Hydrocephalus
 Those primarily disturbing normal homeostatic control of CSF production and CSF flow or drainage.
Those primarily disturbing normal homeostatic control of CSF production and CSF flow or drainage.
 Those principally associated with developmental abnormalities or destructive lesions in the brain (Table 4.1).
Those principally associated with developmental abnormalities or destructive lesions in the brain (Table 4.1).
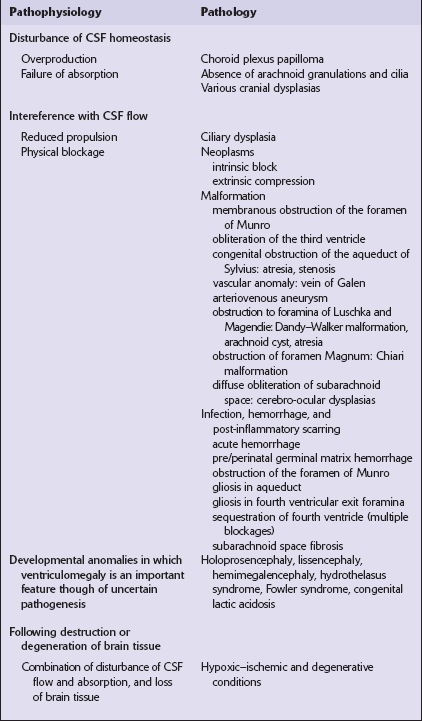
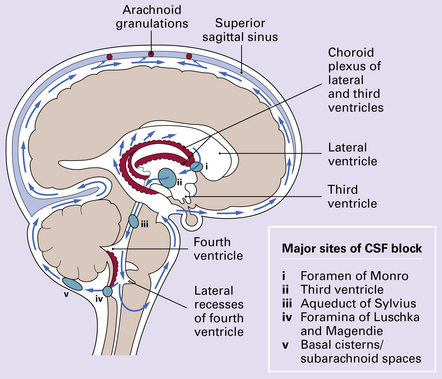
Overproduction of CSF by a choroid plexus papilloma (Fig. 4.1), reduced propulsion consequent upon primary ciliary dysplasia, and reduced absorption due to aplasia of arachnoid granulations or raised venous pressure due to bony dysplasia (Fig. 4.2) are rare causes of hydrocephalus. More commonly hydrocephalus results from interference with CSF flow at various points in the CSF pathway (Fig. 4.3).
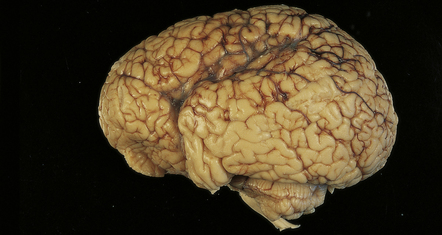
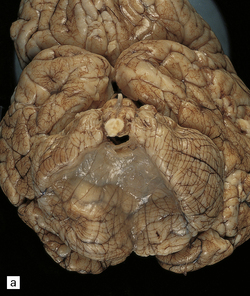
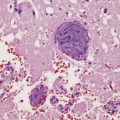
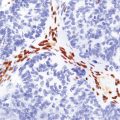
4.1 Choroid plexus papilloma.
These neoplasms may cause hydrocephalus by obstructing CSF flow or by oversecretion.
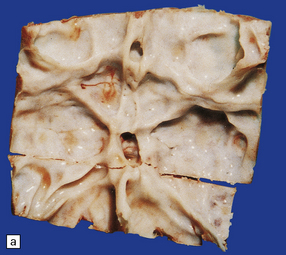
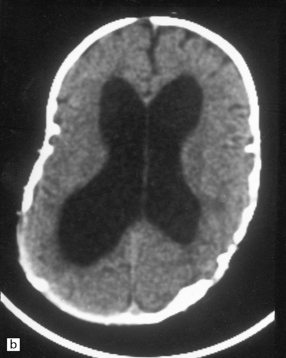
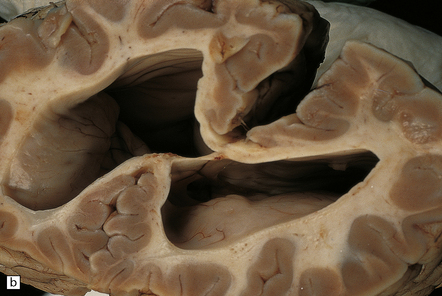
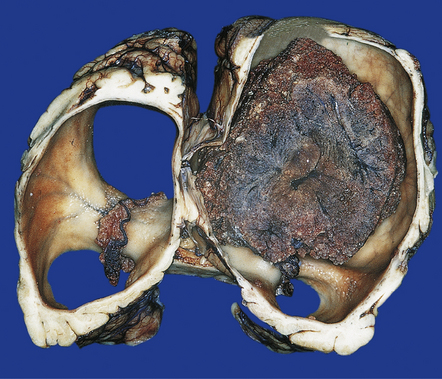
4.2 Hydrocephalus associated with bony dysplasia.
(a) Part of the skull base from an 8-year-old child who presented with hydrocephalus and chronic tonsillar herniation resulting from craniosynostosis, clover-leaf skull, and Klippel–Feil deformity. The bone is alternately thinned or massively thickened into irregular ridges and contains excessively narrow exit foramina, which compromise venous return and reduce CSF reabsorption, thereby causing hydrocephalus. (b) A computerized tomogram of the head of an 18-month-old infant with Crouzon syndrome shows synostosis, distortion of the skull bones, and hydrocephalus.
Many neoplasms can fill and block the ventricles or subarachnoid spaces, notably those growing close to narrow parts of the ventricular system. For example, the foramen of Monro may be blocked by a subependymal giant cell astrocytoma of tuberous sclerosis (Fig. 4.4) or a colloid cyst (see Chapters 34, 35, and 45). Otherwise, obstruction commonly occurs at the aqueduct and the fourth ventricular exit foramina.
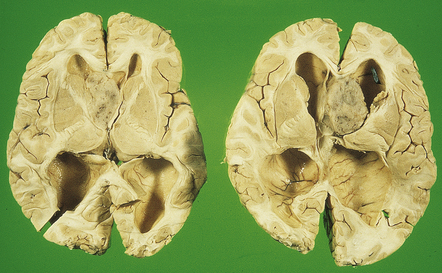
4.4 Obstruction of interventricular foramina of Monro.
In this case the obstruction is caused by a subependymal giant cell astrocytoma in tuberous sclerosis.
OBSTRUCTION OF THE AQUEDUCT OF SYLVIUS
MACROSCOPIC AND MICROSCOPIC APPEARANCES
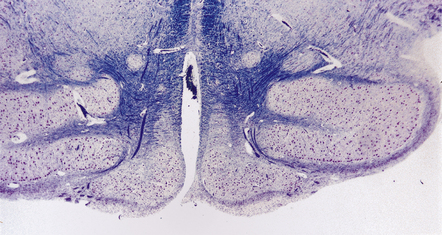
Stenosis may be sporadic, X-linked, or (rarely) autosomal recessive. It is characterized by a tiny lumen in the usual position, a normal ependymal lining, and an absence of gliosis (Fig. 4.5). Experimental and clinical evidence suggests that narrowing can be secondary to compression of the tectal plate by expanding hydrocephalic hemispheres.
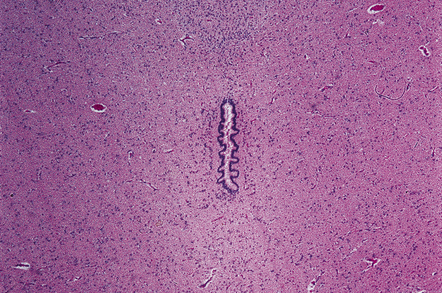
4.5 Aqueduct stenosis.
This is characterized by a very tiny lumen, intact ependymal lining, and normal surrounding midbrain parenchyma.
In atresia (forking) a normal channel is replaced by groups of ependymal cells, small rosettes, or tiny aqueductules irregularly scattered across the midbrain tegmentum. There is no gliosis (Fig. 4.6).
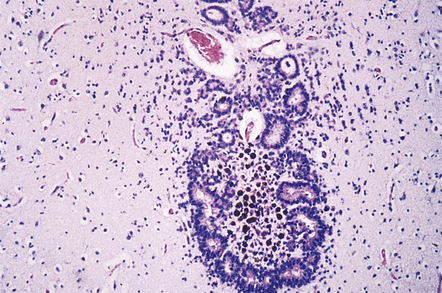
4.6 Aqueduct atresia.
This is characterized by the presence of many tiny canals or aqueductules scattered through the midbrain tegmentum in the absence of overt gliosis.
With gliosis, an outline of the aqueduct is still visible. An interrupted ring of ependymal cells, rosettes, and tubules are surrounded and filled by dense fibrillary gliosis, in which there may be one or two small central channels that lack an ependymal lining (Fig. 4.7).
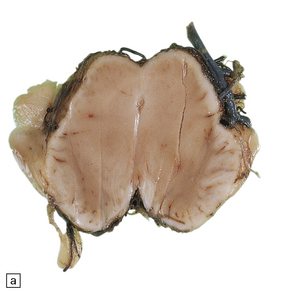
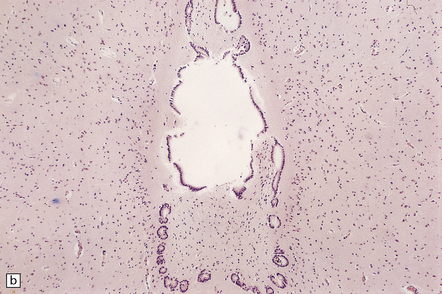
4.7 Aqueduct gliosis.
(a) Macroscopic view of the midbrain. Although the position of the aqueduct can be seen, it appears blocked. (b) Microscopically, the outline of the aqueduct is still visible as an interrupted ring of ependymal canals. Gliotic tissue enmeshes these tubules and fills the aqueductal lumen.
A septum is a rare variant of aqueductal gliosis. A thin translucent glial membrane is surrounded by a ring of ependymal tissue, which interrupts the aqueduct at its caudal end (Fig. 4.8).
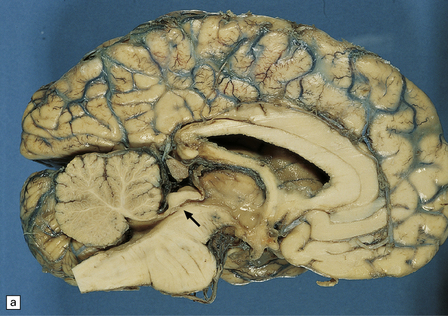
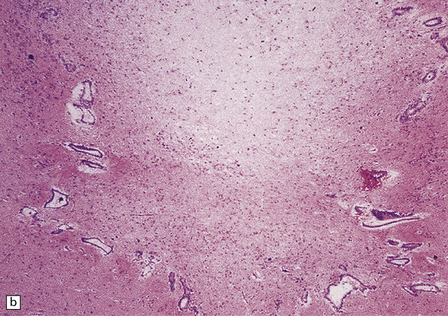
4.8 Septum of the aqueduct.
(a) A narrow band of white tissue occludes the lower end of the aqueduct (arrow) in this sagittal hemisection of the brain. (b) Microscopically, the similarity with aqueduct gliosis is striking. A tenuous glial membrane is stretched across the lumen of the Sylvian canal, outlined by a series of ependymal tubules separated and enmeshed by astroglial tissue.
In X-linked hydrocephalus ventricular dilatation may be present with or without aqueduct stenosis (Fig. 4.9). The cerebrum shows polygyria (excessive gyration with normal histology) (Fig. 4.10), which is a common consequence of hydrocephalus in early life. Absence of the medullary pyramids (Fig. 4.11) is almost invariable. Other findings may be fusion of the thalamic nuclei and agenesis of the corpus callosum.
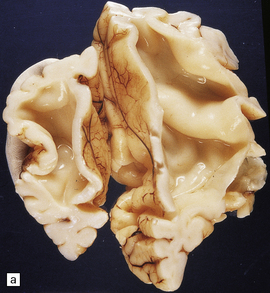
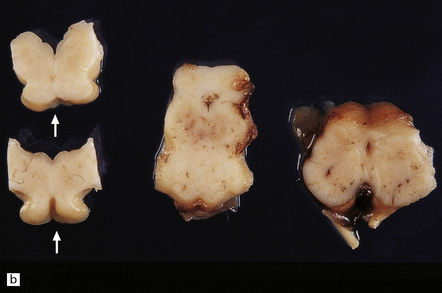
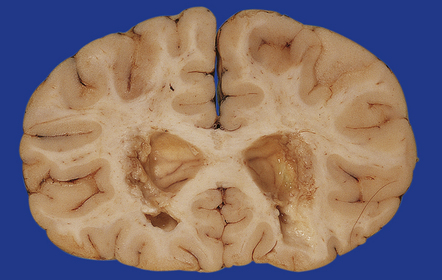
4.9 X-linked hydrocephalus.
(a) Coronal section through the frontal horns showing marked ventriculomegaly. (b) Horizontal section of the hindbrain. The aqueduct is widely patent, but the medullary pyramids are absent (arrows).
OBSTRUCTION OF THE FOURTH VENTRICULAR EXIT FORAMINA
Obstruction may result from Dandy–Walker syndrome, atresia, or arachnoid cysts.
With atresia, the cerebellar foramina are covered by thin glioependymal membranes (Fig. 4.12).
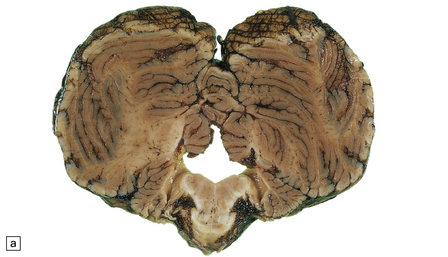
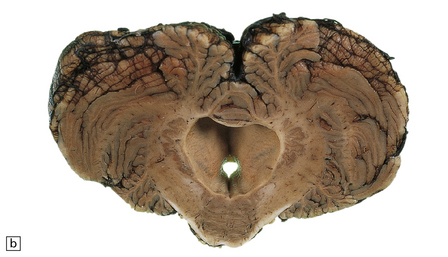
4.12 Atresia of the cerebellar exit foramina.
(a) At the level of the pontomedullary junction the lateral apertures are dilated and closed over by white membranes. (b) At the midpontine level there is marked dilatation of the fourth ventricle and its wall is studded with granular ‘ependymitis’.
Infratentorial arachnoid cysts can obstruct the exit foramina or compress the aqueduct and fourth ventricle (Fig. 4.13).
POST-INFLAMMATORY OR FIBROTIC OBSTRUCTION OF THE CSF
MACROSCOPIC AND MICROSCOPIC APPEARANCES
Intrauterine infection
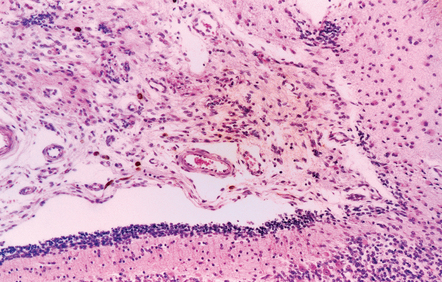
Congenital toxoplasmosis causes a destructive meningoencephalitis. Progressive ventriculomegaly results from parenchymal destruction, acute aqueduct block (by necrotic debris), ependymitis, and chronic gliosis (Fig. 4.14).
Neonatal infection
The exudate of a purulent leptomeningitis caused by Gram-negative enterobacteria, group B streptococci, Pseudomonas, or Candida may block the foramen of Monro, aqueduct, or fourth ventricular exit foramina. Later organization and scarring lead to gliotic occlusion at one or several of these sites (Fig. 4.15). Ventriculitis (Fig. 4.16), infarction, and abscess and cavity formation cause ventricular loculations and diverticula, which may distend and interfere with CSF flow. Chronic adhesive arachnoiditis may block the basal cisterns.
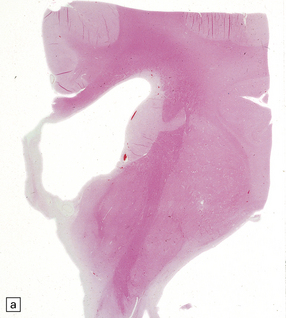
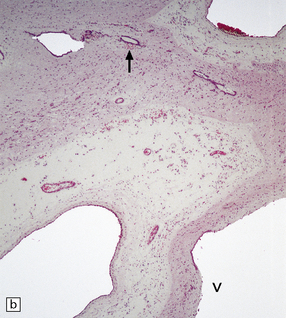
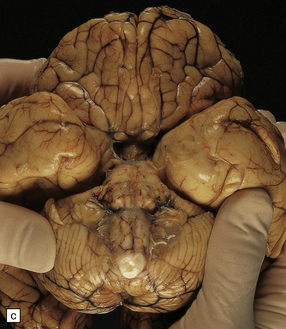
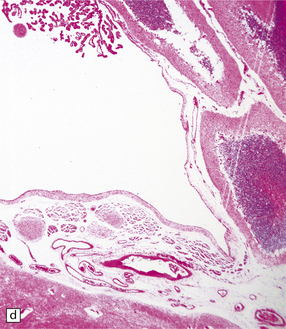
4.15 Post-inflammatory scarring resulting in hydrocephalus in an infant who had suffered neonatal meningitis.
Post-inflammatory scarring may interfere with CSF circulation at several levels. (a) Gliotic occlusion of the foramen of Monro. This low-power histologic section of frontal lobe shows that the foramen of Monro is walled over and the frontal horn is dilated. (b) Within the glial tissue occluding the foramen of Monro, ependymal remnants form canals (arrow). Note the interrupted ependymal lining of the ventricle (V). (c) Gliotic occlusion of the cerebellar foramina. The foramina of Luschka with choroid plexus are sealed over, and covered by membranous pouches which bulge when the cerebellum is compressed. (d) Microscopically, the lateral aperture of (c) is occluded by a delicate sheet of glioependymal tissue, which encloses choroid plexus.
Intraventricular hemorrhage
This can acutely block the aqueduct, foramen of Monro, and fourth ventricular outlets (Fig. 4.17). Later resolution of the hematoma and chronic gliosis may also compromise these foramina, while subarachnoid fibrosis is a common cause of obstructed basal cisterns or fourth ventricular exit foramina (Fig. 4.18).
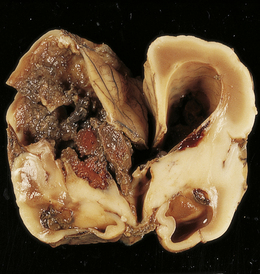
4.17 Dilatation of the right frontal horn following blockage of the foramen of Monro.
In this case the cause of the obstruction is an acute hematoma in a premature infant born at 26 weeks’ gestation. The hematoma resulted from an intraventricular (germinal matrix) hemorrhage and white matter hemorrhagic infarction.
Mucopolysaccharidosis
Hydrocephalus is associated not only with generalized brain atrophy in this condition, but also with blockage of CSF due to leptomeningeal fibrosis (Figs 4.19, 4.20).
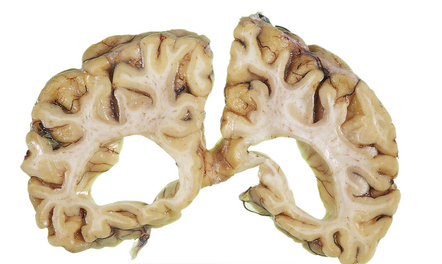
4.19 Marked ventricular dilatation complicating mucopolysaccharidosis.
Coronal slice through the brain of an 11-year-old boy with Sanfillipo A disease (mucopolysaccharidosis type IIIA).
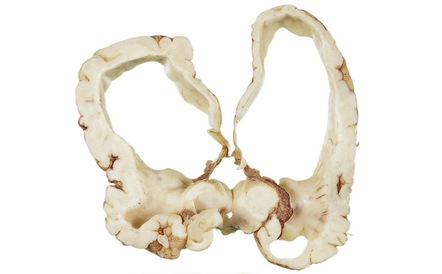
4.20 Congenital cerebral lactic acidosis resulting from pyruvate dehydrogenase deficiency.
A characteristic combination of malformations, including microcephaly, hydrocephalus, olivary heterotopia, and hypoplastic pyramidal tracts is produced. This is an example of one of the developmental disorders in which hydrocephalus is an invariable feature, although its cause remains obscure.
REFERENCES
Baker, D.W., Vinters, H.V. Hydrocephalus with cerebral aqueductal dysgenesis and craniofacial anomalies. Acta Neuropathol.. 1984;63:170–173.
Chahlavi, A., El-Babaa, S.K., Luciano, M.G. Adult-onset hydrocephalus. Neurosurg Clin N Am.. 2001;12:753–760.
Chow, C.H., Halliday, J.L., Anderson, RMcD, et al. Congenital absence of pyramids and its significance in genetic diseases. Acta Neuropathol. 1985;65:313–317.
Danzer, E., Johnson, M.P., Adzick, N.S. Fetal surgery for myelomeningocele: progress and perspectives. Dev Med Child Neurol.. 2012;54:8–14.
Del Bigio, M.R. Pathophysiologic consequences of hydrocephalus. Neurosurg Clin N Am.. 2001;12:639–649.
Friede, R.L. Developmental neuropathology. Berlin: Springer-Verlag; 1989.
Johnson, R.T., Johnson, K. Hydrocephalus following virus infection: the pathology of aqueductal stenosis developing after experimental mumps virus infection. J Neuropath Exp Neurol.. 1968;27:591–606.
Kenwrick, S., Watkins, A., De Angelis, E. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Hum Mol Genet.. 2000;9:879–886.
Masters, C., Alpers, M., Kakulas, B. Pathogenesis of Reovirus Type 1 hydrocephalus in mice. Significance of aqueductal changes. Arch Neurol.. 1977;34:18–28.
Russell, D., Observations on the pathology of hydrocephalus. MRC Special Report Series No. 265. London: HMSO; 1949.
Shaw, C.M., Alvord, E.C., Jr. Hydrocephalus. In: Duckett S., Malvern P.A., eds. Pediatric neuropathology. Baltimore: Williams and Wilkins; 1997:149–211.
Spataro, R.F., Lin, S.R., Horner, F.A., et al. Aqueductal stenosis and hydrocephalus; rare sequelae of mumps virus infection. Neuroradiology.. 1976;12:11–13.
Turnbull, I.M., Drake, C.G. Membranous occlusion of the aqueduct of Sylvius. J Neurol.. 1966;24:24–33.
Weller, R.O., Kida, S., Harding, B.N. Aetiology and pathology of hydrocephalus. In: Schurr P.H., Polkey C.E., eds. Hydrocephalus. Oxford: Oxford University Press; 1993:521–538.
Willems, P.J., Brouwer, O.F., Dijkstra, I., et al. X-linked hydrocephalus. Am J Med Genet.. 1987;27:921–928.
Williams, B. Is aqueduct stenosis a result of hydrocephalus? Brain.. 1973;96:399–412.