant treatment of esophageal cancer. In the first study by Sugimachi and colleagues, 53 patients with squamous cell carcinoma of the thoracic esophagus were randomized to preoperative HT, radiotherapy, and chemotherapy, compared with chemoradiotherapy alone.188 The chemotherapy (bleomycin) and HT were given concurrently 1 hour prior to the irradiation in a 3-week regimen with a total radiotherapy dose of approximately 32 Gy. Clinical complete responses and pathologic responses were significantly improved in the tri-modality arm, with a pCR of 26% combined in the tri-modality group versus 8% in the chemoradiotherapy group (see Table 21-2).
In a follow-up study, an additional 40 patients were treated with chemotherapy alone (bleomycin and cisplatin) or chemotherapy combined with HT.184 No radiotherapy was given in this trial. Again, an improvement in histopathologic response was noted favoring the HT group (19% vs. 41%).
Malignant Melanoma Trials
A major multicenter trial was conducted in patients with metastatic melanoma by Overgaard and colleagues.57 Seventy patients with 134 metastatic or recurrent malignant melanoma lesions were randomized to receive radiotherapy with or without HT. Overall, there was a significant benefit for the addition of HT, with a 2-year local control rate of 46% in the combined group compared with 28% for those receiving radiotherapy alone (see Table 21-2). Quality assurance issues were problematic in this trial, with only 14% of treatments achieving the protocol objective of 43° C for 60 minutes. Despite this, positive benefits were seen.
Glioblastoma Multiforme Trials
A University of California San Francisco study by Sneed and coworkers209 evaluated interstitial HT combined with a brachytherapy boost for selected patients with glioblastoma multiforme. One hundred and twelve patients were entered into this trial, which randomized patients whose tumor was implantable following external beam radiation therapy and chemotherapy to receive brachytherapy with or without HT. Seventy-nine were randomized; 33 patients were dropped from the protocol due to disease progression. Both time to tumor progression and survival times were significantly improved for the HT patients compared with those treated with brachytherapy alone (see Table 21-2). Two-year survival rates in the two groups were 31% and 15%, respectively. Toxicity appeared to be slightly greater in the HT patients, with seven grade 3 toxicities reported compared with one in the brachytherapy-alone group. Thermal dose data showed that good heating was achieved in most patients. No good correlation was seen between thermal dose and response.209
Pelvic Tumor (Cervical, Bladder, and Colorectal) Trials
The Dutch hyperthermia group conducted a study (van der Zee and colleagues)210 in which 358 patients with previously untreated locally advanced pelvic tumors were randomized to receive radiotherapy alone or radiotherapy plus HT (see Table 21-2). HT treatments were given once weekly for a total of five treatments. Generally, thermal goals were not achieved. Detailed thermal dose analyses have not been published. There were approximately equal numbers of patients with bladder, colorectal, and cervical carcinomas. Complete response rates were 39% and 55% after radiotherapy alone and radiotherapy plus HT, respectively (p <.001). Duration of local control was also significantly improved with radiotherapy plus HT. Patients with cervical carcinoma benefited the most. Three-year survival rates were 27% and 51% in the radiotherapy and radiotherapy plus HT groups, respectively (p = .003). This study has been criticized for suboptimal therapy in the control arm, namely, radiotherapy alone as opposed to the combination of radiotherapy and chemotherapy. At the time the trial was initiated in 1990, the role of chemotherapy in cervical carcinoma was not established. Subsequently, a number of studies published from 1999 to 2000 have demonstrated a survival advantage for concurrent cisplatin-based chemotherapy in cervical carcinoma.211–215 Additional phase III trials are needed to establish whether there is an advantage to using thermochemoradiotherapy compared with chemoradiotherapy for locally advanced cervical cancer.
Overview of Phase III Clinical Trials Using Hyperthermia Plus Chemotherapy
Soft Tissue Sarcoma Trials
The European Organization for Research and Treatment of Cancer (EORTC) recently reported on results of a phase III trial by Issels and associates that compared neoadjuvant thermochemotherapy with chemotherapy (etoposide, ifosfamide, and doxorubicin)185 (see Table 21-2). A total of 341 patients were enrolled. Patients received four cycles of neoadjuvant treatment before receiving definitive surgery and/or radiotherapy for the primary tumor. The hazard ratio for local progression-free survival, the primary outcome variable, was 0.58 for patients receiving thermochemotherapy (p = .003). At the 2-year follow-up point, the combined arm had a 15% higher rate of progression-free survival. Disease-free survival analysis showed a hazard ratio for the combined arm of 0.7 (p = .011). For those patients who completed the combined-arm treatment, the hazard ratio for overall survival was 0.66 (p = .038).
Bladder Cancer Trials
Colombo and colleagues216 conducted a randomized phase III trial for recurrent superficial bladder cancer that used intravesicular mitomycin C with and without bladder HT, by means of an intravesicularly placed microwave antenna to heat the bladder contents and wall (see Table 21-2). Eighty-six patients were enrolled and 75 completed therapy and were evaluable. Treatment of the bladder commenced after surgical removal of the tumor. The hazard ratio for chemotherapy alone versus thermochemotherapy was 4.8 (p = .0002). The heated group had a higher rate of complications, particularly related to thermal damage to the posterior bladder wall. Most of this damage resolved quickly, with only one patient experiencing a prolonged several-month recovery period. These results are encouraging, considering the fact that the large majority of these patients had failed mitomycin C therapy previously.
Challenges, Controversies, and Future Possibilities
Technical Challenges
Thermal Dose Prescription
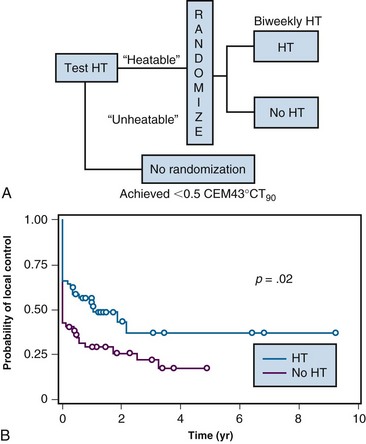
Even if noninvasive thermometry were available, until recently there has been no information on what target temperature ranges are needed to optimize this therapy. Such information is the second key for providing a quantitative assessment of HT treatment adequacy (i.e., it establishes a method for writing an HT prescription). In spite of extensive phase II data suggesting that higher temperatures yield improved likelihood for effective therapy, until recently there have been no successful prospective trials showing that escalation of thermal dose yields improved antitumor effect. However, recently, two clinical trials have been reported in which thermal dose was controlled during application of HT.140,204 These trials have set the stage for establishing realistic goals for how to administer this treatment.
These two trials were designed to perform a “test” HT treatment first, to determine whether the tumor was heatable, as defined by preestablished criteria. If the tumor was “heatable,” then the patient was randomized to receive either a low cumulative thermal dose or a high thermal dose that was at least 10-fold higher than the low dose. The inclusion of the “test” treatment was key in both studies because about 12% of all eligible patients were excluded from randomization because their tumors could not be heated. The first trial was in patients with superficial tumors and the second was in pet dogs with spontaneous soft tissue sarcomas. Both trials demonstrated that cumulative thermal doses of more than 10 CEM43°CT90 yielded improved tumor responses and durations of local control140,204 (Fig. 21-9). To put this into perspective, this cumulative thermal dose is equivalent to achieving a temperature of 40° C for 1 hour, once or twice a week, at the 10th percentile of the temperature distribution. Importantly, this thermal goal was reached in more than 90% of patients who randomized to the high thermal dose group.
Future Directions
Augmentation of Drug Delivery
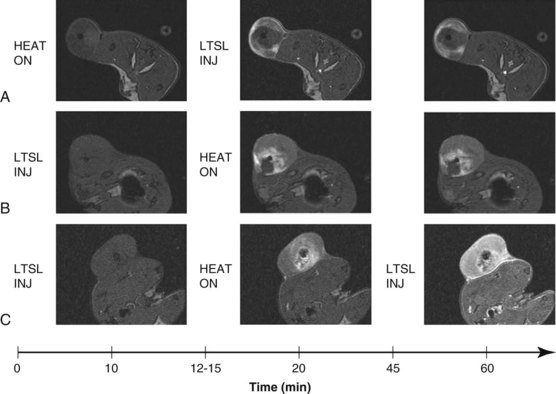
Drug delivery to tumors remains a major challenge due to physiologic barriers such as high interstitial fluid pressure and heterogeneous perfusion. As noted above, HT has been shown to augment macromolecule and nanoparticle drug delivery by increasing blood flow, available volume fraction, and tumor vascular permeability. Furthermore, local HT can be used as a trigger for temperature-sensitive drug delivery systems such as liposomes, polymers, and hydrogels.217,218,219,220 In this manner, site-specific bioavailability can be achieved. For example, a temperature-sensitive liposome containing doxorubicin (ThermoDox) has been developed to release rapidly at clinically achievable temperatures of 40° to 42° C. This formulation has shown dramatic improvement in tumor drug concentration and antitumor efficacy in preclinical studies221,222,223 and is currently being investigated in phase II/III clinical trials for chest wall recurrences of breast cancer and radiofrequency ablation of liver metastases, respectively. As thermal technology and liposomal formulations advance, this method has the potential for precise control of drug delivery independent of tumor phenotype and drug composition. Newer applications of this liposome have contained both drug and MR contrast agents, and this tandem has been used to monitor drug delivery to heated tumors in real time, as the MR contrast agents change the MR signal when they are released from the liposomes224,225,226 (Fig. 21-10).
2 Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787-800.
3 Dewhirst MW, Viglianti BL, Lora-Michiels M. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267-294.
9 Streffer C. Metabolic changes during and after hyperthermia. Int J Hyperthermia. 1985;1(4):305-319.
13 Song CW. Effect of local hyperthermia on blood flow and microenvironment. A review. Cancer Res. 1984;44(10 Suppl):4721s-4730s.
15 Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery. Effect of particle size. Cancer Res. 2000;60(16):4440-4445.
21 Dahl O. Interaction of heat and drugs in vitro and in vivo. In: Seegenschmiedt M, Fessenden P, Vernon C, editors. Thermoradiotherapy and thermochemotherapy. Berlin: Springer-Verlag; 1995:103-155.
25 Dreher MR, Liu W, Michelich CR, et al. Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors. Cancer Res. 2007;67(9):4418-4424.
30 Oleson JR. Eugene Robinson Special Lecture. Hyperthermia from the clinic to the laboratory: a hypothesis. Int J Hyperthermia. 1995;11(3):315-322.
31 Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res. 2001;155(4):515-528.
33 Vujaskovic Z, Poulson J, Gaskin A, et al. Temperature dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia. Int J Radiat Oncol Biol Phys. 2000;46(1):179-185.
58 Gillette SM, Dewhirst MW, Gillette EL, et al. Response of canine soft tissue sarcomas to radiation or radiation plus hyperthermia. A randomized phase II study. Int J Hyperthermia. 1992;8(3):309-320.
65 Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys. 1989;16(3):535-549.
66 Dewhirst MW, Sim DA. The utility of thermal dose as a predictor of tumor and normal tissue responses to combined radiation and hyperthermia. Cancer Res. 1984;44(10 Suppl):4772s-4780s.
67 Gillette EL, McChesney SL, Dewhirst MW, Scott RJ. Response of canine oral carcinomas to heat and radiation. Int J Radiat Oncol Biol Phys. 1987;13(12):1861-1867.
73 Hettinga JV, Konings AW, Kampinga HH. Reduction of cellular cisplatin resistance by hyperthermia—a review. Int J Hyperthermia. 1997;13(5):439-457.
83 Furusawa Y, Tabuchi Y, Takasaki I, et al. Gene networks involved in apoptosis induced by hyperthermia in human lymphoma U937 cells. Cell Biol Int. 2009;33(12):1253-1262.
84 Borkamo ED, Dahl O, Bruland O, Fluge O. Kinetics study on markers of the immune system by gene expression profiling of an in vivo heated tumor. Int J Hyperthermia. 2009;25(1):41-46.
100 Ostberg JR, Kabingu E, Repasky EA. Thermal regulation of dendritic cell activation and migration from skin explants. Int J Hyperthermia. 2007;19(5):520-533.
101 Menoret A, Chaillot D, Callahan M, Jacquin C. Hsp70, an immunological actor playing with the intracellular self under oxidative stress. Int J Hyperthermia. 2002;18:490-505.
102 Manjili MH, Wang XY, Park J, et al. Cancer immunotherapy. Stress proteins and hyperthermia. Int J Hyperthermia. 2002;18:506-520.
103 Multhoff G. Activation of natural killer cells by heat shock protein 70. Int J Hyperthermia. 2002;18:576-585.
104 Shah A, Unger E, Bain MD, et al. Cytokine and adhesion molecule expression in primary human endothelial cells stimulated with fever-range hyperthermia. Int J Hyperthermia. 2002;18:534-551.
106 Hand JR. Physical techniques in clinical hyperthermia. New York: John Wiley and Sons; 1986.
107 Cossett JM. Interstitial, endocavitary, and perfusional hyperthermia. Berlin: Springer-Verlag; 1990.
110 Hynynen K. Ultrasound heating technology. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and thermochemotherapy. Berlin, Heidelberg: Springer-Verlag; 1995:253-277.
112 Stauffer PR. Evolving technology for thermal therapy of cancer. Int J Hyperthermia. 2005;21(8):731-744.
119 Nadobny J, Wlodarczyk W, Westhoff L, et al. Development and evaluation of a three-dimensional hyperthermia applicator with Water-COated Antennas (WACOA). Med Phys. 2003;30(8):2052-2064.
120 Turner PF. Regional hyperthermia with an annular phased array. IEEE Trans Biomed Eng. 1984;31:106-114.
129 Cetas TC. Thermometry. In: Field SB, Hand JW, editors. An introduction to the practical aspects of clinical hyperthermia. London, New York: Taylor & Francis; 1990:423-477.
130 Dewhirst MW, Phillips TL, Samulski TV, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia. Int J Radiat Oncol Biol Phys. 1990;18(5):1249-1259.
140 Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23(13):3079-3085.
162 McDannold N. Quantitative MRI-based temperature mapping based on the proton resonant frequency shift. Review of validation studies. Int J Hyperthermia. 2005;21(6):533-546.
169 Cheng KS, Stakhursky V, Craciunescu OI, et al. Fast temperature optimization of multi-source hyperthermia applicators with reduced-order modeling of ‘virtual sources. Phys Med Biol. 2008;53(6):1619-1635.
177 Gellerman J, Wlodarczyk W, Feussner A, et al. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperthermia. 2005;21(6):497-513.
179 Rieke V, Butts PK. MR thermometry. J Magn Reson Imaging. 2008;27(2):376-390.
185 Issels R, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft tissue sarcoma. A randomised phase III multi-centre study of the EORTC-STBSG and the ESHO. Lancet Oncol. 2010;11(6):561-570.
192 Sneed PK, Dewhirst MW, Samulski T, et al. Should interstitial thermometry be used for deep hyperthermia? [Editorial; comment.]. Int J Radiat Oncol Biol Phys. 1998;40(5):1015-1017.
204 Thrall DE, LaRue SM, Yu D, et al. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res. 2005;11(14):5206-5214.
205 Vernon CC, Hand JW, Field SB, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys. 1996;35(4):731-744.
208 Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 1994;28(1):163-169.
209 Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40(2):287-295.
210 van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours. A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355(9210):1119-1125.
216 Colombo R, Da Pozzo LF, Salonia A, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol. 2003;21(23):4270-4276.
219 Chilkoti A, Dreher MR, Meyer DE. Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv Drug Delivery Rev. 2002;54(8):1093-1111.
221 Kong G, Anyarambhatla G, Petros WP, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model. Importance of triggered drug release. Cancer Res. 2000;60(24):6950-6957.
222 Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia. Characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60(5):1197-1201.
224 Ponce AM, Viglianti BL, Yu D, et al. Magnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effects. J Natl Cancer Inst. 2007;99(1):53-63.
1 Arrhenius S. Uber die reaktionsgeschwindigkeit bei der inversion von rohrzucker durch Sauren. Zeitschr Physik Chem. 1889;4:226-248.
2 Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787-800.
3 Dewhirst MW, Viglianti BL, Lora-Michiels M. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267-294.
4 Morimoto RI, Kroeger PE, Cotto JJ. The transcriptional regulation of heat shock genes. A plethora of heat shock factors and regulatory conditions. Exs. 1996;77:139-163.
5 Raaphorst G. Fundamental aspects of hyperthermic biology. In: Field S, Hand J, editors. An introduction to the practical aspects of clinical hyperthermia. London: Taylor and Francis; 1990:10-54.
6 Coss RA, Linnemans WA. The effects of hyperthermia on the cytoskeleton. A review. Int J Hyperthermia. 1996;12(2):173-196.
7 Pratt WB, Silverstein AM, Galigniana MD. A model for the cytoplasmic trafficking of signalling proteins involving the hsp90-binding immunophilins and p50cdc37. Cell Signal. 1999;11(12):839-851.
8 Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. J Cell Sci. 1997;110(Part 13):1431-1440.
9 Streffer C. Metabolic changes during and after hyperthermia. Int J Hyperthermia. 1985;1(4):305-319.
10 Vidair CA, Doxsey SJ, Dewey WC. Heat shock alters centrosome organization leading to mitotic dysfunction and cell death. J Cell Physiol. 1993;154(3):443-455.
11 Kampinga HH, Dikomey E. Hyperthermic radiosensitization. Mode of action and clinical relevance. Int J Radiat Biol. 2001;77(4):399-408.
12 Dewhirst MW, Sim D, Gross J, Kundrat M. Effects of heating rate on normal and tumor microcirculatory function. In: Diller K, Roemer RB, editors. Heat and mass transfer in the microcirculation of thermally significant vessels, Anaheim. California: ASME; 1986:75-80.
13 Song CW. Effect of local hyperthermia on blood flow and microenvironment. A review. Cancer Res. 1984;44(10 Suppl):4721s-4730s.
14 Reinhold HS. Physiological effects of hyperthermia. Recent Results Cancer Res. 1988;107:32-43.
15 Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery. Effect of particle size. Cancer Res. 2000;60(16):4440-4445.
16 Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001;61(7):3027-3032.
17 Matteucci ML, Anyarambhatla G, Rosner G, et al. Hyperthermia increases accumulation of technetium-99m-labeled liposomes in feline sarcomas. Clin Cancer Res. 2000;6(9):3748-3755.
18 Krol A, Dewhirst MW, Yuan F. Effects of cell damage and glycosaminoglycan degradation on available extravascular space. Int J Hyperthermia. 2003;19(2):154-164.
19 Kong G, Dewhirst MW. Hyperthermia and liposomes. Int J Hyperthermia. 1999;15(5):345-370.
20 Kouloulias VE, Dardoufas CE, Kouvaris JR, et al. Liposomal doxorubicin in conjunction with reirradiation and local hyperthermia treatment in recurrent breast cancer. A phase I/II trial. Clin Cancer Res. 2002;8(2):374-382.
21 Dahl O. Interaction of heat and drugs in vitro and in vivo. In: Seegenschmiedt M, Fessenden P, Vernon C, editors. Thermoradiotherapy and thermochemotherapy. Berlin: Springer-Verlag; 1995:103-155.
22 Curry FE. Determinants of capillary permeability. A review of mechanisms based on single capillary studies in the frog. Circ Res. 1986;59(4):367-380.
23 Hauck M, Zalutsky M, Dewhirst MW. Enhancement of radiolabeled monoclonal antibody uptake in tumors with local hyperthermia. In: Torchilin V, editor. Targeted delivery of imaging agents. Boca raton, Florida: CRC Press; 1995:335-361.
24 Meyer DE, Kong GA, Dewhirst MW, et al. Targeting a genetically engineered elastin-like polypeptide to solid tumors by local hyperthermia. Cancer Res. 2001;61(4):1548-1554.
25 Dreher MR, Liu W, Michelich CR, et al. Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors. Cancer Res. 2007;67(9):4418-4424.
26 Dreher MR, Elas M, Ichikawa K, et al. Nitroxide conjugate of a thermally responsive elastin-like polypeptide for non-invasive thermometry. Med Phys. 2004;31(10):2755-2762.
27 Prescott DM, Charles HC, Sostman HD, et al. Therapy monitoring in human and canine soft tissue sarcomas using magnetic resonance imaging and spectroscopy. Int J Radiat Oncol Biol Phys. 1994;28(2):415-423.
28 Sijens PE, Bovee WM, Seijkens D, et al. Murine mammary tumor response to hyperthermia and radiotherapy evaluated by in vivo 31P-nuclear magnetic resonance spectroscopy. Cancer Res. 1987;47(24 Part 1):6467-6473.
29 Kelleher DK, Engel T, Vaupel PW. Changes in microregional perfusion, oxygenation, ATP and lactate distribution in subcutaneous rat tumours upon water-filtered IR-A hyperthermia. Int J Hyperthermia. 1995;11(2):241-255.
30 Oleson JR. Eugene Robinson Special Lecture. Hyperthermia from the clinic to the laboratory: a hypothesis. Int J Hyperthermia. 1995;11(3):315-322.
31 Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res. 2001;155(4):515-528.
32 Sun X, Xing L, Ling CC, Li GC. The effect of mild temperature hyperthermia on tumour hypoxia and blood perfusion. Relevance for radiotherapy, vascular targeting and imaging. Int J Hyperthermia. 2010;26(3):224-231.
33 Vujaskovic Z, Poulson J, Gaskin A, et al. Temperature dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia. Int J Radiat Oncol Biol Phys. 2000;46(1):179-185.
34 Brizel DM, Scully SP, Harrelson JM, et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 1996;56(23):5347-5350.
35 Jones EL, Prosnitz LR, Dewhirst MW, et al. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res. 2004;10(13):4287-4293.
36 Dewhirst MW, Thrall DE, Palmer G, et al. Utility of functional imaging in prediction or assessment of treatment response and prognosis following thermotherapy. Int J Hyperthermia. 2010;26(3):283-293.
37 Griffin RJ, Ogawa A, Williams BW, Song CW. Hyperthermic enhancement of tumor radiosensitization strategies. Immunol Invest. 2005;34(3):343-359.
38 Okajima K, Griffin RJ, Iwata K, et al. Tumor oxygenation after mild-temperature hyperthermia in combination with carbogen breathing. Dependence on heat dose and tumor type. Radiat Res. 1998;149(3):294-299.
39 Wahl ML, Bobyock SB, Leeper DB, Owen CS. Effects of 42 degrees C hyperthermia on intracellular pH in ovarian carcinoma cells during acute or chronic exposure to low extracellular pH. Int J Radiat Oncol Biol Phys. 1997;39(1):205-212.
40 Coss RA, Storck CW, Wachsberger PR, et al. Acute extracellular acidification reduces intracellular pH, 42 degrees C-induction of heat shock proteins and clonal survival of human melanoma cells grown at pH 6.7. Int J Hyperthermia. 2004;20(1):93-106.
41 Crabtree H. LXI. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929;23:536-545.
42 Newsholme E, Crabtree B, Ardawi M. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985;5:393-400.
43 Leeper DB, Engin K, Thistlethwaite AJ, et al. Human tumor extracellular pH as a function of blood glucose concentration. Int J Radiat Oncol Biol Phys. 1994;28(4):935-943.
44 Prescott DM, Charles HC, Sostman HD, et al. Manipulation of intra- and extracellular pH in spontaneous canine tumours by use of hyperglycaemia. Int J Hyperthermia. 1993;9(5):745-754.
45 Biaglow JE, Manevich Y, Leeper D, et al. MIBG inhibits respiration. Potential for radio- and hyperthermic sensitization. Int J Radiat Oncol Biol Phys. 1998;42(4):871-876.
46 Zhou R, Bansal N, Leeper DB, Glickson JD. Intracellular acidification of human melanoma xenografts by the respiratory inhibitor m-iodobenzylguanidine plus hyperglycemia. A 31P magnetic resonance spectroscopy study. Cancer Res. 2000;60(13):3532-3536.
47 Wachsberger PR, Burd R, Bhala A, et al. Quercetin sensitizes cells in a tumour-like low pH environment to hyperthermia. Int J Hyperthermia. 2003;19(5):507-519.
48 Asea A, Ara G, Teicher BA, et al. Effects of the flavonoid drug quercetin on the response of human prostate tumours to hyperthermia in vitro and in vivo. Int J Hyperthermia. 2001;17(4):347-356.
49 Jones EL, Zhao M, Stevenson MA, Calderwood SK. The 70 kilodalton heat shock protein is an inhibitor of apoptosis in prostate cancer. Int J Hyperthermia. 2004;20(8):835-849.
50 Song CW, Kim GE, Lyons JC, et al. Thermosensitization by increasing intracellular acidity with amiloride and its analogs. Int J Radiat Oncol Biol Phys. 1994;30(5):1161-1169.
51 Nathanson SD, Cerra RF, Hetzel FW, et al. Changes associated with metastasis in B16-F1 melanoma cells surviving heat. Arch Surg. 1990;125(2):216-219.
52 Nathanson SD, Nelson L, Anaya P, et al. Development of lymph node and pulmonary metastases after local irradiation and hyperthermia of footpad melanomas. Clin Exp Metastasis. 1991;9(4):377-392.
53 Hill SA, Denekamp J. Does local tumour heating in mice influence metastatic spread? Br J Radiol. 1982;55(654):444-451.
54 Baker DG, Sager H, Constable WC, Goodchild NT. Influence of ultrasound-induced hyperthermia and X-irradiation on the incidence of metastases from a solid tumor. Invasion Metastasis. 1984;4(2):111-124.
55 Dewhirst MW, Sim DA, Forsyth K, et al. Local control and distant metastases in primary canine malignant melanomas treated with hyperthermia and/or radiotherapy. Int J Hyperthermia. 1985;1(3):219-234.
56 Overgaard J, Gonzalez Gonzalez D, Hulshof MC, et al. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia. 1996;12(1):3-20.
57 Overgaard J, Gonzalez Gonzalez D, Hulshof MC, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet. 1995;345(8949):540-543.
58 Gillette SM, Dewhirst MW, Gillette EL, et al. Response of canine soft tissue sarcomas to radiation or radiation plus hyperthermia. A randomized phase II study. Int J Hyperthermia. 1992;8(3):309-320.
59 Maguire PD, Samulski TV, Prosnitz LR, et al. A phase II trial testing the thermal dose parameter CEM43 degrees T90 as a predictor of response in soft tissue sarcomas treated with pre-operative thermoradiotherapy. Int J Hyperthermia. 2001;17(4):283-290.
60 Pisters WT, O’Sullivan BO, Demetri GD. Sarcomas of nonosseous tissues. In: Bast RC, Kufe DW, Pollack RE, editors. Cancer medicine. 5th ed. Hamilton, Ontario: B.C. Decker; 2000:1906-1930.
61 Engin K, Leeper D, Thistlethwaite A, et al. Tumor extracellular pH as a prognostic factor in thermoradiotherapy. Int J Radiat Oncol Biol Phys. 1994;29:125-132.
62 Mivechi NF, Dewey WC. DNA polymerase alpha and beta activities during the cell cycle and their role in heat radiosensitization in Chinese hamster ovary cells. Radiat Res. 1985;103(3):337-350.
63 Raaphorst GP, Ng CE, Yang DP. Thermal radiosensitization and repair inhibition in human melanoma cells. A comparison of survival and DNA double strand breaks. Int J Hyperthermia. 1999;15(1):17-27.
64 Raaphorst GP, Yang DP, Ng CE. Effect of protracted mild hyperthermia on polymerase activity in a human melanoma cell line. Int J Hyperthermia. 1994;10(6):827-834.
65 Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys. 1989;16(3):535-549.
66 Dewhirst MW, Sim DA. The utility of thermal dose as a predictor of tumor and normal tissue responses to combined radiation and hyperthermia. Cancer Res. 1984;44(10 Suppl):4772s-4780s.
67 Gillette EL, McChesney SL, Dewhirst MW, Scott RJ. Response of canine oral carcinomas to heat and radiation. Int J Radiat Oncol Biol Phys. 1987;13(12):1861-1867.
68 Denman DL, Legorreta RA, Kier AB, et al. Therapeutic responses of spontaneous canine malignancies to combinations of radiotherapy and hyperthermia. Int J Radiat Oncol Biol Phys. 1991;21(2):415-422.
69 Kapp DS, Cox RS, Fessenden P, et al. Parameters predictive for complications of treatment with combined hyperthermia and radiation therapy. Int J Radiat Oncol Biol Phys. 1992;22(5):999-1008.
70 Wiggenraad R, Koning C, Westermann C, et al. Two cases of fatal necrosis of the lesser pelvis in patients treated with combined radiotherapy and hyperthermia for cervical carcinoma. Int J Hyperthermia. 2005;21(3):185-192.
71 Matthews KS, Rocconi RP, Straughn JMJr. Complete uterine necrosis following chemoradiation for advanced cervical cancer. A case report. Gynecol Oncol. 2007;106(1):265-267.
72 Marnitz S, Kohler C, Fuller J, et al. Uterus necrosis after radiochemotherapy in two patients with advanced cervical cancer. Strahlenther Onkol. 2006;182(1):45-51.
73 Hettinga JV, Konings AW, Kampinga HH. Reduction of cellular cisplatin resistance by hyperthermia—a review. Int J Hyperthermia. 1997;13(5):439-457.
74 Laskowitz DT, Elion GB, Dewhirst MW, et al. Hyperthermia-induced enhancement of melphalan activity against a melphalan-resistant human rhabdomyosarcoma xenograft. Radiat Res. 1992;129(2):218-223.
75 Da Silva VF, Feeley M, Raaphorst GP. Hyperthermic potentiation of BCNU toxicity in BCNU-resistant human glioma cells. J Neurooncol. 1991;11(1):37-41.
76 Averill DA, Su C. Sensitization to the cytotoxicity of adriamycin by verapamil and heat in multidrug-resistant Chinese hamster ovary cells. Radiat Res. 1999;151(6):694-702.
77 Herman TS, Teicher BA, Varshney A, et al. Effect of hypoxia and acidosis on the cytotoxicity of mitoxantrone, bisantrene and amsacrine and their platinum complexes at normal and hyperthermic temperatures. Anticancer Res. 1992;12(3):827-836.
78 Teicher BA, Holden SA, Rudolph MB, et al. Effect of environmental conditions (pH, oxygenation and temperature) on the cytotoxicity of flavone acetic acid and its dimethylaminoethyl ester. Int J Hyperthermia. 1991;7(6):905-915.
79 Teicher BA, Herman TS, Holden SA, Rudolph MB. Effect of oxygenation, pH and hyperthermia on RSU-1069 in vitro and in vivo with radiation in the FSaIIC murine fibrosarcoma. Cancer Lett. 1991;59(2):109-117.
80 Holden SA, Teicher BA, Herman TS. Effect of environmental conditions (pH, oxygenation, and temperature) on misonidazole cytotoxicity and radiosensitization in vitro and in vivo in FSaIIC fibrosarcoma. Int J Radiat Oncol Biol Phys. 1991;20(5):1031-1038.
81 Teicher BA, Herman TS, Holden SA. Effect of pH, oxygenation, and temperature on the cytotoxicity and radiosensitization by etanidazole. Int J Radiat Oncol Biol Phys. 1991;20(4):723-731.
82 Tabuchi Y, Takasaki I, Wada S, et al. Genes and genetic networks responsive to mild hyperthermia in human lymphoma U937 cells. Int J Hyperthermia. 2008;24(8):613-622.
83 Furusawa Y, Tabuchi Y, Takasaki I, et al. Gene networks involved in apoptosis induced by hyperthermia in human lymphoma U937 cells. Cell Biol Int. 2009;33(12):1253-1262.
84 Borkamo ED, Dahl O, Bruland O, Fluge O. Kinetics study on markers of the immune system by gene expression profiling of an in vivo heated tumor. Int J Hyperthermia. 2009;25(1):41-46.
85 Borkamo ED, Dahl O, Bruland O, Fluge O. Global gene expression analyses reveal changes in biological processes after hyperthermia in a rat glioma model. Int J Hyperthermia. 2008;24(5):425-441.
86 Nivon M, Richet E, Codogno P, et al. Autophagy activation by NF kappa B is essential for cell survival after heat shock. Autophagy. 2009;5(6):766-783.
87 Chen F, Wang CC, Kim E, Harrison LE. Hyperthermia in combination with oxidative stress induces autophagic cell death in HT-29 colon cancer cells. Cell Biol Int. 2008;32(7):715-723.
88 Pajonk F, van Ophoven A, McBride WH. Hyperthermia-induced proteasome inhibition and loss of androgen receptor expression in human prostate cancer cells. Cancer Res. 2005;65(11):4836-4843.
89 Kullberg M, Mann K, Owens JL. A two-component drug delivery system using Her-2-targeting thermosensitive liposomes. J Drug Targeting. 2009;17(2):98-107.
90 Wuang SC, Neoh KG, Kang ET, et al. HER-2-mediated endocytosis of magnetic nanospheres and the implications in cell targeting and particle magnetization. Biomaterials. 2008;29(14):2270-2279.
91 Sawant RM, Sawant RR, Gultepe E, et al. Nanosized cancer cell-targeted polymeric immunomicelles loaded with superparamagnetic iron oxide nanoparticles. J Nanoparticle Res. 2009;11(7):1777-1785.
92 Huang YF, Sefah K, Bamrungsap S, et al. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir. 2008;24(20):11860-11865.
93 Urano M, Kuroda M, Nishimura Y. For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia. 1999;15(2):79-107.
94 Katschinski DM, Robins HI. Hyperthermic modulation of SN-38-induced topoisomerase I DNA cross- linking and SN-38 cytotoxicity through altered topoisomerase I activity. Int J Cancer. 1999;80(1):104-109.
95 Ng CE, Bussey AM, Raaphorst GP. Sequence of treatment is important in the modification of camptothecin induced cell killing by hyperthermia. Int J Hyperthermia. 1996;12(5):663-678. discussion Int J Hyperthermia 12(5):679-680, 1996
96 Leal BZ, Meltz ML, Mohan N, et al. Interaction of hyperthermia with Taxol in human MCF-7 breast adenocarcinoma cells. Int J Hyperthermia. 1999;15(3):225-236.
97 Cividalli A, Cruciani G, Livdi E, et al. Hyperthermia enhances the response of paclitaxel and radiation in a mouse adenocarcinoma. Int J Radiat Oncol Biol Phys. 1999;44(2):407-412.
98 Vaden SL, Page RL, Williams PL, Riviere JE. Effect of hyperthermia on cisplatin and carboplatin disposition in the isolated, perfused tumour and skin flap. Int J Hyperthermia. 1994;10(4):563-572.
99 Kido Y, Kuwano H, Maehara Y, et al. Increased cytotoxicity of low-dose, long-duration exposure to 5- fluorouracil of V-79 cells with hyperthermia. Cancer Chemother Pharmacol. 1991;28(4):251-254.
100 Ostberg JR, Kabingu E, Repasky EA. Thermal regulation of dendritic cell activation and migration from skin explants. Int J Hyperthermia. 2007;19(5):520-533.
101 Menoret A, Chaillot D, Callahan M, Jacquin C. Hsp70, an immunological actor playing with the intracellular self under oxidative stress. Int J Hyperthermia. 2002;18:490-505.
102 Manjili MH, Wang XY, Park J, et al. Cancer immunotherapy. Stress proteins and hyperthermia. Int J Hyperthermia. 2002;18:506-520.
103 Multhoff G. Activation of natural killer cells by heat shock protein 70. Int J Hyperthermia. 2002;18:576-585.
104 Shah A, Unger E, Bain MD, et al. Cytokine and adhesion molecule expression in primary human endothelial cells stimulated with fever-range hyperthermia. Int J Hyperthermia. 2002;18:534-551.
105 Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptidembearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones. 2000;5(5):425-431.
106 Hand JR. Physical techniques in clinical hyperthermia. New York: John Wiley and Sons; 1986.
107 Cossett JM. Interstitial, endocavitary, and perfusional hyperthermia. Berlin: Springer-Verlag; 1990.
108 Fessenden P, Hand JW. Hyperthermia therapy physics. In: Smith AR, editor. Medical radiology: radiation therapy physics. Berlin, Heidelberg: Springer-Verlag; 1995:315-363.
109 Hand JW. Biophysics and technology of electromagnetic hyperthermia. In: Gautherie M, editor. Methods of external hyperthermia heating. Berlin, Heidelberg: Springer-Verlag; 1990:1-60.
110 Hynynen K. Ultrasound heating technology. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and thermochemotherapy. Berlin, Heidelberg: Springer-Verlag; 1995:253-277.
111 Lee ER. Electromagnetic superficial heating technology. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and thermochemotherapy. Berlin, Heidelberg: Springer-Verlag; 1995:193-217.
112 Stauffer PR. Evolving technology for thermal therapy of cancer. Int J Hyperthermia. 2005;21(8):731-744.
113 Stauffer PR, Diederich CJ, Seegenschmiedt MH. Interstitial heating technologies. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and thermochemotherapy. Vol. 1. Biology, physiology and physics. Berlin, New York: Springer-Verlag; 1995:279-320.
114 Stauffer PR, Diederich CJ, Pouliot J. Thermal therapy for cancer. In: Thomadsen B, Rivard M, Butler W, editors. Brachytherapy Physics. 2nd ed. Joint AAPM/ABS Summer School; 2005:901-932. Med Phys Monograph No. 31
115 Rhee JG, Lee CK, Osborn J, et al. Precooling prevents overheating of subcutaneous fat in the use of RF capacitive heating. Int J Radiat Oncol Biol Phys. 1991;20(5):1009-1015.
116 Kroeze H, van de Kamer JB, de Leeuw AA, et al. Treatment planning for capacitive regional hyperthermia. Int J Hyperthermia. 2003;19(1):58-73.
117 Ohguri T, Imada H, Yahara K, et al. Radiotherapy with 8-MHz radiofrequency-capacitive regional hyperthermia for stage III non-small-cell lung cancer. The radiofrequency-output power correlates with the intraesophageal temperature and clinical outcomes. Int J Radiat Oncol Biol Phys. 2009;73(1):128-135.
118 Maehara Y, Kuwano H, Kitamura K, et al. Hyperthermochemoradiotherapy for esophageal cancer (review). Anticancer Res. 1992;12(3):805-810.
119 Nadobny J, Wlodarczyk W, Westhoff L, et al. Development and evaluation of a three-dimensional hyperthermia applicator with Water-COated Antennas (WACOA). Med Phys. 2003;30(8):2052-2064.
120 Turner PF. Regional hyperthermia with an annular phased array. IEEE Trans Biomed Eng. 1984;31:106-114.
121 van Dijk JDP, Schneider C, van Os R, et al. Results of deep body hyperthermia with large waveguide radiators. Adv Exp Med Biol. 1990;267:315-319.
122 Van Es CA, Wyrdeman HK, de Leeuw AA, et al. Regional hyperthermia of pelvic tumours using the Utrecht “Coaxial TEM” system. A feasibility study. Int J Hyperthermia. 1995;11(2):173-186.
123 Dewhirst MW, Samulski TV. Hyperthermia in the treatment of cancer. (Current Concepts Monograph.). Michigan, Upjohn, Kalamazoo, 1988..
124 Samulski TV, Grant WJ, Oleson JR, et al. Clinical experience with a multi-element ultrasonic hyperthermia system. Analysis of treatment temperatures. Int J Hyperthermia. 1990;6(5):909-922.
125 Moros EG, Fan X, Straube WL. Experimental assessment of power and temperature penetration depth control with a dual frequency ultrasonic system. Med Phys. 1999;26(5):810-817.
126 Novak P, Penagaricano JA, Nahirnyak V, et al. Influence of the SURLAS applicator on radiation dose distributions during simultaneous thermoradiotherapy with helical tomotherapy. Phys Med Biol. 2008;53(10):2509-2522.
127 Hynynen K, Roemer R, Anhalt D, et al. A scanned, focused, multiple transducer ultrasonic system for localized hyperthermia treatments. Int J Hyperthermia. 1987;3(1):21-35.
128 Hynynen K, McDannold N. MRI guided and monitored focused ultrasound thermal ablation methods: a review of progress. Int J Hyperthermia. 2004;20(7):725-737.
129 Cetas TC. Thermometry. In: Field SB, Hand JW, editors. An introduction to the practical aspects of clinical hyperthermia. London, New York: Taylor & Francis; 1990:423-477.
130 Dewhirst MW, Phillips TL, Samulski TV, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia. Int J Radiat Oncol Biol Phys. 1990;18(5):1249-1259.
131 Dewhirst MW. Thermal dosimetry. In: Seegenschmiedt M, Fessenden P, Vernon CC, editors. Thermoradiotherapy and thermochemotherapy. Berlin: Springer-Verlag; 1995:123-128.
132 Edelstein-Keshet L, Dewhirst MW, Oleson JR, Samulski TV. Characterization of tumour temperature distributions in hyperthermia based on assumed mathematical forms. Int J Hyperthermia. 1989;5(6):757-777.
133 Dewhirst MW, Winget JM, Edelstein-Keshet L, et al. Clinical application of thermal isoeffect dose. Int J Hyperthermia. 1987;3(4):307-318.
134 Tilly W, Wust P, Rau B, et al. Temperature data and specific absorption rates in pelvic tumours: predictive factors and correlations. Int J Hyperthermia. 2001;17(2):172-188.
135 Wust P, Gellermann J, Harder C, et al. Rationale for using invasive thermometry for regional hyperthermia of pelvic tumors. Int J Radiat Oncol Biol Phys. 1998;41(5):1129-1137.
136 Waterman FM, Dewhirst MW, Fessenden P, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia administered by ultrasound. Int J Radiat Oncol Biol Phys. 1991;20(5):1099-1107.
137 Emami B, Stauffer P, Dewhirst MW, et al. RTOG quality assurance guidelines for interstitial hyperthermia. Int J Radiat Oncol Biol Phys. 1991;20(5):1117-1124.
138 Sapozink MD, Corry PM, Kapp DS, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia for deep-seated malignancy. Int J Radiat Oncol Biol Phys. 1991;20(5):1109-1115.
139 Hand JW, Machin D, Vernon CC, Whaley JB. Analysis of thermal parameters obtained during phase III trials of hyperthermia as an adjunct to radiotherapy in the treatment of breast carcinoma. Int J Hyperthermia. 1997;13(4):343-364.
140 Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23(13):3079-3085.
141 Kapp DS, Cox RS. Thermal treatment parameters are most predictive of outcome in patients with single tumor nodules per treatment field in recurrent adenocarcinoma of the breast. Int J Radiat Oncol Biol Phys. 1995;33(4):887-899.
142 Oleson JR, Samulski TV, Leopold KA, et al. Sensitivity of hyperthermia trial outcomes to temperature and time: implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys. 1993;25(2):289-297.
143 Seegenschmiedt MH, Martus P, Fietkau R, et al. Multivariate analysis of prognostic parameters using interstitial thermoradiotherapy (IHT-IRT). Tumor and treatment variables predict outcome. Int J Radiat Oncol Biol Phys. 1994;29(5):1049-1063.
144 Sherar M, Liu FF, Pintilie M, et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: data from a phase III trial. Int J Radiat Oncol Biol Phys. 1997;39(2):371-380.
145 Cetas TC. Practical thermometry with a thermographic camera—calibration, transmittance, and emittance measurements. Rev Sci Instrum. 1978;49(2):245-254.
146 Arunachalam K, Maccarini P, Juang T, et al. Performance evaluation of a conformal thermal monitoring sheet sensor array for measurement of surface temperature distributions during superficial hyperthermia treatments. Int J Hyperthermia. 2008;24(4):313-325.
147 Arunachalam K, Maccarini PF, Stauffer PR. A thermal monitoring sheet with low influence from adjacent waterbolus for tissue surface thermometry during clinical hyperthermia. IEEE Trans Biomed Eng. 2008;55(10):2397-2406.
148 Gautherie M, editor. Thermal dosimetry and treatment planning. Berlin, Heidelberg: Springer-Verlag, 1990.
149 Paulsen KD, Moskowitz MJ, Ryan TP, et al. Initial in vivo experience with EIT as a thermal estimator during hyperthermia. Int J Hyperthermia. 1996;12(5):573-591. discussion Int J Hyperthermia 12(5):593-594, 1996
150 Arthur RM, Straube WL, Trobaugh JW, Moros EG. In vivo change in ultrasonic backscattered energy with temperature in motion-compensated images. Int J Hyperthermia. 2008;24(5):389-398.
151 Trobaugh JW, Arthur RM, Straube WL, Moros EG. A simulation model for ultrasonic temperature imaging using change in backscattered energy. Ultrasound Med Biol. 2008;34(2):289-298.
152 Meaney PM, Zhou T, Fanning MW, et al. Microwave thermal imaging of scanned focused ultrasound heating. Phantom results. Int J Hyperthermia. 2008;24(7):523-536.
153 Arunachalam K, Stauffer PR, Maccarini PF, et al. Characterization of a digital microwave radiometry system for noninvasive thermometry using a temperature-controlled homogeneous test load. Phys Med Biol. 2008;53(14):3883-3901.
154 Bardati F, Brown VJ, Tognolatti P. Temperature reconstructions in a dielectric cylinder by multi-frequency microwave radiometry. J Electromagn Waves Appl. 1993;7(11):1549-1571.
155 Dubois L, Sozanski JP, Tessier V, et al. Temperature control and thermal dosimetry by microwave radiometry in hyperthermia. IEEE Trans Microwave Theory Tech. 1996;44(10, Part.2):1755-1761.
156 Hand JW, Van Leeuwen GMJ, Mizushina S, et al. Monitoring of deep brain temperature in infants using multi-frequency microwave radiometry and thermal modelling. Phys Med Biol. 2001;46(7):1885-1903.
157 Jacobsen S, Stauffer P. Non-invasive temperature profile estimation in a lossy medium based on multi-band radiometric signals sensed by a microwave dual-purpose body-contacting antenna. Int J Hyperthermia. 2002;18(2):86-103.
158 Jacobsen S, Stauffer PR. Can we settle with single-band radiometric temperature monitoring during hyperthermia treatment of chestwall recurrence of breast cancer using a dual-mode transceiving applicator? Phys Med Biol. 2007;52(4):911-928.
159 Mizushina S, Shimizu T, Suzuki K, et al. Retrieval of temperature-depth profiles in biological objects from multi-frequency microwave radiometric data. J Electromagn Waves Appl. 1993;7(11):1515-1548.
160 Gellermann J, Wlodarczyk W, Ganter H, et al. A practical approach to thermography in a hyperthermia/magnetic resonance hybrid system. Validation in a heterogeneous phantom. Int J Radiat Oncol Biol Phys. 2005;61(1):267-277.
161 MacFall J, Soher B. From the RSNA refresher courses. MR imaging in hyperthermia. Radiographics. 2007;27:1809-1818.
162 McDannold N. Quantitative MRI-based temperature mapping based on the proton resonant frequency shift. Review of validation studies. Int J Hyperthermia. 2005;21(6):533-546.
163 Soher B, Wyatt C, Reeder S, MacFall J. Noninvasive temperature mapping with MRI using chemical shift water-fat separation. Magn Res Med. 2010;63(5):1238-1246.
164 Wyatt C, Soher B, Maccarini P, et al. Hyperthermia MRI temperature measurement. Evaluation of measurement stabilisation strategies for extremity and breast tumours. Int J Hyperthermia. 2009;25(6):422-433.
165 Craciunescu OI, Stauffer PR, Soher BJ, et al. Accuracy of real time noninvasive temperature measurements using magnetic resonance thermal imaging in patients treated for high grade extremity soft tissue sarcomas. Med Phys. 2009;36(11):4848-4858.
166 Gellermann J, Weihrauch M, Cho CH, et al. Comparison of MR-thermography and planning calculations in phantoms. Med Phys. 2006;33(10):3912-3920.
167 Li Z, Vogel M, Maccarini P, et al. Towards the validation of a commercial hyperthermia treatment planning system (invited paper). Microwave J. 2008;51(12):28-42.
168 Li Z, Vogel M, Maccarini P, et al. Improved thermal planning and treatment control using SAR/temperature simulation and PRFS magnetic resonance thermal imaging. Int J Hyperthermia. 2011;27(1):86-89.
169 Cheng KS, Stakhursky V, Craciunescu OI, et al. Fast temperature optimization of multi-source hyperthermia applicators with reduced-order modeling of ‘virtual sources’. Phys Med Biol. 2008;53(6):1619-1635.
170 Hutchinson E, Dahleh M, Hynynen K. The feasibility of MRI feedback control for intracavitary phased array hyperthermia treatments. Int J Hyperthermia. 1998;14(1):39-56.
171 Stakhursky VL, Arabe O, Cheng KS, et al. Real-time MRI-guided hyperthermia treatment using a fast adaptive algorithm. Phys Med Biol. 2009;54(7):2131-2145.
172 Stauffer P, Craciunescu O, Maccarini P, et al. Clinical utility of magnetic resonance thermal imaging (mrti) for realtime guidance of deep hyperthermia. In: Ryan T, editor. Conference proceedings of the SPIE, 2009. Bellingham Washington: SPIE Press; 2009:7181OI-1-12.
173 Cline HE, Hynynen K, Schneider E, et al. Simultaneous magnetic resonance phase and magnitude temperature maps in muscle. Magn Reson Med. 1996;35(3):309-315.
174 McDannold N, Hynynen K. Quality assurance and system stability of a clinical MRI-guided focused ultrasound system. Four-year experience. Med Phys. 2006;33(11):4307-4313.
175 Kinsey AM, Diederich CJ, Rieke V, et al. Transurethral ultrasound applicators with dynamic multi-sector control for prostate thermal therapy. In vivo evaluation under MR guidance. Med Phys. 2008;35(5):2081-2093.
176 van Rhoon GC, Wust P. Introduction: non-invasive thermometry for thermotherapy. Int J Hyperthermia. 2005;21(6):489-495.
177 Gellerman J, Wlodarczyk W, Feussner A, et al. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperthermia. 2005;21(6):497-513.
178 Kuroda K. Non-invasive MR thermography using the water proton chemical shift. Int J Hyperthermia. 2005;21(6):547-560.
179 Rieke V, Butts PK. MR thermometry. J Magn Reson Imaging. 2008;27(2):376-390.
180 Meyer JL. The clinical efficacy of localized hyperthermia. Cancer Res. 1984;44(10 Suppl):4745s-4751s.
181 Dewhirst MW, Connor WG, Sim DA. Preliminary results of a phase III trial of spontaneous animal tumors to heat and/or radiation. Early normal tissue response and tumor volume influence on initial response. Int J Radiat Oncol Biol Phys. 1982;8(11):1951-1961.
182 Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17(1):1-18.
183 Issels RD, Abdel-Rahman S, Wendtner C, et al. Neoadjuvant chemotherapy combined with regional hyperthermia (RHT) for locally advanced primary or recurrent high-risk adult soft-tissue sarcomas (STS) of adults: long-term results of a phase II study. Eur J Cancer. 2001;37(13):1599-1608.
184 Sugimachi K, Kuwano H, Ide H, et al. Chemotherapy combined with or without hyperthermia for patients with oesophageal carcinoma. A prospective randomized trial. Int J Hyperthermia. 1994;10(4):485-493.
185 Issels R, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft tissue sarcoma. A randomised phase III multi-centre study of the EORTC-STBSG and the ESHO. Lancet Oncol. 2010;11(6):561-570.
186 Herman TS, Teicher BA. Sequencing of trimodality therapy [cis- diamminedichloroplatinum(II)/hyperthermia/radiation] as determined by tumor growth delay and tumor cell survival in the FSaIIC fibrosarcoma. Cancer Res. 1988;48(10):2693-2697.
187 Bornstein BA, Herman TS, Hansen JL, et al. Pilot study of local hyperthermia, radiation therapy, etanidazole, and cisplatin for advanced superficial tumours. Int J Hyperthermia. 1995;11(4):489-499.
188 Sugimachi K, Kitamura K, Baba K, et al. Hyperthermia combined with chemotherapy and irradiation for patients with carcinoma of the oesophagus—a prospective randomized trial. Int J Hyperthermia. 1992;8(3):289-295.
189 Jones EL, Samulski TV, Dewhirst MW, et al. A pilot Phase II trial of concurrent radiotherapy, chemotherapy, and hyperthermia for locally advanced cervical carcinoma. Cancer. 2003;98(2):277-282.
190 Westermann AM, Jones EL, Schem BC, et al. First results of triple-modality treatment combining radiotherapy, chemotherapy, and hyperthermia for the treatment of patients with stage IIB, III, and IVA cervical carcinoma. Cancer. 2005;104(4):763-770.
191 Rau B, Wust P, Gellermann J, et al. [Phase II study on preoperative radio-chemo-thermotherapy in locally advanced rectal carcinoma]. Strahlenther Onkol. 1998;174(11):556-565.
192 Sneed PK, Dewhirst MW, Samulski T, et al. Should interstitial thermometry be used for deep hyperthermia? [Editorial; comment.]. Int J Radiat Oncol Biol Phys. 1998;40(5):1015-1017.
193 van der Zee J, Peer-Valstar JN, Rietveld PJ, et al. Practical limitations of interstitial thermometry during deep hyperthermia [see comments]. Int J Radiat Oncol Biol Phys. 1998;40(5):1205-1212.
194 Lagendijk JJ, Van Rhoon GC, Hornsleth SN, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia. 1998;14(2):125-133.
195 Dewhirst MW, Griffin TW, Smith AR, et al. Intersociety Council on Radiation Oncology essay on the introduction of new medical treatments into practice. J Natl Cancer Inst. 1993;85(12):951-957.
196 Emami B, Scott C, Perez CA, et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors. A prospectively controlled randomized study by the Radiation Therapy Group. Int J Radiat Oncol Biol Phys. 1996;34(5):1097-1104.
197 Perez CA, Gillespie B, Pajak T, et al. Quality assurance problems in clinical hyperthermia and their impact on therapeutic outcome. A Report by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1989;16(3):551-558.
198 Perez CA, Pajak T, Emami B, et al. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the Radiation Therapy Oncology Group. Am J Clin Oncol. 1991;14(2):133-141.
199 Hand JW, Lagendijk JJ, Bach Andersen J, Bolomey JC. Quality assurance guidelines for ESHO protocols. Int J Hyperthermia. 1989;5(4):421-428.
200 Engin K, Tupchong L, Moylan DJ, et al. Randomized trial of one versus two adjuvant hyperthermia treatments per week in patients with superficial tumours. Int J Hyperthermia. 1993;9(3):327-340.
201 Kapp DS, Petersen IA, Cox RS, et al. Two or six hyperthermia treatments as an adjunct to radiation therapy yield similar tumor responses. Results of a randomized trial. Int J Radiat Oncol Biol Phys. 1990;19(6):1481-1495.
202 Thrall DE, Prescott DM, Samulski TV, et al. Radiation plus local hyperthermia versus radiation plus the combination of local and whole-body hyperthermia in canine sarcomas. Int J Radiat Oncol Biol Phys. 1996;34(5):1087-1096.
203 Dewhirst MW, Poulson JM, Yu D, et al. Relation between pO2, 31-P MRS parameters and treatment outcome in patients with high grade soft tissue sarcomas (STS) treated with thermoradiotherapy. Int J Radiat Oncol Biol Phys. 2005;61(2):480-491.
204 Thrall DE, LaRue SM, Yu D, et al. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res. 2005;11(14):5206-5214.
205 Vernon CC, Hand JW, Field SB, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys. 1996;35(4):731-744.
206 Datta NR, Bose AK, Kapoor HK, Gupta S. Head and neck cancers. Results of thermoradiotherapy versus radiotherapy. Int J Hyperthermia. 1990;6(3):479-486.
207 Valdagni R, Amichetti M, Pani G. Radical radiation alone versus radical radiation plus microwave hyperthermia for N3 (TNM-UICC) neck nodes. A prospective randomized clinical trial. Int J Radiat Oncol Biol Phys. 1988;15(1):13-24.
208 Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 1994;28(1):163-169.
209 Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40(2):287-295.
210 van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours. A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355(9210):1119-1125.
211 Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154-1161.
212 Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340(15):1137-1143.
213 Peters WA3rd, Liu PY, Barrett RJ2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606-1613.
214 Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144-1153.
215 Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes. A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17(5):1339-1348.
216 Colombo R, Da Pozzo LF, Salonia A, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol. 2003;21(23):4270-4276.
217 Allen TM, Cullis PR. Drug delivery systems. Entering the mainstream. Science. 2004;303(5665):1818-1822.
218 Mills JK, Needham D. The materials engineering of temperature-sensitive liposomes. Methods Enzymol. 2004;387:82-113.
219 Chilkoti A, Dreher MR, Meyer DE. Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv Drug Delivery Rev. 2002;54(8):1093-1111.
220 Liu XY, Ding XB, Guan Y, et al. Fabrication of temperature-sensitive imprinted polymer hydrogel. Macromol Biosci. 2004;4(4):412-415.
221 Kong G, Anyarambhatla G, Petros WP, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model. Importance of triggered drug release. Cancer Res. 2000;60(24):6950-6957.
222 Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia. Characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60(5):1197-1201.
223 Yarmolenko P, Zhao Y, Landon C, et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumors. Int J Hyperthermia. 2010;26(5):485-498.
224 Ponce AM, Viglianti BL, Yu D, et al. Magnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effects. J Natl Cancer Inst. 2007;99(1):53-63.
225 Viglianti BL, Abraham SA, Michelich CR, et al. In vivo monitoring of tissue pharmacokinetics of liposome/drug using MRI. Illustration of targeted delivery. Magn Reson Med. 2004;51(6):1153-1162.
226 Viglianti BL, Ponce AM, Michelich CR, et al. Chemodosimetry of in vivo tumor liposomal drug concentration using MRI. Magn Reson Med. 2006;56(5):1011-1018.
227 Roizin-Towle L, Pirro JP. The response of human and rodent cells to hyperthermia. Int J Radiat Oncol Biol Phys. 1991;20(4):751-756.
228 Repasky E, Issels R. Physiological consequences of hyperthermia. Heat, heat shock proteins and the immune response. Int J Hyperthermia. 2002;18(6):486-489.
* The original formulation was based on Arrhenius plots derived from rodent studies, which showed a slope below the breakpoint temperature (43° C) of 4 degrees. Historically, this is what has been used, even though the slope for human cells is nearer to 2 degrees below the breakpoint and the breakpoint is probably nearer to 43.5° C.