2
Human embryogenesis
Introduction
In obstetric practice, it is common to use 280 days (40 weeks) from the first day of the last menstrual period (LMP) to estimate the delivery date of a full-term pregnancy. This period has also become a convenient but inaccurate description of the number of weeks of ‘pregnancy’. This is an obvious source of confusion, since embryologists refer to the number of gestational days, i.e. days since fertilization, when discussing early human development. Since conception requires male and female gametes to be in the same place (the ampulla of the fallopian tube) at the same time (within a few hours of ovulation and within 6 days of intercourse), it can be approximated to:
![]() ~ 14 days after the first day of the LMP, i.e. an estimate of the day of ovulation in a woman with a regular 28-day cycle
~ 14 days after the first day of the LMP, i.e. an estimate of the day of ovulation in a woman with a regular 28-day cycle
![]() the day of ovulation as judged by a change in basal body temperature, serum luteinizing hormone (LH), and/or oestrogen-to-progesterone ratios
the day of ovulation as judged by a change in basal body temperature, serum luteinizing hormone (LH), and/or oestrogen-to-progesterone ratios
![]() 6 days prior to the rapid increase in chorionic gonadotrophin levels that is associated with implantation of the embryo.
6 days prior to the rapid increase in chorionic gonadotrophin levels that is associated with implantation of the embryo.
In most pregnancies, only the first of these options is available, and thus 8 weeks of ‘pregnancy’ implies 42 gestational days (GD).
Nomenclature
Embryology is cursed with a dense, classically-based nomenclature. Attempts at standardization have often led to the same structure having at least two different names (e.g. yolk sac and umbilical vesicle; branchial and pharyngeal arches) and/or the same structure changing its name at different stages of development (e.g. allantois → urachus → medial umbilical ligament). This complexity can be disheartening, so only the most up-to-date terms will be used and these will be related to the mature tissues, wherever possible. Some transient structures are important in understanding malformations and these will be highlighted.
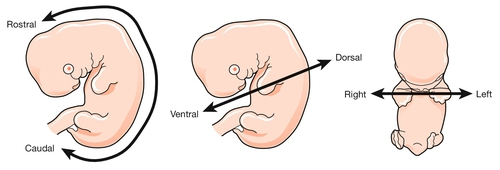
To start with the title of this chapter, the transition from embryonic to fetal life is at ~ 56 GD (i.e. ~ 10 weeks post-LMP). This is an arbitrary but useful boundary that is based on the fact that 90% of adult structures are recognizable at this stage. One of the most important concepts in embryogenesis is the establishment of the three main embryonic axes: (1) rostrocaudal (more correctly termed anteroposterior in early embryogenesis); (2) dorsoventral; (3) left–right (Box 2.1, Fig. 2.1).
Fertilization to implantation (0–6 GD)
In-vitro fertilization has greatly increased our understanding of the early cellular and molecular events in human embryogenesis (Fig. 2.2). Sadly for the male ego, the sperm has only three essential roles in embryogenesis:
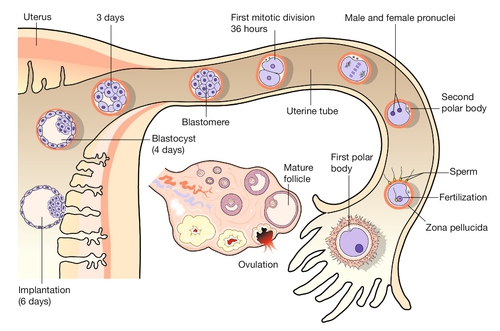
Fig. 2.2Fertilization occurs in the fallopian tube.
The embryo is then transported along the fallopian tube and implants in the uterine wall around 6 GD.
1. to stimulate a change in the zona pellucida that prevents further sperm entering the cell
2. to deliver a paternally imprinted haploid genome (see Box 2.2) in order to reconstitute a diploid chromosome number
3. to stimulate the second meiotic division in the egg with subsequent production of the second polar body.
In contrast to the sperm, the egg is a complex cell with many subcompartments, each with a critical role. At the most basic level, the egg carries a maternally imprinted haploid genome (Box 2.2). It also determines the orientation of at least one of the ‘axes’ in the early embryo (Box 2.1), and it provides all the RNA and protein synthesis requirements until the embryonic genome becomes transcriptionally active at 2–3 days post-fertilization.
The first mitotic division occurs 36 h after fertilization. The next four mitoses are at intervals of ~ 17 h and produce a ball of cells called a blastomere. After the fifth mitotic division, the blastomere becomes polarized as a sphere with a single-cell-layered wall (trophoblast) and an inner cell mass. The inner cell mass of this blastocyst contains the cells that will form the embryo itself. The 128 cells that are present following the seventh mitotic division still occupy the same volume as the initial fertilized egg, i.e. there has been no physical growth. The embryo sheds the zona pellucida at about this time, a process termed fancifully as ‘hatching’. Over these first 5 days, the embryo is transported along the fallopian tube and becomes attached to the uterine wall around 6 GD.
Implantation and formation of the germ layers (7–18 GD)
At implantation the trophoblast buries itself in the endometrium. The embryo thus gains access to the maternal circulation and behaves as an efficient paracytic organism, which enables a very rapid period of growth. The inner cell mass begins to differentiate and the embryo takes on the appearance of a disc consisting of two layers of morphologically distinct cells: the epiblast (the dorsal region) and the hypoblast (the ventral region, Fig. 2.3A). This is the first real evidence of embryonic polarity. The largest embryonic cavity at this stage is the umbilical vesicle, which is lined with hypoblast cells. The amniotic cavity begins to form by 9 GD and is lined with epiblast cells.
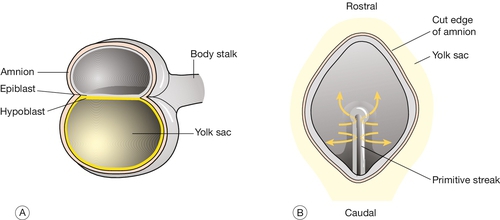
Fig. 2.3Formation of the germ layers.
(A) The inner cell mass takes on the appearance of a disc comprising two layers of morphologically distinct cells: the epiblast (the dorsal region) and the hypoblast (the ventral region). (B) Gastrulation is the process where cells from the epiblast migrate towards and through the primitive streak – a groove that forms in the caudal end of the disc – to form the mesoderm and to replace the hypoblast with the embryonic endoderm.
By day 16, the embryonic disc takes on an oval shape and a second axis (rostrocaudal or head–tail axis) becomes apparent. This is accompanied by a process known as gastrulation, during which cells from the epiblast migrate towards a groove in the caudal end of the disc known as the primitive streak (Fig. 2.3B). The migrating cells pass though the primitive streak and form a new embryonic compartment or ‘germ layer’ called the mesoderm. The dorsal epiblast now becomes the embryonic ectoderm and the hypoblast is replaced by embryonic endoderm. The embryo now has three ‘germ’ layers bounded by the umbilical vesicle ventrally and the amniotic cavity dorsally.
Organogenesis (19–56 + GD)
The outline of organogenesis given here is necessarily brief and is divided into five main areas:
1. neural tube and brain
2. gut tube and derivatives
3. heart and liver
4. craniofacial structures
5. limbs and skeletal muscle.
Neural tube and brain
Formation of the neural tube and primitive brain is the first evidence of organogenesis. It begins at 19 GD, when a midline groove forms on the dorsal ectodermal surface, rostral to the primitive streak. This change in the surface ectoderm is induced by a strip of specialized midline mesodermal cells called the notochord (Fig. 2.4). Two important paired structures form on either side of this groove:
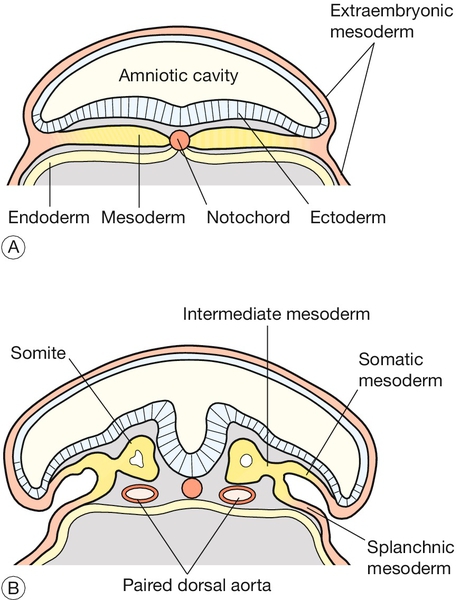
Fig. 2.4Formation of the neural tube.
The notochord – a strip of specialized midline mesodermal cells – induces a midline groove on the ectodermal surface. (A) The edges of this groove will fuse to form the neural tube (B) Failure of this tube to close rostrally results in anencephaly, and failure caudally leads to spina bifida.
![]() the neural folds
the neural folds
![]() the somites.
the somites.
The neural folds grow rapidly and begin to fuse across the midline in the cervical region to form the neural tube by 22 GD. At the same time, paired segmental condensations of the mesoderm (somites) are beginning to form blocks of tissue on either side of the midline (paraxial mesoderm). A new pair of somites appears every 6.6 h in a rostrocaudal direction. The somites are critical for establishing the adult body plan and their formation is an example of repeated segment (metameric) pattern formation during embryogenesis (Box 2.3).
Fusion of the neural tube proceeds rapidly in both rostral and caudal directions. This apparently simple tube will give rise to the entire central nervous system (CNS). At either end of the embryo, the neural tube remains open for a short time, and these openings are referred to as the rostral and caudal neuropores. The rostral neuropore closes by 23 GD, and failure of this process results in one of the most severe of human malformations, anencephaly. The caudal neuropore is the last part of the neural tube to close at 26 GD, and failure of this closure leads to spina bifida.
The rostral half of the neural tube is more complex and forms the future brain. The caudal part forms the spinal cord. In common with many other embryological structures, the formation of the CNS is best viewed as a tube which is segmented along the rostrocaudal long axis, each linear segment then differentiates dorsoventrally to produce the final adult form. At day 26 the CNS is a tube with four main segments:
1. forebrain – prosencephalon
2. midbrain – mesencephalon
3. hindbrain – rhombencephalon
4. spinal cord.
The forebrain
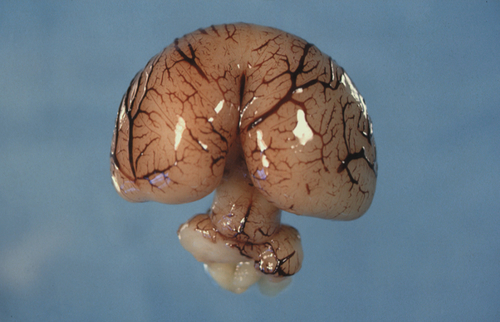
The prosencephalon is initially subdivided into three segments. The most rostral is the telencephalon medium, then come the D1 and D2 compartments of the diencephalon. All three segments surround the future third ventricle (Fig. 2.6). D1 will give rise to the bilateral optic evaginations by day 28 (see Craniofacial structures, below) and D2 will form the thalamus, hypothalamus, pineal gland and part of the pituitary (neurohypophysis). The telencephalon medium forms as a rostral out-pouching of the third ventricle. By day 32, a midline crest separates the two future cerebral hemispheres. Failure of this process results in a malformation called holoprosencephaly (Fig. 2.7). Subsequent development of telencephalic structures is complex and mostly occurs during fetal and postnatal life. In addition to the cerebral hemispheres, the telencephalon gives rise to the caudate and lentiform nuclei (derivatives of the corpus striatum) and the connections between the hemispheres derived from the lamina terminalis (anterior commissure, hippocampal commissure and corpus callosum).
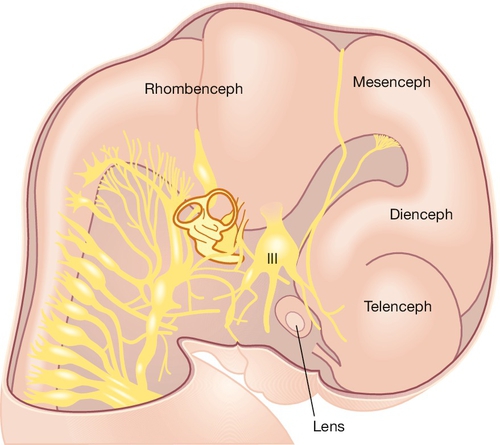
Fig. 2.6Development of the CNS.
The main brain segments – forebrain (telencephalon and diencephalon), midbrain (mesencephalon) and hindbrain (rhombencephalon) – are shown in a 48–51 GD human embryo. The lens is located within the optic vesicle.
The midbrain
The mesencephalon is less complicated than the forebrain. It segments dorsally to form the superior and inferior colliculi (roles in integrating visual and auditory signals, respectively) and ventrally, to form the tegmentum (oculomotor nuclei of cranial nerves III and IV). This typifies a general pattern within the neural tube, where dorsal grey matter has a sensory function and ventral grey matter has a motor function. Later in development the cerebral peduncles occupy a significant proportion of the midbrain.
The hindbrain
The rhombencephalon is highly segmented into eight regions known as rhombomeres. The exact function of each rhombomere is not clear but they appear to have a role in forming the motor components of cranial nerves V, VII, IX and X. More rostrally, the rhombencephalon forms the pons and the cerebellum. Like the cerebral hemispheres, the cerebellum continues to develop throughout intrauterine life.
The spinal cord
The spinal cord itself is unsegmented along the rostrocaudal axis. The adjacent somites produce the repeated pattern of spinal nerve roots that is seen in adult life. The cord is patterned in the dorsoventral axis and maintains the dorsal/sensory, ventral/motor pattern for grey matter function mentioned above.
Gut tube and its derivatives
Initially, the primitive gut is simply a concavity within the embryo that is lined with embryonic endoderm and is open to the umbilical vesicle (Fig. 2.8). As development progresses, the gut becomes a tube with a fundamental role in the development of many organs. The most important of these, in rostrocaudal order, are:
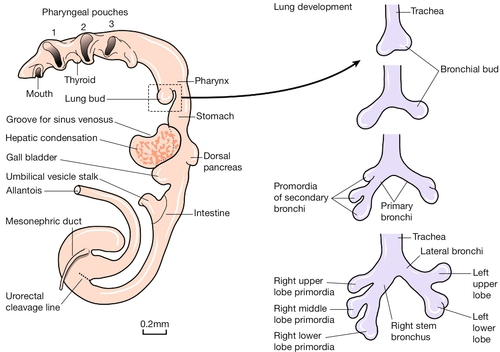
Fig. 2.8Development of the gut tube and its derivatives.
In the left panel is a drawing of the developing gut. Initially, the primitive gut is simply a concavity within the embryo lined with embryonic endoderm, with a large direct connection to the umbilical vesical. As development progresses, the gut becomes a tube with a fundamental role in the development of the gastrointestinal tract, the thyroid and pituitary glands, the lungs, the pancreas, the bile ducts, gall bladder and the urogenital system. The right panel shows the development of the lungs – from top to bottom – at 28 days; at 32 days; at 33 days; and at the end of the 5th week.
![]() the thyroid and pituitary glands
the thyroid and pituitary glands
![]() the lungs
the lungs
![]() the pancreas
the pancreas
![]() the bile ducts and gall bladder (covered in the section on Heart and liver, below)
the bile ducts and gall bladder (covered in the section on Heart and liver, below)
![]() the urogenital system.
the urogenital system.
All these structures arise as invaginations of the gut tube endoderm into the underlying mesoderm. The gut tube, like the neural tube, is strictly patterned along the rostrocaudal long axis with complex dorsoventral specification. Morphogenesis broadly proceeds in rostrocaudal temporal sequence, again like the neural tube.
Thyroid and pituitary glands
The tongue develops from a bud of tissue on the ventral wall of the gut tube. Just caudal to this, the thyroid primordium is evident from day 20 as a midline ventral invagination of endoderm. The base of the invagination hypertrophies and differentiates to form the thyroid gland itself. By 40 GD the thyroid gland lies at the level of the second and third tracheal cartilage. Terms such as ‘descended’ are used in morphogenesis; however, most commonly, differential growth of surrounding tissue, rather than active burrowing of the structure, results in the change in position within the embryo. The stalk of the thyroid invagination (thyroglossal duct) usually regresses, apart from a small pit at the base of the tongue, called the foramen caecum. Remnants of the thyroglossal ducts can persist in the form of ectopic thyroid tissue and thyroglossal cysts.
The pituitary is formed by the meeting of two different invaginations, one from the dorsal wall of the rostral gut tube (Rathke’s pouch) and the other from the floor of the diencephalon. Neoplastic change in the stalk of the former results in the rare hormone-secreting tumour craniopharyngioma.
The lungs
The respiratory diverticulum is first seen as an invagination of the ventral wall of the gut tube at 26 GD. This diverticulum elongates so that the trachea lies immediately ventral to the oesophagus. The development of each of these structures is intimately related, and developmental problems may result in tracheo-oesophageal fistulae and/or oesophageal atresia. The development of the airways proceeds by a process known as branching morphogenesis, in which there is bifurcation of the invaginating tubelike structures at regular intervals. The earliest branching events show evidence of the left–right asymmetry apparent in adult life (Fig. 2.8).
The pancreas
The pancreas develops as separate dorsal and ventral invaginations in the gut tube, caudal to the developing stomach. The ventral pancreas develops in the same region as the hepatic ducts and gall bladder (see below), and their ductal systems are connected. The dorsal pancreas is larger and its duct drains directly into the duodenum. At some point after 42 GD the dorsal and ventral pancreas fuse and the ducts of both structures anastomose.
The urogenital system
The development of the renal, adrenal, gonadal, genital and anorectal systems are closely related. The paired primitive kidney (mesonephros) develops from intermediate mesoderm on either side of the gut tube. The mesonephros is connected to a specialized midline region of the caudal gut (the cloaca) via the metamerically patterned mesonephric (Wolffian) ducts. At the caudal end of the mesonephric duct is a diverticulum called the ureteric bud that induces the surrounding mesenchyme to form the mature kidney (metanephros) (Fig. 2.9). Failure of this process results in renal agenesis. The primitive gonad develops at the rostral end of the mesonephric duct at the level of the stomach. At 46 GD, a second paired-duct system, the paramesonephric (Müllerian) duct, forms parallel to the mesonephric duct (Fig. 2.10). In male embryos, a cascade of gene activation initiated by SRY protein causes the primitive gonad to become a testis and the mesonephric duct forms the vas deferens; the paramesonephric duct regresses. In female embryos, the primitive gonad becomes the ovary, the mesonephric duct regresses, and the paramesonephric ducts form the fallopian tubes, uterus and upper vagina. This sexually dimorphic development occurs during late embryogenesis and early fetal life, and molecular analysis of this system has been very useful in understanding intersex states (Chapter 6). The mesonephros regresses in both sexes.
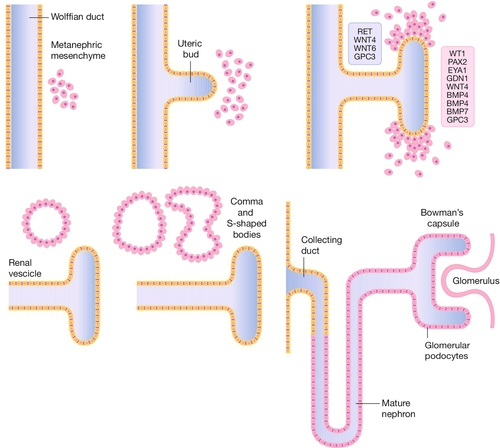
Fig. 2.9Development of the kidney.
The ureteric bud forms as an outpouching of the Wolffian (mesonephric duct) and induces a particular set of genes in the surrounding mesenchyme which leads to a cascade of branching morphogenesis in the bud and differentiation of the mesenchyme to form comma and S-shaped bodies, which are precursors of the glomeruli of the mature kidney (metanephros).
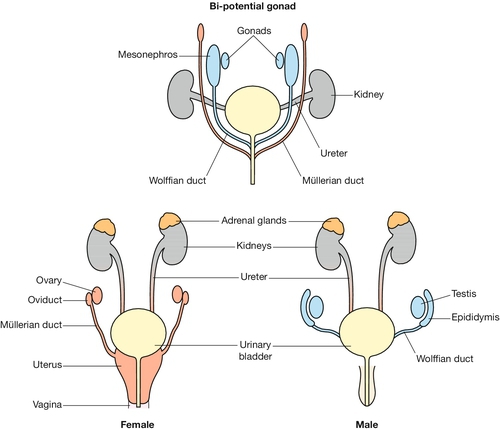
Fig. 2.10 Development of the gonads.
At 46 GD, a paired duct system, the paramesonephric (Müllerian) duct, forms parallel to the mesonephric duct. In male embryos, a cascade of gene activation initiated by SRY protein causes the primitive gonad to become a testis and the mesonephric duct to form the vas deferens; the paramesonephric duct regresses. In female embryos, the primitive gonad becomes the ovary, the mesonephric duct regresses, and the paramesonephric ducts form the fallopian tubes, uterus and upper vagina.
The cloaca separates dorsally to form the rectum and ventrally to form the bladder, urethra and the lower part of the vagina. The external genitalia develop from swellings on the ectodermal surface of the embryo: the midline genital tubercle which forms the penis and clitoris; the paramedial genital fold which forms part of the penile urethra and the labia minora; and the paired lateral genital swellings which form the scrotum and labia majora. Development of the external genitalia is a late event and it is often difficult for a non-expert to correctly assign the sex of a mid-gestation fetus by external morphology.
Heart and liver
The heart and liver are derived from a condensation of mesenchyme that forms by day 20 in the very rostral part of the embryo, between the forebrain and the endoderm. This mesenchyme forms the heart tube, which then shows highly programmed regional growth over the next 2 days to become asymmetrically looped. This ‘cardiac looping’ is the first evidence of a left–right axis in the embryo. The heart tube (like the neural tube and gut tube) is highly organized along its long axis to form, in a caudorostral direction, the atria, the left ventricle, the right ventricle and the conotruncal region. The subsegmental growth of the heart tube continues until four chambers are recognizable but retain a common connection (Fig. 2.11). Separation of the chambers and the formation of AV valves is dependent on the fusion of ridges of tissue within the heart (the endocardial cushions). Failure of this process results in a heart malformation not infrequently found in Down syndrome, an AV canal defect. The aorta at this stage consists of a ventral root that feeds four paired vessels (aortic arches), which supply the rapidly growing pharyngeal arches (see below). A combination of regression and differential growth of these arches eventually shapes the mature thoracic aorta. The right side of the fourth arch, for example, becomes the right subclavian artery, whereas the left side of the fourth arch becomes part of the aortic arch.
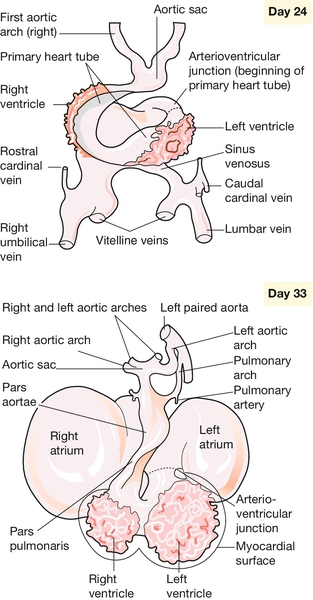
Fig. 2.11Development of the heart.
The primary cardiac loop forms, in a caudorostral direction, the atria, the left ventricle, the right ventricle and the conotruncal region. Growth of the heart tube continues until four chambers are recognizable.
The first evidence of liver formation is on day 22 as a condensation of mesenchyme in the cardiac region overlying a thickened region of gut endoderm (hepatic plate). The ductal system of the liver and the gall bladder is derived from an invagination of the hepatic plate, whereas the hepatocytes are mesenchymal in origin.
Craniofacial structures
The face develops around the primitive oral cavity at the rostral end of the gut tube. The major early morphogenic events involve:
![]() the paired sensory placodes
the paired sensory placodes
![]() the facial processes.
the facial processes.
The paired sensory placodes
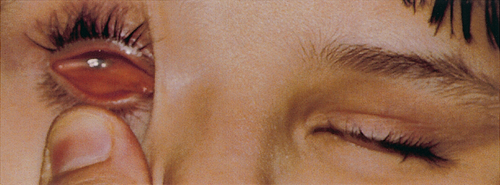
The otic, nasal and optic placodes are defined thickenings on the surface ectoderm. The otic placode is the first to appear at 20 GD on the lateral wall of the hindbrain. This placode gradually invaginates to become the otic cyst and then undergoes complex morphogenesis to form the semicircular canals and cochlea. The external ear develops from mounds of tissue (auricular hillocks) on the first and second pharyngeal arches (see below). The middle ear develops from the tissue between these arches. The nasal placode is first evident on the frontonasal process by 30 GD and invaginates to form the nasal air passages. The optic placode is induced at ~ 28 GD by signals emanating from the bilateral optic evagination (optic stalks) arising from the diencephalon. The optic placode invaginates to form the optic vesicle that will ultimately become the lens of the eye (Fig. 2.12). The cupped end of the optic stalk becomes the neural retina, and the stalk itself, the optic nerve. Failure of any part of this process will result in anophthalmia.
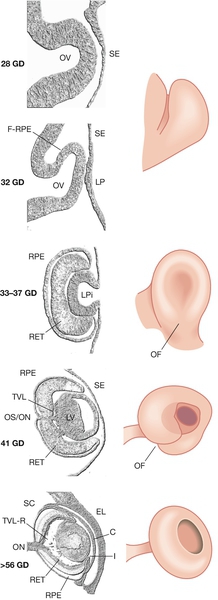
Fig. 2.12Development of the eye.
The invaginating lens placode becomes separated from the surface ectoderm. The retina is formed from an outpouching of the forebrain (the optic cup). C, cornea; GD, gestational days; (F-)RPE (future) retinal pigment epithelium; I, iris; LP, lens placode; LPi, lens pit; LV, lens vesicle; OF, optic fissure; OV, optic vesicle; RET, retina; SE, surface ectoderm; TVL(-R), tunica vasculosa lentis (regressing); OS/ON, optic stalk/optic nerve; SC, Schlemm’s canal; EL, eyelid.
The facial processes
By 22 GD paired tubes of tissue form ventral to the hindbrain. These ‘pharyngeal arches’ have a fundamental role in the morphogenesis of the head and neck. There are usually only three pharyngeal arches visible in the embryo, and the cells that form the arches contain a significant number of migratory neural crest cells (Box 2.4). The first pharyngeal arch is the most important in face development, and through differential growth, this tube of tissue becomes C-shaped; the top arm forms the maxillary process and the bottom arm the mandibular process (Fig. 2.13). The upper lip is formed at ~ 40 GD by fusion of the maxillary process with the midline unpaired frontonasal process. Failure to fuse, results in unilateral or bilateral cleft lip (Fig. 2.14A). The lower jaw is formed by fusion of the mandibular processes in the midline. The secondary palate forms from outgrowths of palatal shelves from the maxillary process within the oral cavity. These initially grow down beside the tongue and then elevate to fuse in the midline. Failure to do so, results in cleft palate (Fig. 2.14B).
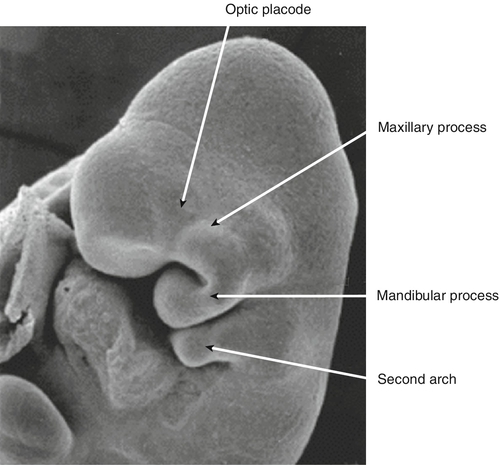
By 22 GD, paired tubes of tissue form ventral to the hindbrain – the ‘pharyngeal arches’. The first pharyngeal arch becomes C-shaped. The top arm of the ‘C’ forms the maxillary process, and the bottom arm the mandibular process.
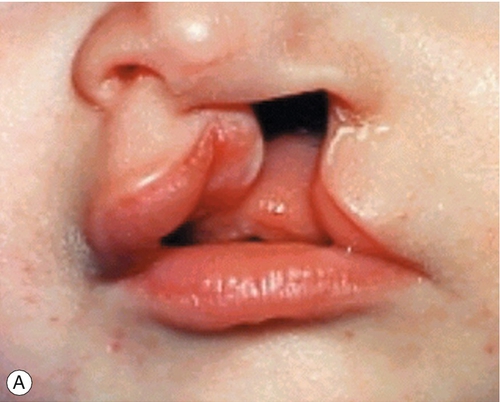
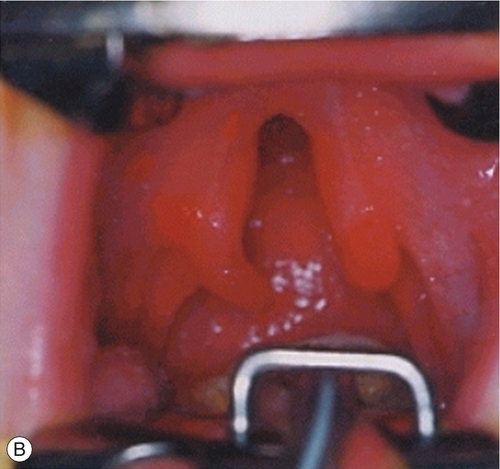
Fig. 2.14Formation of the lips and palate.
(A) The upper lip is formed by fusion of the maxillary process with the midline unpaired frontonasal process. Failure of this process results in unilateral or bilateral cleft lip. (B) The lower jaw is formed by fusion of the mandibular processes in the midline. Failure of this process results in cleft palate.
Limbs and skeletal muscle
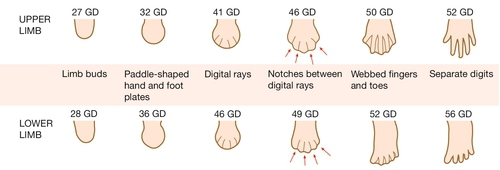
The upper limbs develop first, but the sequence of events is similar in both the upper and lower limbs. Limb formation begins as a ridge of cells on the lateral aspect of the embryo opposite somites 8–10 at ~ 26 GD. Rapid division of cells under this apical ectodermal ridge results in paired limb buds (the lower limb bud is visible by 28 GD). These limb buds elongate and a terminal hand plate becomes apparent by 33 GD. By 41 GD, finger rays can be seen. The fingers are then formed by a remarkable process of apoptosis, or programmed cell death, in the interdigital spaces (Fig. 2.15). Failure of this process results in syndactyly. The molecular signals that pattern the hand plate are reminiscent of those that pattern the neural tube, as well as other structures in the embryo (Box 2.3). Failure of this patterning can lead to polydactyly (Fig. 2.16).
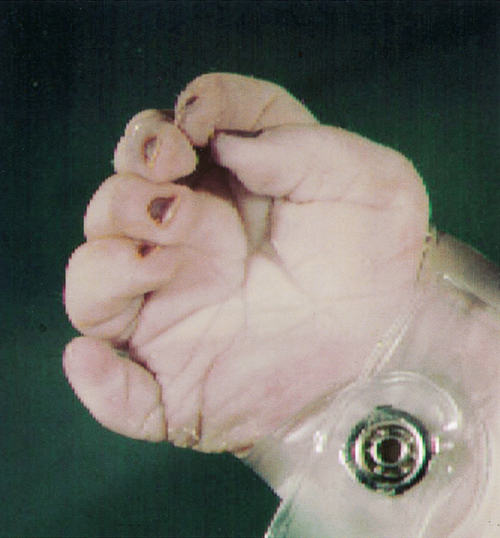
Fig. 2.16Extra digit (polydactyly).
This results from the failure of normal pattern formation in the hand plate.
The cells that form the muscle of the trunk and the limbs are also patterned and migrate from the paraxial mesoderm of the somites. Their fate is determined by their somite of origin. Other tissues derived from the paraxial mesoderm include the vertebrae and the dermis.