Chapter 14 Histamine and Antihistamines
| Abbreviations | |
|---|---|
| CNS | Central nervous system |
| Epi | Epinephrine |
| IgE | Immunoglobulin E |
Therapeutic Overview
Histamine is synthesized, stored, and released primarily by mast cells and has profound effects on many organs. It is an important mediator of immediate hypersensitivity reactions and acute inflammatory responses and is a primary stimulator of gastric acid secretion. It is also an important neurotransmitter in the central nervous system (CNS) (see Chapter 27).
H1 and H2 receptors have been most widely characterized and mediate well-defined responses in humans, as summarized in Table 14-1. Most responses are mediated by H1 receptors, such as bronchoconstriction, and are selectively antagonized by classical antihistamines, more accurately described as selective H1 receptor blocking drugs such as diphenhydramine. Antihistamines are widely used to treat allergic reactions, motion sickness, and emesis and as over-the-counter sleeping aids. H2-mediated responses such as gastric acid secretion are selectively antagonized by specific H2 receptor blocking drugs such as cimetidine. The H2 receptor antagonists are discussed extensively in Chapter 18; this chapter focuses on histamine and H1 receptor antagonists.
TABLE 14–1 Histamine Receptor Subtypes Mediating Selected Responses in Humans
| Subtype | Responses |
|---|---|
| H1 receptor only | Basilar, pulmonary, coronary artery constriction; increased permeability of postcapillary venules; contraction of bronchiolar smooth muscle; stimulation of vagal sensory nerve endings promoting bronchospasm and coughing; gastrointestinal smooth muscle relaxation and contraction; Epi release from adrenal medulla |
| H2 receptor only | Acid and pepsin secretion from oxyntic mucosa; facial cutaneous vasodilation; pulmonary and carotid artery relaxation; increased rate and force of cardiac contraction; relaxation of bronchial smooth muscle; inhibition of IgE-dependent degranulation of basophils |
| H1 and H2 (?) receptors | Decreased total peripheral resistance; increased forearm blood flow; increased cardiac atrial and ventricular automaticity; stimulation of cutaneous nerve endings causing pain and itching |
Clinical uses of histamine antagonists are summarized in the Therapeutic Overview Box.
| Therapeutic Overview |
|---|
| Histamine and Histamine Receptor Agonists |
| No significant clinical use |
| Antihistamines (H1 Receptor Antagonists) |
| Allergic reactions |
| Motion sickness |
| Insomnia |
| Nausea and vomiting |
| H2 Receptor Antagonists |
| Peptic ulcers and gastroesophageal reflux disease (see Chapter 18) |
Mechanisms of Action
Synthesis and Metabolism of Histamine
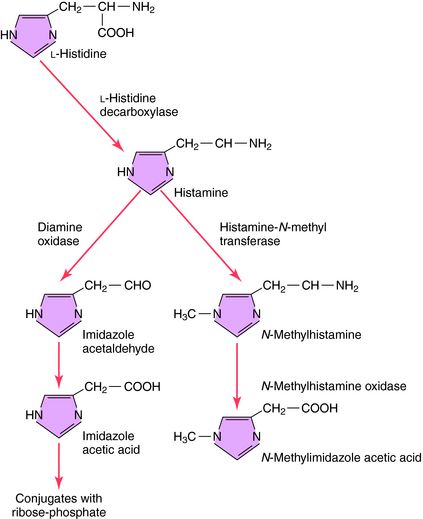
The synthesis and catabolism of histamine are depicted in Figure 14-1. Histamine is synthesized by decarboxylation of the amino acid L-histidine by histidine decarboxylase. Most histamine is stored in an inert form at its site of synthesis, and very little is freely diffusible. After synthesis and release from its storage sites, histamine acts at its targets and is rapidly metabolized through two primary pathways. Oxidative deamination leads to formation of imidazole acetic acid, while methylation, which predominates in the brain, leads to the formation of N-methylimidazole acetic acid. Both metabolites are inactive and subject to further biotransformation.
Storage and Release of Histamine by Mast Cells and Basophils
Histamine is stored and released primarily by mast cells. Although basophils and central neurons also use histamine, their role is not fully understood. Histamine is widely distributed, with the highest concentrations in the skin, lungs, and gastrointestinal tract mucosa, consistent with mast cell densities in these tissues.
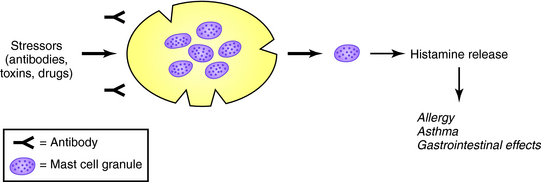
In mast cell granules, histamine exists as an ionic complex with a proteoglycan, chiefly heparin sulfate, but also chondroitin sulfate E. In basophils, histamine is also stored in granules as an ionic complex, predominantly with proteoglycans. The release of histamine and other mediators from mast cells and basophils is common during allergic reactions but also can be induced by drugs and endogenous compounds to produce pseudoallergic, anaphylactoid reactions as shown in Figure 14-2. The role of mast cells in immediate and delayed hypersensitivity reactions and nonallergic disorders explains the therapeutic utility of antihistamines and degranulation inhibitors.
A summary of agents that release histamine is presented in Table 14-2. Histamine concentrations of 0.2 to 1.0 ng/mL in humans produce mild signs and symptoms, including metallic taste, headache, and nasal congestion. Concentrations exceeding 1 ng/mL produce moderate effects, including skin reactions, cramping, diarrhea, flushing, tachycardia, cardiac dysrhythmias, and hypotension. Life-threatening hypotension, ventricular fibrillation, and bronchospasm leading to cardiopulmonary arrest can occur when concentrations approach 12 ng/mL.
TABLE 14–2 Common Causal Factors Involved in Anaphylactic and Anaphylactoid Reactions
| IgE-Mediated Anaphylaxis | Non–IgE-Mediated Anaphylactoid Reactions |
|---|---|
| Food | Drugs |
| Peanuts, seafood, eggs, milk products, grains | Anesthesia related: neuromuscular blocking agents, opioids, plasma expanders |
| Drugs | |
| Antibiotics: penicillins, cephalosporins, sulfonamides |
Inhibitors of Degranulation and Histamine Release
Because many mediators are released from mast cells and basophils during noncytolytic degranulation, agents that can prevent this reaction are of therapeutic value. Cromolyn sodium is used to treat asthma and bronchospastic diseases and is discussed in Chapter 16.
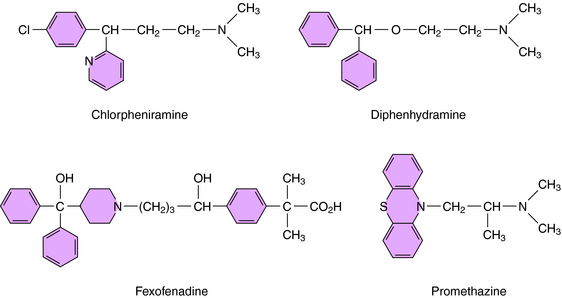
H1 antagonists have little structural resemblance to histamine (Figs. 14-1 and 14-3). A common feature, however, is a substituted ethylamine containing a nitrogen atom in an alkyl chain or ring. H1 antagonists are classified by the chemical group containing the substituted ethylamine.
Other Antiallergic Properties of Antihistamines
Other effects have also been observed for second-generation antihistamines, including inhibition of allergen-induced migration of eosinophils, basophils, and neutrophils and an inhibition of platelet-activating factor-induced eosinophil accumulation in skin. The clinical significance of these effects remains to be established.
Pharmacokinetics
The pharmacokinetic parameters of selected H1 receptor antagonists are given in Table 14-3. Those of cromolyn are presented in Chapter 16, and those of H2 receptor antagonists are presented in Chapter 18.
TABLE 14–3 Pharmacokinetic Parameters of Selected H1 Receptor Antagonists
| Drug | t1/2 (hrs) | Disposition |
|---|---|---|
| Cetirizine | 7–9 | R, N |
| Chlorpheniramine | ~20 | M |
| Desloratadine | ~27 | M, N |
| Diphenhydramine | –8 | M |
| Fexofenadine | 14 | R, N |
| Loratadine | 2–15 | M, A, N |
| Promethazine | 7–15 | M |
M, Metabolism; A, active metabolite that contributes to therapeutic effect; N, non-sedating; R, renal elimination.
Relationship of Mechanisms of Action to Clinical Response
The effects of histamine on the systemic vasculature are complex, and different vascular beds show different responses (see Table 14-1). The predominant action is vasodilation resulting from relaxation of arteriolar smooth muscle, precapillary sphincters, and muscular venules mediated by H1 and H2 receptors. Vasodilation produced by H1 receptors occurs at lower doses and is transient, whereas that caused by H2 receptors occurs at higher doses and is sustained. Both H1 and H2 antagonists are required to completely block the hypotensive response.
Histamine may also produce a modest relaxation of contracted bronchial smooth muscle through H2 receptors, although this is not usually clinically significant. Histamine also acts on H1 receptors to increase secretion of airway fluid and electrolytes, which may produce pulmonary edema and contribute to bronchial obstruction in patients with extrinsic asthma (see Chapter 16).
An important physiological function of histamine is its role as a primary mediator in the secretion of gastric acid, discussed in Chapter 18.
Headaches, nausea, and vomiting have also been observed in human volunteers receiving histamine. In high doses histamine stimulates catecholamine release from the adrenal medulla, an effect particularly pronounced in patients with pheochromocytoma, a catecholamine-secreting tumor of the adrenal medulla.
Inhibitors of Mast Cell Degranulation
Cromolyn Na+ inhibits the degranulation of mast cells and activation of inflammatory cells. It is used in the treatment of asthma and bronchospastic disorders (see Chapter 16).
The effectiveness of H1 receptor antagonists in the treatment of motion sickness and extrapyramidal symptoms may result from their antimuscarinic actions. They may promote sleep by blocking central H1 receptors and muscarinic receptors. The drugs with antiemetic activity are largely those in the phenothiazine class, and they may work primarily by acting as dopamine D2 receptor antagonists. Local anesthetic actions result from the blockade of Na+ channels. Thus the actions of antihistamines at sites other than H1 receptors are the source of both therapeutic and adverse effects. Pharmacological properties of representative H1 antagonists are summarized in Table 14-4.
| Chemical Class/Agents | Comments |
|---|---|
| Alkylamines | |
The release of histamine from mast cells and basophils is accompanied by the release of many other mediators of the immediate hypersensitivity response. Drugs that antagonize H1 receptors are useful as monotherapy in mild pseudoallergic or true allergic reactions and as adjunct agents in the treatment of severe reactions. The treatment of severe allergic reactions requires the use of a physiological antagonist such as an adrenergic agonist, commonly Epi (see Chapter 11), which will reverse the hypotension, laryngeal edema, and bronchoconstriction produced by mast cell mediators.
H1 receptor antagonists are not primary drugs for treating bronchial asthma (see Chapter 16) but may be useful in patients with asthma who require therapy for allergic rhinitis, allergic dermatoses, or urticaria.
H1 receptor antagonists that enter the CNS are used in prophylaxis of motion sickness. Promethazine and diphenhydramine are most potent but are also associated with a high incidence of sedation. Promethazine is an effective antiemetic that can reduce vomiting stemming from a variety of causes. Meclizine, cyclizine, and dimenhydrinate (a salt of diphenhydramine) are used in over-the-counter preparations for the prevention of motion sickness. However, none is as effective as the antimuscarinic agent scopolamine (see Chapter 10). These agents also are used in symptomatic treatment of vertigo.
Combination of H1 and H2 Receptor Antagonists
Combinations of H1 and H2 receptor antagonists are used in the prophylaxis and treatment of severe allergic reactions. Such a combination is more effective than either antagonist alone in reducing histamine-induced vasodilatation, hypotension, mucus secretion, decrease in diastolic blood pressure, and widening of pulse pressure that occur in this setting. Because many mediators are involved, other antiallergic agents are often required, including corticosteroids (see Chapter 39).
Pharmacovigilance: Side Effects, Clinical Problems, and Toxicity
Clinical problems with the H1 receptor antagonists are listed in the Clinical Problems Box. Problems associated with H2 receptor antagonists are covered in Chapter 18, and those with cromolyn are covered in Chapter 16.
Most adverse effects of H1 receptor antagonists stem from their antimuscarinic and sedative actions. CNS depression is common with the older agents and occurs in 25% to 50% of patients. Antihistamines with antimuscarinic activity may cause dry mouth, blurred vision, urinary retention, constipation, and other symptoms typical of muscarinic receptor blockade (see Chapter 10). Additional effects include insomnia, nervousness, tremors, and euphoria. Appetite stimulation and weight gain have been reported for some drugs. Paradoxical excitation can occur in children; nausea, vomiting, diarrhea, and epigastric distress are also reported. Over-the-counter cough and cold medicines containing H1 receptor antihistamines in pediatric formulations have raised significant concerns about lack of efficacy and excessive adverse effects in children.
The incidence of CNS depression and antimuscarinic effects is less in patients receiving second-generation agents and is comparable to those produced by placebo. The incidence of true allergic responses is low for drugs administered systemically. However, allergic responses are relatively common after repeated topical use; therefore such use is discouraged. Teratogenic effects of piperazine derivatives are observed in experimental animals, and although there is no evidence of this in humans, antihistamines are not recommended during pregnancy.
Anonymous. Drugs for allergic disorders. Treat Guidel Med Lett. 2007;60:71-80.
Esbenshade TA, Fox GB, Cowart MD. Histamine H3 receptor antagonists: Preclinical promise for treating obesity and cognitive disorders. Mol Interv. 2006;6:77-88.
Miyoshi K, Das AK, Fujimoto K, et al. Recent advances in molecular pharmacology of the histamine systems: Regulation of histamine H1 receptor signaling by changing its expression level. J Pharmacol Sci. 2006;101:3-6.