Hyperbaric oxygen therapy
Klaus D. Torp, MD, Neil G. Feinglass, MD, FASE, FCCP and Timothy S.J. Shine, MD
All gases follow fundamental gas laws:
Boyle’s law: A volume of gas is inversely proportional to pressure at a constant temperature:
< ?xml:namespace prefix = "mml" />
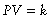
where P is absolute pressure, V is volume, and k is a constant representative of the pressure and volume of the system.
Dalton’s law: The total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases.
Henry’s law: At constant temperature, the amount of gas dissolved in a liquid is directly proportional to the partial pressure of that gas in equilibrium with the liquid.
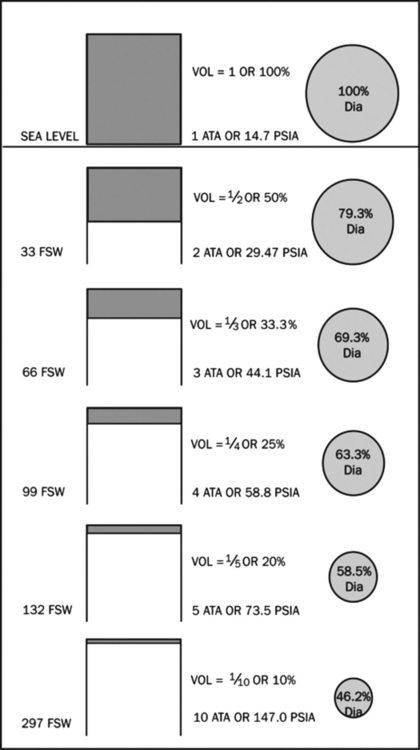
In clinical medicine, the liquid of interest is blood and the dissolved gas is O2. The driving pressure of O2 into blood is the partial pressure of O2 in alveoli (PAO2). Note that it is the partial pressure of O2 and not the percentage of O2 that is responsible for its effects (Table 224-1). As the PaO2 increases in arterial blood, the saturation of hemoglobin approaches 100% (at PaO2 ∼100 mm Hg). Above this level, all additional O2-carrying capacity of blood comes from the oxygen dissolved in the plasma. HBOT can therefore increase O2 content in the face of severe anemia and increase O2 delivery in areas of partial obstruction to blood flow. In addition, the increased barometric pressure can reduce intravascular air bubbles in patients with decompression sickness or air embolism, improving perfusion and increasing the removal of N from the blood (Figure 224-1).
Table 224-1
Expected Gas Tensions and Arterial Blood O2 Content at Various Ambient Pressures in a Normal Individual*
| Atm | FIO2 | Inspired PO2 (mm Hg) | PAO2 (mm Hg) | PaO2 (mm Hg) | CaO2 (mL/dL) | PaCO2 (mm Hg) | |
| Total | Dissolved | ||||||
| 1 | 0.21 | 150 | 102 | 87 | 18.7 | 0.3 | 40 |
| 1 | 1.0 | 713 | 673 | 572 | 21.2 | 1.7 | 40 |
| 2 | 1.0 | 1473 | 1433 | 1218 | 23.1 | 3.7 | 40 |
| 3 | 1.0 | 2233 | 2193 | 1864 | 25.1 | 5.6 | 40 |
| 6 | 0.21 | 898 | 848 | >750 | 21.8 | 2.3 | 40 |
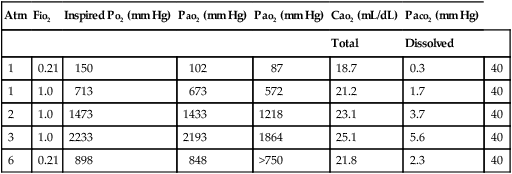
Modified, with permission, from Moon RE, Camporesi EM. Clinical care in altered environments: At high and low pressure and in space. In: Miller RD, ed. Anesthesia. 6th ed. Philadelphia: Churchill Livingstone; 2005:2665-2701.
The effectiveness of HBOT has been established for several indications, and the basic mechanisms for its effect on the body have been demonstrated. Table 224-2 lists conditions that are recommended for HBOT by the Undersea and Hyperbaric Medical Society, as well as those that are reimbursed by the Centers for Medicare and Medicaid Services. For any other indications, one should first examine how the basic mechanisms of HBOT (Box 224-1) will affect the underlying pathophysiology of the disease.
Table 224-2
Recommended (UHMS) and Reimbursed (CMS) Indications for Hyperbaric O2 Therapy
| Indication | UHMS | CMS |
| Air or gas embolism | X | X |
| Carbon monoxide poisoning | X | X |
| Carbon monoxide poisoning complicated by cyanide poisoning | X | X |
| Clostridial myositis and myonecrosis (gas gangrene) | X | X |
| Crush injury, compartment syndrome, and other acute traumatic ischemias | X | X |
| Decompression sickness | X | X |
| Enhancement of healing in selected problem wounds | X | |
| Exceptional blood loss (anemia) | X | |
| Intracranial abscess | X | |
| Necrotizing soft tissue infections | X | X |
| Osteomyelitis (refractory) | X | X |
| Delayed radiation injury (soft tissue and bony necrosis) | X | X |
| Skin grafts and flaps (compromised) | X | X |
| Thermal burns | X | |
| Acute peripheral arterial insufficiency | X | X |
| Refractory actinomycosis | X | |
| Diabetic wounds of the lower extremities in patients who meet certain criteria | X | |
| Idiopathic Sudden Sensorineural Hearing Loss | X | |
| Central Retinal Artery Occlusion | X |
CMS, Centers for Medicare and Medicaid Services; UHMS, Undersea and Hyperbaric Medical Society.





