Chapter 1 History of Spine Injections
Neuraxial Injections
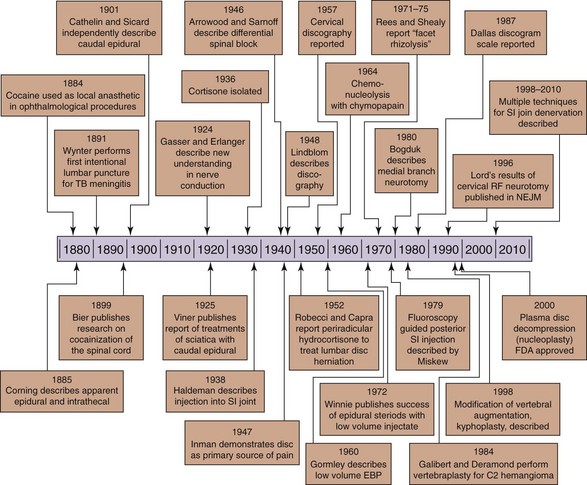
In review of the history of spinal injections for pain management, it is clear that the procedures have been developed and expanded from their original use in anesthesia (Fig. 1-1). After the publication of evidence in 1884 that cocaine could be used to render the cornea insensate for ophthalmologic procedures,1 interest in the ability to anesthetize only the region to be operated upon grew. In 1885, the neurologist James Leonard Corning first described spinal anesthesia,2 which was interestingly a result of a misunderstanding of the anatomy and physiology of the spine and its contents. His intention was to inject cocaine into the interspinal blood vessels, so that it could be delivered to the spine via the communicating vessels in the spinal cord. No such vessels exist, although the correct anatomy had been described in Gray’s Anatomy by 1870.3
Corning used a hypodermic needle with the goal of injecting cocaine into the interspinous vessels. He wrote: “I hoped to produce artificially a temporary condition of things analogous in its physiological consequences to the effect observed in transverse myelitis or after total section of the cord.”2
In his report, he describes injecting 120 mg of cocaine into a male human subject and 13 mg of cocaine into a dog. By description of onset and effects, it appears that the injections probably resulted in epidural anesthesia in the man and spinal anesthesia in the dog. The doses used far exceeded the potential toxic doses, but fortunately, there were no significant complications. Corning had been searching for treatment for neurological diseases but noted that the procedure certainly could have surgical implications.4
Documentation of intentional dural puncture was introduced by Dr. Essex Wynter in 1891. Using a Southey’s tube and trocar, he placed the tube between the lumbar vertebrae after making an incision in the skin for the purpose of draining the fluid in tuberculous meningitis. He noted temporary relief and no complications with the procedure, although none of the patients survived the tuberculous meningitis.5
Six months later, Heinrich Irenaeus Quincke wrote “Die Lumbalpunction des Hydrocephalus.”6 He based his approach on the knowledge of the lumbar anatomy of the continuous subarachnoid space and the end of the spinal cord at approximately L2, which allows for the introduction of a needle below that point, avoiding spinal cord injury. The procedure was introduced for the treatment of hydrocephalus. Quincke improved the technique by the use of needles that were 0.5 to 1.2 mm in diameter, including a stylet in the larger needles. The initial description is a paramedian approach, starting 5 to 10 mm from midline.
The application of the procedure for spinal anesthesia rather than a therapeutic option was developed by a surgeon, Dr. August Bier. He published his findings in 1899 with the title “Versuche uber Cocainisirung des Ruckenmarkes” (“Research on Cocainization of the Spinal Cord”).7 His goal was to use minimal amounts of medication to anesthetize a large region. News and promise of the technique spread quickly, and by October of 1899 Drs. Dudley Tait and Guido Caglieri had tried the approach in San Francisco, becoming the first to do so in the United States.8 By January 1901, a report in The Lancet stated that there were already almost 1000 published reports of spinal anesthesia.9
In 1901, Fernand Cathelin demonstrated the ability to gain access to the epidural space via the caudal approach. He noted that fluids rose in a fashion that was proportional to the volume and speed of the injection.10 Both Cathelin and Dr. John Sicard presented a paper on epidural injections the same year (1901), but the two physicians were working independently.
De Pasquier and Leri attempted intrathecal injections of 5 mg of cocaine at the lumbar level but noted in their results “toxic cocaine accidents . . . to the bulbar and cerebral centers.” Using a rubber band “gently tightened around the neck,” they tried to prevent the flow of cocaine to the brain but were unsuccessful. They claimed a better level of success with sacral epidural injections.11
W. Stoeckel12 published his experience in obstetrical care with the caudal epidural method in 1909 after modifying the method by using the less toxic procaine rather than cocaine. He was interested in the possible spread of medication in the epidural space from a caudal injection and used colored fluid in cadavers to document the extensive spread, including through the sacral foramina.
In 1925, Dr. Norman Viner13 published his experience in treating intractable sciatica with caudal epidural injections. He described his technique of injecting first 20 cc of 1% Novocain followed by 50 to 100 cc of sterile Ringer’s solution, normal saline, or liquid petrolatum. These injections were typically repeated three to four times at weekly intervals. He notes that “liquid petrolatum is frowned on by some on account of the remote possibility of fatty embolism” but goes on to note the overall low risk. He concludes his paper by suggesting the procedure be tried with many other conditions because he believed it could be very successful in the treatment of sciatica.
Cortisone (called Compound E) was discovered in 1936.14,15 Hench et al16,17 reported in a 1950 publication that it could treat rheumatoid arthritis, rheumatic fever, and other conditions as well. A longer acting steroid, Compound F (hydrocortisone) was noted by Hollander18 to reduce the synovial membrane inflammation histologically but even then the author was cautious to state that the action of the steroid was palliative, not curative. The use of steroids to treat many conditions became common during this period.
Claiming that patients’ sciatic pain was a result of inflammation, Robecchi and Capra19 reported using “periradicular” hydrocortisone to treat lumbar disc herniation in 1952. Lievre et al20 described caudal epidural injections as being effective when five of 20 patients improved. No data were reported more than 3 weeks after the injections, and no control subjects were used, so placebo and the natural history of the problem were not addressed. The popularity of caudal epidural injections appears to have increased after this report.
“Pressure caudal anesthesia” was advocated by Brown,21 who used 50 to 70 cc of mixtures of lidocaine, normal saline, and steroid. He noted improved success (100% vs. 32 of 38) when a steroid was added to the normal saline and local anesthetic. Again, however, the lack of control subjects and structured follow-up is notable.
The first clinical description of the technique for a paramidline lumbar approach is credited to Pagés in 1921.22 The procedure then modified to include the loss of resistance technique, introduced by Dogliotti in 1933.23 Gutierrez suggested the hanging drop technique by using the negative pressure of the epidural space in the same year.24 Dogliotti was also the first to describe an epidural injection into the cervical region.23
During the middle of the twentieth century, investigators experimented with treatments using both intrathecal and epidural injections. In the 1950s, there was interest in treating patients with multiple sclerosis with intrathecal steroids, but no control subjects or follow-up were included in these trials.25–27 In later reports,28,29 excitement about the procedure waned as persistent improvement was seen in only a limited portion of the patients.
In the early 1960s, Gardner et al30 tried high-volume epidural injections (20 cc of 1% procaine and 125 mg hydrocortisone) in 239 patients with sciatica. About half of the subjects had failed to obtain relief with surgery. After 57% of the patients failed to get pain relief with the epidural injections, the investigators started using an intrathecal approach with 80 mg of methylprednisolone acetate and 40 mg of procaine. Sixty percent of the 75 subjects noted relief of the sciatic pain for more that 4 months. By 1963, Sehgal and Gardner31 and Sehgal et al32 had treated more than 1000 patients with intrathecal steroids for diverse conditions, but no improvement data or control group was reported.
The transition back to epidural injections began in response to data published by Winnie et al in 1972.33 At the time, there continued to be controversy as to the aspect of the procedure that produced pain relief. The theories proposed were therapeutic benefit from the injections resulted from lysis of adhesions by large volumes of injectate, interruption of the sympathetic reflex mechanisms by the local anesthetic, or the antiinflammatory effect of the steroid. By demonstrating success of epidurals with low-volume injectate, Winnie et al33 proposed that the effect seemed to be from the steroid itself. They further suggested that the success of an injection seemed to be related to the proximity of the injection to the pathology causing the patients’ complaint.
The more recent modifications of epidural injections have occurred as a result of the concern regarding accurate delivery of the medication to the site of pathology. Multiple studies34,35 show that the loss of resistance technique in a lumbar epidural steroid injection results in inaccurate needle placement up to 30% to 40% of the time. The use of fluoroscopy has been encouraged by some to improve accuracy in epidural injections for chronic pain in recent years.36,37 Fredman and Nun38 reported a lower incidence of inaccurate placement into the epidural space during “blind” epidural injections (8.3% failure rate) than previous reports but noted that the intended level of the injection was missed in 53% of the cases. Interestingly, in the cases in which the needle placement was correct, the contrast reached the level of the pathology in only 26% of the patients, largely because of altered anatomy. This study was done on patients with failed back surgery syndrome and highlights the potential difficulty of injections in this population.
The other major modification of the procedure is the transforaminal approach to the epidural space. This technique mandates the use of fluoroscopy. This technique was developed with the recognition that in caudal and translaminar approaches, the medication is delivered into the dorsal aspect of the spinal canal. The dorsal median epidural septum can stop the spread of the medication to the contralateral side.39 The translaminar technique delivers the medication to the ventral aspect of the nerve root sleeve and to the dorsal aspect of the disc herniation.40,41 Although the transforaminal technique is commonly used, one complication that has been particularly concerning is inadvertent arterial injection of particulate steroid, which has resulted in devastating consequences. To decrease the risk, nonparticulate steroid and digital subtraction imaging can be used. Some practitioners have abandoned the procedure altogether because of this risk, particularly in the cervical region.
Epidural Blood Patch
It is interesting to note that the development of a dural puncture headache was described very early in the development of spinal procedures. Quincke,6 while noting some improvement of patients with hydrocephalus after lumbar puncture, also reported that some patients complained of a pattern of pain for several days that would seem consistent with a postdural puncture headache (PDPH). Multiple punctures with a large-bore needle had been used. The observation of edema in the surrounding tissues seems to be evidence of continued cerebrospinal fluid (CSF) leaks.
It was not, however, until 1898 that August Bier42 clearly made the association between dural punctures and subsequent headaches that appeared to have unique characteristics. He reported that three of his first six patients in whom he performed the procedure complained of a headache shortly after the procedure. As an experiment, Dr. Bier and his clinical assistant went on to perform spinal anesthesia on themselves and then developed classical symptoms of PDPH. They documented their personal experience in what makes both interesting and somewhat comical reading today.
After performing these experiments on our own bodies we proceeded without feeling any symptoms to dine and drink wine and smoke cigars. I went to bed at 11 p.m., slept the whole night, awoke the next morning hale and hearty and went for an hour’s walk. Towards the end of the walk I developed a slight headache, which gradually got worse as I went about my daily business. By 3 p.m. I was looking pale and my pulse was fairly weak though regular and about 70 beats per minute. In addition, I had a feeling of very strong pressure on my skull and became rather dizzy when I stood up rapidly from my chair. All these symptoms vanished at once when I lay down flat, but returned when I stood up. Towards the evening I was forced to take to bed and remained there for nine days, because all the manifestations recurred as soon as I got up. … The symptoms finally resolved nine days after the lumbar puncture.42
Treatment of PDPH historically can be viewed as using one of several different approaches. One approach focused on replacing the lost CSF volume to restore the intracranial pressure. Infusions of normal saline into the intrathecal space were attempted, which tended to provide temporary relief but also produced a second dural puncture. The intracranial hypotension would return with the painful symptoms shortly after the infusion was stopped with redistribution of the fluid and pressure.43–47 Such efforts were abandoned in the 1950s. Attempts to increase CSF production by using hypotonic intravenous saline infusions and intramuscular pituitary extract resulted in perhaps some relief for a portion of patients but again did not produce consistent or dramatic results.48,49
In attempt to produce a “splint” type of effect, the second of the approaches, epidural infusions were used.50 This technique avoided the second dural hole in theory but failed to produce long-term pain relief because again the pain would return with redistribution of the fluid shortly after the infusion was stopped.51
The third approach is the one that we are still working with today. The principle is to plug to hole in the dura that is allowing for escape of the CSF. Dr. James Gormley was a general surgeon in the truest sense in a time (1950s and 1960s) when surgeons were also directly involved in the anesthetic care of the patient. Spinal anesthesia was attractive because the surgeon could perform the block and allow the patient to maintain his or her own airway while performing surgery and supervising the nurse for management of vital signs. One of his observations was that bloody taps seemed to result in a lower incidence of PDPH. Also important was the idea that blood in the central neuraxis did not appear to result in disaster as previously believed.4 He published a report of seven cases in which 2 to 3 mL of autologous blood was injected into the epidural space for the treatment of PDPH in 1960.52 He was actually one of these subjects who presented with a PDPH after a myelogram. Although later studies have refuted the notion that bloody taps decrease the incidence of PDPH, it was a fortunate mistaken idea.
In 1960, Dr. Anthony DiGiovanni, having just read Gormley’s letter in Anesthesiology, was asked to help in the care of a woman on the obstetric ward who had a severe headache after a spinal anesthetic. Because the anesthesiologist who did the initial procedure had attempted the injection at multiple levels and could not remember the level of the successful block, Dr. DiGiovanni decided to use a volume of 10 mL of autologous blood, thinking that the higher volume could possibly cover several levels. This resulted in the resolution of the patient’s headache, and Dr. DiGiovanni continued to treat patients presenting with PDPH with this volume as a result of this initial success.4 In subsequent years, he trained many other anesthesiologists in this technique and published his experience with the procedure in 1970.53
This procedure, quite understandably, was met with resistance by many in the field, particularly because of concerns about safety. Animal studies as well as prospective data accumulated with time and suggested that the technique was not only effective but also very safe.54 This led to the general acceptance of the blood patch in the treatment of PDPH.
Given the long history and the well-accepted practice of performing blood patches for PDPH, it is interesting to note the relative lack of evidence from randomized, controlled clinical trials. Van Kooten et al55 published such a study in 2007 that strongly supports the current practice and is interesting to review.
Differential Spinal Blockade
Clinically, the etiology of a patient’s pain is sometimes difficult to define. This has remained true despite recent advances in medicine. In the 1920s, Gasser and Erlanger56,57 published some groundbreaking work in the area of neural conduction. Although incorrect about the site of conduction (mistaking it to be within the axoplasm), they established the idea that fiber size was related to conduction velocity and fiber function. They were able to define three classes (A, B, and C) of nerve fibers and subdivided class A fibers into 4 groups (α, β, γ, and δ). Working with cocaine, they were able to demonstrate that the fibers types appeared to have different sensitivities to local anesthetic.
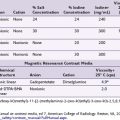
This understanding was the basis for the differential spinal block developed by Sarnoff and Arrowood.58,59 Noting prior animal experimentation suggesting that a low concentration of procaine could selectively abolish the carotid sinus reflex without affecting respiration or motor function, they proceeded to test this principle in patients with varying diagnoses (residual limb pain, herpes zoster, sciatic nerve pain, and inguinal hernia repair pain). The 1948 publication is focused on patients with stump pain or phantom limb pain, with the goal of the study to decipher if the pain was of a local origin or whether it was related to a projection from the sensory cortex. If it were found to be of local origin, the investigators wanted to know if interruption of the sympathetic nervous system would result in pain relief. An initial bolus of 0.2% procaine was injected into the subarachnoid space. This was followed by an infusion of the same concentration, and observations were made regarding pain relief and neurological examination results. The results of the procedure were intended to aid in surgical planning. Table 1-1 shows their results, demonstrating their ability to block some nerve fibers and spare others.
Table 1-1 Fibers That Are and Those That Are Not Blocked by the Introduction of 0.2% Procaine Hydrochloride in Large Amount into the Subarachnoid Space
| Differential Spinal Block | |
|---|---|
| Fibers Blocked | Fibers Spared |
| Vasomotor | Touch |
| Sudomotor | Position sense |
| Visceromotor | Vibration sense |
| Pinprick sensation | Pain, types other than pinprick |
| Stretch afferents | Somatic motor |
If the smaller fibers were successfully blocked without relief of pain, full spinal anesthesia was induced to test if the pain had a local origin. This technique was further modified to the conventional technique as described by Winnie and Candido.60 This technique involved four sequential injections (normal saline, 0.25% procaine, 0.5% procaine, and 5% procaine). If the patient responded to the normal saline, the pain was classified as “psychogenic.” Response to 0.25% procaine was interpreted to mean that the pain was sympathetically mediated because the concentration is usually sufficient to block B fibers but not A-δ and C fibers. No response to the first two injections but pain relief with 0.5% procaine was interpreted as consistent with a somatic pain diagnosis as such a concentration is usually able to block B, A-δ and C fibers without blocking A-α, A-β, and A-γ fibers. The solution of 5% procaine blocked all fiber types, and failure to respond to that solution was interpreted as having a “central mechanism,” the possibilities of which include a central lesion, psychogenic pain, encephalization, and malingering.
Because this type of investigation is clearly time consuming and made with the assumption of a “typical” minimum blocking concentration response for each patient when clinically there is variation, a modification was proposed.61–64 The newer technique requires only two injections, the first with normal saline and the second with 2 cc of 5% procaine. The pain response and neurological examination are then followed with the return of function of the different nerve fibers. This decreases the time for the procedure and does not rely on an average minimal concentration response of the nerve fibers. After a patient recovers sensation, only the sympathetic fibers remain blocked. Pain relief that remains after recovery of sensation suggests a sympathetically mediated pain.
Raj65 presented a similar differential block strategy using the epidural space in 1977. The technique is limited, however, because of the even slower onset of the blockade and even less clear distinctions of the appropriate dose and concentration of local anesthetic for any particular patient compared with the intrathecal approach. In theory, however, the technique has the advantage of avoiding dural puncture.
The theory behind the differential spinal block was challenged by other investigators, including Fink.66 He found that the size of the fiber did not truly explain the differential blockade and proposed the “bathed length principle.” To block conduction of a nerve, at least three consecutive nodes must have adequate local anesthetic exposure. He reasoned that thicker axons have larger intermodal distances, and this decreases the likelihood of blocking the larger axons compared with the smaller fibers. He was also able to explain the differential block of the sympathetic nerves was a result of decremental block. Fink66 explained some of the phenomenon noted during a spinal epidural differential block and contributed to a better understanding of the clinical observations noted during a differential spinal block.
The true utility of these blocks have been questioned in recent years, and the use of the technique has certainly declined. There is a significant range of conduction speed and fiber size within a fiber type. A lack of correlation of size and necessary anesthetic concentration for blockade within a group creates an overlap of the fiber types that seems to “negate any possibility of obtaining steady state differential interruption” by local anesthetics.67,68 The vulnerability of the fiber type to diffusion of the local anesthetic also seems to play a significant role in explaining the timing of the neural blockade.69 The clinical result of the overlap is that a partial block of the A fibers has already occurred by the time C fiber activity is blocked.70
The complex nature of pain often makes interpretation of even well-designed techniques difficult. The differential spinal blocks are a good reminder of not only the complexity of the nervous system but also the important role of performer bias, reliable and valid measurement, placebo response, and patient expectations. Although some authors60 continue to promote the use of this procedure to establishing more accurate diagnosis, others70,71 suggest significant caution in their use and applications.
Injections and Procedures Targeting the Zygapophysial (Facet) Joints
Lumbar medial branch blocks (MBBs) were first described in the late 1970s and were supported by anatomical studies showing that these branches of the lumbar dorsal rami were a valid and accessible target.72–75 The sole purpose of the lumbar MBB is to determine if the patient’s pain is relieved by anesthetizing the nerves targeted. Because the lumbar zygapophysial joints (Z-joints) account for 15% to 40% of low back pain76 and because the lumbar medial branches send an intraarticular branch that supplies these joints, by convention a positive response to the MBB suggests the pain is arising from the Z-joints (facet joints). Although there was a flurry of literature describing intraarticular facet joint corticosteroid injections in the 1980s, the literature suggests that these did not provide lasting relief in the majority of studies. Studies by Dreyfuss et al77 and Kaplan et al78 showed that lumbar MBBs were target specific and a valid test of zygapophysial joint pain. Lumbar medial branch neurotomy has emerged as the treatment of choice for patients with pain arising from the lumbar zygapophysial joints.
Radiofrequency (RF) neurotomy has been used successfully for the treatment of trigeminal neuralgia since the pioneering work by White and Sweet in 1969.79 Early studies by Rees80,81 and Shealy80–85 reporting neurotomy or rhizolysis of the “facets” sparked interest in the Z-joint as a source of pain and target for treatment. Subsequent analysis of their technique, however, lead to conclusions that they were unsuccessful in severing the nerves to the lumbar zygapophysial joints.72,73,86 After this, a modified technique targeting the correct nerve locations was reported in 1980,87and subsequent studies showed good benefit. Analysis of the lesion created with RF neurotomy88 led to a modification in technique that placed the needle and therefore lesion parallel to the target nerve.89 A study using controlled diagnostic blocks as a diagnostic step and lesions created parallel to the target nerve demonstrated significant benefit for patients with chronic lumbar zygapophysial joint pain.90 Pulsed RF (PRF) treatment has also been proposed as an alternative to conventional RF, although overall, there is less supportive evidence.
In the cervical spine, the premise for cervical MBBs as a diagnostic test for cervical zygapophysial joint pain also appears justified. The technique of selective blockade of the cervical dorsal rami was first suggested in 1980.91 Anatomical studies again led to further refinement and description of blockade of the cervical medial branches92 rather than their parent nerve. Diagnostic utility of cervical MBBs was established with studies for head pain and neck pain beginning in 1985. Pain referral maps were created that enabled practitioners to better predict the segmental level of painful joints.93,94 In the early 1990s, a series of papers argued the importance of comparative diagnostic blockade and the shortcomings of single diagnostic blocks. Epidemiological studies that followed reported a high prevalence of pain arising from the cervical zygapophysial joints, especially in patients with head or neck pain from whiplash injuries. Injecting cervical zygapophysial joints with corticosteroids did not provide any additional benefit over anesthetizing the joint.
The first descriptions of cervical medial branch neurotomy appeared in the 1970s in papers focused predominantly on low back pain, and the first studies focusing exclusively on neck pain appeared in the early 1980s. Over the following decade, several more studies appeared, but similar to the lumbar treatments, the selection criteria and techniques varied, resulting in only fair overall results. The publications between 1995 and 2003 on cervical medial branch RF neurotomy demonstrated significant and prolonged benefit, most notably with the publications by Lord et al88 and Govind et al95 on patients with neck and head pain, respectively.
Pain arising from the thoracic zygapophysial joints account for 34% to 48% of chronic thoracic pain.96–99 Thoracic MBBs have also been described as analogs to the diagnostic blocks in the cervical and lumbar regions, but few studies have described their application in clinical practice. In one study of 46 patients with chronic thoracic pain, 48% had relief with the diagnostic blocks.98 A subsequent study by the same authors showed that 71% had relief that persisted for several months or even years with or without the inclusion of corticosteroids.99 There is great variability in the location of the medial branch nerves, especially in the midthoracic region (T5-T8). Two papers have reported thoracic referred pain patterns.100,101 Thoracic intraarticular zygapophysial joint blocks were first reported for relief of chronic thoracic pain in 1987.102 One prospective study showed significant pain relief persisting for 12 months after thoracic RF medial branch neurotomy.103
Sacroiliac Joint Injections and Procedures
Appreciation that the sacroiliac joint could be a source of low back pain fluctuated throughout the twentieth century. There were no validated tests to confirm the diagnosis of pain arising from the sacroiliac joint. The first description of injection of medication into the sacroiliac joint for diagnosis and treatment was described by Haldeman and Soto-Hall in 1938.104 Later studies105 suggested that the likelihood of medication entering the sacroiliac joint with a blind injection was approximately 22%. The first description of using fluoroscopic guidance to secure entry in the sacroiliac joint was described in 1979.106 Three years later in the same journal, Hendrix et al107 described the use of contrast medium to confirm intraarticular spread of injectate. Both of these descriptions involved using a posterior approach to the joint, which has since been replaced with the recommended inferior approach to the joint. This inferior approach was first described in 1992108 with numerous modifications and descriptions until the simplified approach that is currently used in clinical practice was described in 2000.109 With the ability to confirm needle entry into the sacroiliac joint with use of fluoroscopy and contrast, it was now possible to more accurately diagnose sacroiliac joint pain. Sacroiliac joint pain has now become a recognized source of pain in the low back with an estimated incidence of 13% to 19%110,111 based on response to controlled diagnostic blocks. Pain referral patterns after injections were created to better elicit which patients were likely to possess sacroiliac joint pain based on history and physical examination findings, including use of provocative maneuvers. Numerous studies have demonstrated that no single clinical feature is predictive to response of diagnostic blockade.112–114
The sacroiliac joint has a diffuse and variable innervation that cannot be reliably blocked using selective nerve blocks. The exact pattern of innervation is disputed but likely involves a possible anterior component from the ventral rami of L5-S2 and via branches from the sacral plexus and a posterior component from the lateral branches of the S1-S4 dorsal rami and possibly involving the L5 and even L4 dorsal rami. Numerous studies have targeted the sacral lateral branches as diagnostic tests and for RF ablation of these same nerves. Others have targeted extraarticular structures, including the deep interosseus ligament and the posterior sacroiliac ligaments.115–117 In fact, targeting these structures has shown promise for both RF ablation procedures and for corticosteroid injections.
After the sacroiliac joint has been confirmed as the source of the patient’s pain, then the most common treatment involves injection of corticosteroid in the same fashion as the diagnostic block. Injections of corticosteroids into the sacroiliac joint have been shown to be efficacious in the treatment of sacroiliitis caused by various spondyloarthropathies.118–121 Other studies demonstrate efficacy with extraarticular corticosteroid injections104,122–124 or a combination of both intra- and extraarticular techniques.116 One study reported moderate relief of sacroiliac joint pain after injection of phenol (6%) into the sacroiliac joint.125
Numerous techniques have been described for RF denervation of the sacroiliac joint and contributing structures. Ferrante et al126 described performing bipolar strip lesions along the inferior pole of the sacroiliac joint. This provided significant benefit to only a small percentage of patients. Because of the variable innervation to the joint, the treatments also offer some variability but generally target the lateral branches of S1-S3 and may include the lateral branch of S4 and the dorsal rami of L5 and even L4.114,127–129 The most recent innovations that show promising results include cooled RF treatment130–132 and use of a single multilesion RF probe.133 Both of these techniques involve creating larger sized lesions than has been reported with conventional RF approaches directed at the sacroiliac joint and contributing structures.
Disc Stimulation (Provocation Discography)
Lumbar disc stimulation was developed in the late 1940s as a technique for diagnosing herniation of lumbar intervertebral discs, and the first published description appeared in 1948.134 This corresponded with the published belief in 1947 that the disc could be a primary source of pain,135 a notion that was supported by subsequent intraoperative studies.136–138 Nevertheless, the disc as a primary source of pain ran contrary to conventional wisdom until the 1980s.
Despite a key paper by Massie & Stevens139 in 1967 reinforcing that the pain reproduction is the essential element in distinguishing symptomatic discs from similarly degenerated ones, there remained skepticism and controversy surrounding the procedure. Again, many of the critics who offered negative reviews failed to recognize that it was the stimulation portion that was critical to identifying the symptomatic disc.140 This led to an executive statement from a major spine society, the North American Spine Society,141 in 1988, again emphasizing that the pain response to disc stimulation is the key component to the procedure. Studies demonstrated that discography did improve surgical results when interpreted and performed correctly.142–145 At the same time, there was an explosion of studies between 1980 and 1992 showing that the disc can be innervated and a source of pain.
After the reports of computed tomography discography in 1986 to 1987,146,147 the Dallas discogram scale was reported148 and subsequently modified.149 Studies correlated pain reproduction with the extent of annular disruption,150 and the term internal disc disruption emerged.151 Use of manometry led to a classification system based on observational studies.145,152 Risks associated and reported with the procedure include infection (discitis) and reaction to medication. A recent prospective study demonstrated accelerated progression of degenerative changes in the lumbar disc 7 to 10 years after “discography” compared with those who did not undergo the procedure.153
The history of disc stimulation in the cervical spine parallels that of the lumbar region. The technique for cervical discography was first published in 1957154 and was followed by more published reports over subsequent years. Intraoperative disc stimulation (mechanical and electrical) verified the notion that the cervical disc itself could be a source of pain and may by mediated by sinuvertebral nerves,155 a notion that was confirmed by anatomical studies.156–159 Many of the same critics of lumbar discography argued that the morphological changes seen on discography did not correlate with the reproduction of pain.160,161 They again missed the notion that the primary objective of discography is to detect reproduction of concordant pain. A 1996 study demonstrated that stimulation of cervical discs in asymptomatic volunteers is either painless or minimally painful.162 A prior study163 had shown a “false-positive” response to cervical disc stimulation in patients who had positive relief with diagnostic blockade of the cervical zygapophysial joints. Grubb and Kelly164 showed that many patients had positive responses at multiple levels and argued that disc stimulation should be performed at all levels from C2-C3 to C6-C7 when technically feasible. Others152 had modified this approach to exclude C2-C3 if head pain was not a major component based on pain referral distribution maps based on prior studies.162,164 Observational studies suggest that cervical discography does help surgeons select (and avoid) segmental levels that should (not) be fused and may lead to avoidance of surgery altogether if multilevel disease is present.164
Risks and complications associated with cervical disc stimulation are similar to the lumbar spine with the noted difference that high pressures may accentuate disc bulging or prolapse, especially in patients with spinal stenosis or impingement on the spinal cord.165,166 Additionally, the larynx may obstruct access to the disc at C2-C3, and the apex of the lung may intervene at C7-T1. Before utilization of antibiotic prophylaxis, the incidence of discitis reported was 0.64% per patient,165 possibly related to the proximity of the pharynx and esophagus. A recent review167 reported an overall incidence of discitis of 0.44%, but there were no cases of discitis noted in the only two studies (2140 patients) that consistently gave intradiscal antibiotics.
Thoracic disc pathology is far less common than in the lumbar or cervical regions. Accordingly, there is less reporting on thoracic provocation discography. The first published series of 100 patients was published in 1994,168 and this was followed by a prospective study in 1999.169 The principles are the same as for the lumbar and cervical regions, but thoracic provocation discography is a technically challenging procedure with the added risk of pleural puncture that should only be performed by expert physicians.
Anesthetic discography was described by Roth in 1976170 but has gained little attention until recently. A subsequent study reported that the authors only achieved an anesthetic response to anesthetizing the disc in seven of 34 patients with painful cervical discs.163 Functional anesthetic discography (FAD) is an emerging new technique for establishing pain reproduction and evaluating potential relief after injection of local anesthetic into the disc. The clinical utility and safety of this test are yet to be determined; however, preliminary presentations suggest that it may further stratify patients with positive pain provocation with conventional disc stimulation into those who do or do not gain relief from FAD.171,172
Intradiscal Treatments
Numerous intradiscal treatments have been reported for patients with discogenic pain or disc herniation or protrusion. These include chemonucleolysis with chymopapain173,174 and intradiscal injection of corticosteroid,175,176 ozone,177 hypertonic dextrose,178 etanercept,179 and methylene blue.180 Additionally, mechanical and electrical means have been used, including high-voltage intradiscal PRF,181 intradiscal RF,182 intradiscal electrothermal annuloplasty (IDET),183–194 RF annuloplasty,191,195 intradiscal biacuplasty,196–199 percutaneous lumbar discectomy,200 and plasma disc decompression (nucleoplasty).201–211
IDET is a treatment in which a flexible electrode is introduced into a lumbar intervertebral disc and delivers heat to the annulus fibrosus in an attempt to relieve pain stemming from the disc. The mechanism of action remains unclear but may work to strengthen the collagen and seal radial tears or by denervating nerve endings near painful fissures, thereby sealing the fissures against fresh exudates entering from the nucleus pulposus. Saal and Saal183,184 first presented the IDET treatment in 1999, and several observational studies were reported over the first few years of introduction. The procedure has demonstrated good benefit in a number of observational studies185–191 and in one of two placebo-controlled trials.192,193 A meta-analysis demonstrated compelling evidence for the efficacy and safety of the procedure.194 Outcomes from IDET have been reported to be similar to those from surgical fusion but with fewer complications.190 Other thermal treatments have also been performed with similar goals of denervating symptomatic nerve endings. One such treatment termed RF posterior annuloplasty has been performed but with far less benefit compared with IDET, including a head-to-head study of the two treatments. In that study, both treatments provided benefit, but the pain and disability scores were both significantly better in the IDET group.191,195 A new treatment termed intradiscal biacuplasty has been performed for patients with internal disc disruption.196 This treatment consists of placing bilateral RF probes in the posterolateral annulus and delivering bipolar cooled RF energy to create a precise and reproducible lesion. By placing the probes directly into the annulus, this treatment avoids having to navigate a thermoelastic coil around the annulus, which can prove difficult. A few early studies have been quite promising in demonstrating benefits in pain scores in patients with discogenic pain who underwent intradiscal biacuplasty.196–199
The second area of focus for intradiscal procedures is for contained disc protrusions or herniations causing radicular or axial pain complaints (or both). Chemonucleolysis with chymopapain was initially described in 1964 as a management option for contained disc herniations without sequestration or extrusion.173 Chymopapain is a proteolytic enzyme that was derived from papaya and is thought to catalyze hydrolysis of proteins in the nucleus pulposus. A number of studies have demonstrated benefit compared with placebo but possibly inferior results when compared with surgical discectomy. A meta-analysis of 22 eligible clinical trials found that chemonucleolysis with chymopapain was superior to placebo and was as effective as collagenase in the treatment of lumbar disc prolapse. The summary data comparing chemonucleolysis with surgery were heterogenous, showing both options to be equivalent in their effectiveness.174 After a number of patients were reported to have developed anaphylaxis and died after chymopapain injection, the substance was banned for a short time in the mid-1970s by the Food and Drug Administration, and reinjection continues to be prohibited in the United States for fear of sensitization and anaphylaxis. Collagenase has also been associated with allergic reactions.
Newer techniques have focused on percutaneous manual decompression by mechanical (automated percutaneous lumbar discectomy [APLD], DeKompressor) or electrical (plasma disc decompression) means. Use of APLD was first published in the early 1990s and showed mixed results. Later, a mechanical high-RPM device (DeKompressor probe) was introduced. It was designed to extract the nuclear material through an introducer cannula using an auger-like device that rotates at high speeds. A number of studies demonstrated improvement in pain and function,200 but overall, the evidence was limited. Percutaneous disc decompression (PDD) with nucleoplasty (coblation technology) is performed with RF energy to dissolve nuclear material through molecular dissociation with resultant intradiscal pressure reduction.201 The proposed advantage of the coblation technology is production of a controlled and highly localized ablation with minimal thermal damage to surrounding tissues. Additionally, there is avoidance of injection of chemicals that may predispose the patient to allergic reaction or anaphylaxis as is seen with chemonucleolysis. Although a number of prospective studies have demonstrated benefit,202–208 overall the evidence is limited. This treatment has also been proposed as an alternative to IDET for patients with discogenic pain, although the results appear less dramatic than for radicular pain.209 Cervical PDD has also been reported with good benefit,210 including a recent randomized trial.211
Vertebral Augmentation
In the early twentieth century, chemist Otto Röhm developed and marketed a substance with unique structural properties and good biocompatibility called polymethyl methacrylate (PMMA)212,213 registered under the brand name Plexiglas. In 1936, commercially viable production of acrylic safety glass began. The acrylic glass was used for submarine periscopes, windshields, and gun turrets for airplanes in World War II.214 The biocompatibility of the substance was noted when splinters from the side windows of the Supermarine Spitfire fighters (made of PMMA) caused almost no rejection reaction in the eyes of soldiers compared with the glass splinters of aircraft such as the Hawker Hurricane.215 Sir John Charnley started using PMMA as bone cement for fixation of the femur and acetabulum in total hip arthroplasty in the 1960s. The same substance has been used for decades in dentistry as part of dentures and in filling materials. PMMA is used as a grout material to fill in the gaps between the prosthesis and bone. The substance has been found to be stable for long-term implantation.212,213
PMMA has been used extensively in the spine as well. Historically, it has been used to stabilize motion segments with posterior applications,216 fill defects in open corpectomy procedures for spinal tumors,217,218 and improve hardware stability in osteoporotic bone.219
The first percutaneous use of PMMA, however, was not until 1984, when Deramond et al220 used this material as part of a treatment for a patient with an aggressive C2 hemangioma. Treatment of aggressive hemangiomas was the first major indication for the procedure, now known as vertebroplasty. PMMA was then injected in a similar percutaneous manner with fluoroscopic guidance into vertebral compression fractures (VCFs) secondary to osteoporosis.221 After the initial experience was documented in Europe, the procedure was introduced and expanded by the neuroradiology interventionalists at the University of Virginia starting in 1994.222 Jensen et al222 published the results of the treatment of 47 painful vertebral fractures related to osteoporotic fractures a few years later in an English language journal, concluding that vertebroplasty could provide pain relief and early mobilization in appropriately selected patients. The paradigm of “benign neglect” of VCFs to active intervention started to shift.223
The mechanism in which pain relief occurs with the introduction of PMMA into fractured vertebral bodies has been debated for years. The polymerization of the cement is exothermic, and temperatures can reach 122° C.224,225 Some investigators have suggested that thermal necrosis and chemotoxicity of the intraosseous pain receptors as well as restored mechanical stability could be responsible for the pain relief. Animal data, however, suggests that PMMA causes relatively little necrotic exothermic effect.226
The complication rate of vertebroplasty is low, with most of the concerns focused on leakage of the PMMA into nearby structures. The majority of cases of cement extravasations are asymptomatic and occur in areas of cortical destruction, fracture lines, or into the epidural and paravertebral venous complexes.227,228 Murphey and Deramond229 divided the risk by indication for the procedure and found a complication rate of 1.3% for osteoporosis, 2.5% for hemangiomas, and 10% for neoplastic disease. Another issue of concern has been an increased risk for fracture in an adjacent vertebral body,230 but some data from cadaveric studies have suggested that it is the result of the natural progression of the disease rather than a result of vertebral augmentation.231
Another technique modification to decrease the extravasations risk was a modified use of an angioplasty balloon, a technique first performed by Mark Reiley, an orthopedic surgeon, in 1998.232 In this procedure, called kyphoplasty, the inflatable balloon is used to create a cavity in the fractured vertebral body. The cavity is then filled with PMMA. This allows for a lower pressure injection and the use of a more viscous cement mixture than is possible with vertebroplasty. The additional benefit of kyphoplasty over vertebroplasty is the possibility of partial restoration of the height of the vertebral body. This normalization of the vertebral column could potentially decrease the complications of vertebral fractures such as pulmonary dysfunction.
The consensus statement from 2009233 concluded that vertebroplasty resulted in significant pain reduction and improved function and quality of life in the setting of osteoporotic fractures and vertebral fractures related to metastatic cancer. Although fewer studies have investigated the efficacy of kyphoplasty compared with vertebroplasty, the position of the committee was that the efficacy appeared equivalent. There was no evidence of additional benefit of either procedure in regard to pain relief, vertebral height restoration, or complication rate.
1 Koller C. On the use of cocaine for producing anaesthesia on the eye. Lancet. 1884;2:990.
2 Corning JL. Spinal anaesthesia and local medication of the cord with cocaine. NY Med J. 1885;42:483.
3 Gray H. Anatomy: descriptive and surgical, ed 5. Philadelphia: Henry C. Lea; 1870. pp 572-574
4 Harrington BE. Postdural puncture headache and the development of the epidural blood patch. Reg Anesth Pain Med. 2004;29:136-163.
5 Wynter WE. Four cases of tubercular meningitis in which paracentesis of the theca vertebralis was performed for the relief of fluid pressure. Lancet. 1891;1:981-982.
6 Quincke H. Die lumbalpunction des hydrocephalus. Berliner Klinische Wochenschrift. 1891;28:929-933.
7 Bier A. Versuche uber Cocainisrung des Ruckenmarkes. Dtsch Z Chir. 1899;5151:361.
8 Tait D, Caglieri G. Experimental and clinical notes on the subarachnoid space. Trans Med Soc State Cal. 1900;30:266-271.
9 Anonymous. Surgical anaesthesia by the injection of cocaine into the lumbar sub-arachnoid space. Lancet. 1901;12:137-138.
10 Cathelin MF. Mode d’action de la cocaine injectee dans l’espace epidural par le procede du canal sacre. CR Soc Biol (Paris). 1901;53:478-479.
11 De Pasquier M, Leri M. Injections intra et extra-durales de cocaine a dose minime dans le traitement de la sciatique. Valeur comparee des deux methods: resultats immediats et Tardifs. Bull Gen Ther. 1901;142:192-223.
12 Stoeckel W. Ueber sakrale Anasthesie. Zentralbl. Gynaekol. 1909;33:1.
13 Viner N. Intractable sciatica: the sacral epidural injection: an effective method of giving relief. Can Med Assoc J. 1925;15:630-634.
14 Mason HL, Myers CS, Kendall EC. The chemistry of crystalline substances isolated from the suprarenal gland. J Biol Chem. 1936;114:613-631.
15 Mason HL, Myers CS, Kendall EC. Chemical studies of the suprarenal cortex II. The definition of a substance which possesses the qualitative action of cortin; its conversion into a diketone closely related to androstenedione. J Biol Chem. 1936;116:267-276.
16 Hench PS, Kendall EC, Slocumb CH, et al. Effects of cortisone acetate and pituitary ACTH on rheumatic fever and certain other conditions. Arch Intern Med. 1950;85:545-566.
17 Hench PS, Kendall EC, Slocumb CH, et al. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocortisone: compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis; preliminary report. Proc Staff Meet Mayo Clin. 1949;24:181-197.
18 Hollander JL. The local effects of compound F (hydrocortisone) injected into joints. Bull Rheum Dis. 1951;2:3-4.
19 Robecchi A, Capra R. [Hydrocortisone (compound F); first clinical experiment in the field of rheumatology]. Minerva Med. 1952;2:1259-1263.
20 Lievre JA, Block-Michel H, Pean G, et al. L’hydrocortisone en injection locale. Revue du Rhumatisme et des Maladies Osteo-articulares. 1953;20:310-311.
21 Brown JH. Pressure caudal anesthesia and back manipulation. Northwest Med. 1960;59:905-909.
22 Pagés E. Anestesia metamerica. Rev Sanid Mil Madr. 1921;11:351.
23 Dogliotti AM. Segmental peridural anesthesia. Am J Surg. 1933;20:107.
24 Gutierrez A. Valor de la aspiracion liquada en al espacio peridural en la anesthesia peridural. Rev Circ. 1933;12:225.
25 Kamen GF, Erdman GL. Subdural administration of hydrocortisone in multiple sclerosis: effect of ACTH. J Am Geriatr Soc. 1953;1:794-804.
26 Boines GJ. Remissions in multiple sclerosis following intrathecal methylprednisolone acetate. Del Med J. 1961;33:231-235.
27 Boines GJ. Predictable remissions in multiple sclerosis. Del Med J. 1963;35:200-203.
28 Goldstein NP, McKenzie BF, McGuckin WF, et al. Experimental intrathecal administration of methylprednisolone acetate in multiple sclerosis. Trans Am Neurol Assoc. 1970;95:243-244.
29 Nelson DA, Vates TS, Thomas RB. Complications from intrathecal steroid therapy in patients with multiple sclerosis. Acta Neurol Scand. 1973;49:176-188.
30 Gardner WJ, Goebert HW, Sehgal AD. Intraspinal corticosteroids in the treatment of sciatica. Trans Am Neurol Assoc. 1961;86:214-215.
31 Sehgal AD, Gardner WJ. Place of intrathecal methylprednisolone acetate in neurological disorders. Trans Am Neurol Assoc. 1963;88:275-276.
32 Sehgal AD, Tweed DC, Gardner WJ, et al. Laboratory studies after intrathecal corticosteroids. Arch Neurol. 1963;9:64-68.
33 Winnie AP, Hartman JT, Meyers HL, et al. Pain Clinic II: intradural and extradural corticosteroids for sciatica. Anesth Analg. 1972;51:990-999.
34 White AH, Derby R, Wynne G. Epidural injections for the diagnosis and treatment of low back pain. Spine. 1980;5:78-86.
35 Weinstein SM, Herring SA, Derby R. Contemporary concepts in spine care: epidural steroid injections. Spine. 1995;20:1842-1846.
36 el-Khoury G, Ehara S, Weinstein JN, et al. Epidural steroid injection: a procedure ideally performed with fluoroscopic control. Radiology. 1988;168:554-557.
37 Stojanovic MP, Vu T, Caneris O, et al. The role of fluoroscopy in cervical epidural steroid injections: an analysis of contrast dispersal patterns. Spine. 2002;27:509-514.
38 Fredman B, Nun MB, et al. Epidural steroids for treating “failed back surgery syndrome”: is fluoroscopy really necessary? Anesth Analg. 1999;88:367-372.
39 O’Neil C, Derby R, Knederes L. Precision injection techniques for the diagnosis and treatment of lumbar disc disease. Semin Spine Surg. 1999;11:104-118.
40 Derby R, Bogduk N, Kine G. Precision percutaneous blocking procedures for localizing spinal pain: part 2. The lumbar neuroaxial compartment. Pain Diag. 1993;3:175-188.
41 Derby R, Kine G, Saal JA, et al. Responses to steroid and duration of radicular pain as predictors of surgical outcome. Spine. 1992;17(suppl):176-183.
42 Bier A. Versuche ueber cocainisirung des rueckenmarkes. Deutsche Zeitschrift fuer Chirurgie. 1899;51:361-368.
43 Jacobaeous HC, Frumerie K. About the leakage of the cerebrospinal fluid after lumbar puncture. Acta Med Scandinav. 1923;58:102-108.
44 Pickering GW. Experimental observations on headache. Br Med J. 1939;1:907-912.
45 Ahearn RE. Management of severe postlumbar puncture headache. NY State J Med. 1948;48:1495-1498.
46 Ekstrom T. Treatment of headache after spinal anesthesia with intraspinal injection of physiological saline solution. Acta Chir Scand. 1951;101:450-456.
47 Glesne OG. Lumbar puncture headaches. Anesthesiology. 1950;11:702-708.
48 Solomon HC. Raising cerebrospinal fluid pressure. JAMA. 1924;82:1512-1515.
49 Weed LH, Cushing H. Studies on cerebrospinal fluid. VIII. The effect of pituitary extract upon its secretion (choroidorrhoea). Am J Physiol. 1915;36:77-103.
50 Heldt TJ, Moloney JC. Negative pressure in epidural space. Preliminary studies. Am J Med Sci. 1928;175:371-376.
51 Heldt TJ. Lumbar puncture headache. Med J Rec. 1929;129:13613-13619.
52 Gormley JB. Treatment of postspinal headache. Anesthesiology. 1960;21:565-566.
53 DiGiovanni AJ, Dunbar BS. Epidural injections of autologous blood for postlumbar-puncture headache. Anesth Analg. 1970;49:268-271.
54 DiGiovanni AJ, Galbert MW, Wahle WM. Epidural injection of autologous blood for postlumbar-puncture headache. II. Additional clinical experiences and laboratory investigation. Anesth Analg. 1972;51:226-232.
55 Van Kooten F, Oedit R, Bakker SLM, Dippel DWJ. Epidural blood patch in post dural puncture headache: a randomized, observer-blind, controlled clinical trial. J Neurol Neurosurg Psychiatry. 2008;79:553-558.
56 Gasser HS, Erlanger J. The compound nature of the action current of nerve as disclosed by the cathode ray oscilloscope. Am J Physiol. 1924;70:624.
57 Gasser HS, Erlanger J. The role played by the size of the constituent fibers of a verve trunk in determining the form of its action potential wave. Am J Physiol. 1927;80:522.
58 Sarnoff SJ, Arrowood JG. Differential spinal block. I. Surgery. 1946;20:150-159.
59 Sarnoff SJ, Arrowood JG. Differential spinal block. V. Use in the investigation of pain following amputation. Anesthesiology. 1948;9:614-622.
60 Winnie AJ, Candido KD. Differential neural blockade for the diagnosis of pain. In: Waldman SD, editor. Pain management. Philadelphia: Saunders; 2007:155-166.
61 Akkineni SR, Ramamurthy S: Simplified differential spinal block. Presented at the Annual Meeting of the American Society of Anesthesiologists, New Orleans, October 15-19, 1977.
62 Winnie AP. Differential diagnosis of pain mechanisms. ASA Refresher Courses in Anesthesiology. 1978;6:171.
63 Ramamurthy S, Winnie AP. Diagnostic maneuvers in painful syndromes. Int Anesth Clin. 1983;21:47.
64 Ramamurthy S, Winnie AP. Regional anesthetic techniques for pain relief. Semin Anesth. 1985;4:237.
65 Raj PP: Sympathetic pain mechanism and management. Presented at the Second Annual Meeting of the American Society of Regional Anesthesia, Hollywood, FL, March 10-11, 1977.
66 Fink BR. Mechanisms of differential axial blockade in epidural and subarachnoid anesthesia. Anesthesiology. 1989;70:851-858.
67 Fink BR, Cairnes AM. Lack of size related differential sensitivity to equilibrium conduction block among mammalian myelinated axons exposed to lidocaine. Anesth Analg. 1987;66:948-953.
68 Raymond SA. Subblocking concentrations of local anesthetic: effects on impulse generation and conduction in single myelinated sciatic nerve axons in frog. Anesth Analg. 1992;75:906-921.
69 Torebjork HE, Hallin RG. Perceptual changes accompanying controlled preferential blocking of A and C fibre responses in intact human skin nerves. Exp Brain Res. 1973;16:321-332.
70 Hogan QH, Abram SE. Neural blockade for diagnosis and prognosis: a review. Anesthesiology. 1997;86:216-241.
71 Raja SN. Nerve blocks in the evaluation of chronic pain: a plea for caution in their use and interpretation. Anesthesiology. 1997;86:4-6.
72 Bogduk N, Long DM. The anatomy of the so-called ‘articular nerves’ and their relationship to facet denervation in the treatment of low back pain. J Neurosurg. 1979;51:172-177.
73 Bogduk N, Long DM. Percutaneous lumbar medial branch neurotomy. A modification of facet denervation. Spine. 1980;5:193-200.
74 Bogduk N, Wilson AS, Tynan W. The human dorsal rami. J Anat. 1982;134:383-397.
75 Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286-293.
76 Schwarzer AC, Wang S, Bogduk N, et al. Prevalence and clinical features of lumbar zygapophysial joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54:100-106.
77 Dreyfuss P, Schwarzer AC, Lau P, et al. Specificity of lumbar medial branch and L5 dorsal ramus blocks: a computed tomographic study. Spine. 1997;22:895-902.
78 Kaplan M, Dreyfuss P, Halbrook B, et al. The ability of lumbar medial branch blocks to anesthetize the zygapophysial joint. Spine. 1998;23:1847-1852.
79 White JC, Sweet WH. Pain and the neurosurgeon. Springfield, IL: CC Thomas; 1969. pp 193-197
80 Rees WES. Multiple bilateral subcutaneous rhizolysis of segmental nerves in the treatment of intervertebral disc syndrome. Ann Gen Pract. 1971;16:126-127.
81 Rees WES. Multiple bilateral percutaneous rhizolysis. Med J Aust. 1975;1:536-537.
82 Shealy CN. Facets in back and sciatic pain. Minn Med. 1974;57:199-203.
83 Shealy CN. The role of the spinal facets in back and sciatic pain. Headache. 1974;14:101-104.
84 Shealy CN. Percutaneous radiofrequency denervation of spinal facets. J Neurosurg. 1975;43:448-451.
85 Shealey CN. Facet denervation in the management of back sciatic pain. Clin Orthop. 1976;115:157-164.
86 Bogduk N, Colman RRS, Winer CER. An anatomical assessment of the “percutaneous rhizolysis” procedure. Med J Aust. 1977;1:397-399.
87 King JS, Lagger R. Sciatica viewed as a referred pain syndrome. Surg Neurol. 1976;5:46-50.
88 Lord SM, Barnsley L, Wallis B, et al. Percutaneous radiofrequency neurotomy for chronic cervical zygapophyseal joint pain. N Eng J Med. 1996;335:1721-1726.
89 Bogduk N, Macintosh J, Marsland A. Technical limitations to the efficacy of radiofrequency neurotomy for spinal pain. Neurosurgery. 1987;20:529-535.
90 Dreyfuss P, Halbrook B, Pauza K, et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine. 2000;25:1270-1277.
91 Sluijter ME, Koetsveld-Baart CC. Interruption of pain pathways in the treatment of the cervical syndrome. Anesthesia. 1980;35:302-307.
92 Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine. 1982;7:319-330.
93 Dwyer A, April C, Bogduk N. Cervical zygapophyseal joint pain patterns I: a study in normal volunteers. Spine. 1990;15:453-457.
94 April C, Dwyer A, Bogduk N. Cervical joint pain patterns II: a clinical evaluation. Spine. 1990;15:458-461.
95 Govind J, King W, Bailey B, et al. Radiofrequency neurotomy for the treatment of third occipital headache. J Neurol Neurosurg Psychiatry. 2003;74:88-93.
96 Manchikanti L, Boswell MV, Singh V, et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15.
97 Manchukonda R, Manchikanti KN, Cash KA, et al. Facet joint pain in chronic spinal pain: an evaluation of prevalence and false positive rate of diagnostic blocks. J Spinal Disord Tech. 2007;20(7):539-545.
98 Manchikanti L, Singh V, Pampati V, et al. Evaluation of the prevalence of facet joint pain in chronic thoracic pain. Pain Physician. 2002;5:354-359.
99 Manchikanti L, Manchikanti KN, Manchukonda R, et al. Evaluation of therapeutic thoracic medial branch block effectiveness in chronic thoracic pain: a prospective outcome study with minimum 1-year follow up. Pain Physician. 2006;9(2):97-105.
100 Dreyfuss P, Tibiletti C, Dryer SJ. Thoracic zygapophyseal joint pain patterns. A study in normal volunteers. Spine. 1994;19:807-811.
101 Fukui S, Ohseto K, Shiotani M. Patterns of pain induced by distending the thoracic zygapophyseal joints. Reg Anesth. 1997;22:332-336.
102 Wilson PR. Thoracic facet syndrome—a clinical entity? Pain Suppl. 1987;4(suppl):S87.
103 Stolker RJ, Vervest ACM, Groen GJ. Percutaneous facet denervation in chronic thoracic spinal pain. Acta Neurochir. 1993;122:82-90.
104 Haldeman KO, Soto-Hall R. The diagnosis and treatment of sacroiliac condition by the injection of procaine. J Bone Joint Surg. 1938;20:675-685.
105 Rosenberg J, Quint T, de Rosayro A. Computerized tomographic localization of clinically-guided sacroiliac joint injections. Clin J Pain. 2000;16:18-21.
106 Miskew DB, Block RA, Witt PF. Aspiration of infected sacro-iliac joints. J Bone Joint Surg Am. 1979;61(suppl A):1071-1072.
107 Hendrix RW, Lin PP, Kane BJ. Simplified aspiration or injection technique for the sacroiliac joint. J Bone Joint Surg Am. 1982;64(suppl A):1249-1252.
108 April CN. The role of anatomically specific injections into the sacroiliac joint. In: Vleeming A, Mooney V, Snijders C, Dorman T, editors. First Interdisciplinary World Congress on Low Back Pain and its Relation to the Sacroiliac Joint. San Diego: Rotterdam, ECO; 1992:373-380. November 5-6, 1992
109 Dussault RG, Kaplan PA, Anderson MW. Fluoroscopy-guided sacroiliac joint injections. Radiology. 2000;214:273-277.
110 Schwarzer AC, April CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20:31-37.
111 Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21:1889-1892.
112 Fortin JD, Dwyer AD, West S, et al. Sacroiliac joint pain: pain referral maps upon applying a new injection/arthrogram technique. Part I: asymptomatic volunteers. Spine. 1994;19:1475-1482.
113 Fortin JD, April CN, Ponthieux B, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part II: clinical evaluation. Spine. 1994;19:1483-1489.
114 Dreyfuss P, Michaelson M, Pauza K, et al. The value of history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21:2594-2602.
115 Yin W, Willard F, Carreiro J, et al. Sensory stimulation-guided sacroiliac joint radiofrequency neurotomy: technique based on neuroanatomy of the dorsal sacral plexus. Spine. 2003;28(20):2419-2425.
116 Borowsky CD, Fagen G. Sources of sacroiliac region pain: insights gained from a study comparing standard intra-articular injection with a technique combining intra- and peri-articular injection. Arch Phys Med Rehabil. 2008;89:2048-2056.
117 Dreyfuss P, Henning T, Malladi N, et al. The ability of multi-site, multi-depth sacral lateral branch blocks to anesthetize the sacroiliac joint complex. Pain Med. 2009;10(4):679-688.
118 Maugers Y, Mathis C, Vilon P, et al. Corticosteroid injection of the sacroiliac joint in patients with seronegative spondyloarthropathy. Arthritis Rheum. 1992;35:564-568.
119 Maugers Y, Mathis C, Berthelot J. Assessment of the efficacy of sacroiliac corticosteroid injections in spondyloarthropathies: a double-blind study. Br J Rheum. 1996;35:767-770.
120 Braun J, Bollow M, Seyrekbasan F, et al. Computed tomography guided corticosteroid injection of the sacroiliac joint in patients with spondyloarthropathy with sacroiliitis: clinical outcome and follow up by dynamic magnetic resonance imaging. J Rheumatol. 1996;23:659-664.
121 Bollow M, Braun J, Taupitz M, et al. CT-guided intra-articular corticosteroid injection into the sacroiliac joints in patients with spondyloarthropathy: indication and follow up with contrast enhanced MRI. J Comput Assist Tomo. 1996;20:512-521.
122 Luukkainen R, Nissila M, Asikiainen E, et al. Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondyloarthropathy. Clin Exp Rheum. 1999;17:88-90.
123 Norman GF. Sacroiliac disease and its relationship to lower abdominal pain. Am J Surg. 1968;116:54-56.
124 Schuchmann JA, Cannon CL. Sacroiliac strain syndrome: diagnosis and treatment. Tex Med. 1986;82:33-36.
125 Ward S, Jenson M, Royal M, et al. Fluoroscopy-guided sacroiliac joint injections with phenol ablation for persistent sacroiliitis: a case series. Pain Pract. 2002;2(4):332-335.
126 Ferrante FM, King LF, Roche EA, et al. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Reg Anesth Pain Med. 2001;26(2):137-142.
127 Bujis E, Kamphuis E. Radiofrequency treatment of sacroiliac joint-related pain aimed at the first three sacral dorsal rami: a minimal approach. Pain Clin. 2004;16(2):139-146.
128 Cohen SP, Abdi S. Lateral branch blocks as a treatment for sacroiliac joint pain: a pilot study. Reg Anesth Pain Med. 2003;28(2):113-119.
129 Burnham RS, Yasui Y. An alternate method of radiofrequency neurotomy of the sacroiliac joint: a pilot study on the effect on pain, function and satisfaction. Reg Anesth Pain Med. 2007;32(1):12-19.
130 Kapural L, Nageeb F, Kapural M, et al. Cooled radiofrequency (RF) system for the treatment of chronic pain from sacroiliitis: the first case-series. Pain Pract. 2008;8(5):348-354.
131 Cohen SP, Hurley RW, Buckenmaier CCIII, et al. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
132 Kapural L, Stojanovic M, Bensitel T, et al. Cooled radiofrequency (RF) of L5 dorsal ramus for RF denervation of the sacroiliac joint: technical report. Pain Med. 2010;11:53-57.
133 Starr B, Dahle N, Vorenkamp KE. Radiofrequency lesioning of the SI joint with the Simplicity III probe: a case series. Proceedings of the American Society of Regional Anesthesia and Pain Medicine. San Antonio, TX: Annual Fall Pain Meeting. 2009.
134 Lindblom K. Diagnostic disc puncture of intervertebral discs in sciatica. Acta Orthop Scandinav. 1948;17:231-239.
135 Inman VT, Saunders JB. Anatomicophysiological aspects of injuries to the intervertebral disc. J Bone Joint Surg Am. 1947;29:461-475.
136 Falconer MA, McGeorge M, Begg AC. Observations on the cause and mechanism of symptom-production in sciatica and low-back pain. J Neurol Neurosurg Psychiatry. 1948;11:13-26.
137 Wiberg G. Back pain in relation to the nerve supply of intervertebral discs. Octa Orthop Scandinav. 1949;19:211-221.
138 Hirsch C, Ingelmark BE, Miller M. The anatomical basis for low back pain. Acta Orthop Scandinav. 1963;33:1-17.
139 Massie WK, Stevens DB. A critical evaluation of discography. J Bone Joint Surg Am. 1967;49(suppl A):1243-1244.
140 Holt EP. The question of lumbar discography. J Bone Joint Surg Am. 1968;50(suppl A):720-725.
141 Executive Committee of the North American Spine Society. Position statement on discography. Spine. 1988;13:1343.
142 Colhoun E, McCall IW, Williams L, et al. Provocation discography as a guide to planning operations on the spine. J Bone Joint Surg Am. 1988;70(suppl B):267-271.
143 Grubb SA, Lipscomb HJ, Guilford WB. The relative value of lumbar roentgenograms, metrizamide myelography, and discography in the assessment of patients with chronic low-back-syndrome. Spine. 1987;12:282-286.
144 McFadden JW. The stress lumbar discogram. Spine. 1988;13:931-933.
145 Derby R, Howard MW, Grant JM, et al. The ability of pressure-controlled discography to predict surgical and non-surgical outcomes. Spine. 1999;24:364-372.
146 McCutcheon ME. CT scanning of lumbar discography: a useful diagnostic adjunct. Spine. 1986;11:257-259.
147 Videman T, Malmivaara A, Mooney V. The value of the axial view in assessing discograms: an experimental study with cadavers. Spine. 1987;12:299-304.
148 Sachs BL, Vanharta H, Spivey MA, et al. Dallas discogram description: a new classification of CT/discography in low-back disorders. Spine. 1987;12:287-294.
149 April C, Bogduk N. High intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361-369.
150 Vanharanta H, Sachs BL, Spivey MA, et al. The relationship of pain provocation to lumbar disc deterioration as seen by CT/discography. Spine. 1987;12:295-298.
151 Crock HV. Internal disc disruption: a challenge to disc prolapse fifty years on. Spine. 1986;11:650-653.
152 Bogduk N, editor. Practice guidelines for spinal diagnostic and treatment procedures. San Francisco: International Spinal Intervention Society. 2004:20-46.
153 Carragee E, Don A, Hurwitz E, et al. Does discography cause accelerated progression of degeneration changed in the lumbar disc: a ten-year matched cohort study. Spine. 2009;43(21):2338-2345.
154 Smith GW, Nichols P. The technic of cervical discography. Radiology. 1957;68:718-720.
155 Cloward RB. The clinical significance of the sinu-vertebral nerve of the cervical spine in relation to the cervical disk syndrome. J Neurol Neurosurg Psychiatry. 1960;23:321-326.
156 Bogduk N, Windsor M, Inglis A. The innervation of the cervical intervertebral discs. Spine. 1989;13:2-8.
157 Groen GJ, Baljert B, Drukker J. Nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990;188:282-296.
158 Mendel T, Wink CS, Zimny ML. Neural elements in human cervical intervertebral discs. Spine. 1992;17:132-135.
159 Stuck RM. Cervical discography. Am J Roentgenol Radium Ther Nucl Med. 1961;86:975-982.
160 Sneider SE, Winslow OP, Pryor JH. Cervical diskography: is it relevant? JAMA. 1963;185:163-165.
161 Holt EP. The fallacy of cervical discography. JAMA. 1964;188:799-801.
162 Schellhas KP, Smith MD, Gundry CR, et al. Cervical discogenic pain. Prospective correlation of magnetic resonance imaging and discography in asymptomatic subjects and pain sufferers. Spine. 1996;21:300-312.
163 Bogduk N, Aprill C. On the nature of neck pain, discography and cervical zygapophysial joint blocks. Pain. 1993;54:213-217.
164 Grubb SA, Kelly CK. Cervical discography: clinical implications from 12 years of experience. Spine. 2000;25:1382-1389.
165 Connor PM, Darden BV. Cervical discography complications and clinical efficacy. Spine. 1993;18:2034-2038.
166 Laun A, Lorenz R, Agnoli AL. Complications of cervical discography. J Neurosurg Sci. 1981;25:17-20.
167 Kapoor SG, Huff J, Cohen SP. Systematic review of the incidence of discitis after cervical discography. Spine J. 2010;10(8):739-745.
168 Schellhas KP, Pollei SR, Dorwart RH. Thoracic discography: a safe and reliable technique. Spine. 1994;19:2103-2109.
169 Wood KB, Schellhas KP, Garvey TA, et al. Thoracic discography in healthy individuals. A controlled prospective study of magnetic resonance imaging and discography in asymptomatic and symptomatic individuals. Spine. 1999;24:1548-1555.
170 Roth DA. Cervical analgesic discography. A new test for the definitive diagnosis of the painful-disk syndrome. JAMA. 1976;235:1713-1714.
171 Anitescu M, Patel A, Simon A: Benefit of functional anesthetic discography, a double-blinded, prospective surgery outcome study. Proceedings of the American Society of Anesthesiology 2009 Annual Meeting, New Orleans, 2009.
172 Luchs J, Cho M, Demoura A. Preliminary experience with (functional anesthetic discography) FAD. Proceedings of the NASS 22nd Annual Meeting, Orlando, FL. Spine J, 7;suppl. 635:2007.
173 Smith L. Enzyme dissolution of nucleus pulposus in humans. JAMA. 1964;187:137-140.
174 Couto JMC, de Castilho EA, Menezes PR. Chemonucleolysis in lumbar disc herniation: a meta-analysis. Clinics (São Paulo). 2007;62(2):175-180.
175 Feffer HL. Treatment of low-back and sciatic pain by the injection of hydrocortisone into degenerated intervertebral discs. J Bone Joint Surg Am. 1956;38(suppl A):585-590.
176 Khot A, Bowditch M, Powell J, et al. The use of intradiscal steroid therapy for lumbar spinal discogenic pain: a randomized controlled trial. Spine. 2004;29:833-836.
177 Buric J, Molino Lova R. Ozone chemonucleolysis in non-contained lumbar disc herniations: a pilot study with 12 months follow-up. Acta Neurochir Suppl. 2005;92:93-97.
178 Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115-121.
179 Cohen SP, Wenzell D, Hurley RW, et al. A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology. 2007;107:99-105.
180 Peng B, Zhang Y, Hou S, et al. Intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Eur Spine J. 2007;16:33-38.
181 Teixeira A, Sluijter ME. Intradiscal high-voltage, long-duration pulsed radiofrequency for discogenic pain: a preliminary report. Pain Med. 2006;7(5):424-428.
182 Van Kleef M, Barendse GAM, Wilmink JT. Percutaneous intradiscal radiofrequency thermocoagulation in chronic non-specific low back pain. Pain Clin. 1996;9:259-268.
183 Saal JS, Saal JA: Intradiscal electrothermal annuloplasty (IDET) for chronic disc disease: outcome assessment with minimum one year follow-up. Proceedings of the 14th Annual Meeting of the North American Spine Society, 1999, pp 75-76.
184 Saal JA, Saal JS: Intradiscal electrothermal annuloplasty (IDET) for chronic multi-level discogenic pain: prospective one year follow-up outcome study. Proceedings of the 14th Annual Meeting of the North American Spine Society, 1999, pp 78-79.
185 Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain. A prospective outcome study with minimum 1-year follow-up. Spine. 2000;25:2622-2627.
186 Derby R, Eek B, Chen Y, et al. Intradiscal electrothermal annuloplasty(IDET): a novel approach for treating chronic discogenic back pain. Neuromodulation. 2000;3:82-88.
187 Singh V. Intradiscal electrothermal therapy: a preliminary report. Pain Physician. 2000;3:367-373.
188 Kapural L, Mekhail N, Korunda Z, et al. Intradiscal thermal annuloplasty for the treatment of lumbar discogenic pain in patients with multilevel degenerative disc disease. Anesth Analg. 2004;99:472-476.
189 Assietti R, Morosi M, Block JE. Intradiscal electrothermal therapy for symptomatic internal disc disruption: 24-month results and predictors of clinical success. J Neurosurg Spine. 2010;12(3):320-326.
190 Andersson GB, Mekhail NA, Block JE. Treatment of intractable discogenic low back pain. A systematic review of spinal fusion and intradiscal electrothermal therapy (IDET). Pain Physician. 2006;9:237-248.
191 Kapural L, Hayek S, Malak O, et al. Intradiscal thermal annuloplasty versus intradiscal radiofrequency ablation for the treatment of discogenic pain: a prospective matched control trial. Pain Med. 2005;6:425-431.
192 Pauza KJ, Howell S, Dreyfuss P, et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27-35.
193 Freeman BJ, Fraser RD, Cain CM, et al. A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine. 2005;30:2369-2377.
194 Appleby D, Anderson G, Totta M. Meta-analysis of the efficacy and safety of intradiscal electrothermal therapy (IDET). Pain Med. 2006;7:308-316.
195 Kvarstein G, Måwe L, Indahl A, et al. A randomized double-blind controlled trial of intra-annular radiofrequency thermal disc therapy—a 12-month follow-up. Pain. 2009;145:279-286.
196 Kapural L, Ng A, Dalton J, et al. Intervertebral disc biacuplasty for the treatment of lumbar discogenic pain: results of a six-month follow-up. Pain Med. 2008;9(1):60-67.
197 Kapural L. Letter to the editor: intervertebral disk cooled bipolar radiofrequency (intradiskal biacuplasty) for the treatment of lumbar diskogenic pain: 12 month follow up of the pilot study. Pain Med. 2008;9:407-408.
198 Kapural L, Cata JP, Narouze S. Successful treatment of lumbar discogenic pain using intradiscal biacuplasty in previously discectomized disc. Pain Pract. 2009;9(2):130-134.
199 Kapural L, Sakic K, Boutwell K. Intradiscal biaculoplasty (IDB) for the treatment of thoracic discogenic pain. Clin J Pain. 2010;26(4):354-357.
200 Alò KM, Wright RE, Sutcliff J, et al. Percutaneous lumbar discectomy: clinical response in an initial cohort of fifty consecutive patients with chronic radicular pain. Pain Pract. 2004;4(1):19-29.
201 Chen YC, Lee SH, Chen D. Intradiscal pressure study of percutaneous disc decompression with nucleoplasty in human cadavers. Spine. 2003;28:661-665.
202 Sharps LS, Isaac Z. Percutaneous disc decompression using nucleoplasty. Pain Physician. 2002;5:121-126.
203 Singh V, Piryani C, Liao K, et al. Percutaneous disc decompression using coblation (nucleoplasty) in the treatment of chronic discogenic pain. Pain Physician. 2002;5:250-259.
204 Gerszten PC, Welch WC, King JTJr. Quality of life assessment in patients undergoing nucleoplasty-based percutaneous discectomy. J Neurosurg Spine. 2006;4:36-42.
205 Masala S, Massari F, Fabiano S, et al. Nucleoplasty in the treatment of lumbar diskogenic back pain: one year follow-up. Cardiovasc Intervent Radiol. 2007;30:426-432.
206 Mirzai H, Tekin I, Yaman O, et al. The results of nucleoplasty in patients with lumbar herniated disc: a prospective clinical study of 52 consecutive patients. Spine J. 2007;7:88-92.
207 Yakovlev A, Tamimi MA, Liang H, et al. Outcomes of percutaneous disc decompression utilizing nucleoplasty for the treatment of chronic discogenic pain. Pain Physician. 2007;10:319-328.
208 Gertszten P, Smuck M, Rathmell JP, et al. Plasma disc decompression compared with fluoroscopy-guided transforaminal epidural steroid injections for symptomatic contained lumbar disc herniation: a prospective, randomized, controlled trial. J Neurosurg Spine. 2010;12(4):357-371.
209 Gerges FJ, Lipsitz SR, Nedeljkovic SS. A systematic review on the effectiveness of the Nucleoplasty procedure for discogenic pain. Pain Physician. 2010;13(2):117-132.
210 Nardi PV. Percutaneous cervical nucleoplasty using coblation technology. Clinical results in fifty consecutive cases. Acta Neurochir Suppl. 2005;92:73-78.
211 Cesaroni A, Nardi PV. Plasma disc decompression for contained cervical disc herniation: a randomized, controlled trial. Eur Spine J. 2010;19(3):477-486.
212 Mathis JM, Maroney M, Fenton DC, et al: Evaluation of bone cements for use in percutaneous vertebroplasty (abstract). In Proceeding of the 13th Annual Meeting of the North American Spine Society, San Francisco, CA, October 28-31, 1998, Rosemont, IL, North American Spine Society, 1998, pp 210-211.
213 DiMaio FR. The science of bone cement: a historical review. Orthopedics. 2002;25(12):1399-1407.
214 . Acrylic plastic: how products are made. accessed from http://www.enotes.com/how-products-encylopedia/acrylic-plastic.080515enotes.com
215 Basak SK. Birth centenary of Sir Harold Ridley (10th July1906-25th May 2001). Indian J Ophthalmol. 2006;54:219-220.
216 Panjabi MM, Goel VK, Clark CR, et al. Biomechanical study of cervical spine stabilization with methylmethacrylate. Spine. 1985;10:198-203.
217 King GJ, Kostuik JP, McBroom RJ, Richardson W. Surgical management of metastatic renal carcinoma of the spine. Spine. 1991;16:265-271.
218 McAfee PC, Zdeblick TA. Tumors of the thoracic and lumbar spine: surgical treatment via the anterior approach. J Spinal Disorders. 1989;2:145-154.
219 Kostuik JP, Errico TJ, Gleason TF. Techniques of internal fixation for degenerative conditions of the lumbar spine. Clin Orthop. 1986;203:203-231.
220 Deramond H, Depriester C, Galibert P, Le Gars D. Percutaneous vertebroplasty with polymethylmethacrylate: technique, indications, and results. Radiol Clin North Am. 1998;36:533-546.
221 Bascoulergue Y, Duquesnel J, Leclercq R, et al. Percutaneous injection of methy methacrylate in the vertebral body for the treatment of various diseases: percutaneous vertebroplasty [abstract]. Radiology. 1988;169P:372.
222 Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of vertebral body compression fractures: technical aspects. Am J Neuroradiol. 1997;18:1877-1904.
223 Lewiecki M. Augmentation procedures for osteoporotic vertebral fractures—an ongoing experiment or emerging standard of care. Southern Med J. 2006;99:449-450.
224 San Millan Ruiz D, Burkhardt K, Jean B, et al. Pathology findings with acrylic implants. Bone. 1999;25(2 suppl):85S-90S.
225 Jefferiss CD, Lee AFC, Ling RSM. Thermal aspects of self-curing polymethylmethacrylate. J Bone Joint Surg. 1975;57B(4):511-518.
226 Togawa D, Kovacic J, Bauer TW, et al. Radiographic and histologic findings of vertebral augmentation using polymethylmethacrylate in the primate spine: percutaneous vertebroplasty versus kyphoplasty [abstract]. Spine. 2006;31:E4-E10.
227 Cotton A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastasis and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200:525-530.
228 Jenson ME, Dion JE. Percutaneous vertebroplasty in osteoporotic compression fractures. Neuroimaging Clin North Am. 2000;10:547-568.
229 Murphy KJ, Deramond H. Percutaneous vertebroplasty in benign and malignant disease. Neuroimaging Clin N Am. 2000;10:535-545.
230 Grados F, Depriester C, Cayrolle G, et al. Long-term observations of vertebral osteoporosis fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford). 2000;39:1410-1414.
231 Ananthakrishnan D, Berven S, Deviren V, et al. The effect on anterior column loading due to different vertebral augmentation techniques. Clin Biomech. 2005;20:25-31.
232 Garfin SR, Yan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511-1515.
233 Jensen ME, McGraw JK, Cardella JF, et al. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, American Association of Neurological Surgeons/Congress of Neurological Surgeons, and American Society of Spine Radiology. J Neuro Intervent Surg. 2009;1:181-185.