Histoplasmosis
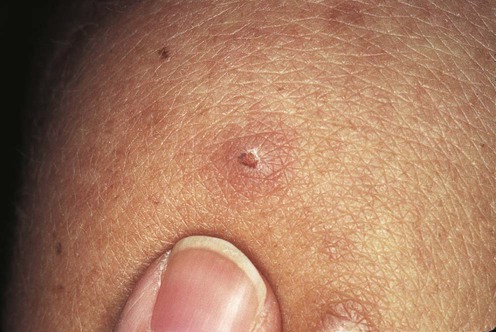
(From Lebwohl, M.G., 2003. The Skin and Systemic Disease: A Color Atlas, 2nd edn. Churchill Livingstone, with permission of Elsevier.)
First-line therapies
Second-line therapies

(From Lebwohl, M.G., 2003. The Skin and Systemic Disease: A Color Atlas, 2nd edn. Churchill Livingstone, with permission of Elsevier.)
