Chapter 26 Hormonal Contraception
INTRODUCTION
Despite the continued development of effective contraceptive methods and increased educational efforts, unintended pregnancies continue to be a major problem in the United States. According to the National Survey of Family Growth, half of a total of 6.3 million pregnancies in the United States in 1994 were unintended, and 1 million of these pregnancies occurred while using oral contraceptives.1,2 Clearly, the need remains for development of improved contraceptive methods.
HISTORY
The history of the development of “the pill” is fascinating. At the turn of the 20th century, an Austrian professor of physiology, Ludwig Haberlandt, demonstrated that pregnancy could be prevented in mice by giving them oral extracts from mice ovaries.3 In the 1940s, Dr. Carl Djerassi, working for the pharmaceutical company Syntex, discovered that the removal of the 19 carbon from yam-derived progesterone increased its progestational activity. This discovery led to the synthesis of norethindrone, an orally active progestin, in 1951.3,4
Modern Hormonal Contraceptives
A broad range of contraceptive hormones and delivery systems are presently available in the United States today (Table 26-1). Several of the newer hormonal delivery systems are parenteral, and some of the preparations no longer need to be taken daily. The most frequently used and best studied are the combination oral contraceptives that contain a synthetic estrogen and one of several progestins. Three progestin-only oral contraceptives are currently available. In addition, a vaginal patch, a vaginal ring, a single rod implant, and an injectable medroxyprogesterone are commercially available.
Table 26-1 Formulations of Some Modern Hormonal Contraceptives
| Delivery System | Estrogens | Progestins* |
|---|---|---|
| Oral contraceptives | Ethinyl estradiol (dose: 20–50 μg) | Estranes: |
| Combination (constant dose) | Mestranol (dose: 50 μg) |
* Sequential oral contraceptives have changing doses of estrogens, progestins, or both throughout the cycle.
ORAL CONTRACEPTIVES
Patient Selection
Oral contraceptives are an excellent contraceptive choice for many patients who are willing and able to consistently take a daily pill. In some cases, oral contraceptives may be recommended to patients because of their noncontraceptive benefits, such as treatment of dysmenorrhea or acne. The vast majority of healthy women can take oral contraceptives with an extremely low risk of serious side effects or risks. However, there are several standard contraindications to use of hormonal contraceptive methods that contain estrogen (Table 26-2). Many of these women are good candidates for progestin-only contraceptives. The remainder should be counseled to use an alternative nonhormonal method.
Table 26-2 Contraindications to Use of Combination Oral Contraceptives
| Contraindication (current or past history) | Combination (estrogen-containing) | Progestin-only |
|---|---|---|
| Absolute Contraindications | ||
| Suspected pregnancy | X | X |
| Undiagnosed uterine bleeding | X | X |
| Liver disease | X | X |
| Symptomatic gall bladder disease | X | X |
| Breast cancer | X | X |
| Estrogen-induced liver tumor | X | |
| Thromboembolism | X | |
| Cerebral vascular disease | X | |
| Coronary heart disease | X | |
| Estrogen-dependent tumors | X | |
| Seriously impaired liver function | X | |
| Relative Contraindications | ||
| Age > 35 years with any cardiovascular risk factors: | X | |
| Cigarette smoking | ||
| Hypertension | ||
| Abnormal lipid profile | ||
| Diabetes mellitus | ||
| Known cardiovascular disease | X | |
| Severe headaches: vascular or migraine | X | |
| Hypertension | X | |
| Diabetes | X | |
| Gallstones | X | X |
| Within 3 weeks of childbirth | X | |
| Breastfeeding (especially first 6 weeks) | X | |
| History of cholestasis of pregnancy | X | |
| Systemic lupus erythematosus | X | |
| Abdominal or lower extremity surgery contemplated within 4–6 weeks | X | |
| Lower leg cast | X | |
| Hypertriglyceridemia | X | |
| Use of drugs that interact with oral contraceptives (e.g., rifampin) | X | |
Estrogens and Progestins
Estrogens
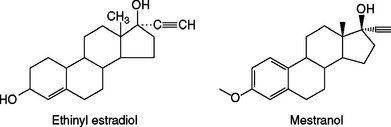
Combination oral contraceptive pills contain one of two synthetic estrogens: ethinyl estradiol or, less commonly, mestranol. Both of these steroids are created by adding a 17-α ethinyl group to estradiol to increase potency and enhance oral activity by impeding hepatic degradation (Fig. 26-1). Natural estrogens are relatively weak when given by mouth due to their rapid clearance from blood by the liver (i.e., the first-pass effect). As a result of decreased degradation, both of these synthetic estrogens are 10 to 20 times more active than estradiol when given orally, but have similar potency when given intravenously.
The original formulation for oral contraceptive pills contained as much as 150 μg of synthetic estrogen each. When studies found an association between the estrogen content of early oral contraceptives and venous thromboembolism, researchers found that the estrogen component could be significantly reduced with no loss of contraceptive efficacy. As a result, most modern oral contraceptive pills now contain between 25 and 35 μg of ethinyl estradiol, and a few contain as little as 20 μg.5 Although reducing the daily estrogen dose to either 30 or 35 μg has been shown to reduce the risk of venous thromboembolism, no study has shown that further decreasing the estrogen dose reduces the risk further.
Progestins
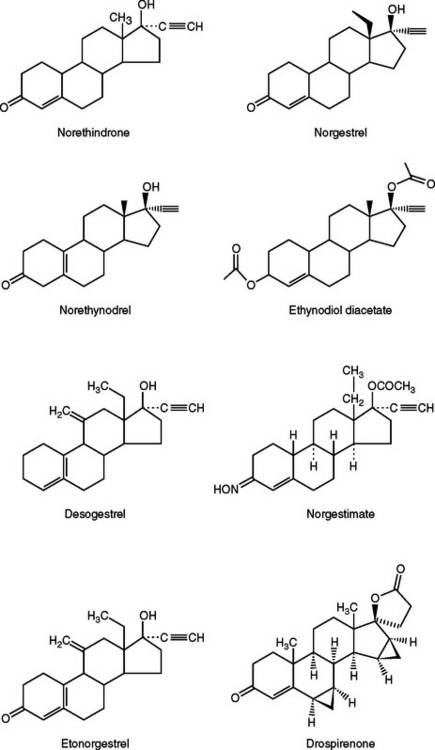
The prototype progestin used in modern oral contraceptive pills is norethindrone (an estrane), which is created from progesterone by adding both a 19-carbon methyl group and a 17-α ethinyl group similar to ethinyl estradiol (Fig. 26-2). Norgestrel (a gonane) is the 18-carbon-ethyl derivative of norethindrone and is a racemic mixture of dextronorgestrel and levonorgestrel. Levonorgestrel is the biologically active component of norgestrel.
Recently, drospirenone, a somewhat unique progestin, has been introduced in the United States.6 This anti-androgenic analogue of spironolactone has a high binding affinity for progesterone and mineralocorticoid receptors, but a low binding affinity for the androgen receptors. Oral contraceptives containing this progestin are associated with less fluid retention but have a slight theoretical risk of hyperkalemia.7 Women with renal insufficiency, hepatic dysfunction, or adrenal insufficiency should not use oral contraceptives containing drospirenone.
COMBINATION ORAL CONTRACEPTIVE PILLS
The majority of oral contraceptive pills on the market today contain both an estrogen and progestin in tablets that are taken daily for 21 days out of each 28-day cycle (see Table 26-1). During the remaining 7 days, women are instructed to take either inert pills (to assist the patient in maintaining the daily habit of taking a contraceptive pill) or no pills at all. At least one modern contraceptive pill gives women a lower dose of estrogen alone during 5 of these remaining 7 days. Extended-use oral contraceptives refers to an approach where active combination oral contraceptive pills are taken daily for 84 days followed by a 7-day hormone-free period.
Efficacy
Approximately 7% of women using combination oral contraceptives will have an unintended pregnancy during the first 12 months of use.8 Rates reported in different clinical trials can be highly variable because of factors, including methodology, demographics, various types of bias, and methods of calculating rates.9–12 Two of the most common methods are the Pearl index and life table analysis.
Life table analysis is more effective for comparing failure rates than the Pearl index because a separate failure rate is determined for each month of use.12 Life table analysis also allows separate evaluation of both method and user failure rates. Method failure rates refer to pregnancy rates that occur when the method has been used correctly, in a consistent manner, and according to the instructions in the package insert. User failure rates (i.e., typical failure rates) refer to pregnancies that occur when the method is not used correctly. The reported method failure rates for oral contraceptive pills are between 1% and 3% and user failure rates are approximately 7%.11 Both method and user failure rates are similar for women over age 30 who are in higher socioeconomic groups. In contrast, the user failure rate is much higher for teenagers and unmarried women, sometimes surpassing 30%.
NONCONTRACEPTIVE BENEFITS OF COMBINATION ORAL CONTRACEPTIVES
Decreased Risk of Ectopic Pregnancies
Combination oral contraceptives reduce the risk of ectopic pregnancy by 90%.13 The likely mechanism is through suppression of ovulation, an effect that obviously prevents all types of pregnancy. In contrast, some studies have indicated that women using progestin-only oral contraceptives are at higher risk for an ectopic pregnancy than the general population.
Decreased Risk of Pelvic Inflammatory Disease
Several studies have shown that oral contraceptive use reduces the risk of pelvic inflammatory disease (PID) by 50% to 80% compared to women who use no method or a barrier method for contraception.13–15 However, one recent multi-center study failed to show a protective effect of oral contraceptives.16
Decreased Risk of Malignancies
Endometrial Cancer
Multiple case-control and cohort studies have shown that oral contraceptives protect against endometrial cancer.17–19 The overall reduction in risk is up to 50% and begins 1 year after initiation of use. This protection increases with the duration of use and persists for up to 20 years after oral contraceptives are discontinued. The strength of the protective effect varies according to the existence of other risk factors, such as obesity and nulliparity. The purported protective mechanism of action is a reduction in the mitotic activity of endometrial cells by the action of the progestin component of oral contraceptives.
Ovarian Cancer
Multiple case-control and cohort studies have shown that the use of oral contraceptives protects against the development of ovarian cancer.20–22 The overall risk is decreased by 40% to 80%. The effect begins about 1 year after initiating use and decreases by 10% to 12% for each year of use. Protection persists for 15 to 20 years after discontinuation of the oral contraceptives.
Several mechanisms have been proposed by which oral contraceptives might reduce ovarian cancer risk. Suppression of ovulation has been hypothesized to reduce the frequency of “injury” to the ovarian capsule that occurs during ovulation. Suppression of gonadotropins by hormonal contraceptives might also play a role. Finally, data from a primate model suggests that hormonal contraceptives can induce ovarian apoptosis, which in turn eliminates surface epithelium inclusion cysts, thus reducing ovarian cancer risk.23
Colorectal Cancer
Multiple studies have demonstrated up to a 40% reduction in colon and rectal cancer among women who have used oral contraceptives.24–26 However, one study did not find a protective effect of oral contraceptives.27 Theoretical protective mechanisms include a reduction in bile acid production and concentration, and effects on colonic mucosa or flora.28
Decreased Menstrual Flow and Dysmenorrhea
Up to 75% of young women will experience some degree of dysmenorrhea, and 15% to 20% will have severe pain.29,30 Multiple studies have shown that combination oral contraceptives reduce both menstrual flow and dysmenorrhea.29–34
Two possible mechanisms have been proposed to explain how oral contraceptives reduce dysmenorrhea. Women taking oral contraceptives have been shown to have reduced prostaglandins in the menstrual fluid. It is hypothesized that by decreasing prostaglandin production by the uterus, oral contraceptives decrease dysmenorrhea by decreasing either endometrial vasoconstriction and ischemia or uterine contractile activity.35,36
Decreased Benign Breast Disease
Several studies have shown a 30% to 50% decrease in the incidence of benign fibrocystic breast changes in women using oral contraceptives.37,38 One of the clearest effects is a decrease in the occurrence of fibroadenomas among current and recent long-term users of oral contraceptives under age 45. The most likely mechanism is through suppression of ovulation and therefore inhibition of the breast cell proliferation that normally occurs in the first half of an ovulatory menstrual cycle.
Ovarian Cysts
Cyst Formation
It has been commonly accepted that moderate- and high-estrogen oral contraceptives (≥50 μg ethinyl estradiol) protect against cyst formation, although the data supporting this are somewhat inconsistent. Unfortunately, current low-estrogen (≤35 μg) formulations appear to have no effect on ovarian cyst formation.39–41 However, these studies examined cysts found on ultrasound rather than symptomatic cysts. It remains to be seen whether low-estrogen (≤35 μg) oral contraceptives decrease the risk of symptomatic ovarian cysts. Interestingly, progestin-only oral contraception may actually increase the frequency of functional ovarian cysts, according to one small study.42
Cyst Resolution
Many clinicians prescribe combination oral contraceptives to women found to have an ovarian cyst in an effort to accelerate its spontaneous resolution, presumably by decreasing gonadotropin secretion centrally. However, there are no data to support the use of oral contraceptives to expedite the resolution of existing functional ovarian cysts. One cross-sectional study found that ovarian cysts resolved independently of treatment with oral contraceptives.43
Improved Acne
Randomized, placebo-controlled clinical trials have demonstrated that some combination oral contraceptives will reduce acne lesions by as much as 50%.44 Several combination oral contraceptives containing new-generation progestins (e.g., norgestimate, desogestrel) or progestational anti-androgens (e.g., drospirenone in the United States, cyproterone acetate overseas) have been shown to be able to effectively reduce acne. Although it is likely that many other pill formulations will reduce acne, most formulations have not been studied for this outcome.
Increased Bone Density
The majority of studies indicate that oral contraceptives have a positive effect on bone mineral density, and no studies have demonstrated a negative effect.45 Oral contraceptives appear to delay or prevent the decline in bone mass that begins between ages 30 and 40. Women who use oral contraceptives for 5 to 10 years or longer are afforded the greatest protection. A population-based, case-control study revealed a 25% reduction in hip fracture risk in these women.46
Improvement in Rheumatoid Arthritis
Oral contraceptives appear to diminish both the severity and symptomatology of rheumatoid arthritis but do not prevent this disease. A large meta-analysis of nine hospital and population-based studies suggested that oral contraceptives prevent the progression of rheumatoid arthritis to more severe varieties.47 However, the protective effect was noted in the hospital- based studies only, suggesting the possibility of some unexplained bias.
METABOLIC CHANGES RELATED TO COMBINATION ORAL CONTRACEPTIVES
Insulin Resistance
The effect of combination oral contraceptives on carbohydrate metabolism has been studied for several decades. It is now recognized that both estrogens and progestins affect glucose metabolism by increasing peripheral resistance to insulin. However, studies of women on current low-estrogen (≤35 μg) formulations have shown minimal changes in glucose and insulin levels that are of no clinical significance.48 Further, there is no evidence that combination preparations increase the risk of subsequently developing diabetes in women at risk, such as those with prior gestational diabetes.49
Women with polycystic ovary syndrome who usually demonstrate some degree of insulin resistance benefit from oral contraceptives. Although oral contraceptives may slightly increase their insulin resistance, they also reduce associated symptoms such as acne and hirsutism, regulate menses, and protect the endometrium against development of endometrial malignancy.50
Insulin-dependent diabetic women can use oral contraceptives with little fear of affecting fasting plasma glucose, insulin requirements, or glycosylated hemoglobin levels.51 However, one should avoid oral contraceptives in diabetics with evidence of vascular disease because of their increased risk of cardiovascular disease.
Changes in Lipids
The estrogen component of combination oral contraceptives causes elevations in serum triglycerides but has a favorable change on the other two major lipids by elevating high-density lipoprotein cholesterol (HDL-C) while lowering low-density lipoprotein cholesterol (LDL-C) levels.52 Contraceptive formulations containing progestins with low or no androgenicity (e.g,. desogestrel, drospirenone, or norgestimate) lower LDL-C and elevate HDL-C and triglycerides. In contrast, contraceptive formulations containing more androgenic progestins may change lipids in an unfavorable direction by lowering HDL-C levels and elevating LDL-C levels.6,52–54 It is important to be aware of these differences when prescribing oral contraceptives to women with risk factors for cardiovascular disease or to those who have strong family histories of ischemic heart disease or substantial lipid abnormalities.
Coagulation Changes
Low-estrogen (≤35 μg) oral contraception formulations are associated with clinically insignificant changes in both procoagulant and anticoagulant factors.55,56 However, women with risk factors for venous thromboembolism, such as factor V Leiden mutations, should avoid combination oral contraceptives.
MAJOR RISKS OF COMBINATION ORAL CONTRACEPTIVES
Cardiovascular Risk
Venous Thromboembolism
Multiple studies have established that women taking combination oral contraceptives containing 50 to 100 μg of ethinyl estradiol are at a small but increased risk of venous thromboembolism.57,58 It soon became clear that the risk correlates with the estrogen dose. When compared to women taking low-estrogen (≤35 μg) pills with an age-adjusted relative risk of venous thromboembolism of 1, women taking intermediate-estrogen (50 μg) pills are found to have a relative risk of 1.5, and women taking high-estrogen (>50 μg) pills have a risk of 1.7.59 However, even in the highest risk group, the absolute risk of venous thromboembolism was extremely low, at only 10 events per 10,000 women-years of use.
There is some evidence that two of the newer generation progestins might also increase the risk of venous thromboembolism. Two studies published a decade ago suggested gestodene and desogestrel had a greater risk for venous thromboembolism than previously used progestins, such as levonorgestrel.60–63 Subsequent analyses and reviews have failed to reach consensus about whether these finding are real or somehow spurious.64–71
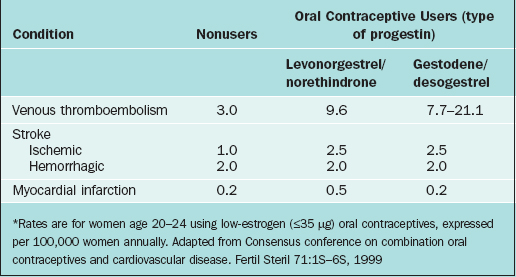
The relative incidence of venous thromboembolism in young women taking low-estrogen (≤35μg) pills with different progestins is given in Table 26-3. At worst, the attributable risk of using oral contraceptives containing gestodene or desogestrel is only about 18 additional cases annually per 100,000 users compared to nonusers. Other risk factors for venous thromboembolism include age, obesity, pregnancy, trauma, smoking, immobilization, recent surgery, medical conditions such as cancer or collagen vascular disorders, and inherited coagulation disorders. Surprisingly, there is no evidence that cigarette smoking or varicose veins appreciably increases oral contraceptive users’ relative risk of venous thromboembolism.72
The mortality due to venous thromboembolism is low in reproductive-age women using oral contraceptives. Age significantly increases this risk of mortality, such that women age 44 have twice the mortality as women age 35.
Factor V Leiden Mutation
The most common genetic cause of primary and recurrent venous thromboembolism in women is factor V Leiden mutation. The identification of the factor V Leiden mutation in 1993 has led to new insights into the relationship between oral contraceptive use and venous thromboembolism.73 White women have approximately 5% prevalence of a homozygous mutation; African-American and Asian women have a much lower prevalence. Women with this mutation not using oral contraceptives have a risk of venous thromboembolism of approximately 5.7 events per 10,000 women-years. In contrast, women with this mutation using oral contraceptives have an increased risk of venous thromboembolism of 28.5 events per 10,000 women-years.
Screening women who desire oral contraceptives for factor Leiden V mutation would not be a cost-effective strategy in light of the low absolute risk of venous thromboembolism in women with this mutation. It has been calculated that screening 1 million potential oral contraceptive users for all known coagulation factor deficiencies or mutations would identify approximately 50 women at risk but also would result in approximately 62,000 false-positive results.69 However, screening women with a strong family history of thromboembolic events for factor V Leiden mutation remains appropriate. Certainly, a known homozygous or heterozygous factor V Leiden mutation is a relative contraindication to estrogen-containing hormonal contraceptives, although the absolute risk of thromboembolism in these patients is still relatively low.
Stroke
Ischemic Stroke
Low-estrogen (≤35 μg) oral contraceptives do not increase the risk of ischemic stroke in women with no additional risk factors (i.e., nonsmoking women younger than age 35 without hypertension).74 However, women with risk factors do have a somewhat increased risk. Mortality from oral contraceptive-related strokes is less than 2 per 100,000 cases.
The risk of ischemic stroke in oral contraceptive users is directly proportional to estrogen dose, in a manner analogous to the risk of venous thromboembolism.72,74–77 The risk of ischemic stroke is increased fivefold in women using higher estrogen (≥50μg) compared to low-estrogen (≤35μg) oral contraceptives.78 This risk is not influenced by progestin type or duration of pill use.72,74
Age is the most important independent risk factor for stroke. The relative risk of ischemic stroke doubles as women reach age 40 to 44.74 Stroke mortality also increases by about sixfold once women reach age 35 to 44.72
Other important risk factors include hypertension, cigarette smoking, and migraine headaches. These risk factors appear to interact with oral contraceptive use to substantially increase the risk of ischemic stroke.78–81
Hemorrhagic Stroke
Low-estrogen (≤35 μg) oral contraceptives do not increase the risk of hemorrhagic stroke in women with no additional risk factors, such as age greater than 35 years, cigarette smoking, or hypertension (see Table 26-3).74,77,82–86 The risk of hemorrhagic stroke is increased twofold in women using higher estrogen (≥50μg) compared to low-estrogen (≤35μg) oral contraceptives. This risk is not related to the type of the progestin or duration of oral contraception use.
In contrast, oral contraceptive users with additional risk factors, including age greater than 35 years, cigarette smoking, or hypertension, are at slightly increased risk of hemorrhagic stroke.86 Women older than age 35 have a twofold increased risk, cigarette smokers have a threefold increased risk, and those with a history of hypertension have a 10- to 15-fold increased risk of hemorrhagic stroke while using oral contraceptives.86 Although the risk of hemorrhagic stroke for reproductive-age women is extremely low, mortality can be as high as 25%.
Myocardial Infarction
Myocardial infarction is a rare occurrence in reproductive-age women (see Table 26-3), but the fatality rate is near 30%.72 The risk of myocardial infarction is not increased in women using low-estrogen (≤35μg) oral contraceptives who have no other risk factors such as cigarette smoking, diabetes, or hypertension. One study suggested that oral contraceptives containing gestodene or desogestrel might actually reduce risk of myocardial infarction compared to preparations containing levonorgestrel, although this finding awaits confirmation by other studies.61
Age is an important risk factor for myocardial infarction. Risk increases exponentially with age, such that women age 40 to 44 have an incidence of myocardial infarction of 30 cases per 100,000 annually.72,74 Fortunately, low-estrogen (≤35μg) oral contraceptives do not increase the relative risk of myocardial infarction further.72 Likewise, the risk of myocardial infarction is not increased by the duration or a past history of oral contraceptive use.72,79,87–89
Cigarette smoking increases the risk of myocardial infarction regardless of whether or not women use oral contraceptives. For women who do not use oral contraceptives, cigarette smoking increases the risk of myocardial infarction by threefold to 10-fold in direct proportion to the number of cigarettes smoked daily.72 For women who use low-dose oral contraceptives, light smoking (<15 cigarettes/day) increases the risk of myocardial infarction threefold and heavy (15 cigarettes/day) increased this risk 20-fold compared to nonsmokers.90
Hypertension also increases the risk of myocardial infarction in reproductive-age women. The relative risk of myocardial infarction among oral contraceptive users with hypertension is at least threefold higher than in those with normal blood pressure.
Cancer Risk
Breast Cancer
Oral contraceptives appear to slightly increase the risk of breast cancer, and this increase persists for 10 years and then disappears. According to a meta-analysis of 54 studies encompassing 53,297 women with breast cancer and 100,239 controls, the relative risk of breast cancer for current users of oral contraceptives is approximately 1.24 compared to never-users.91 Breast cancers detected in oral contraceptive users tend to be localized, with a relative risk of metastases of 0.88 compared to nonusers. The increased risk of breast cancer associated with oral contraceptives is not related to hormone dose, specific formulation, duration of use, age at first use, age at time of cancer diagnosis, or family history of breast cancer.
It is not universally accepted that oral contraceptives increase the risk of breast cancer, and many investigators attribute positive studies to detection bias. A recent case-control study found no increased in the risk of breast cancer for women currently taking oral contraceptives or for these same women later in life, where the risk is highest.92 This multicenter, population-based study, which involved 4575 breast cancer subjects and 4682 controls, found a relative risk of breast cancer of 1.0 (95% CI, 0.8–1.3) among current users of oral contraceptives and 0.9 (95% CI, 0.8–1.0) among former users. The relative risk did not increase with race, earlier age of starting oral contraceptives, higher estrogen doses, or positive family history of breast cancer.
Cervical Cancer
Oral contraceptives appear to be associated with an increased risk of cervical cancer, especially in women with evidence of human papillomavirus infections.93 This cervical cancer risk appears to be related to duration of oral contraceptive use.94–96 With less than 5 years of oral contraceptive use, the relative risk of cervical cancer is 1.1 (95% CI, 1.1–1.2), whereas with 10 or more years of use the relative risk increases to 2.2 (95% CI, 1.9–2.4). The risk appears to be roughly the same for squamous carcinoma versus adenocarcinoma, as well as for in situ versus invasive disease.
Reproductive Risks
Birth Defects
When women using oral contraceptives accidentally conceive, they often inadvertently continue taking oral contraceptives for a short time early in the first trimester. Despite some early concerns, the vast preponderance of available evidence indicates that these women do not have an increased risk of fetal anomalies compared to the general population. Multiple prospective studies have failed to find an association between oral contraceptives and birth defects, in agreement with the results of most of the better-designed case-control studies.97
Future Fertility
The return of menses and the achievement of a pregnancy may be slightly delayed after discontinuation of oral contraceptives, but fertility rates return to normal within 1 year.98 There is no relationship between oral contraceptive use and pituitary adenomas or subsequent amenorrhea.
COMMON SIDE EFFECTS OF COMBINATION ORAL CONTRACEPTIVES AND THEIR MANAGEMENT
Oral contraceptives are associated with bothersome side effects in some patients. Approximately one third of women will discontinue contraceptives within 6 months of starting, and in almost half of these women, the cause will be side effects.99
Headache
If a migraine headache is suspected, combination oral contraceptives should be used with care. Although there are no conclusive data on differences in stroke risk for migraine with or without aura, oral contraceptive use in the former circumstance should be considered only if other contraceptive methods cannot be used and only after detailed counseling. If migraine occurs without aura in the absence of other risk factors, most women can be given a trial of oral contraceptives. Follow-up should be initiated within the first 2 to 3 months to assess frequency and severity of headaches. If there is an increase in frequency during the trial, a change in formulation might be considered. If headache occurs more frequently in the hormone-free interval, the physician might consider the use of extended-use oral contraceptives without a hormone-free interval. However, if the headaches become significantly more severe or frequent, oral contraceptives should be discontinued.
Irregular Bleeding
As many as 39% of women starting oral contraceptives with previously normal cycles will experience irregular bleeding, midcycle spotting, especially within the first 3 months.100 Women with a history of irregular menses may be even more likely to experience heavy or irregular bleeding initially. Long-term users sometimes develop amenorrhea. It is important to forewarn patients to expect changes in bleeding and to reassure them that this should resolve within three cycles and is not a sign of cancer.
Drug Interactions with Oral Contraceptives
A small number of drugs may interact with oral contraceptives (Table 26-4). There is no substantive evidence that commonly used antibiotics (e.g., tetracyclines, penicillins, or cephalosporins) or analgesics (e.g., aspirin or acetaminophen) decrease the efficacy of oral contraceptives.
Table 26-4 Drugs and Their Potential Interactions with Oral Contraceptives
| Drug | Action | Effects |
|---|---|---|
| Rifampin | Induces cytochrome P450 enzyme | Increased steroid metabolism; decreased contraceptive efficacy |
| Griseofulvin | Induces hepatic enzymes | Increased steroid metabolism; decreased contraceptive efficacy |
Special Situations
Breastfeeding
The existing randomized, controlled trials are insufficient to establish an effect of hormonal contraception, if any, on milk quality and quantity.101 Based on the best available data, it appears that hormonal oral contraceptives can be used immediately postpartum. Many clinicians prefer to give progestin-only contraceptives during the first 6 months after delivery because they have no apparent deleterious effect on breastfeeding.102
ALTERNATIVE FORMULATIONS AND REGIMENS
Extended-use Oral Contraceptives
Extended-use oral contraceptives offer an approach whereby active combination oral contraceptive pills are taken daily for 84 days followed by a 7 day hormone-free period, rather than the usual 21 days of active pills followed by a 7-day hormone-free period used for monthly regimens. The purpose is to limit the number of withdrawal menstrual periods to four per year. This is an effective approach for the treatment of any medical condition that causes dysmenorrhea or menorrhagia or is exacerbated by menstruation, including hemorrhagic diathesis, endometriosis, uterine leiomyoma, migraine headaches, and epilepsy.103–105 It can also be used for women who electively desire to reduce their days of menstrual bleeding.
Originally, standard low-estrogen (≤35 μg) combination oral contraceptive pills were used for this method by having the patient discard the inert pills from four monthly packages and take one of the remaining 84 active pills daily. There is now an FDA-approved prepackaged system commercially available containing 30 μg of ethinyl estradiol and 0.15 mg levonorgestrel in each pill (see Table 26-1).
Efficacy
This regimen appears to have an increased contraceptive efficacy compared to monthly regimens and may be less likely to result in unintended pregnancies when a pill is missed.105
Side Effects
This approach minimizes predictable withdrawal bleeding but irregular breakthrough bleeding can occur. Accordingly, the number of bleeding days are reduced but not eliminated by this approach.106 Breakthrough bleeding occurred primarily in the initial cycles of extended-use contraception, with 59% of users still experiencing unscheduled bleeding in the fourth cycle of use. Approximately 8% of women will discontinue extended-use contraception due to irregular bleeding, compared to 2% women using a monthly pill cycle. An additional 33% will discontinue extended-use contraception due to weight gain, acne, and mood swings.
Progestin-only Contraceptive Pills
Mechanisms of Action
Progestin-only pills do not appear to dependably inhibit ovulation.107 The contraceptive effects of this formulation are related to the ability of progestins to thin the endometrium, to thicken the cervical mucus, and possibly to interfere with tubal transport. Approximately 40% of women ovulate normally when using a progestin-only pill, even when no pills are missed.
Efficacy
The reported user failure rates for progestin-only pills are from 1% to 10% per 100 women in the first year of use.10
Breastfeeding
The progestin-only pill is the oral contraceptive of choice during the first 6 months of breastfeeding, because this formulation has been shown to have no measurable adverse effect on milk volume or infant growth.102,108 Another advantage of this estrogen-free contraceptive method is that the already elevated risk of venous thromboembolism in the immediate postpartum period is not affected. Many lactating women using this type of contraception will have prolonged amenorrhea. When breastfeeding is stopped, it is reasonable to change to a combination estrogen/progestin pill if irregular bleeding becomes a problem.
Emergency Contraception
Emergency contraception is an approach to prevent pregnancy after unprotected intercourse or known failure of a contraceptive method such as a broken or leaking condom.109 Two oral hormonal methods are currently available.
Levonorgestrel-Only
The levonorgestrel-only regimen, available commercially as Plan B, consists of administration of two doses of 0.75mg of levonorgestrel 12 hours apart. The initial dose should be taken within 72 hours of intercourse, although recent data suggests that the first dose can be taken as long as 5 days after intercourse.110 Further, there is data to suggest that a single dose of 1.5mg of levonorgestrel may be as effective as the two-dose regimen.110
Efficacy
Both emergency contraception formulations prevent pregnancy by delaying or inhibiting ovulation or by disrupting the function of the corpus luteum regardless of the day of the menstrual cycle.111,112 Both methods prevent almost 75% of expected pregnancies.113 One episode of unprotected intercourse near midcycle carries a pregnancy risk of about 8%. Use of the combination hormonal method or the levonorgestrel-only method reduces this rate to 2% and 1%, respectively. The earlier emergency contraception is initiated, the more effective it is.11,110
Side Effects
Using the Yuzpe regimen, approximately 50% of women will experience nausea, and 20% will have vomiting.110 Levonorgestrel emergency contraception is associated with substantially less nausea (23%) and vomiting (6%). These symptoms can be minimized by administrating an antiemetic (e.g., meclizine) 1 hour before taking the first pills.114
TRANSDERMAL CONTRACEPTIVE PATCH
Formulation
The transdermal contraceptive patch is a three-layer matrix system consisting of an outer polyester protective layer, a middle layer containing an adhesive and the contraceptive steroids, and an inner clear polyester liner that is removed before application to the skin.115 Its size is 20 cm2, roughly the size of a matchbook.
After each patch has been worn for 7 days, it is removed and a new one is applied to another skin site. Three consecutive 7-day patches are applied in a typical cycle followed by a 7-day patch-free period to allow withdrawal bleeding. Application sites that have been determined to be therapeutically equivalent in clinical trials include the buttocks, lower abdomen, upper outer arm, and upper torso except for the breasts.116
Efficacy
The pregnancy rate for the transdermal contraceptive patch is less than 1% per 100 women-years.115,117,118 These rates are comparable to pregnancy rates achieved with current oral contraceptives. There is also data to indicate that it is easier for women to use this method correctly compared to oral contraceptives.119 The rate of perfect compliance for the patch did not vary by age, whereas younger women pill users had lower compliance rates. Among the pregnancies reported, about one-third occurred in women weighing more than 90 kg. However, a Food and Drug Administration (FDA) report has identified a potential increase in the risk of venous thromboembolism.123
Metabolic Changes
The contraceptive patch changes serum lipids and lipoproteins in a manner similar to that seen with oral contraceptives. Contraceptive patch users have some increase in total cholesterol, HDL-C, LDL-C, and triglycerides, but the LDL-C to HDL-C ratio is unaffected.120
The transdermal patch, much like combination oral contraceptives, increase conversion of prothrombin to thrombin, which appears to be balanced by increased fibrinolysis.121 Both the patch and oral contraceptives increase the coagulation activity marker prothombin fragment 1+2, markers for fibrinolysis, fibrin degradation products, and plasma α2-antiplasmin.
Major Risks
Serious adverse events have been reported that are potentially attributable to the patch, including nonfatal pulmonary embolism, migraine headache, cholecysititis, and carcinoma in situ of the cervix.122 Although the rates of serious risks remain unknown, these rates may be similar to those for combination oral contraceptives because the transdermal patch contains the same hormones. However, a Food and Drug Administration (FDA) report has identified a potential increase in risk of venous thromboembolism.123
Side Effects
The most frequently reported side effects of the transdermal patch include irregular bleeding, breast symptoms, headache, nausea, dysmenorrhea, and application site reactions.122 There is no evidence that use of the patch influences body weight.
Management Issues
Patch Adherence
All transdermal delivery systems must remain adherent to be effective. Approximately 1.8% of transdermal patches will become completely detached and 2.9% partially detached prematurely.122 The contraceptive patch is designed to remain in place during activities such as saunas, whirlpool baths, and strenuous exercise. Skin adherence does not appear to be adversely affected by a vigorous, athletic lifestyle or warm, humid climates.
When patches detach, users should attempt to re-attach them if possible but should not attempt to use ancillary adhesives or tape. If detachment has occurred for less than 24 hours, the cycle continues as usual with the patch being changed on the previously determined change day. If detachment has occurred for more than 24 hours, a new patch should be applied, backup contraception should be used for 1 week, and the day that the new patch is applied now becomes the patch change day.
CONTRACEPTIVE VAGINAL RING
Formulation
The contraceptive vaginal ring is flexible, is composed of ethylene vinyl acetate copolymer, and is approximately 5 cm in diameter and 4 mm in thickness. Contact of the ring with the vaginal wall leads to release of ethinyl estradiol at a rate of 15μg daily and etonogestrel (the active metabolite of desogestrel) at 120μg daily.124 After vaginal insertion, maximum serum hormone concentrations are reached in about 1 week for progestin, and in 2 or 3 days for estrogen.123,124 After reaching maximum serum concentrations, these serum levels slowly decline. The vaginal ring results in a relatively steady serum hormone concentration similar to that seen with the contraceptive patch, in contrast to the concentration peaks and troughs seen with oral contraceptives.
Mechanism of Action
The vaginal ring provides contraception by inhibiting ovulation with ovarian suppression similar to that achieved by oral contraceptives. This has been documented by transvaginal ultrasound measurements of follicular activity and measurements of luteinizing hormone and progesterone.124
Efficacy
The reported pregnancy rate for the vaginal ring is 0.65% per 100 women-years.125 The ring maintains its efficacy even if removed for up to 3 hours, although it is designed to be left in place continuously, even during intercourse. If removed for longer than 3 hours, a backup method for contraception should be used until the ring has been in place for 7 more days. The ring has not been studied extensively in heavier women, and thus the effect of weight on efficacy is unknown.
Side Effects
Approximately 10% to 15% of users report vaginal-related symptoms, such as slight discomfort, a sensation of a foreign body, leukorrhea, vaginitis, or coital problems. Systemic side effects are similar to those seen with users of combination oral contraceptives. However, the vaginal ring appears to have a low frequency of irregular bleeding and spotting, which occurs in only 5% of cycles. This compares favorably to the incidence of irregular bleeding seen with oral contraceptives, which occurs in 5% to 39%.100,125 The irregular bleeding pattern is relatively constant for all cycles, in contrast to the declining incidence seen after several cycles of oral contraceptives.
Specific Management Issues
Overall acceptance of the ring is high, with 95% of users indicating satisfaction and 97% indicating they would recommend it to others.126 Major reasons cited for liking the ring include ease of remembering how to use (45%) and overall ease of use (27%). Approximately 90% of users report using the ring according to the instructions, a rate similar to that seen with the transdermal patch.
INJECTABLE CONTRACEPTION
Formulation
Depot medroxyprogesterone acetate (DMPA) is an aqueous suspension of 17-acetoxy-6-methyl progesterone. It is the only injectable contraception currently available in the United States.127 Although utilized as a contraceptive for at least 40 years, the FDA did not approve its use for contraception until 1992. The usual dose is 150 mg every 3 months, administered intramuscularly into either the gluteus maximus or deltoid muscles. DMPA is also available as a subcutaneous injection of 104 mg every 3 months.
Mechanism of Action
The primary mechanism of action of DMPA is suppression of ovulation by suppressing the surge of gonadotropins.128 Despite suppression of gonadotropins, the ovary continues to produce estradiol at levels found in the early follicular phase of the menstrual cycle.129 Accordingly, women using DMPA do not exhibit signs or symptoms of estrogen deficiency. Other actions include thickening cervical mucus to impede ascent of sperm and thinning of the endometrium such that implantation of a blastocyst is less likely.
Efficacy
The reported method failure rate for DMPA injections is 0.3% (0.3 pregnancies per 100 women-years), whereas the typical user failure rate is 3%.1 The contraceptive effectiveness of a DMPA injection persists for at least 16 weeks. This is longer than the manufacturer’s stated effectiveness of only 13 weeks after an injection, allowing for at least a 3-week margin of safety when administration of a subsequent injection is delayed.130
Noncontraceptive Benefits
A number of gynecologic health benefits are associated with use of DMPA.130–132 The risk of ectopic pregnancy is significantly lower among users compared to women not using contraception. Similar to oral contraceptives, DMPA reduces the risk of endometrial cancer by as much as 80%.133 The risk reduction is long-term and is greater for women who use DMPA for prolonged periods. Although DMPA does not protect against ovarian or breast cancer, neither does it increase risk of these malignancies.
DMPA has nongynecologic health benefits as well. Sickle cell crises are reduced in frequency by as much as 70%, although the mechanism for this effect remains unknown.134 DMPA also appears to reduce the risk of seizure activity among women with epilepsy.
Metabolic Changes
The metabolic changes associated with DMPA are for the most part minor. Although there are small changes in coagulation factors, these are not felt to be clinically significant.135 There are no increases in procoagulant factors, probably related to the fact that DMPA does not increase hepatic globulin production.136 Relative to lipids and lipoproteins, studies have shown either small declines in HDL-C levels and increases in LDL-C or no changes at all.135,137,138 As with most progestins, there is evidence that DMPA impairs glucose tolerance in some users, although these changes are not clinically significant.139,140
Major Risks
Reduced Bone Mineral Density
The most significant potential risk associated with DMPA use is a reduction in bone mineral density.130,141 DMPA decreases serum estrogen levels, which can lead to loss in bone mineral density. In women who use the drug for 5 years, bone mineral density in the spine and hip is reduced by 5% to 6% below baseline.141 Loss of bone mineral density is greatest in the first 2 years of use. In all age groups, when DMPA is discontinued bone density is usually restored to normal within 2 to 3 years.
Cardiovascular Disease
There is no evidence that DMPA or other progestin-only contraceptives increase cardiovascular risks, such as venous thrombosis, myocardial infarction, and stroke.142
Malignancies
There is no evidence that DMPA increases the risk of endometrial, ovarian, or cervical cancer.130 Earlier case-control studies examining the risk of DMPA and breast cancer found that although overall women who used DMPA had no increased risk of breast cancer, a subset of women who had started using DMPA within the previous 5 years had a twofold increase in risk.143 However, a more recent study conducted in the United States failed to show any significant risk of breast cancer among DMPA users.144
Side Effects
Menstrual Pattern Alterations
For the first 6 months of DMPA use, irregular spotting and prolonged menstrual flow is common. For women with troublesome irregular bleeding, the use of short courses of nonsteroidal anti-inflammatory agents is often helpful. With continued use, many women experience amenorrhea. The amenorrhea rate is 20% by 3 months of use and 70% after 1 year.130
Delayed Fertility
Return to fertility is delayed in patients who have used DMPA for contraception. When DMPA users stop injections in an effort to achieve pregnancy, the return to baseline fertility takes an average 10 months.145
CONTRACEPTIVE IMPLANTS
Implanon is the only contraceptive implant approved in the United States.148 It is a single implant 40mm in length and 2mm in diameter with an ethinylene vinyl acetate core that contains 68mg of etonogestrel, the major metabolite of desogestrel. The progestin is released at rates that vary from 60μg per day initially and slowly decreases to 30μg daily after two years. The device is placed using a preloaded applicator in the superficial subdermal tissue on the inner aspect of the non dominant upper arm and removed after three years.
SUMMARY
Safe and effective contraceptive methods are an important component of modern society. Currently, several hormonal methods are available, each with distinct advantages and disadvantages. There are several standard contraindications to use of hormonal contraceptive methods that utilize estrogen (see Table 26-4). For many women with these contraindications, progestin-only hormonal methods are an important alternative contraception option. Successful use of hormonal contraceptive methods requires that patients be counseled on proper use of the methods, expected risks and side effects, and any potential noncontraceptive health benefits. Finally, emergency contraception using oral hormones is a highly effective backup method when other methods have failed or been used incorrectly.
1 Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30:24-29.
2 Piccinino LJ, Mosher WD. Trends in contraceptive use in the United States: 1982–1995. Fam Plann Perspect. 1998;30:4-10.
3 Goldzieher JW. Hormonal contraception—whence, how and whither? In: Givens J, editor. Clinical Uses of Steroids. Chicago: Yearbook Medical Publishers, 1980.
4 Medvei VC. A History of Endocrinology. Hingham, Mass.: MTB Press, 1982.
5 Burkman RT. Handbook of Contraception and Abortion. Boston: Little, Brown and Co., 1989.
6 Parsey KS, Pong A. An open-label, multicenter study to evaluate Yasmin, a low-dose combination oral contraceptive containing drospirenone, a new progestogen. Contraception. 2000;61:105-111.
7 Sangthawan M, Taneepanichskul S. A comparative study of monophasic oral contraceptives containing either drospirenone 3 mg or levonorgestrel 150 μg on premenstrual symptoms. Contraception. 2005;71:1-7.
8 Trussell J, Vaughan B. Contraceptive failure, method-related discontinuation and resumption of use: Results from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:64-72.
9 Potter L, Oakley D, de Leon-Wong E, Canamar R. Measuring compliance among oral contraceptive users. Fam Plann Perspect. 1996;28:154-158.
10 Trussell J, Kost K. Contraceptive failure in the United States: A critical review of the literature. Stud Fam Plann. 1987;18:237-283.
11 Trussell J, Rodriguez G, Ellertson C. Updated estimates of the effectiveness of the Yuzpe regimen of emergency contraception. Contraception. 1999;59:147-151.
12 Trussel LJ, Hatcher RA, Cates W, et al. A guide to interpreting contraceptive efficacy studies. Obstet Gynecol. 1990;76:558-567.
13 Peterson HB, Lee NC. The health effects of oral contraceptives: Misperceptions, controversies, and continuing good news. Clin Obstet Gynecol. 1989;32:339-355.
14 Mishell DRJr. Noncontraceptive health benefits of oral steroidal contraceptives. Am J Obstet Gynecol. 1982;142:809-816.
15 Burkman RTJr. Noncontraceptive effects of hormonal contraceptives: Bone mass, sexually transmitted disease and pelvic inflammatory disease, cardiovascular disease, menstrual function, and future fertility. Am J Obstet Gynecol. 1994;170:1569-1575.
16 Ness RB, Soper DE, Holley RL, et al. Hormonal and barrier contraception and risk of upper genital tract disease in the PID Evaluation and Clinical Health (PEACH) study. Am J Obstet Gynecol. 2001;185:121-127.
17 Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives. A practitioner’s guide to meta-analysis. Hum Reprod. 1997;12:1851-1863.
18 Sherman ME, Sturgeon S, Brinton LA, et al. Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomas. Mod Pathol. 1997;10:963-968.
19 Vessey MP, Painter R. Endometrial and ovarian cancer and oral contraceptives—findings in a large cohort study. Br J Cancer. 1995;71:1340-1342.
20 Hankinson SE, Colditz GA, Hunter DJ, et al. A quantitative assessment of oral contraceptive use and risk of ovarian cancer. Obstet Gynecol. 1992;80:708-714.
21 Piver MS, Baker TR, Jishi MF, et al. Familial ovarian cancer. A report of 658 families from the Gilda Radner Familial Ovarian Cancer Registry 1981–1991. Cancer. 1993;71:582-588.
22 Risch HA, Marrett LD, Jain M, Howe GR. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol. 1996;144:363-372.
23 Rodriguez GC, Walmer DK, Cline M, et al. Effect of progestin on the ovarian epithelium of macaques: Cancer prevention through apoptosis? J Soc Gynecol Investig. 1998;5:271-276.
24 Martinez ME, Grodstein F, Giovannucci E, et al. A prospective study of reproductive factors, oral contraceptive use, and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:1-5.
25 Potter JD, McMichael AJ. Large bowel cancer in women in relation to reproductive and hormonal factors: A case-control study. J Natl Cancer Inst. 1983;71:703-709.
26 Fernandez E, La Vecchia C, Franceschi S, et al. Oral contraceptive use and risk of colorectal cancer. Epidemiology. 1998;9:295-300.
27 Troisi R, Schairer C, Chow WH, et al. A prospective study of menopausal hormones and risk of colorectal cancer (United States). Cancer Causes Control. 1997;8:130-138.
28 Crandall CJ. Estrogen replacement therapy and colon cancer: A clinical review. J Womens Health Gend Based Med. 1999;8:1155-1166.
29 Klein JR, Litt IF. Epidemiology of adolescent dysmenorrhea. Pediatrics. 1981;68:661-664.
30 Wilson CA, Keye WRJr. A survey of adolescent dysmenorrhea and premenstrual symptom frequency. A model program for prevention, detection, and treatment. J Adolesc Health Care. 1989;10:317-322.
31 van Hooff MH, Hirasing RA, Kaptein MB, et al. The use of oral contraceptives by adolescents for contraception, menstrual cycle problems or acne. Acta Obstet Gynecol Scand. 1998;77:898-904.
32 Weng LJ, Xu D, Zheng HZ, et al. Clinical experience with triphasic oral contraceptive (Triquilar) in 527 women in China. Contraception. 1991;43:263-271.
33 Milman N, Clausen J, Byg KE. Iron status in 268 Danish women aged 18–30 years: Influence of menstruation, contraceptive method, and iron supplementation. Ann Hematol. 1998;77:13-19.
34 Davis A, Lippman J, Godwin A, et al. Triphasic norgestimate/ethinyl estradiol oral contraceptive for the treatment of dysfunctional uterine bleeding. Obstet Gynecol. 2000;95:S84.
35 Dawood MY. Dysmenorrhea. Clin Obstet Gynecol. 1990;33:168-178.
36 Chan WY, Dawood MY. Prostaglandin levels in menstrual fluid of nondysmenorrheic and of dysmenorrheic subjects with and without oral contraceptive or ibuprofen therapy. Adv Prostaglandin Thromboxane Res. 1980;8:1443-1447.
37 Brinton LA, Vessey MP, Flavel R, Yeates D. Risk factors for benign breast disease. Am J Epidemiol. 1981;113:203-214.
38 Charreau I, Plu-Bureau G, Bachelot A, et al. Oral contraceptive use and risk of benign breast disease in a French case-control study of young women. Eur J Cancer Prev. 1993;2:147-154.
39 Lanes SF, Birmann B, Walker AM, Singer S. Oral contraceptive type and functional ovarian cysts. Am J Obstet Gynecol. 1992;166:956-961.
40 Young RL, Snabes MC, Frank ML, Reilly M. A randomized, double-blind, placebo-controlled comparison of the impact of low-dose and triphasic oral contraceptives on follicular development. Am J Obstet Gynecol. 1992;167:678-682.
41 Holt VL, Cushing-Haugen KL, Daling JR. Oral contraceptives, tubal sterilization, and functional ovarian cyst risk. Obstet Gynecol. 2003;102:252-258.
42 Tayob Y, Adams J, Jacobs HS, Guillebaud J. Ultrasound demonstration of increased frequency of functional ovarian cysts in women using progestogen-only oral contraception. BJOG. 1985;92:1003-1009.
43 Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
44 Redmond GP, Olson WH, Lippman JS, et al. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: A randomized, placebo-controlled trial. Obstet Gynecol. 1997;89:615-622.
45 DeCherney A. Bone-sparing properties of oral contraceptives. Am J Obstet Gynecol. 1996;174:15-20.
46 Michaelsson K, Baron JA, Farahmand BY, et al. Oral contraceptive use and risk of hip fracture: A case-control study. Lancet. 1999;353:1481-1484.
47 Spector TD, Hochberg MC. The protective effect of the oral contraceptive pill on rheumatoid arthritis: An overview of the analytic epidemiological studies using meta-analysis. J Clin Epidemiol. 1990;43:1221-1230.
48 Gaspard UJ, Lefebvre PJ. Clinical aspects of the relationship between oral contraceptives, abnormalities in carbohydrate metabolism, and the development of cardiovascular disease. Am J Obstet Gynecol. 1990;163:334-343.
49 Kjos SL, Shoupe D, Douyan S, et al. Effect of low-dose oral contraceptives on carbohydrate and lipid metabolism in women with recent gestational diabetes: Results of a controlled, randomized, prospective study. Am J Obstet Gynecol. 1990;163:1822-1827.
50 Guzick DS. Polycystic ovary syndrome. Obstet Gynecol. 2004;103:181-193.
51 Kjos SL, Peters RK, Xiang A, et al. Contraception and the risk of type 2 diabetes mellitus in Latina women with prior gestational diabetes mellitus. JAMA. 1998;280:533-538.
52 LaRosa JC. Effects of oral contraceptives on circulating lipids and lipoproteins: Maximizing benefit, minimizing risk. Int J Fertil. 1989;34(Suppl):71-84.
53 Chapdelaine A, Desmarais JL, Derman RJ. Clinical evidence of the minimal androgenic activity of norgestimate. Int J Fertil. 1989;34:347-352.
54 Lobo RA, Skinner JB, Lippman JS, Cirillo SJ. Plasma lipids and desogestrel and ethinyl estradiol: A meta-analysis. Fertil Steril. 1996;65:1100-1109.
55 Speroff L, DeCherney A. Evaluation of a new generation of oral contraceptives. The Advisory Board for the New Progestins. Obstet Gynecol. 1993;81:1034-1047.
56 Conard J. Biological coagulation findings in third-generation oral contraceptives. Hum Reprod Update. 1999;5:672-680.
57 Stadel BV. Oral contraceptives and cardiovascular disease (first of two parts). NEJM. 1981;305:612-618.
58 Stadel BV. Oral contraceptives and cardiovascular disease (second of two parts). NEJM. 1981;305:672-677.
59 Gerstman BB, Piper JM, Tomita DK, et al. Oral contraceptive estrogen dose and the risk of deep venous thromboembolic disease. Am J Epidemiol. 1991;133:32-37.
60 Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, et al. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet. 1995;346:1593-1596.
61 Lewis MA, Heinemann LA, Spitzer WO, et al. The use of oral contraceptives and the occurrence of acute myocardial infarction in young women. Results from the Transnational Study on Oral Contraceptives and the Health of Young Women. Contraception. 1997;56:129-140.
62 Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
63 Spitzer WO. Bias versus causality: Interpreting recent evidence of oral contraceptive studies. Am J Obstet Gynecol. 1998;179:S43-S50.
64 Lawrenson R, Farmer R. Venous thromboembolism and combined oral contraceptives: Does the type of progestogen make a difference? Contraception. 2000;62:21S-28S.
65 Spitzer WO. Oral contraceptives and cardiovascular outcomes: Cause or bias? Contraception. 2000;62:3S-9S.
66 Farmer RD, Williams TJ, Simpson EL, Nightingale AL. Effect of 1995 pill scare on rates of venous thromboembolism among women taking combined oral contraceptives: Analysis of general practice research database. BMJ. 2000;321:477-479.
67 Jick H, Kaye JA, Vasilakis-Scaramozza C, Jick SS. Risk of venous thromboembolism among users of third generation oral contraceptives compared with users of oral contraceptives with levonorgestrel before and after 1995: Cohort and case-control analysis. BMJ. 2000;321:1190-1195.
68 Skegg DC. Pitfalls of pharmacoepidemiology. BMJ. 2000;321:1171-1172.
69 Winkler UH. Hemostatic effects of third- and second-generation oral contraceptives: Absence of a causal mechanism for a difference in risk of venous thromboembolism. Contraception. 2000;62:11S-20S.
70 Vandenbroucke JP, Rosing J, Bloemenkamp KW, et al. Oral contraceptives and the risk of venous thrombosis. NEJM. 2001;344:1527-1535.
71 Kemmeren JM, Algra A, Grobbee DE. Third generation oral contraceptives and risk of venous thrombosis: Meta-analysis. BMJ. 2001;323:131-134.
72 WHO Scientific Group. Cardiovascular Disease and Steroid Hormone Contraception. Geneva: World Health Organization, 1998.
73 Dahlback B, Carlsson M, Svensson PJ. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: Prediction of a cofactor to activated protein C. Proc Natl Acad Sci USA. 1993;90:1004-1008.
74 Consensus conference on combination oral contraceptives and cardiovascular disease. Fertil Steril. 1999;71:1S-6S.
75 Heinemann LA, Lewis MA, Thorogood M, et al. Case-control study of oral contraceptives and risk of thromboembolic stroke: Results from International Study on Oral Contraceptives and Health of Young Women. BMJ. 1997;315:1502-1504.
76 Lidegaard O. Oral contraception and risk of a cerebral thromboembolic attack: Results of a case-control study. B. 1993;306:956-963.
77 Vessey MP, Lawless M, Yeates D. Oral contraceptives and stroke: Findings in a large prospective study. Clin Res Ed. 1984;289:530-531.
78 Ischaemic stroke and combined oral contraceptives. Results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348:498-505.
79 Sidney S, Siscovick DS, Petitti DB, et al. Myocardial infarction and use of low-dose oral contraceptives: A pooled analysis of 2 U.S. studies. Circulation. 1998;98:1058-1063.
80 Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833.
81 Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: Case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
82 Petitti DB, Sidney S, Bernstein A, et al. Stroke in users of low-dose oral contraceptives. NEJM. 1996;335:8-15.
83 Hannaford PC, Croft PR, Kay CR. Oral contraception and stroke. Evidence from the Royal College of General Practitioners’ Oral Contraception Study. Stroke. 1994;25:935-942.
84 Schwartz SM, Siscovick DS, Longstreth WTJr, et al. Use of low-dose oral contraceptives and stroke in young women. Ann Intern Med. 1997;127:596-603.
85 Thorogood M, Mann J, Murphy M, Vessey M. Fatal stroke and use of oral contraceptives: Findings from a case-control study. Am J Epidemiol. 1992;136:35-45.
86 Haemorrhagic stroke, overall stroke risk, and combined oral contraceptives: Results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348:505-510.
87 Rosenberg L, Palmer JR, Lesko SM, Shapiro S. Oral contraceptive use and the risk of myocardial infarction. Am J Epidemiol. 1990;131:1009-1016.
88 Stampfer MJ, Willett WC, Colditz GA, et al. A prospective study of past use of oral contraceptive agents and risk of cardiovascular diseases. NEJM. 1988;319:1313-1317.
89 Croft P, Hannaford PC. Risk factors for acute myocardial infarction in women: Evidence from the Royal College of General Practitioners’ oral contraception study. BMJ. 1989;298:165-168.
90 Basdevant A, Conard J, Pelissier C, et al. Hemostatic and metabolic effects of lowering the ethinyl estradiol dose from 30 μg to 20 μg in oral contraceptives containing desogestrel. Contraception. 1993;48:193-203.
91 Collaborative Group on Hormonal Factors in Breast Cancer (CGoHFiB). Breast cancer and hormonal contraceptives: Collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
92 Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. NEJM. 2002;346:2025-2032.
93 World Health Organization. Cervical cancer, oral contraceptives, and parity. Geneva: WHO, 2002.
94 Smith JS, Green J, Berrington de Gonzalez A, et al. Cervical cancer and use of hormonal contraceptives: A systematic review. Lancet. 2003;361:1159-1167.
95 Green J, Berrington de Gonzalez A, Sweetland S, et al. Risk factors for adenocarcinoma and squamous cell carcinoma of the cervix in women aged 20–44 years: The UK National Case-Control Study of Cervical Cancer. Br J Cancer. 2003;89:2078-2086.
96 Moreno V, Bosch FX, Munoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multicentric case-control study. Lancet. 2002;359:1085-1092.
97 Bracken MB. Oral contraception and congenital malformations in offspring: A review and meta-analysis of the prospective studies. Obstet Gynecol. 1990;76:552-557.
98 Huggins GR, Cullins VE. Fertility after contraception or abortion. Fertil Steril. 1990;54:559-573.
99 Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: A prospective evaluation of frequency and reasons. Am J Obstet Gynecol. 1998;179:577-582.
100 Bjarnadottir RI, Tuppurainen M, Killick SR. Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. Am J Obstet Gynecol. 2002;186:389-395.
101 Truitt ST, Fraser AB, Grimes DA, et al. Combined hormonal versus nonhormonal versus progestin-only contraception in lactation. Cochrane Database Sys Rev. 2003. CD003988.
102 Kelsey JJ. Hormonal contraception and lactation. J Hum Lact. 1996;12:315-318.
103 Sulak PJ, Cressman BE, Waldrop E, et al. Extending the duration of active oral contraceptive pills to manage hormone withdrawal symptoms. Obstet Gynecol. 1997;89:179-183.
104 Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol. 2002;186:1142-1149.
105 Wiegratz I, Kuhl H. Long-cycle treatment with oral contraceptives. Drugs. 2004;64:2447-2462.
106 Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
107 Moghissi KS, Syner FN, McBride LC. Contraceptive mechanism of microdose norethindrone. Obstet Gynecol. 1973;41:585-594.
108 Tankeyoon M, Dusitsin N, Chalapati S, et al. Effects of hormonal contraceptives on milk volume and infant growth. WHO Special Programme of Research, Development and Research Training in Human Reproduction Task force on oral contraceptives. Contraception. 1984;30:505-522.
109 Westhoff C. Clinical practice. Emergency contraception. NEJM. 2003;349:1830-1835.
110 Dunn S, Guilbert E, Lefebvre G, et al. Emergency contraception. J Obstet Gynaecol Can. 2003;25:673-687.
111 Piaggio G, von Hertzen H, Grimes DA, Van Look PF. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Task Force on Postovulatory Methods of Fertility Regulation. Lancet. 1999;353:721.
112 Trussell J, Rodriguez G, Ellertson C. New estimates of the effectiveness of the Yuzpe regimen of emergency contraception. Contraception. 1998;57:363-369.
113 Grimes DA, Raymond EG. Emergency contraception. Ann Intern Med. 2002;137:180-189.
114 Raymond EG, Creinin MD, Barnhart KT, et al. Meclizine for prevention of nausea associated with use of emergency contraceptive pills: A randomized trial. Obstet Gynecol. 2000;95:271-277.
115 Audet MC, Moreau M, Koltun WD, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive: A randomized controlled trial. JAMA. 2001;285:2347-2354.
116 Abrams LS, Skee DM, Natarajan J, et al. Pharmacokinetics of a contraceptive patch (Evra/Ortho Evra) containing norelgestromin and ethinyloestradiol at four application sites. Br J Clin Pharmacol. 2002;53:141-146.
117 Smallwood GH, Meador ML, Lenihan JP, et al. Efficacy and safety of a transdermal contraceptive system. Obstet Gynecol. 2001;98:799-805.
118 Hedon B, Helmerhorst FM, Cronje HS, Shangold G, Fisher AC, Creasy G. Comparison of efficacy, cycle control, compliance, and safety in users of a contraceptive patch vs. an oral contraceptive. Int J Obstet Gynecol. 2000;70:78.
119 Archer DF, Bigrigg A, Smallwood GH, et al. Assessment of compliance with a weekly contraceptive patch (Ortho Evra/Evra) among North American women. Fertil Steril. 2002;77(Suppl 2):27-31.
120 Creasy G, Fisher AC, Hall N, Shangold G. Effect of a contraceptive patch vs. placebo (PBO) on serum lipid profile. Fertil Steril. 2000;74(Suppl):S185.
121 Kluft C. Renewed interest in haemostasis changes induced by oral contraceptives (OCs). Thromb Haemost. 2000;84:1-3.
122 Sibai BM, Odlind V, Meador ML, et al. A comparative and pooled analysis of the safety and tolerability of the contraceptive patch (Ortho Evra/Evra). Fertil Steril. 2002;77(Suppl):S19-S26.
123 United States Food and Drug Administration. Press release, September 21, 2006.
124 Timmer CJ, Mulders TM. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokin. 2000;39:233-242.
125 Roumen FJ, Apter D, Mulders TM, Dieben TO. Efficacy, tolerability and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyl oestradiol. Hum Reprod. 2001;16:469-475.
126 Novak A, de la Loge C, Abetz L, van der Meulen EA. The combined contraceptive vaginal ring, NuvaRing: An international study of user acceptability. Contraception. 2003;67:187-194.
127 Kaunitz AM. Current concepts regarding use of DMPA. J Reprod Med. 2002;47:785-789.
128 Mishell DRJr, Kletzky OA, Brenner PF, et al. The effect of contraceptive steroids on hypothalamic-pituitary function. Am J Obstet Gynecol. 1977;128:60-74.
129 Jeppsson S, Gershagen S, Johansson ED, Rannevik G. Plasma levels of medroxyprogesterone acetate (MPA), sex-hormone binding globulin, gonadal steroids, gonadotrophins and prolactin in women during long-term use of depot-MPA (Depo-Provera) as a contraceptive agent. Acta Endocrinol (Copenh). 1982;99:339-343.
130 Kaunitz AM. Injectable contraception. New and existing options. Obstet Gynecol Clin North Am. 2000;27:741-780.
131 Cullins VE. Noncontraceptive benefits and therapeutic uses of depot medroxyprogesterone acetate. J Reprod Med. 1996;41:428-433.
132 Westhoff C. Depot-medroxyprogesterone acetate injection (Depo-Provera): A highly effective contraceptive option with proven long-term safety. Contraception. 2003;68:75-87.
133 Thomas D, Ray RM. Depot-medroxyprogesterone acetate (DMPA) and risk of endometrial cancer. The WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Int J Cancer. 1991;49:186-190.
134 De Ceulaer K, Gruber C, Hayes R, Serjeant GR. Medroxyprogesterone acetate and homozygous sickle-cell disease. Lancet. 1982;2:229-231.
135 Fahmy K, Khairy M, Allam G, et al. Effect of depot-medroxyprogesterone acetate on coagulation factors and serum lipids in Egyptian women. Contraception. 1991;44:431-444.
136 Fraser IS, Weisberg E. A comprehensive review of injectable contraception with special emphasis on depot medroxyprogesterone acetate. Med J Aust. 1981;1:3-19.
137 Kremer J, de Bruijn HW, Hindriks FR. Serum high density lipoprotein cholesterol levels in women using a contraceptive injection of depot-medroxyprogesterone acetate. Contraception. 1980;22:359-367.
138 Westhoff C. Depot medroxyprogesterone acetate contraception. Metabolic parameters and mood changes. J Reprod Med. 1996;41:401-406.
139 Liew DF, Ng CS, Yong YM, Ratnam SS. Long-term effects of Depo-Provera on carbohydrate and lipid metabolism. Contraception. 1985;31:51-64.
140 Kamau RK, Maina FW, Kigondu C, Mati JK. The effect of low-oestrogen combined pill, progestogen-only pill and medroxyprogesterone acetate on oral glucose tolerance test. East Afr Med J. 1990;67:550-555.
141 Wooltorton E. Medroxyprogesterone acetate (Depo-Provera) and bone mineral density loss. Can Med Assoc J. 2005;172:746.
142 Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives. Results of an international, multicenter, case-control study. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Contraception. 1998;57:315-324.
143 Skegg DC, Noonan EA, Paul C, et al. Depot medroxyprogesterone acetate and breast cancer. A pooled analysis of the World Health Organization and New Zealand studies. JAMA. 1995;273:799-804.
144 Strom BL, Berlin JA, Weber AL, et al. Absence of an effect of injectable and implantable progestin-only contraceptives on subsequent risk of breast cancer. Contraception. 2004;69:353-360.
145 Schwallie PC, Assenzo JR. The effect of depot-medroxyprogesterone acetate on pituitary and ovarian function, and the return of fertility following its discontinuation: A review. Contraception. 1974;10:181-202.
146 Kaunitz AM. Long-acting hormonal contraception: Assessing impact on bone density, weight, and mood. Int J Fertil Womens Med. 1999;44:110-117.
147 Pelkman C. Hormones and weight change. J Reprod Med. 2002;47:791-794.
148 Meckstroth KR, Darney PD. Implant contraception. Semin Reprod Med. 2001;19:339-354.