CHAPTER 10 Hormones
their action and measurement in gynaecological practice
Introduction
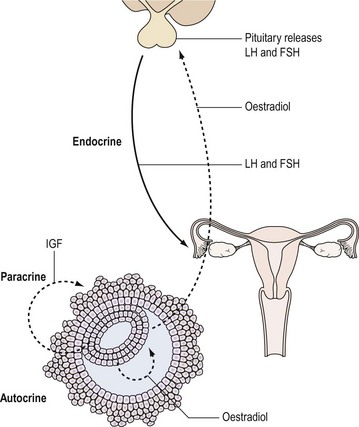
Hormones are one of the important means by which cells communicate with each other. They ensure that the body’s physiological systems are coordinated appropriately, and if this is impaired, as occurs with hormone deficiency or excess, function is compromised. Classically, hormones are synthesized by specialized cells, which may exist as distinct endocrine glands or be located as single cells within other organs, and secreted into the blood stream and transported through the circulation to a distant site of action (endocrine effect). However, autocrine or paracrine actions may also be prevalent and indeed modulate the function of the original endocrine cell type (Figure 10.1).
Hormones: Types and Action
There are three major groups of hormones according to biochemical structure and method of synthesis:
Peptide hormones
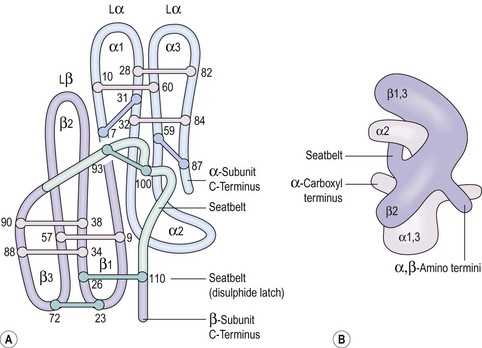
Some peptide hormones have complex tertiary structures; for example, glycoproteins are composed of more than one peptide chain. Oxytocin and arginine vasopressin, two of the posterior pituitary hormones, have ring structures linked by disulphide bridges. Despite being remarkably similar in structure, they have very different physiological roles. The gonadotrophins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), TSH and human chorionic gonadotrophin (hCG) also have two chains (Figure 10.2). However, these α- and β-subunits are synthesized quite separately from separate genes. The α-subunit of each is shared; it is the distinctive β-subunit of each that confers biological specificity.
Steroids
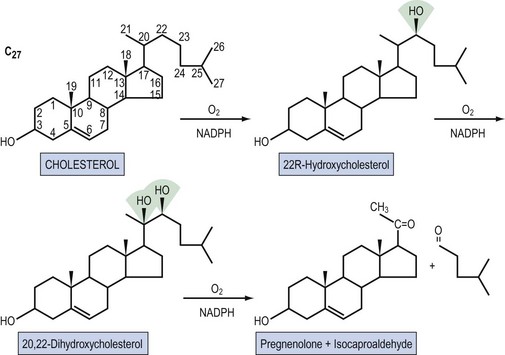
Steroid hormones are lipid-soluble molecules derived from modification of the four-carbon ring structure of cholesterol (Figure 10.3). Steroids can be classified according to the number of carbon atoms they possess: C-21 (progestogens, cortisol, aldosterone), C-19 (androgens) and C-18 (oestrogens). The common names used for steroids also adhere to a loose convention. The suffix -ol indicates an important hydroxyl group, as in cholesterol or cortisol, whereas the suffix -one indicates an important ketone (e.g. testosterone). The extra presence of -di, as in diol (e.g. oestradiol) or dione (e.g. androstenedione), reflects two of these groups, respectively; -ene (androstenedione) within the name indicates a significant double bond in the steroid nucleus.
Regulation of Hormone Secretion and Action
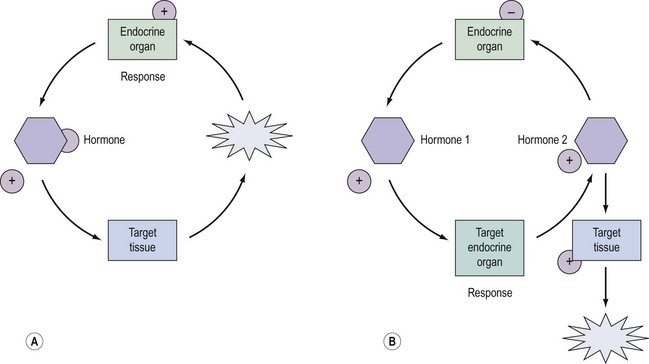
Regulation and control of hormones is essential, and underpins not only the function of many endocrine systems but also their clinical investigation (Figure 10.4). Hormones utilize a variety of differential regulatory mechanisms.
Availability of the hormone to a cell
A hormone can be present in the blood stream but unavailable to the target cell. A hormone circulating in the blood stream, not attached to a binding protein, readily enters cells by simple diffusion. However, most of the steroid hormones circulate bound to protein carriers, such as sex-hormone-binding globulin (SHBG), because their cholesterol origin makes them hydrophobic (King 1988). Consequently, they are generally unavailable, and alterations in the amount of circulating binding globulins can modulate the biological activities of each of their respective hormones. For example, approximately 40% of circulating oestrogen is bound to SHBG, and the remainder is bound to albumin and membrane proteins (Hovarth 1992). As testosterone is largely (80%) bound to SHBG and 19% is loosely bound to albumin, its androgenicity is mainly dependent upon the unbound fraction and partly upon the fraction associated with albumin. SHBG production in the liver is decreased by androgens, hence the binding capacity in men is lower than in normal women. In a hirsute woman, the SHBG level is depressed by the excess androgen, and the percentage of free and therefore active testosterone is elevated (Nestler et al 1991).
Tissue distribution
The tissue distribution of hormone receptors also determines the scope of hormone action, and the presence or absence of a given receptor determines whether a cell will respond to a given class of steroids. For example, the TSH receptor is limited to the thyroid and therefore TSH action is restricted to the thyroid. A hormone can also modify its own and/or another steroid hormone’s activity by regulating the concentration of receptors in a cell. This has the biological effect of increasing tissue response to the hormone if the receptor number is increased, and vice versa if the receptor number is decreased. Oestrogen, for example, increases target tissue responsiveness to itself by increasing the concentration of FSH receptors in granulosa cells (Hillier 1994). This process is important in the selection and maintenance of the dominant ovarian follicle in the menstrual cycle. In order to respond to the ovulatory surge and become a ‘successful’ corpus luteum, the granulosa cells must acquire LH receptors. FSH induces LH receptor development on the granulosa cells of large antral follicles with oestrogen acting as chief coordinator. Progesterone, on the other hand, limits the tissue response to oestrogen by reducing, over time, the concentration of oestrogen receptors, hence its use in the prevention of endometrial hyperplasia and carcinoma (Studd and Wadsworth 2000).
Hormone Receptors
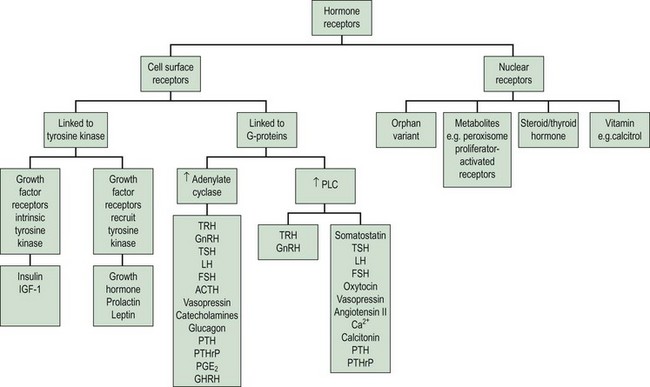
The clinical effects of hormones are mediated through the interaction of a hormone with its receptors. There are two principal superfamilies of hormone receptors. This broad classification is based on their cellular location and their characteristic features (Figure 10.5).
Cell surface receptors
Cell surface receptors are composed of three components, each with structural features, which reflect its location and function (Figure 10.6). The hormone acts as a ligand, with a ligand-binding pocket in the extracellular domain of the receptor being comparatively rich in cysteine residues that form internal disulphide bonds and repeated loops to ensure correct folding. This extracellular domain may also be cleaved for some hormones, and function as a binding protein in the circulation, thus limiting bioavailability (e.g. GH). The α-helical membrane-spanning domain is rich in the hydrophobic and uncharged amino acids, enabling it to be secured with the lipid bilayer. The C-terminal cytoplasmic domain either contains, or links to, separate catalytic systems which initiate the intracellular signals after hormone binding.
Stage 1. Availability of the receptor (up- and downregulation)
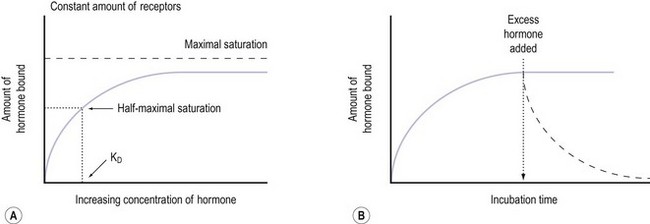
The cell’s mechanism for sensing the low concentration of circulating trophic hormone is to have an extremely large number of receptors but to require only a very small percentage (as little as 1%) to be occupied by the hormone for its action to be evident (Sairam and Bhargavi 1985). Positive and negative modulation of receptor numbers by hormones is known as up- and downregulation. The mechanism of upregulation is unclear, but prolactin and GnRH, for example, can increase the concentration of their own receptors in the cell membrane (Katt et al 1985).
In downregulation, an excess concentration of a trophic hormone, such as LH or GnRH, results in a loss of receptors on the cell membranes and therefore a decrease in biological response. This process occurs by internalization of the receptors, and is the main biological mechanism by which the activity of polypeptide hormones is limited (Ron-El et al 2000). Thus the formation of the hormone–receptor complex on the cell surface initiates the cellular response, and the internalization of the complex (with eventual degradation of the hormone) terminates the response. It therefore appears that the principal reason for the pulsatile secretion of trophic hormones is to avoid downregulation and to maintain adequate receptor numbers. The pulse frequency, therefore, is a key factor in regulating receptor number.
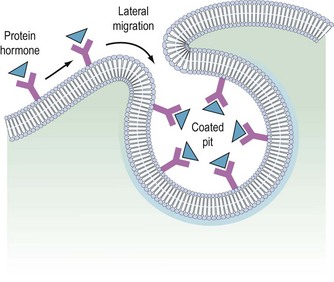
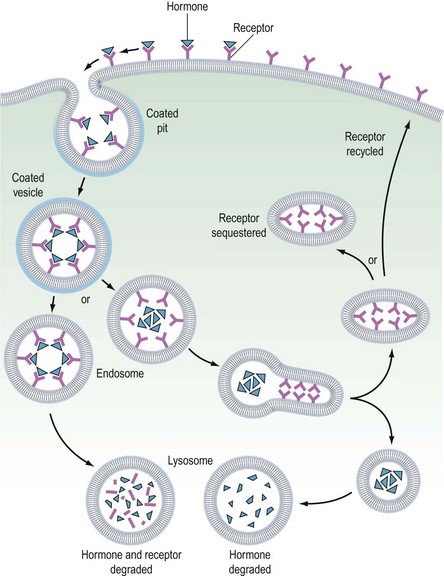
It is believed that receptors are randomly inserted into the cell membrane after intracellular synthesis. They have two important sites — an external binding site, which is specific for a polypeptide hormone, and an internal site, which plays a role in the process of internalization (Kaplan 1981). When a hormone binds to the receptor and a high concentration of the hormone is present in the circulation, the hormone–receptor complex moves laterally in the cell membrane by a process called ‘lateral migration’ to a specialized area, the coated pit, the internal margin of which has a brush border (Goldstein et al 1979). Lateral migration, which takes minutes rather than seconds, thus concentrates hormone–receptor complexes in the coated pit, a process referred to as ‘clustering’ (Figure 10.7). When fully occupied, the coated pit invaginates, ‘pinches off’ and enters the cell as a vesicle. The coated vesicle is delivered to the lysosomes where it undergoes degradation, releasing the hormone receptors. The receptors may be recycled to the cell membranes and used again, or the receptor and hormone may be metabolized, thus decreasing the hormone’s biological activity. This process is called ‘receptor-mediated endocytosis’ (Goldstein et al 1979; Figure 10.8).
As well as downregulation of polypeptide hormone receptors, the process of internalization can be utilized for other cellular metabolic events, including the transfer of vital substances into the cell, such as iron and vitamins. Hence, cell membrane receptors can be separated into two classes. Class I receptors are distributed in the cell membrane and transmit information to modify cell behaviour for these receptors. Internalization is a method for downregulation and recycling is not usually a feature. Hormones which utilize this category of receptor include FSH, LH, hCG, GnRH, TSH and insulin (Kaplan 1981). Class II receptors are located in the coated pits. Binding leads to internalization, which provides the cell with the required ligands or removes noxious agents from the biological fluid bathing the cell. These receptors are spared from degradation and can be recycled. Examples of this category include low-density lipoproteins, which supply cholesterol to steroid-producing cells (Parinaud et al 1987), and transfer of immunoglobulins across the placenta to provide fetal immunity.
Stage 2. Binding of the hormone to the receptor
Notably hormone–receptor systems demonstrate standard kinetics and are saturable and reversible (Figure 10.9). Consequently, the dissociation rate of the hormone and its receptor is an important component of the biological response, as the biological activity of the hormone is only maintained while the hormone–receptor complex is maintained. Hormones that are structurally similar may have some overlap in biological activity. For example, the similarity in structure between GH and prolactin means that GH has a lactogenic action whilst prolactin has some growth-promoting activity and stimulates somatomedin production.
The glycoprotein hormones (LH, FSH, TSH and hCG) share an identical α-chain and require another portion, the β-chain, to confer the specificity inherent in the relationship between hormones and their receptors. The β-subunits differ in both amino acid and carbohydrate content, and the chemical composition may be altered under certain conditions, thereby affecting the affinity of the hormone and its receptor (Willey 1999).
Stage 3. Signal transduction
On binding of a hormone to its cell surface receptor, protein phosphorylation and the generation of ‘second messengers’ mediates a cascade of cytoplasmic and cellular responses. This multistep cascade facilitates amplification of the cellular response to the hormone binding to a receptor, as many second messenger molecules are produced for each hormone–receptor complex. The cell surface receptors may be classified into two groups according to the second messenger and signalling cascades that are stimulated: (1) tyrosine kinase receptors and (2) G-protein coupled receptors (Figure 10.5).
Tyrosine kinase receptors
The subfamily of receptors which recruit tyrosine kinase activity includes those that bind GH, prolactin and leptin, and also includes those for numerous cytokines and erythropoietin. The basic receptor composition, with major homology between family members in the extracellular domain, is the same for the other membrane-spanning receptors (Figure 10.6). Notably, similar mechanisms govern GH and prolactin receptor binding and signal transduction. Two different sites on the hormone are capable of binding receptors. The hormone–receptor interaction of the first leads to the binding of the second to another receptor molecule. It is the close proximity of the two receptor molecules which leads to dimerization of their cytoplasmic regions and signal transduction. With the exception of the receptor for erythropoietin, other cytokine receptors tend to form heterodimers with diverse partner proteins.
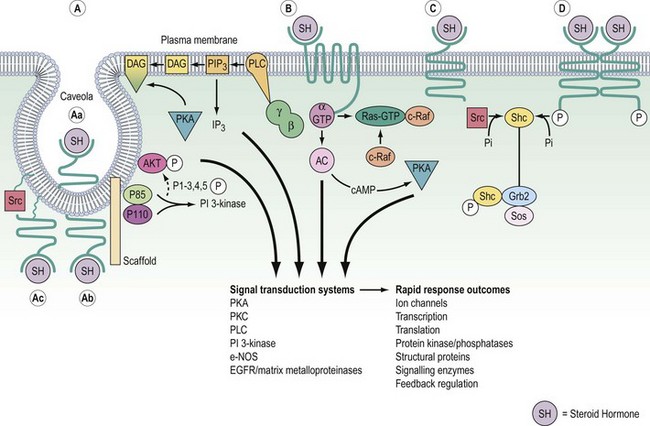
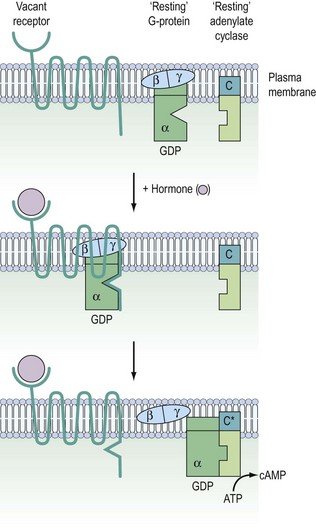
G-protein coupled receptors
Signalling of G-protein receptors is mediated by hydrolysis of GTP (guanosine triphosphate) to GDP (guanosine diphosphate) (Figure 10.10). In their resting state, the G-proteins exist in the cell membrane as heterotrimeric complexes with α-, β- and γ-subunits. In practice, the β- and γ-subunits associate with such affinity that the functional units are Gα and Gβ/γ. When the hormone binds to the receptor, this causes a conformational change in receptor structure, which in turn causes a conformational change in the α-subunit allowing it to dissociate from the heterotrimeric complex and bind to a downstream catalytic unit, either adenylate cyclase facilitating the generation of cAMP or phospholipase C (PLC) for the production of DAG/IP3. The energy to activate these targets comes from the cleavage of phosphate from GTP, thereby regenerating Gα-GDP. This ensures that it is no longer associated with adenylate cyclase or PLC, thereby switching off the cascade and recycling the Gα-GDP back to the start.
Nuclear receptors
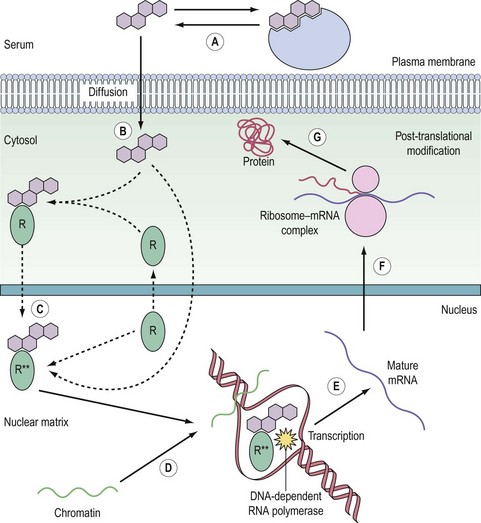
The nuclear receptors are classified by their ligands, which are small lipophilic molecules that diffuse across the plasma membrane of target cells. The receptors, encoded by a single gene, typically bind DNA and function as transcription factors (Figure 10.11). Distinct polypeptide-encoding regions can be identified, for which evolutionary conservation can be as high as 60–90%. Many nuclear receptors exist for which no endogenous ligand has been identified, and these are termed ‘orphan’ nuclear receptors. In addition, some variant receptors have atypical DNA-binding domains and potentially function via indirect interaction with the genome.
The receptor family predominantly resides in the nucleus, although increasingly nuclear import and export appears to be an important regulatory mechanism, as has been long recognized for the glucocorticoid receptor. The receptors have their primary effect by regulating the expression of specific target genes. Consequently, this need for transcription and translation to elicit a biological effect means that cellular responses are relatively slow compared with cell surface receptor signalling. For example, high midcycle levels of oestradiol from the ovary lead to the increase in LH synthesis and secretion by the anterior pituitary that results in ovulation (Hillier 1994).
Hormone specificity of the receptor
This is the most important factor which determines specificity of action and occurs at two levels (King 1988). The presence or absence of a given receptor determines whether or not a cell will respond to a given class of steroids, whilst hormone specificity controls which particular compound is active. For example, the oestrogen receptor, which has the greatest specificity, binds oestradiol 10 times more efficiently than oestrone and about 1000 times more efficiently than the androgen, testosterone, whilst progesterone and cortisol are not recognized at all (Garcia and Rochefort 1977). This recognition specificity is a reflection of the different affinities that the receptor has for the different hormone structures. In biological terms, this means that oestradiol is more active than oestrone, whilst testosterone can have oestrogenic effects but only at pharmacological concentrations.
Androgen, glucocorticoid, progestogen and mineralocorticoid receptors are less precise in their binding requirements than the oestrogen receptor (Beato and Klug 2000). For example, many progestogens, especially the synthetic ones, bind to both progestogen and androgen receptors when present in pharmacological concentrations. This dual specificity is reflected in the biological activities of the compounds. For example, the practice of giving synthetic progestogens to pregnant women to prevent miscarriage resulted in some of their offspring having clinical features associated with androgen exposure (Aarskog 1979). The androgenic side-effects of the synthetic progestogens used in the oral contraceptive pill are another example.
Clinical Assays
This section considers assay methodology for the endocrinological investigations commonly used in gynaecological practice. An understanding of basic methodology permits the clinician to have confidence in the selection of specific laboratory tests, and leads to a greater understanding of the assay for better interpretation of results (Chard 1987).
Binding assays
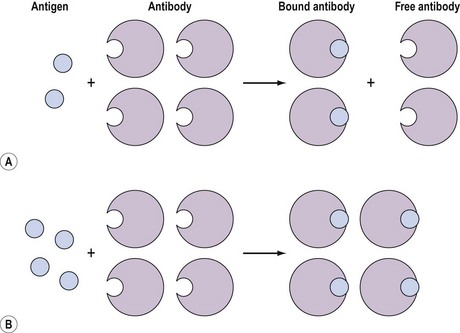
These assays involve the combination of the compound, for example, an antigen, with a binding substance, for example, an antibody, which is added in a fixed amount to the solution. The distribution of the antigen between the bound and free phases is directly related to the total amount of antigen present, and provides a means for quantifying the latter (Figure 10.12). Binding assays can be further subdivided into three groups: receptor assays, competitive protein-binding assays and immunoassays.
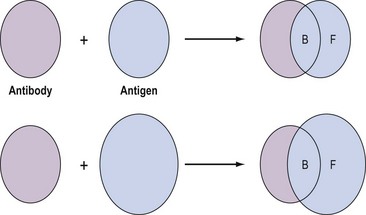
Figure 10.12 Distribution of free (F) and bound (B) antigen in the presence of a fixed amount of antibody.
Immunoassays
The basic binding reaction can be subdivided into two categories, based on whether the antibody is present in a limited concentration or whether it is present in excess. If varying concentrations of the labelled antigen react with an excess amount of antibody (Figure 10.13), it is termed an ‘immunometric assay’. The principle of an immunometric assay is that a constant amount of antibody is added with increasing known amounts of the reference hormone. After incubation, the amount of hormone bound to the antibody is detected by adding, to all the tubes, an excess amount of a second labelled anti-hormone antibody. The second antibody is directed against a different antigenic site on the hormone from the first antibody. Thus, a triple complex is formed, with the hormone sandwiched between the two antibodies. The unbound fraction of the second antibody is removed, leaving the amount of triple complex to be determined by quantifying the bound label; for example, by measuring the radioactivity or fluorescence emitted. This emission is plotted against the increasing known amount of hormone standard to generate a calibration curve. To determine the standard curve with precision, against which patient samples can be interpolated, usually five to eight different concentrations are used. The immunometric assay is only suitable when the hormone to be measured is large enough to permit the discrete binding of two antibodies.
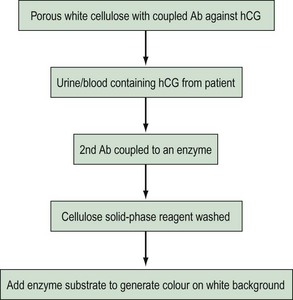
Enzymoimmunoassay, enzymoimmunometric assay and enzyme-labelled immunosorbent assay are currently the most widely used non-isotopic labels. Small quantities of antigen can be quantified by studying enzymatic substrate conversion that leads to a colour change. The colour formation can be a simple yes/no answer (Figure 10.14) as in a rapid pregnancy test, or the intensity of colour can be used to quantify the patient sample. The second marker antibody will not be bound, so following the wash step, it will not be present to react with its substrate.
Measurement and Action of Individual Hormones
Anterior pituitary hormones
Follicle-stimulating hormone
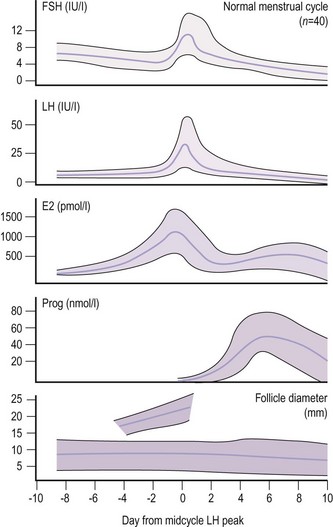
Due to the marked fluctuations in FSH levels in a normal ovulatory cycle, timing of the blood sample in relation to ovulation is required to interpret results (Figure 10.15). The level can be as low as 0.5 IU/l at the luteal phase nadir and as high as 20 IU/l at the midcycle peak. However, values below 1 IU/l are associated with hypothalamic pituitary failure, and values of 20 IU/l or above indicate ovarian failure, as in the menopause.
Luteinizing hormone
As for FSH, timing the sample in relation to the phase of the ovulatory cycle is vital (Figure 10.15). For example, the ovulatory surge is taken as above 20 IU/l. However, in polycystic ovary disease, a raised LH (13–25 IU/l) with normal FSH values is characteristic. Therefore, a raised LH in the early to midfollicular phase is of more significance than a midcycle raised LH. The LH level is within the normal range in a significant number of women with polycystic ovary disease, and the diagnosis requires the use of other tests. As the prospective timing of ovulation using the LH peak presents the difficulty that the peak cannot be identified until the next value significantly lower than the peak is observed, clinical action is taken on the basis of the first definite rise in LH values (LH surge) rather than on the peak, which usually occurs a day later. This initial rise in LH underlies the mechanism of home ovulation kits using urine rather than blood samples.
Ovarian hormones
Oestradiol
Serum levels of oestradiol in spontaneous ovulation in relation to changes in FSH, LH, progesterone and the size of the dominant follicle are shown in Figure 10.15 (Macklon and Fauser 2000). There is a variation in oestradiol levels throughout the menstrual cycle. Results therefore need to be interpreted in relation to the timing of the sample in the cycle. Serum oestradiol levels in pregnancy range from 2 to 10 nmol/l in the first trimester and from 20 to 80 nmol/l in the third trimester. The major oestrogen in pregnancy is oestriol.
Progesterone
Variations throughout the menstrual cycle can lead to difficulties in interpretation if the result is taken at an unidentified time in the cycle, particularly if a single progesterone level is taken as a marker of ovulation (Figure 10.15). Normally, results taken 5–8 days prior to the next menstruation are suitable for the detection of ovulation, so the patient should be asked to keep a record of the timing of the blood sample and her next menstrual period. A result >30 nmol/l indicates ovulation, even if timing is not known. Levels in the mother during pregnancy increase in parallel with the growth of the placenta, being 30–45, 70–150 and 150–600 nmol/l in the first, second and third trimesters, respectively. Maternal progesterone levels are related to the weight of the fetus and placenta at term.
Inhibin
In the female, inhibin is synthesized predominantly by ovarian granulosa cells. Inhibin levels increase in the late follicular phase, acting synergistically with oestradiol to inhibit FSH synthesis and release, although this is predominantly over-ridden by the preovulatory LH and FSH surge (Bevan and Scanlan 1998). Inhibins A and B are dimeric proteins capable of suppressing FSH,with inhibin B being the principal form produced by the Sertoli cells in the testis which inhibits pituitary FSH secretion. Activins A, B and AB are dimeric proteins which share β-subunits with inhibin but stimulate FSH (Groome et al 2001).
Anti-Müllerian hormone
Anti-Müllerian hormone (AMH, Müllerian-inhibiting substance), a member of the transforming-growth factor-β family, has the primary role of regression of the Müllerian duct in the male fetus during early testis differentiation. However, expression of AMH persists after completion of the reproductive duct system in males, and furthermore commences in females, where it is produced by ovarian granulosa cells from early fetal life (Modi et al 2006). In females, AMH appears to have inhibitory effects upon the recruitment of primordial follicles (Durlinger et al 2002), and it may decrease the sensitivity of large preantral and small antral follicles to FSH (Figure 10.16) (Durlinger et al 2001). However, recent analyses of human follicles examining AMH receptor expression suggests that inhibitory effects at the earliest stages of development are unlikely (Rice et al 1997). Although AMH is initially observed in granulosa cells of primary follicles, maximal expression occurs in preantral and small antral follicles (Laven et al 2004, Weenen et al 2004). AMH expression declines as antral follicles increase in size, with nominal expression restricted to the granulosa cells of the cumulus (Weenen et al 2004). This loss of AMH expression during the FSH-dependent final stages of follicular growth, and the lack of expression by atretic follicles, suggests that basal levels of AMH may more accurately reflect the total developing follicular cohort, and consequently the potential ovarian response to FSH. Furthermore, AMH has been shown to be relatively stable across the menstrual cycle (La Marca et al 2010), consistent with its role reflecting the continuous, non-cyclic growth of small follicles in the ovary.
In harmony with the established relationship between age and declining ovarian reserve, AMH falls with increasing age (de Vet et al 2002). The clinical utility of AMH is its strong ability to predict ovarian response to FSH in cycles of assisted conception (Nelson et al 2007, 2009) and onset of the menopause (Sowers et al 2008).
Placenta
Human chorionic gonadotrophin
This is secreted by the blastocyst and appears in maternal blood shortly after implantation and then rises rapidly until 8 weeks’ gestation. Levels show little change at 8–12 weeks’ gestation, then decline to 18 weeks’ gestation and remain fairly constant until term. There is some short-term variation in blood hCG levels but no circadian rhythm. At term, the levels in the female fetus are substantially higher than those in the male (Obiekwe and Chard 1983). The mechanisms which determine the levels of hCG in maternal blood are unknown.
A patient can continue to have a positive pregnancy test for at least 1 week after a miscarriage or therapeutic abortion due to the prolonged half-life of hCG; hCG may be detected up to 20 weeks later if sensitive assays are used. hCG measurements in blood and urine are used for the early detection of ectopic pregnancy, an increased risk of miscarriage, risk of embryos or fetuses with aneuploidy, prediction of pre-eclampsia or fetal growth restriction, and diagnosis or monitoring of trophoblast disease. The choice of assay for measurement of the prevalent form or proportion in relation to the intact hCG in any particular clinical setting is critical, as the complex cascade of enzymes involved in hCG secretion results in heterogeneous molecular forms (de Medeiros and Norman 2009). The synthesis of α- and β-subunits and their further glycosylation require different enzymes. After assembly, hCG reaches the cell surface and is secreted as a bioactive heterodimer. The hCG molecules are metabolized differently by the liver, ovary and kidney, but most of the hCG forms are excreted in the urine. It is clear that specific assays for intact hCG and its variants, α-hCG, β-hCG, hyperglycosylated hCG, nicked hCG and core fragment of β-hCG, have a wide spectrum of clinical implications (Cole 2009).
Pregnancy-associated plasma protein A
There is evidence that pregnancy-associated plasma protein A (PAPP-A) proteolyses insulin-like growth factor binding protein-4, thereby regulating local insulin-like growth factor availability. In addition, PAPP-A can no longer be regarded as ‘pregnancy specific’ (Boldt and Conover 2007). In pregnancy, PAPP-A can be detected in the maternal blood consistently from 6 to 8 weeks’ gestation, with concentrations rising steadily throughout the pregnancy, but the mechanisms which regulate the synthesis of PAPP-A are not yet known. However, depressed serum levels of PAPP-A are seen in association with some fetal chromosomal abnormalities (trisomy 21, trisomy 18 and trisomy 13), Cornelia de Lange syndrome and ectopic pregnancy (El-Farra and Grudzinskas 1995, Kagan et al 2008). Maternal serum screening by maternal age, fetal nuchal translucency thickness, fetal heart rate, and maternal serum-free β-hCG and PAPP-A in the first trimester of pregnancy (11–13 weeks) will detect approximately 95% of pregnancies affected by trisomy 31, trisomy 18 and trisomy 13 (Kagan et al 2008). Serum PAPP-A levels are elevated in acute coronary syndromes and may be associated with adverse outcome (Schoos et al 2008).
Fetus
α-Fetoprotein
The interpretation of normality of levels therefore depends upon gestation, and incorrect assessment of gestation could lead to an erroneous conclusion on the normality of the fetus. Levels are also raised in obstetric problems (threatened miscarriage, intrauterine growth restriction, perinatal death and antepartum haemorrhage) and some congenital abnormalities (exomphalos, nephrosis, Turner’s syndrome, trisomy 13), but are depressed in trisomy 21 (Canick et al 2003). The introduction of more effective maternal serum markers and better-resolution fetal ultrasound has led to the diminished use of isolated AFP measurements.
KEY POINTS
Aarskog D. Maternal progestin as a possible cause of hypospadias. New England Journal of Medicine. 1979;300:75.
Beato M, Klug J. Steroid hormone receptors: an update. Human Reproduction Update. 2000;6:225-236.
Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Hormone and IGF Research. 2007;17:10-18.
Canick JA, Kellner LH, Bombard AT. Prenatal screening for open neural tube defects. Clinics in Laboratory Medicine. 2003;23:385-394.
Chard T. An Introduction to Radioimmunoassay and Related Techniques. Amsterdam: Elsevier; 1987.
Coccia ME, Rizzello F. Ovarian reserve. Annals of the New York Academy of Sciences. 2008;1127:27-30.
Cole L. New discoveries on the biology and detection of human chorionic gonadotrophin. Reproductive Biology and Endocrinology. 2009;7:8.
De Kretser DM, Menhardt A, Meehan T, Phillips DJ, O’Bryan K, Loveland A. The roles of inhibin and related peptides in gonadal function. Molecular and Cellular Endocrinology. 2000;161:43-46.
de Medeiros SF, Norman RJ. Human choriogonadotrophin protein core and sugar branches heterogeneity: basic and clinical insights. Human Reproduction Update. 2009;15:69-95.
de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertility and Sterility. 2002;77:357-362.
Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891-4899.
Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076-1084.
El-Farra K, Grudzinskas JG. Will PAPP-A be a biochemical marker for screening of Down’s syndrome in the first trimester? Early Pregnancy. 1995;1:4-12.
Garcia M, Rochefort H. Androgens on the oestrogen receptor. II. Correlation between nuclear translocation and uterine protein synthesis. Steroids. 1977;29:11.
Goldstein JL, Anderson RGW, Brown MS. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979;279:679.
Groome NP, Tsigou A, Cranfield M, Knight PG, Robertson DM. Enzyme immunoassays for inhibin, activin and follistatin. Molecular and Cellular Endocrinology. 2001;180:73-77.
Hillier SG. Current concepts in the roles of follicle stimulating hormone and luteinising hormone in folliculogenesis. Human Reproduction. 1994;9:188-191.
Holt RIG, Hanley NA. Essential Endocrinology and Diabetes, 5th edn. Massachusetts: Blackwell Publishing; 2007.
Hovarth PM. Sex steroids: physiology and metabolism. In: Swarz DP, editor. Hormone Replacement Therapy. Baltimore: Williams and Wilkins; 1992:1-10.
Kagan KO, Wright D, Valencia C, Maiz N, Nicolaides KH. Screening for trisomies 21, 18 and 13 by maternal age, fetal nuchal translucency, fetal heart rate, free beta-hCG and pregnancy-associated plasma protein-A. Human Reproduction. 2008;23:1968-1975.
Kaplan J. Polypeptide-binding membrane receptors: analysis and classification. Science. 1981;212:14.
Katt JA, Duncan JA, Herbon L, et al. The frequency of gonadotrophin-releasing hormone stimulation determines the number of pituitary gonadotrophin-releasing hormone receptors. Endocrinology. 1985;116:2113.
La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the human menstrual cycle. Human Reproduction. 2006;21:3103-3107.
La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Human Reproduction Update. 2010;16(2):113-130.
Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. Journal of Clinical Endocrinology and Metabolism. 2004;89:318-323.
Macklon NS, Fauser BCJM. Regulation of follicle development and novel approaches to ovarian stimulation for IVF. Human Reproduction Update. 2000;6:307-312.
Modi D, Bhartiya D, Puri C. Developmental expression and cellular distribution of Müllerian inhibiting substance in the primate ovary. Reproduction. 2006;132:443-453.
Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles — implications for individualization of therapy. Human Reproduction. 2007;22:2414-2421.
Nelson SM, Yates RW, Lyall H et al 2009 Anti-Müllerian hormone-based approach to controlled ovarian stimulation for assisted conception. Human Reproduction 2009 Jan 10 [Epub ahead of print].
Nestler JE, Powers LP, Matt DW. A direct effect of hyperinsulinaemia on serum SHBG in obese women with PCOS. Journal of Clinical Endocrinology and Metabolism. 1991;72:83-89.
Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nature Review Drug Discovery. 2004;3:27-41.
Obiekwe BC, Chard T. Placental proteins in late pregnancy: relation to fetal sex. Journal of Obstetrics and Gynaecology. 1983;3:163.
Parinaud J, Perret B, Ribbes H, et al. High density lipoprotein and low density lipoprotein utilization by human granulosa cells for progesterone synthesis in serum-free culture: respective contributions of free and esterified cholesterol. Journal of Clinical Endocrinology and Metabolism. 1987;64:409.
Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrine Reviews. 2004;25:947-970.
Rasmussen H. The calcium messenger system. New England Journal of Medicine. 1986;314:1094.
Rice S, Ojha K, Whitehead S, Mason H. Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-Müllerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. Journal of Clinical Endocrinology and Metabolism. 2007;92:1034-1040.
Rodbell M. The role of hormone receptors and GTP-regulatory protein in membrane transduction. Nature. 1980;284:17.
Rojas RJ, Asch RH. Effects of luteinizing hormone-releasing hormone agonist and calcium upon adenyl cyclase activity of human corpus luteum membranes. Life Sciences. 1985;36:841.
Ron-El R, Raziel A, Schachter M, Strassburger D, Kasterstein E, Friedler S. Induction of ovulation after GNRH antagonists. Human Reproduction. 2000;6:318-321.
Sairam MR, Bhargavi GN. A role for glycosylation of the alpha subunit in transduction of biological signal in glycoprotein hormone. Science. 1985;229:65.
Schoos M, Iversen K, Teisner A, et al. Release patterns of pregnancy-associated plasma protein A in patients with acute coronary syndromes assessed by an optimized monoclonal antibody assay. Scandinavian Journal of Clinical and Laboratory Investigation. 2008;14:1-7.
Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-Müllerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. Journal of Clinical Endocrinology and Metabolism. 2008;93:3478-3483.
Studd J, Wadsworth F. Hormone replacement therapy. In: O’Brien PMS, editor. The Yearbook of Obstetrics and Gynaecology. London: RCOG Press; 2000:374-391.
Weenen C, Laven JS, Von Bergh AR, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Molecular Human Reproduction. 2004;10:77-83.
Willey KP. An elusive role for glycosylation in the structure and function of reproductive hormones. Human Reproduction Update. 1999;5:330-355.