Chapter 49 Hypertension
Introduction
Hypertension is the leading risk factor for cardiovascular disease (CVD) and mortality worldwide,1 with a projected number of 1.56 billion individuals with hypertension by 2025.2
Hypertension has profound effects on both the structure and function of the vasculature in the eye. The retinal, choroidal, and optic nerve circulations undergo a range of pathophysiological changes in response to elevated blood pressure resulting in a spectrum of clinical signs known as hypertensive retinopathy, choroidopathy, and optic neuropathy, respectively.3 Hypertension is also a major risk factor for many other eye diseases, including the development and progression of diabetic retinopathy,4 retinal vein occlusion,5 retinal arterial macroaneurysm,6 and possibly age-related macular degeneration and glaucoma.3,7
Hypertensive retinopathy
Definition and classification
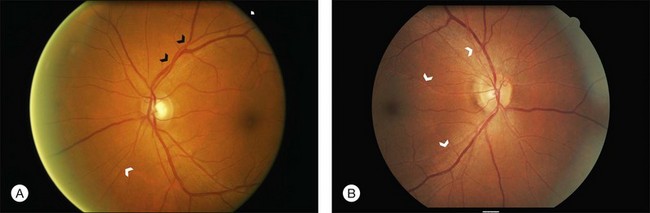
Retinopathy is the most common manifestation of hypertension which develops due to acute and/or chronic elevations in blood pressure. Hypertensive retinopathy is broadly divided into different stages.8 The initial response to elevated blood pressure is vasospasm and an increase in vasomotor tone, with consequent narrowing of retinal arterioles to control for optimal blood volume (“vasoconstrictive” phase). This stage is seen clinically as generalized or diffuse retinal arteriolar narrowing.
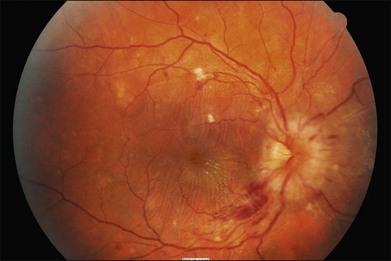
Very severe hypertension (i.e. “malignant hypertension” phase) may lead to optic disc swelling which may reflect underlying hypertensive encephalopathy with raised intracranial pressure.3,7–9
The above phases of hypertensive retinopathy are not always sequential. For example, in patients with acutely raised blood pressure, signs of retinopathy reflecting the “exudative” stage (e.g. retinal hemorrhage) may be present without features of the “sclerotic” stage (e.g. arteriovenous nicking). Furthermore, elevated blood pressure does not fully explain all the pathophysiological mechanisms of hypertensive retinopathy. Other processes involved in the pathogenesis of hypertensive retinopathy signs include inflammation,10 endothelial dysfunction,11 abnormal angiogenesis,12 and oxidative stress.13 In fact, hypertensive retinopathy signs are detected frequently in persons without a known history of hypertension.14
There have been many different classifications for hypertensive retinopathy. Traditionally, the Keith–Wagener–Baker system classifies patients with hypertension into four groups of increasing severity.15 However, it is difficult to distinguish early retinopathy grades (e.g. group 1 signs are not easily distinguished from group 2 signs).9,16 A simplified classification of hypertensive retinopathy based on prognosis of different signs from recent population-based data has been proposed9:
2. Mild: Generalized arteriolar narrowing, focal arteriolar narrowing, arteriovenous nicking, arteriolar wall opacification (silver or copper wiring), or a combination of these signs (Fig. 49.1).
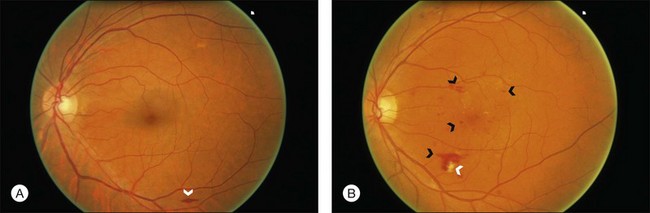
3. Moderate: Hemorrhages (blot, dot, or flame-shaped), microaneurysms, cotton-wool spots, hard exudates, or a combination of these signs (Fig. 49.2).
4. Malignant: Signs of moderate retinopathy in combination with optic disc swelling, in the presence of severely elevated blood pressure (Fig. 49.3).
Recently, the application of digital retinal photography and imaging software has allowed measurements of retinal vessel widths to quantify generalized arteriolar narrowing objectively.17,18 Studies using such methods show that generalized retinal arteriolar narrowing is strongly related to blood pressure and risk of hypertension.19,20 There is also evidence that retinal venular diameter may convey independent prognostic information.21 However, the measurement of retinal vessel width using these methods require specialized computer software and trained technicians and is thus not yet widely available for clinical use.
It has been argued that the clinical assessment of hypertensive retinopathy signs is of limited additional value in the management of patients with hypertension.22 Most international hypertension management guidelines, however, including those of the US Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), the British Society of Hypertension and the European Society of Hypertension (ESH), and the European Society of Cardiology (ESC),23–25 still emphasize that hypertensive retinopathy, with left ventricular hypertrophy and renal impairment, is an indicator of target organ damage, and that its presence should be an indication for a more aggressive approach in managing these hypertensive patients.24 Whether the retinal examinations should be performed by physicians using the direct ophthalmoscope, by ophthalmologists, or via standardized assessment using digital retinal photography remains unclear.
Epidemiology
In the past 30 years, epidemiological studies that have used retinal photography and standardized assessment methods to document and define hypertensive retinopathy have contributed to a greater understanding of the epidemiology, risk factors, and systemic associations of hypertensive retinopathy signs in the general population with different racial samples.26
With the exception of optic disc swelling, hypertensive retinopathy signs are generally common in persons 40 years of age or older, even in the absence of diabetes mellitus, with prevalence ranges from 2 to 17%.27–33 These studies also demonstrate that hypertensive retinopathy signs increase with age, and may vary by race/ethnicity (Chinese have a higher prevalence of hypertensive retinopathy than Caucasian whites) and possibly gender (men have higher rates than women).
While it is well established that hypertensive retinopathy signs are strongly correlated with blood pressure levels,26,34,35 new epidemiological studies show three particularly interesting features. First, there is now good evidence that some signs, particularly generalized retinal arteriolar narrowing, may precede the development of hypertension.19,20,36 In some studies, normotensive persons with this sign were more likely to develop hypertension and, among those with mild hypertension, were more likely to develop the severe stages of hypertension.37 Thus, generalized retinal arteriolar narrowing, possibly reflecting more widespread systemic peripheral vasoconstriction, may be an early preclinical marker of hypertension.
Second, new studies in children have demonstrated that the association between retinal arteriolar narrowing and elevated blood pressure can be observed even in children as young as 4–5 years of age. These findings suggest that the impact of elevated blood pressure on the retinal microcirculation occurs in early life,38,39 which may then “track” through to adulthood, even before the onset of overt hypertension.
Third, there is now evidence to show that the patterns of associations of specific retinopathy signs vary with current and past blood pressure levels. Generalized retinal arteriolar narrowing and arteriovenous nicking, for example, are related not only to current blood pressure levels, but also to blood pressure levels measured in the past, suggesting these two retinal signs reflect the cumulative effects of long-standing hypertension and are persistent markers of chronic hypertensive damage. In contrast, focal arteriolar narrowing, retinal hemorrhages, microaneurysms and cotton-wools spots are related only to concurrently measured blood pressure, mirroring the effects of short-term blood pressure changes.35
Finally, retinal venular diameter, not traditionally considered part of the spectrum of hypertensive retinopathy signs, may convey additional information regarding the state of the retinal vasculature and systemic health. Studies found that retinal venular widening or dilation is also related to elevated blood pressure levels,20,21,34,40 suggesting that the venule may exhibit different optimal flow characteristics across the vascular network compared with arterioles in the presence of hypertension.41 Whether retinal venular dilation should be included as part of the classification of hypertensive retinopathy remains unclear at this time.
Relationship with stroke
In one large multicenter US study, middle-aged, generally healthy persons with moderate hypertensive retinopathy signs were more likely to have subclinical MRI-defined cerebral infarction, cerebral white matter lesions, and cerebral atrophy than those without these signs.42–45 Furthermore, persons with moderate hypertensive signs at baseline were more likely to develop an incident clinical stroke,46 incident lacunar stroke,47 cognitive impairment,48 and cognitive decline49 than persons without these signs, even controlling for traditional risk factors. Another large cohort study based in Rotterdam, Netherlands, have further reported associations of larger retinal venular diameter with incidence of hemorrhagic stroke and the development of dementia.50,51
Some recent studies further demonstrated that hypertensive retinopathy may allow further refinement and subtyping of stroke. In a multicenter study of patients with acute stroke, different hypertensive retinopathy signs were associated with specific stroke subtypes.52 For example, retinal arteriolar narrowing was associated with lacunar stroke, while retinal hemorrhages were linked with cerebral hemorrhages. These findings suggest that hypertensive signs reflect specific cerebral microvasculopathy and may further help to understand the underlying pathologic mechanisms.47,52–54
Relationship with coronary heart disease
The presence of hypertensive retinopathy signs is associated with multiple markers of subclinical atherosclerotic diseases, including coronary artery calcification,55 aortic stiffness,56 left ventricular hypertrophy,57 and carotid intima-media thickness.58 There is also evidence that hypertensive retinopathy signs are predictive of clinical coronary artery disease events and congestive heart failure; however, the results of these studies show less consistent associations than with stroke.59–61 In one study, persons with moderate hypertensive retinopathy were three times more likely to develop congestive heart failure than those without retinopathy, while controlling for the presence of other cardiovascular risk factors.62
Hypertensive retinopathy has also been associated with increased risk of CVD mortality, stroke mortality, and coronary heart disease mortality.14,63,64 In one study, persons with moderate hypertensive retinopathy were more likely to die from coronary heart disease than persons without this sign, with an equivalent risk similar to that of diabetes.63 These data suggest that hypertensive retinopathy may convey additional prognostic information than other risk measures of CVD.
Relationship with other end-organ damage of hypertension
The significance of hypertensive retinopathy signs as risk indicators has long been recognized in patients with renal disease.65 Retinopathy signs have also been associated with other indicators of hypertensive target organ damage, such as microalbuminuria and renal impairment.66,67 Such association was independent of blood pressure, diabetes, and other risk factors, and was also seen in persons without diabetes or hypertension. Furthermore, hypertensive retinopathy was correlated with left ventricular hypertrophy, even in patients with mild-to-moderate hypertensive retinopathy, suggesting that its presence is an indicator of other target organ damage.68–70
Hypertensive choroidopathy
Hypertensive choroidopathy is less well recognized compared with hypertensive retinopathy. The underlying mechanism of hypertensive choroidopathy is related to choroidal ischemia which has effects on the retinal pigment epithelium and retina. Like the retinal vessels, the choroidal vessels may also undergo fibrinoid necrosis at the level of the choroidal capillaries in the presence of elevated blood pressure, leading to hypertensive choroidopathy signs that include Elschnig spots (round, deep, and gray-yellow patches at the level of the retinal pigment epithelium) and Siegrist streaks (linear hyperpigmented streaks along choroidal arteries). In severe cases, there may also be serous retinal detachment which can lead to vision loss.71–73
Hypertensive optic neuropathy
Bilateral optic disc swelling or papilloedema is commonly caused by accelerated or malignant hypertension, representing the “malignant hypertensive retinopathy” stage in the above classification. The pathogenesis of optic disc swelling secondary to accelerated hypertension remains controversial. Ischemia, raised intracranial pressure and hypertensive encephalopathy are all possible mechanisms that can result in papilloedema.73 Bilateral disc swelling is strongly correlated with CVD risk and mortality,14,15,64 and these patients need urgent antihypertensive management.9
Future directions
There are several areas of research in the field of hypertensive retinopathy. First, advances in digital retinal imaging and computer software analysis have provided the opportunity to quantify and monitor hypertensive retinopathy signs in a more objective manner. In addition to the measurement of retinal vascular caliber used in previous studies, new research has identified a number of other retinal vascular features, such as branching angles, bifurcation, fractal dimension, tortuosity, vascular length-to-diameter ratio, and wall-to-lumen ratio, that may also be related to hypertension.74–78 These newer, quantitatively measured retinal vascular changes may offer increasingly accurate and reliable parameters reflecting early and subtle retinal vascular abnormalities, which potentially provide additional predictive value of CVD risk outcomes.
Second, genetic epidemiology studies have provided clues to new vascular pathophysiological processes linked to hypertensive retinopathy signs.40 For example, a recent population-based genome-wide association study demonstrated four novel loci associated with retinal venular caliber, an endophenotype of the microcirculation associated with clinical CVD.79 These genetic studies may allow understanding of the contribution and biological mechanisms of microcirculatory changes that underlie CVD.
Retinal vasculature assessment also allows the study of new therapies for hypertension. Studies have demonstrated regression of hypertensive retinopathy signs in response to blood pressure reduction and that regression patterns are different in response to different antihypertensive regimens (e.g., angiotensin-converting enzyme inhibitors appear to have a more favorable effect on the retinal vasculature).80–82 Further prospective controlled trials are required to clarify whether specific reduction of hypertensive retinopathy also reduces the morbidity and mortality associated with CVD.
1 Lawes CM, Vander HS, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371(9623):1513–1518.
2 Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223.
3 Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369(9559):425–435.
4 Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136.
5 Wong TY, Scott IU. Clinical practice. Retinal-vein occlusion N Engl J Med. 2010;363(22):2135–2144.
6 Panton RW, Goldberg MF, Farber MD. Retinal arterial macroaneurysms: risk factors and natural history. Br J Ophthalmol. 1990;74:595–600.
7 Bhargava M, Ikram MK, Wong TY. How does hypertension affect your eyes? J Hum Hypertens. 2011.
8 Tso MO, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89(10):1132–1145.
9 Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351(22):2310–2317.
10 Klein R, Sharrett AR, Klein BE, et al. Are retinal arteriolar abnormalities related to atherosclerosis?: The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20(6):1644–1650.
11 Delles C, Michelson G, Harazny J, et al. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke. 2004;35(6):1289–1293.
12 Tsai WC, Li YH, Huang YY, et al. Plasma vascular endothelial growth factor as a marker for early vascular damage in hypertension. Clin Sci (Lond). 2005;109(1):39–43.
13 Coban E, Alkan E, Altuntas S, et al. Serum ferritin levels correlate with hypertensive retinopathy. Med Sci Monit. 2010;16(2):CR92–CR95.
14 Wong TY, Klein R, Klein BE, et al. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46(1):59–80.
15 Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1939;197(3):332–343.
16 Dodson PM, Lip GY, Eames SM, et al. Hypertensive retinopathy: a review of existing classification systems and a suggestion for a simplified grading system. J Hum Hypertens. 1996;10(2):93–98.
17 Wong TY, Knudtson MD, Klein R, et al. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111(6):1183–1190.
18 Cheung CY, Hsu W, Lee ML, et al. A new method to measure peripheral retinal vascular caliber over an extended area. Microcirculation. 2010;17(7):495–503.
19 Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140(4):248–255.
20 Ikram MK, Witteman JC, Vingerling JR, et al. Retinal vessel diameters and risk of hypertension: the Rotterdam Study. Hypertension. 2006;47(2):189–194.
21 Wong TY, Kamineni A, Klein R, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med. 2006;166(21):2388–2394.
22 van den Born BJ, Hulsman CA, Hoekstra JB, et al. Value of routine funduscopy in patients with hypertension: systematic review. BMJ. 2005;331(7508):73.
23 Williams B, Poulter NR, Brown MJ, et al. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ. 2004;328(7440):634–640.
24 Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572.
25 Mansia G, De Backer G, Dominiczak A, et al. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press. 2007;16(3):135–232.
26 Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–2280.
27 Klein R. Retinopathy in a population-based study. Trans Am Ophthalmol Soc. 1992;90:561–594.
28 Jeganathan VS, Cheung N, Tay WT, et al. Prevalence and risk factors of retinopathy in an Asian population without diabetes: the Singapore Malay Eye Study. Arch Ophthalmol. 2010;128(1):40–45.
29 Peng XY, Wang FH, Liang YB, et al. Retinopathy in persons without diabetes: the Handan Eye Study. Ophthalmology. 2010;117(3):531–537. 537
30 Chao JR, Lai MY, Azen SP, et al. Retinopathy in persons without diabetes: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2007;48(9):4019–4025.
31 Wong TY, Liew G, Tapp RJ, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet. 2008;371(9614):736–743.
32 Ojaimi E, Nguyen TT, Klein R, et al. Retinopathy signs in people without diabetes: the multi-ethnic study of atherosclerosis. Ophthalmology. 2011;118(4):656–662.
33 Wong TY, Klein R, Duncan BB, et al. Racial differences in the prevalence of hypertensive retinopathy. Hypertension. 2003;41(5):1086–1091.
34 Cheung CY, Tay WT, Mitchell P, et al. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J Hypertens. 2011;29(7):1380–1391.
35 Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Br J Ophthalmol. 2002;86(9):1007–1013.
36 Klein R, Klein BE, Moss SE, et al. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2006;104:98–107.
37 Smith W, Wang JJ, Wong TY, et al. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: the Blue Mountains Eye Study. Hypertension. 2004;44(4):442–447.
38 Mitchell P, Cheung N, de Haseth K, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49(5):1156–1162.
39 Li LJ, Cheung CY, Liu Y, et al. Influence of blood pressure on retinal vascular caliber in young children. Ophthalmology. 2011;118(7):1459–1465.
40 Sun C, Wang JJ, Mackey DA, et al. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54(1):74–95.
41 Patton N, Aslam T, Macgillivray T, et al. Asymmetry of retinal arteriolar branch widths at junctions affects ability of formulae to predict trunk arteriolar widths. Invest Ophthalmol Vis Sci. 2006;47(4):1329–1333.
42 Kawasaki R, Cheung N, Mosley T, et al. Retinal microvascular signs and 10-year risk of cerebral atrophy: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2010;41(8):1826–1828.
43 Wong TY, Klein R, Sharrett AR, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288(1):67–74.
44 Cooper LS, Wong TY, Klein R, et al. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke. 2006;37(1):82–86.
45 Cheung N, Mosley T, Islam A, et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain. 2010;133(Pt 7):1987–1993.
46 Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–1140.
47 Yatsuya H, Folsom AR, Wong TY, et al. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke. 2010;41(7):1349–1355.
48 Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the Atherosclerosis Risk in Communities Study. Stroke. 2002;33(6):1487–1492.
49 Lesage SR, Mosley TH, Wong TY, et al. Retinal microvascular abnormalities and cognitive decline: the ARIC 14-year follow-up study. Neurology. 2009;73(11):862–868.
50 de Jong FJ, Schrijvers EM, Ikram MK, et al. Retinal vascular caliber and risk of dementia: the Rotterdam study. Neurology. 2011;76(9):816–821.
51 Wieberdink RG, Ikram MK, Koudstaal, et al. Retinal vascular calibers and the risk of intracerebral hemorrhage and cerebral infarction: the Rotterdam Study. Stroke. 2010;41(12):2757–2761.
52 Lindley RI, Wang JJ, Wong MC, et al. Retinal microvasculature in acute lacunar stroke: a cross-sectional study. Lancet Neurol. 2009;8(7):628–634.
53 Baker ML, Hand PJ, Wong TY, et al. Retinopathy and lobar intracerebral hemorrhage: insights into pathogenesis. Arch Neurol. 2010;67(10):1224–1230.
54 Baker ML, Hand PJ, Liew G, et al. Retinal microvascular signs may provide clues to the underlying vasculopathy in patients with deep intracerebral hemorrhage. Stroke. 2010;41(4):618–623.
55 Wong TY, Cheung N, Islam FM, et al. Relation of retinopathy to coronary artery calcification: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2008;167(1):51–58.
56 Cheung N, Sharrett AR, Klein R, et al. Aortic distensibility and retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension. 2007;50(4):617–622.
57 Cheung N, Bluemke DA, Klein R, et al. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2007;50(1):48–55.
58 Kawasaki R, Cheung N, Islam FM, et al. Is diabetic retinopathy related to subclinical cardiovascular disease? Ophthalmology. 2011;118(5):860–865.
59 Michelson EL, Morganroth J, Nichols CW, et al. Retinal arteriolar changes as an indicator of coronary artery disease. Arch Intern Med. 1979;139(10):1139–1141.
60 Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287(9):1153–1159.
61 Duncan BB, Wong TY, Tyroler HA, et al. Hypertensive retinopathy and incident coronary heart disease in high risk men. Br J Ophthalmol. 2002;86(9):1002–1006.
62 Wong TY, Rosamond W, Chang PP, et al. Retinopathy and risk of congestive heart failure. JAMA. 2005;293(1):63–69.
63 Liew G, Wong TY, Mitchell P, et al. Retinopathy predicts coronary heart disease mortality. Heart. 2009;95(5):391–394.
64 Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case–control study. Ophthalmology. 2003;110(5):933–940.
65 Gunn RM. Ophthalmoscopic evidence of (1) arterial changes associated with chronic renal diseases and (2) of increased arterial tension. Trans Ophthalmol Soc UK. 1982;12:124–125.
66 Saitoh M, Matsuo K, Nomoto S, et al. Relationship between left ventricular hypertrophy and renal and retinal damage in untreated patients with essential hypertension. Intern Med. 1998;37(7):576–580.
67 Wong TY, Coresh J, Klein R, et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol. 2004;15(9):2469–2476.
68 Kim GH, Youn HJ, Kang S, et al. Relation between grade II hypertensive retinopathy and coronary artery disease in treated essential hypertensives. Clin Exp Hypertens. 2010;32(7):469–473.
69 Cuspidi C, Meani S, Valerio C, et al. Prevalence and correlates of advanced retinopathy in a large selected hypertensive population. The Evaluation of Target Organ Damage in Hypertension (ETODH) study. Blood Press. 2005;14(1):25–31.
70 Tikellis G, Arnett DK, Skelton TN, et al. Retinal arteriolar narrowing and left ventricular hypertrophy in African Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am J Hypertens. 2008;21(3):352–359.
71 Luo BP, Brown GC. Update on the ocular manifestations of systemic arterial hypertension. Curr Opin Ophthalmol. 2004;15(3):203–210.
72 Bourke K, Patel MR, Prisant LM, et al. Hypertensive choroidopathy. J Clin Hypertens (Greenwich ). 2004;6(8):471–472.
73 Chatterjee S, Chattopadhyay S, Hope-Ross M, et al. Hypertension and the eye: changing perspectives. J Hum Hypertens. 2002;16(10):667–675.
74 Witt N, Wong TY, Hughes AD, et al. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47(5):975–981.
75 Cheung CY, Zheng Y, Hsu W, et al. Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology. 2011;118(5):812–818.
76 Liew G, Wang JJ, Cheung N, et al. The retinal vasculature as a fractal: methodology, reliability, and relationship to blood pressure. Ophthalmology. 2008;115(11):1951–1956.
77 Hughes AD, Martinez-Perez E, Jabbar AS, et al. Quantification of topological changes in retinal vascular architecture in essential and malignant hypertension. J Hypertens. 2006;24(5):889–894.
78 Ritt M, Schmieder RE. Wall-to-lumen ratio of retinal arterioles as a tool to assess vascular changes. Hypertension. 2009;54(2):384–387.
79 Ikram MK, Sim X, Jensen RA, et al. Four novel Loci (19q13, 6q24, 12q24, and 5q14) influence the microcirculation in vivo. PLoS Genet. 2010;6(10):e1001184.
80 Hughes AD, Stanton AV, Jabbar AS, et al. Effect of antihypertensive treatment on retinal microvascular changes in hypertension. J Hypertens. 2008;26(8):1703–1707.
81 Dahlof B, Stenkula S, Hansson L. Hypertensive retinal vascular changes: relationship to left ventricular hypertrophy and arteriolar changes before and after treatment. Blood Press. 1992;1(1):35–44.
82 Thom S, Stettler C, Stanton A, et al. Differential effects of antihypertensive treatment on the retinal microcirculation: an Anglo-Scandinavian cardiac outcomes trial substudy. Hypertension. 2009;54(2):405–408.