Chapter 6 Hip and Thigh Ultrasound
![]() Additional videos for this topic are available online at www.expertconsult.com.
Additional videos for this topic are available online at www.expertconsult.com.
Hip and Thigh Anatomy
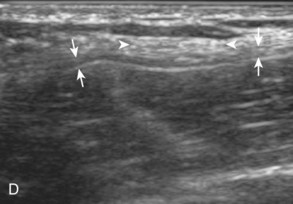
The hip joint is a synovial articulation between the acetabulum of the pelvis and the proximal femur. The joint recess extends from the acetabulum over the femur to the level of the intertrochanteric line, just beyond the femoral neck. The joint capsule becomes thickened from the iliofemoral, ischiofemoral, and pubofemoral ligaments (Fig. 6-1A) and a reflection of the joint capsule extends proximally along the femoral neck.1 The femoral head is covered by hyaline cartilage, whereas the acetabulum is lined by hyaline cartilage in an inverted U shape with a fibrocartilage labrum attached to the acetabular rim.
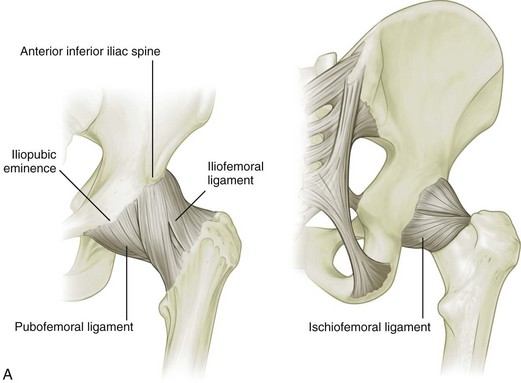
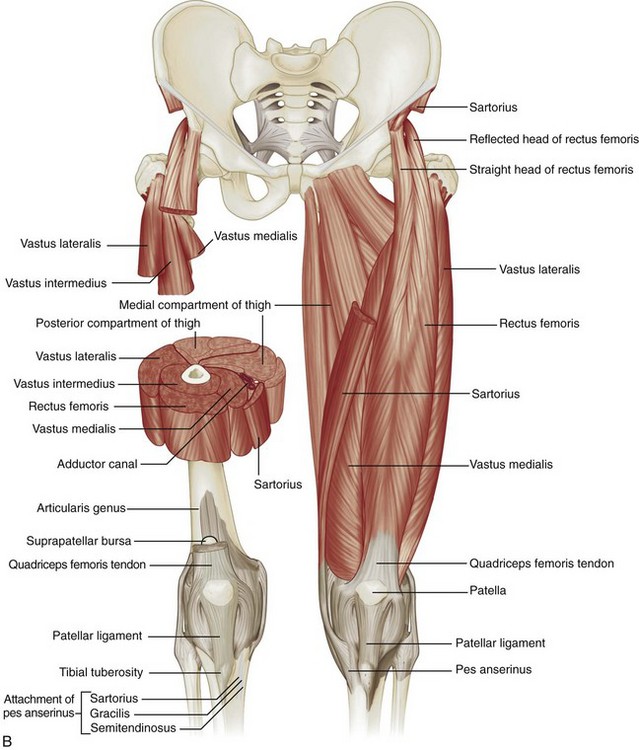
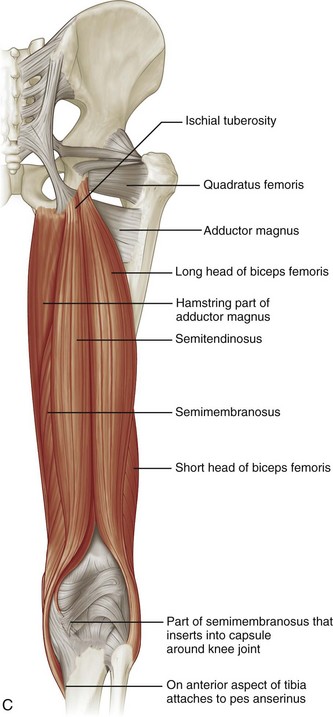
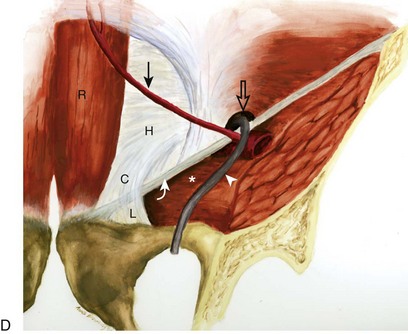
FIGURE 6-1 Hip and thigh anatomy.
(A to C, From Drake R, Vogl W, Mitchell A: Gray’s anatomy for students, Philadelphia, 2005, Churchill Livingstone; D, from Jamadar DA, Jacobson JA, Morag Y, et al: Sonography of inguinal region hernias. AJR Am J Roentgenol 187:185–190, 2006.)
Several muscles originate from the pelvis and extend across the hip joint, and others originate from the femur itself (see Fig. 6-1B and C). Muscles that originate from the posterior surface of the ilium are the gluteus minimus (which inserts on the anterior facet of the greater trochanter), the gluteus medius (which inserts on the lateral and superoposterior facets of the greater trochanter), and the gluteus maximus (which inserts on the posterior femur gluteal tuberosity below the trochanters and iliotibial tract).2 Posteriorly, the piriformis originates from the sacrum and extends inferior and lateral to insert onto the greater trochanter. Other muscles inferior to the piriformis that extend from the ischium to the proximal femur include the superior gemellus, obturator internus, inferior gemellus, and quadratus femoris.
At the anterior aspect of the hip joint, the iliopsoas can be seen as a continuation of the iliacus and psoas muscles, which inserts on the lesser trochanter. Other anterior muscles include the sartorius (which originates from the anterior superior iliac spine of the pelvis and inserts on the medial aspect of the proximal tibia) and the tensor fasciae latae (which originates from the posterolateral aspect of the ilium and inserts on the iliotibial tract, which, in turn, inserts on the proximal tibia). The rectus femoris has two origins: a direct or straight head, which originates from the anterior inferior iliac spine; and an indirect or reflected head, which originates inferior and posterior to the anterior inferior iliac spine from the superior acetabular ridge.3 Distally, the direct tendon forms an anterior superficial tendon with unipennate architecture, whereas the indirect tendon forms the central tendon with bipennate architecture.4 The rectus femoris distally combines with the vastus medialis, vastus lateralis, and vastus intermedius musculature (which all originate from the femur) to form the quadriceps tendon, which inserts on the patella and, to a lesser extent, the tibial tuberosity by way of the patellar tendon.
Medially, the adductor musculature includes the adductor longus, the adductor brevis, and the adductor magnus, which originate from the ischium and pubis of the pelvis and insert on the femur at the linea aspera and, in the case of the adductor magnus, the adductor tubercle as well. Superficial and medial to the adductors, the gracilis muscle extends from the inferior pubic ramus to the proximal tibia as part of the pes anserinus. From medially to laterally, the posterior thigh consists of the semimembranosus, the semitendinosus (both of which originate from the ischial tuberosity and insert on the proximal tibia, with the semitendinosus being part of the pes anserinus), and the biceps femoris (with long head origin from the ischial tuberosity and short head origin from the femur; the biceps femoris inserts on the fibula and lateral tibial condyle). Proximally, the semimembranosus tendon is located anterior to the conjoint tendon of the biceps femoris long head and semitendinosus and the semitendinosus muscle belly; the semimembranosus origin on the ischium is anterolateral to the conjoint tendon origin.5
Other important structures of the anterior thigh include (medial to lateral) the femoral nerve, artery, and vein (use the mnemonic NAVEL for nerve, artery, vein, empty space, lymphatic). The sciatic nerve is seen posteriorly adjacent to the biceps femoris muscle, where it bifurcates as the tibial nerve and the common peroneal nerve laterally. Several bursae are located around the hip joint. The iliopsoas bursa is located anteriorly along the medial aspect of the iliopsoas tendon, has a convex lateral shape, and normally communicates with the hip joint in up to 15% of the population.6 The trochanteric (or subgluteus maximus) bursa is located posterolateral over the posterior and lateral facets of the greater trochanter deep to the gluteus maximus and iliotibial tract, whereas smaller subgluteus medius and subgluteus minimus bursae are located between the lateral facet and gluteus medius and the anterior facet and gluteus minimus, respectively.2 Other possible bursae include the obturator externus bursa, located medially and inferior to the femoral neck, which may communicate with the posteroinferior hip joint.7
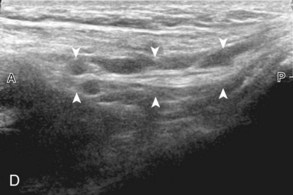
In the inguinal region, the inguinal canal represents a triangular, elongated passage in the lower abdominal wall located just superior to the inguinal ligament (see Fig. 6-1D). The inguinal canal’s posterior opening, the deep inguinal ring, is located laterally, whereas the anterior opening, called the superficial inguinal ring, is located medially near the pubis. The contents of the inguinal canal include the ilioinguinal nerve and the spermatic cord in males and the round ligament in females. The deep inguinal ring is located just lateral to the origin of the inferior epigastric artery from the external iliac artery. The inguinal (or Hesselbach) triangle is demarcated by the lateral margin of the rectus abdominis medially, the inguinal ligament inferiorly, and the superior epigastric artery laterally.8 Another structure near the inguinal ligament is the lateral femoral cutaneous nerve. This peripheral nerve exits the pelvis to extend over the lateral thigh in a somewhat variable manner—it may course across the iliac crest, within the sartorius tendon, within the inguinal ligament, or under the inguinal ligament.9 The lateral femoral cutaneous nerve may also branch proximal to the inguinal ligament.
Ultrasound Examination Technique
Table 6-1 is a checklist for hip and thigh ultrasound examination. Examples of diagnostic hip ultrasound reports are available online at www.expertconsult.com (see eBox 6-1 and 6-2).
| Location | Structures of Interest |
|---|---|
| Hip: anterior | Hip joint, iliopsoas, rectus femoris, sartorius, pubic symphysis |
| Hip: lateral | Greater trochanter, bursae |
| Hip: posterior | Sacroiliac joints, piriformis, hip abductors |
| Inguinal region | Deep inguinal ring, Hesselbach triangle, femoral artery region |
| Thigh: anterior | Rectus femoris, vastus medialis, vastus intermedius, vastus lateralis |
| Thigh: medial | Femoral artery and nerve, sartorius, gracilis, adductors |
| Thigh: posterior | Semimembranosus, semitendinosus, biceps femoris, sciatic nerve |
eBox 6-1 Sample Diagnostic Hip Ultrasound Report
Normal
Examination: Ultrasound of the Right Hip
History: Hip pain, evaluate for bursitis
Findings: The hip joint is normal without effusion or synovial hypertrophy. Limited evaluation of the anterior labrum is unremarkable. No evidence of iliopsoas bursal distention or snapping iliopsoas tendon with dynamic imaging. The remaining anterior tendons, including the rectus femoris and sartorius, as well as the adductors, are normal.
Evaluation of the lateral hip is normal. No evidence of abnormal bursal distention around the greater trochanter. The gluteus minimus and medius tendons are normal. No abnormal snapping with dynamic evaluation.
eBox 6-2 Sample Diagnostic Hip Ultrasound Report
Abnormal
Examination: Ultrasound of the Right Hip
History: Hip pain, evaluate for tendon tear
Findings: There is a partial tear of the adductor longus origin at the pubis. No evidence of full-thickness tear or tendon retraction. The common aponeurosis and rectus abdominis tendon are normal, as is the pubic symphysis.
The hip joint is normal without effusion or synovial hypertrophy. There is a possible tear of the anterior labrum. No paralabral cyst. No evidence of iliopsoas bursal distention or snapping iliopsoas tendon with dynamic imaging.
Evaluation of the lateral hip is normal. No evidence of abnormal bursal distention around the greater trochanter. The gluteus minimus and medius tendons are normal. No abnormal snapping with dynamic evaluation.
Hip Evaluation: Anterior
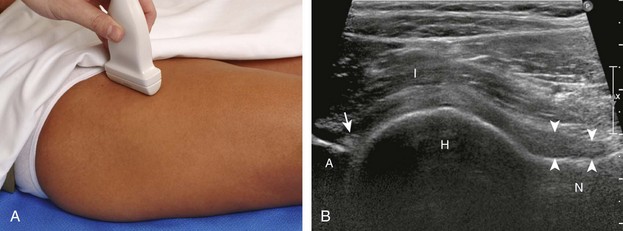
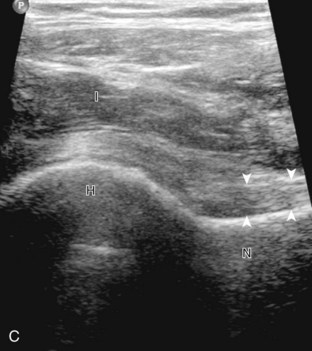
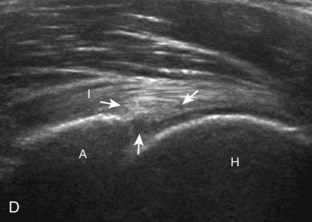
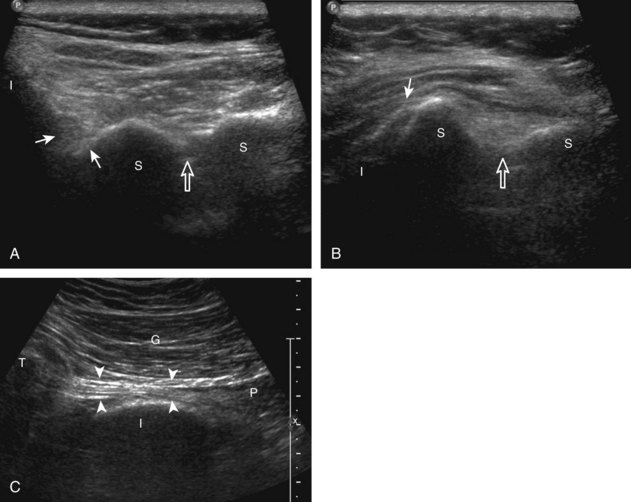
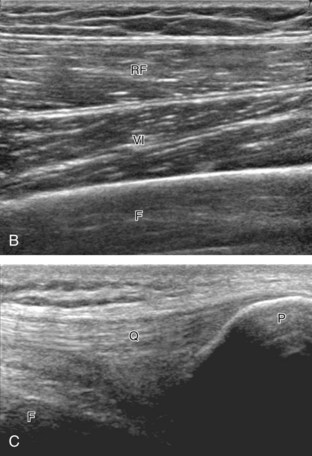
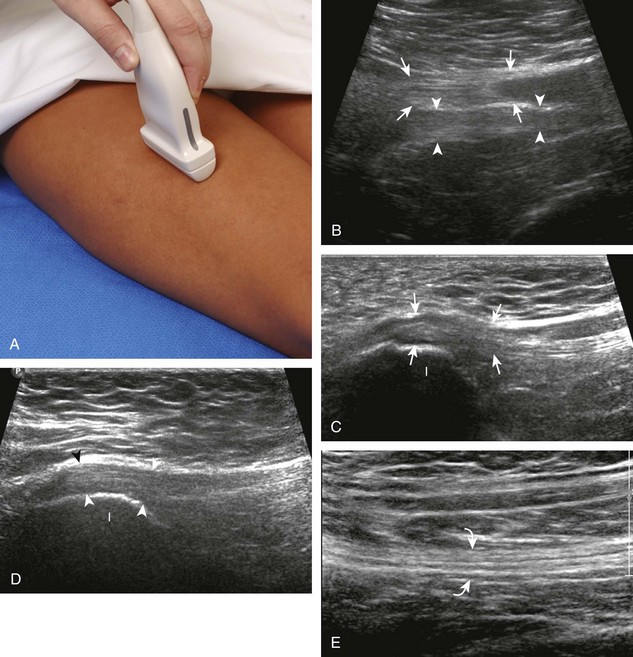
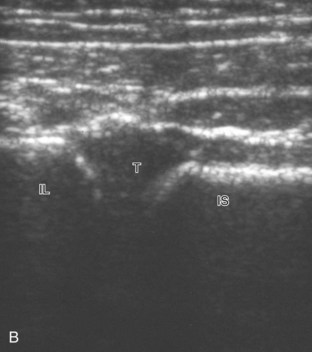
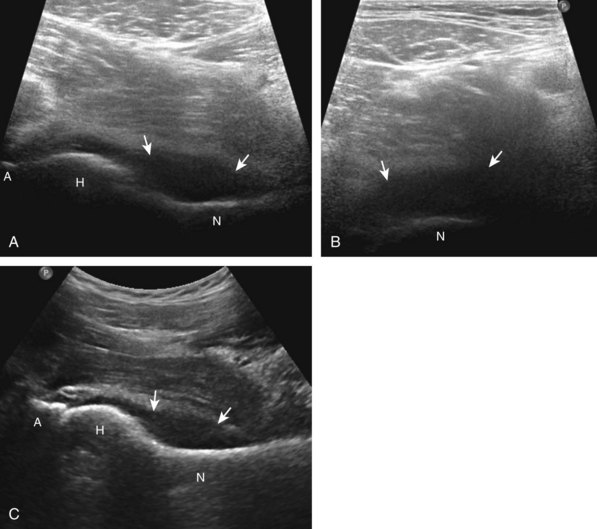
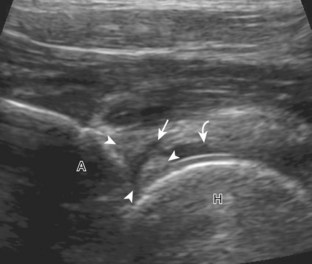
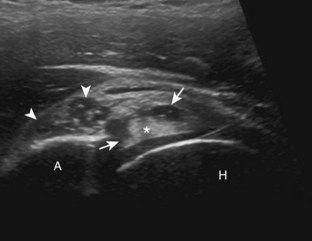
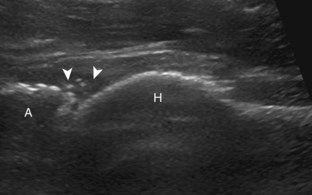
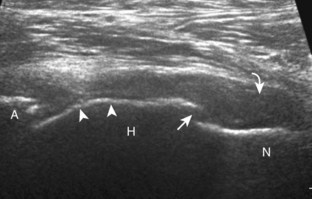
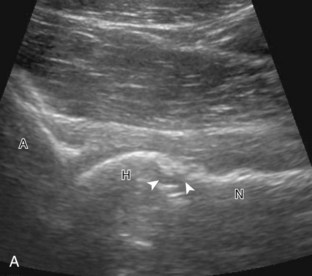
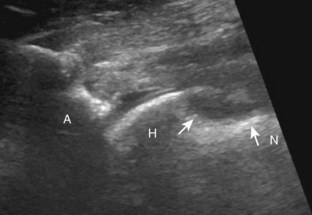
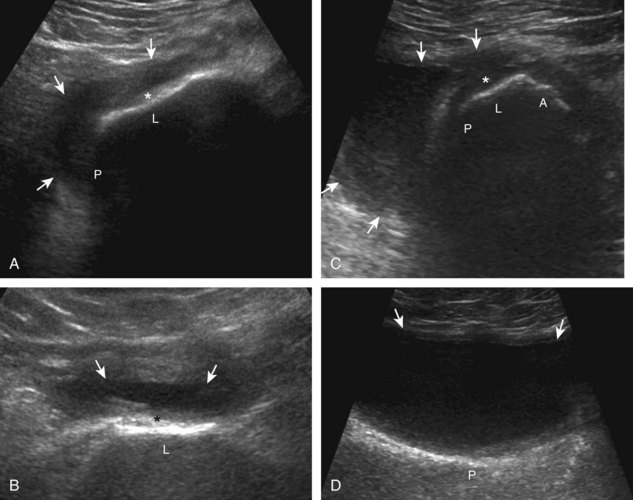
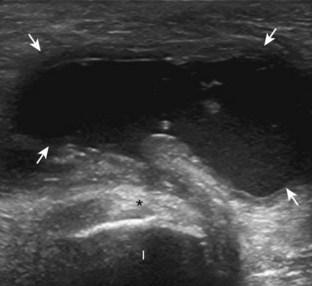
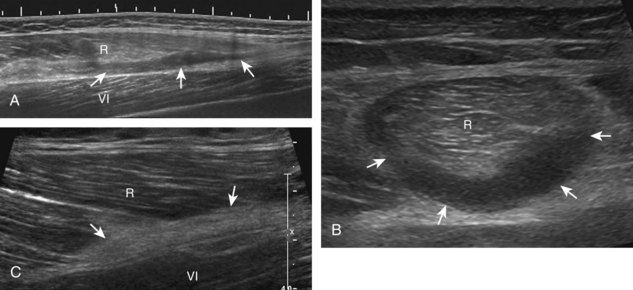
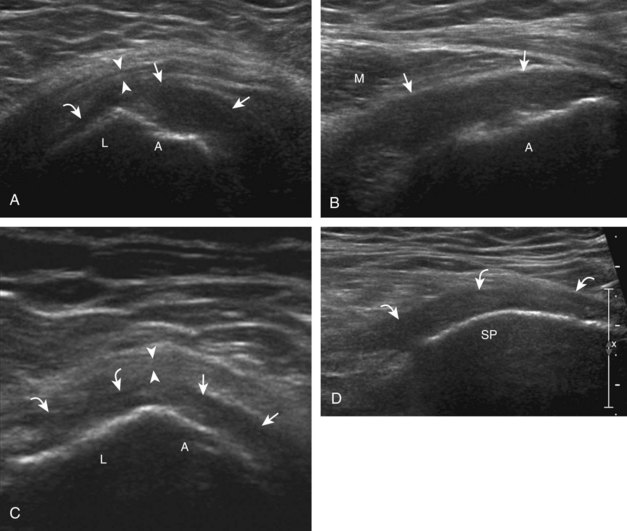
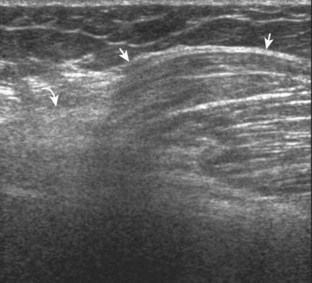
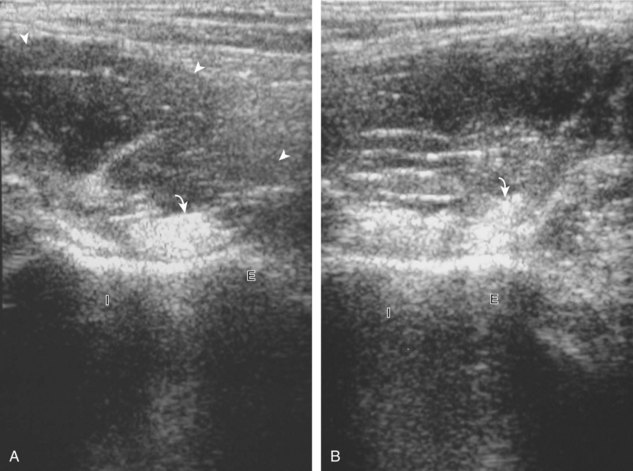
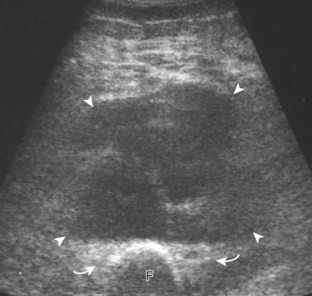
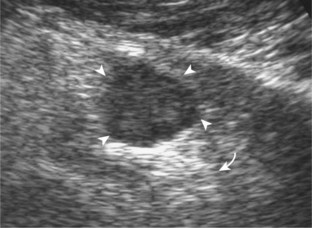
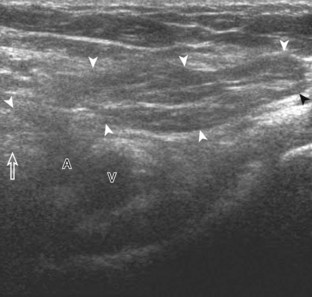
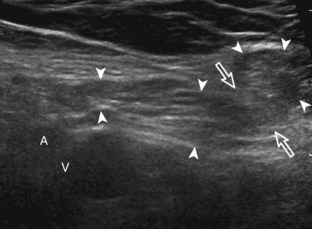
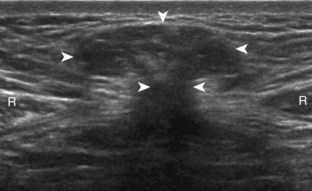
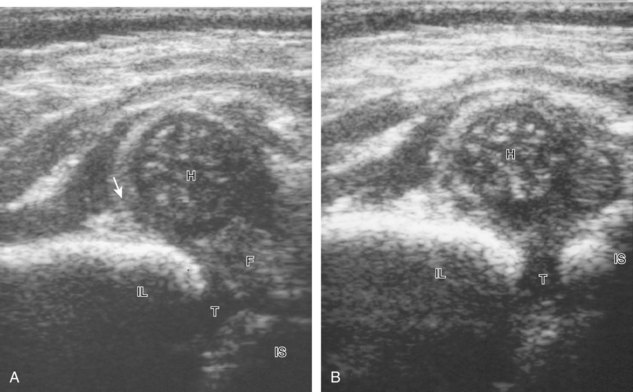
The primary structures evaluated include the hip joint and recess, iliopsoas tendon and bursa, proximal thigh musculature origin in the hip region (rectus femoris and sartorius), and pubic symphysis region. Depending on patient history and symptoms, all of these structures should be considered in the evaluation because symptoms may be referred and etiology multifactorial. Evaluation begins with the anterior hip with the transducer long axis to the femoral neck, which is in the oblique-sagittal plane (Fig. 6-2A). To find the femoral neck, one may initially image transversely over the femoral shaft to locate the curved and echogenic surface of the femur and then move the transducer proximally; once the bony protuberances of the greater and lesser trochanter are identified, the transducer is turned to the sagittal-oblique plane parallel to the femoral neck. The hip joint may also be located lateral to the femoral vasculature. The hip joint is identified long axis to the femoral neck by the characteristic bone contours of the femoral head, acetabulum, and femoral neck (see Fig. 6-2B to D). It is at this location superficial to the femoral neck where the anterior joint recess is evaluated for fluid or synovial abnormalities.1
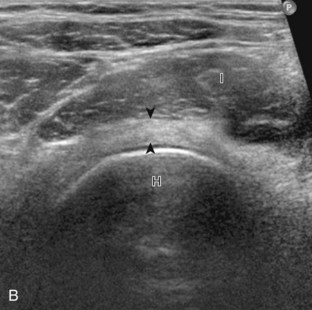
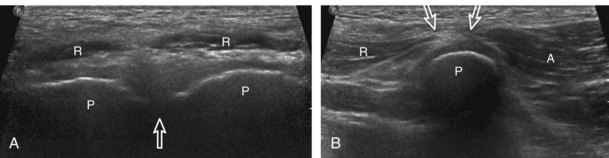
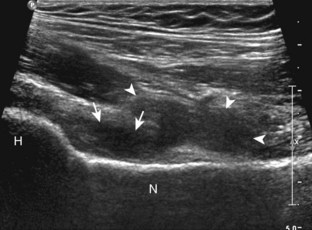
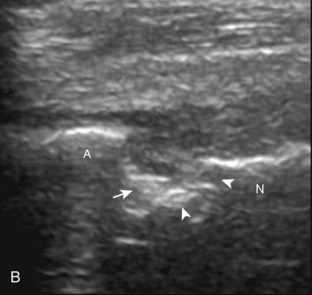
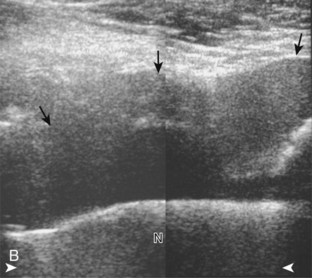
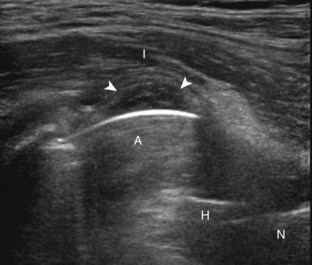
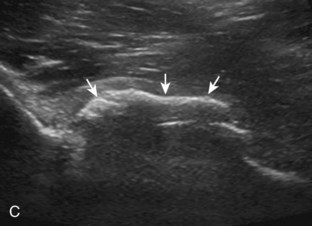
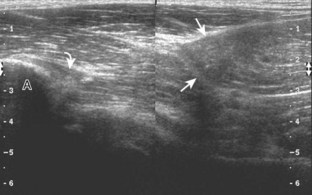
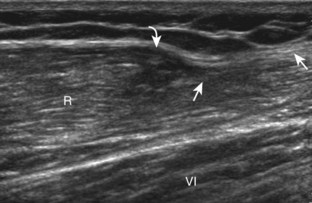
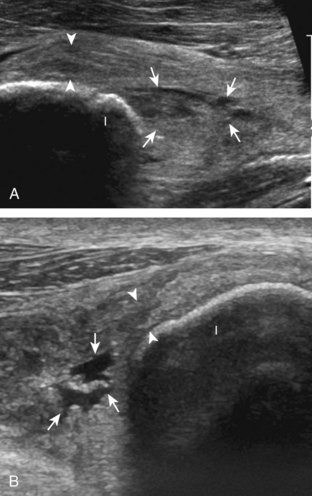
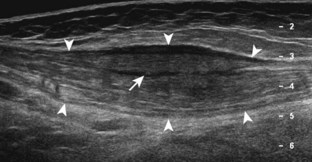
The anterior recess of the hip joint over the femoral neck is normally about 4 to 6 mm thick, and this can be explained anatomically.1 The anterior joint capsule extends inferiorly from the labrum and inserts at the intertrochanteric line; however, some fibers are reflected superiorly along the femoral neck to attach at the femoral head-neck junction (Fig. 6-3). Both the anterior and posterior layers measure 2 to 3 mm each in thickness; physiologic fluid between these layers should measure less than 2 mm, and typically no fluid is identified in the normal situation.1 The anterior capsule layer may be slightly thicker than the posterior layer as a result of capsular thickening from ligaments and the zona orbicularis, which encircles the capsule at the femoral head-neck junction. The posterior layer may demonstrate focal thickening at its attachment at the femoral head-neck junction. The normal anterior joint recess is usually concave or flat anteriorly, rather than convex. The true hyperechoic and fibrillar appearance of the joint capsule and its reflection is best appreciated when the femoral neck is perpendicular to the sound beam (see Fig. 6-2C); if imaged obliquely, the joint capsule may artifactually appear hypoechoic and may simulate fluid in echogenicity, especially in a patient with a large body habitus (see Fig. 6-2B). The femoral head and neck should be smooth, and the visualized portion of the hypoechoic hyaline cartilage that covers the femoral head should be uniform. The fibrocartilage labrum is hyperechoic and triangular and extends from the margins of the acetabulum (see Fig. 6-2D). The femoral head and neck are also evaluated in short axis to the femoral neck (Fig. 6-4).
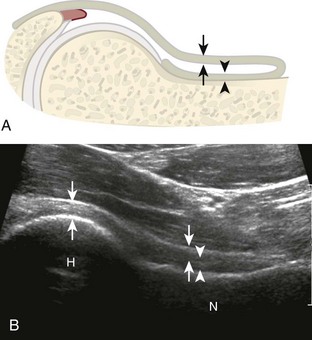
FIGURE 6-3 Anterior hip joint recess.
(Modified from an illustration by Carolyn Nowak, Ann Arbor, Mich. http://www.carolyncnowak.com/MedTech.html.)
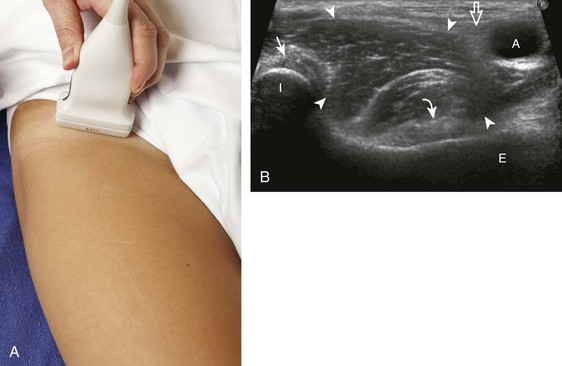
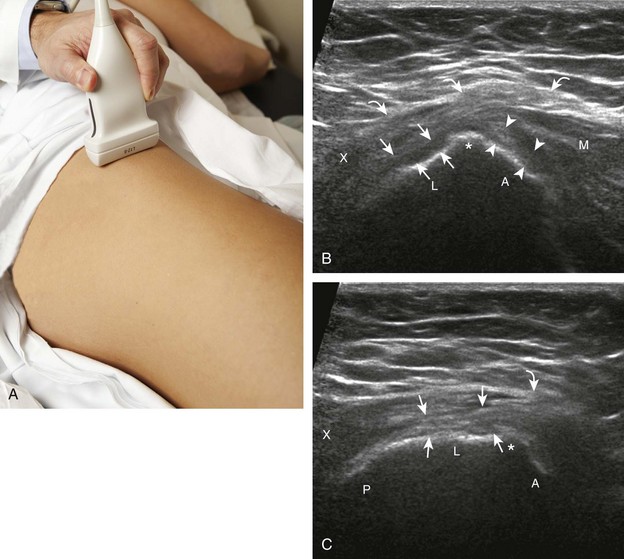
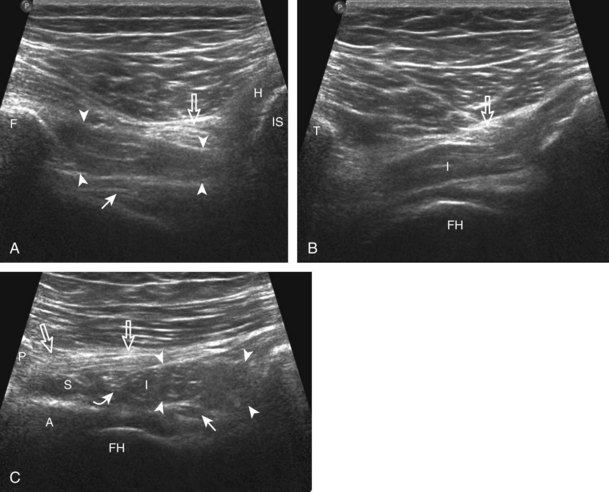
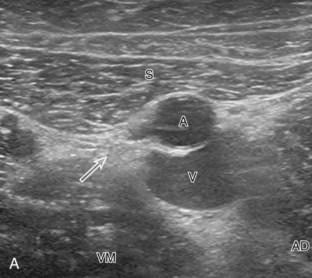
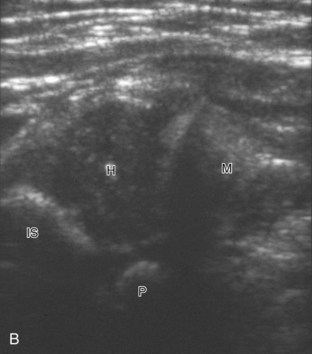
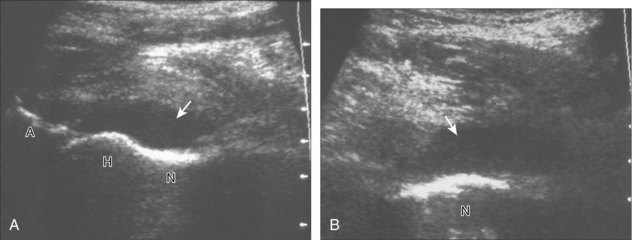
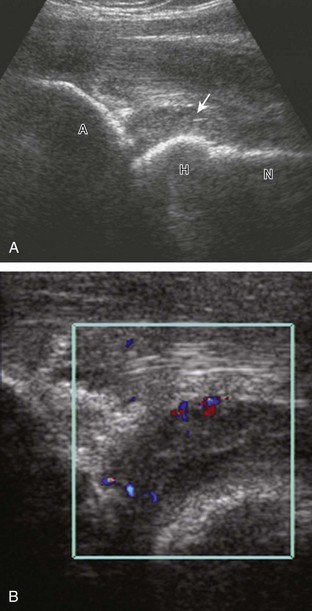
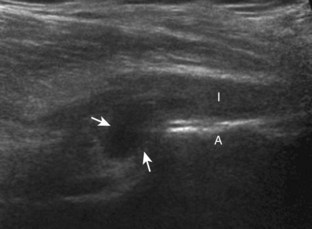
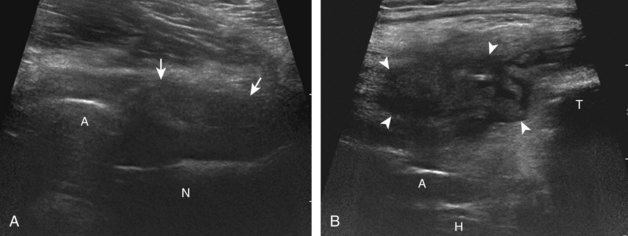
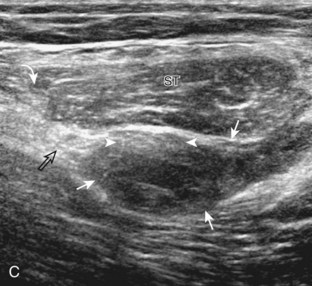
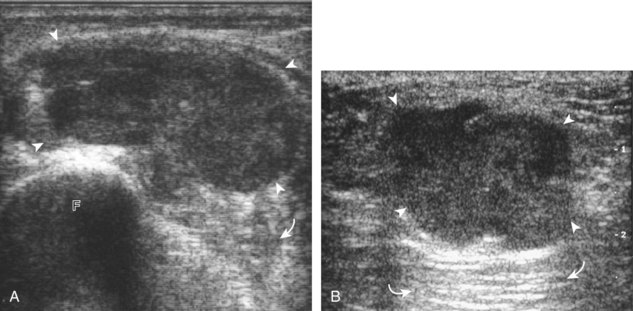
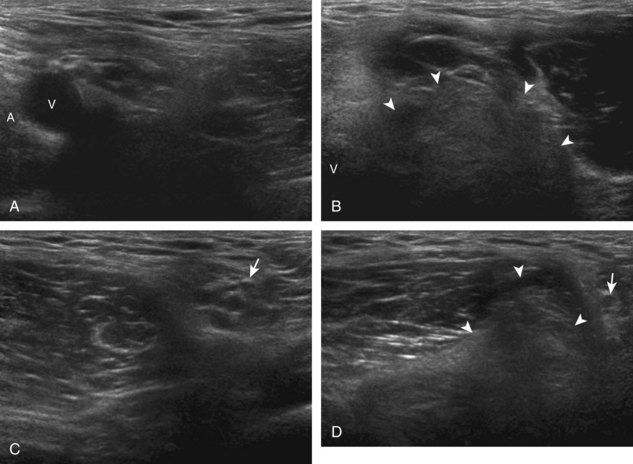
To evaluate the iliopsoas region, the transducer is first placed in the transverse plane over the femoral head because this bone landmark is easy to identify (see Fig. 6-4B). The transducer is then moved superiorly and angled parallel to the inguinal ligament (Fig. 6-5). The characteristic bone contours are seen along with the iliopsoas muscle and tendon, the rectus femoris origin at the anterior inferior iliac spine, and the external iliac vessels. As with imaging any tendon in short axis, toggling the transducer is often helpful to visualize the tendon as hyperechoic, especially because the iliopsoas normally courses deep toward the lesser trochanter and is oblique to the sound beam. The iliopsoas should be evaluated dynamically for tendon snapping (see Snapping Hip Syndrome later in the chapter). The anterior hip is also evaluated for iliopsoas bursa, which originates at the level of the femoral head and typically extends medial and possibly deep to the iliopsoas tendon. The transducer is also rotated 90 degrees to evaluate the iliopsoas tendon in long axis (see Fig. 6-2).
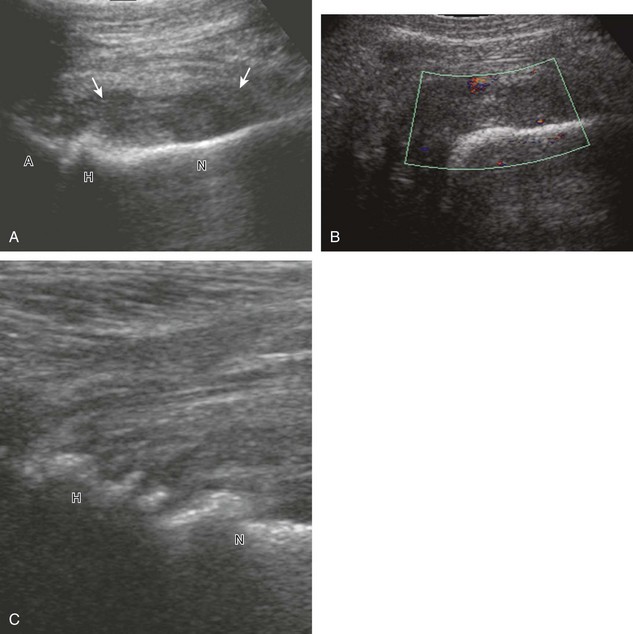
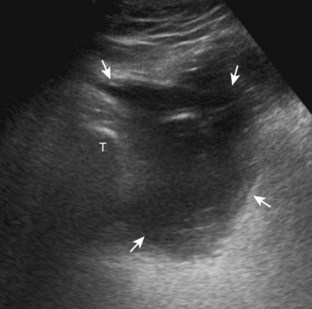
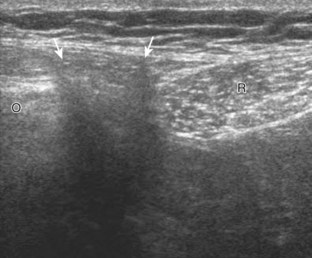
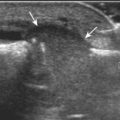
To further evaluate the rectus femoris origin, the transducer is positioned over the anterior inferior iliac spine in the transverse plane. The direct head is seen directly superficial to the anterior inferior iliac spine, whereas the indirect head is at the lateral aspect of the acetabulum (Fig. 6-6). When evaluating the direct head in long axis (see Fig. 6-6B), moving the transducer slightly laterally will show the indirect head coursing proximal and deep, appearing hypoechoic from anisotropy, and producing a characteristic refraction shadow (see Fig. 6-6C) (Video 6-1)![]() . The transducer can be rotated in plane with the indirect head and moved over the lateral hip to identify the origin of the indirect head without artifact (see Fig. 6-6D) (Video 6-2)
. The transducer can be rotated in plane with the indirect head and moved over the lateral hip to identify the origin of the indirect head without artifact (see Fig. 6-6D) (Video 6-2)![]() . The transducer is then returned to short axis relative to the rectus femoris direct head and moved proximally and laterally to visualize the sartorius and its origin on the anterior superior iliac spine (Fig. 6-7).
. The transducer is then returned to short axis relative to the rectus femoris direct head and moved proximally and laterally to visualize the sartorius and its origin on the anterior superior iliac spine (Fig. 6-7).
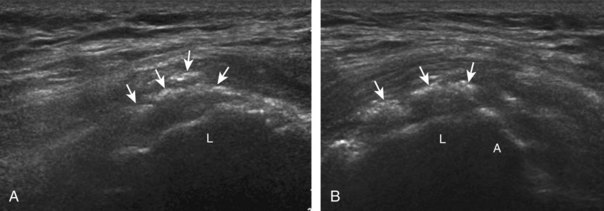
Evaluation for the lateral femoral cutaneous nerve begins with the transducer in the transverse plane over the proximal sartorius near the anterior superior iliac spine.10 As the transducer is moved distally, the lateral femoral cutaneous nerve can be seen as several nerve fascicles coursing over the sartorius from medial to lateral (Fig. 6-8A). More distally, the lateral femoral cutaneous nerve is identified in a triangular hypoechoic fatty space at the lateral aspect of the sartorius (see Fig. 6-8B) (Video 6-3![]() ).11 The transducer is then moved proximally to evaluate for potential nerve entrapment at the inguinal ligament (see Fig. 6-8C and D).12 The lateral femoral cutaneous nerve may branch proximal to the inguinal ligament and has a variable course; it may cross over the iliac crest, through the sartorius tendon, through the inguinal ligament, or under the inguinal ligament.9
).11 The transducer is then moved proximally to evaluate for potential nerve entrapment at the inguinal ligament (see Fig. 6-8C and D).12 The lateral femoral cutaneous nerve may branch proximal to the inguinal ligament and has a variable course; it may cross over the iliac crest, through the sartorius tendon, through the inguinal ligament, or under the inguinal ligament.9
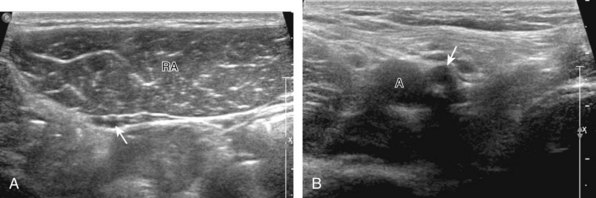
Although thigh evaluation is considered separately, patients with hip pain (especially sports-related pain) may have abnormalities at the adductor tendon origin and the rectus abdominis insertion, with possible abnormalities directly associated with the pubic symphysis.13 The transducer is placed in midline over the pubic symphysis, identified by its characteristic bone contours (Fig. 6-9A). The transducer is turned 90 degrees to evaluate the rectus abdominis in long axis and then rotated toward the adductors to evaluate the common aponeurosis and adductor tendon origin (see Fig. 6-9B).
Hip Evaluation: Lateral
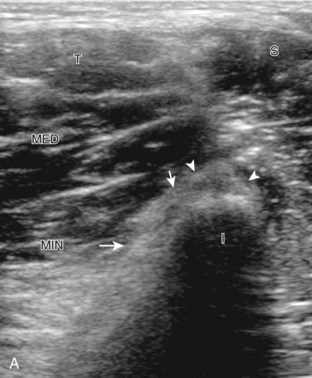
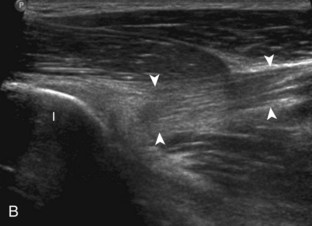
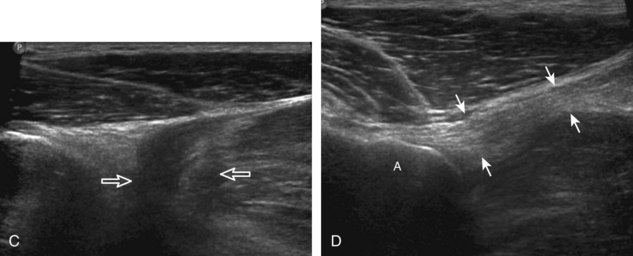
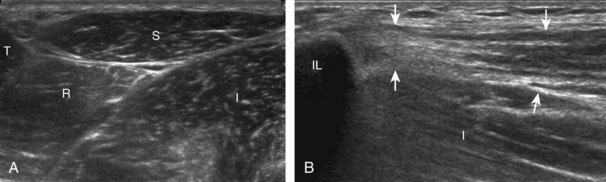
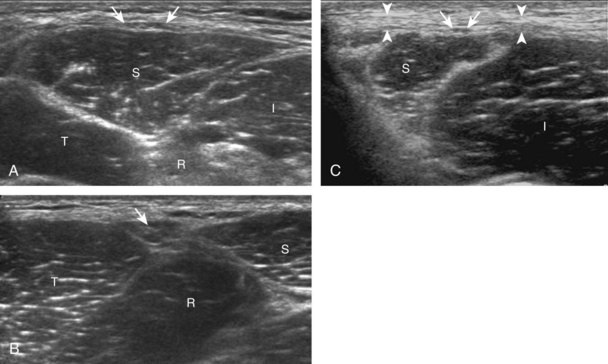
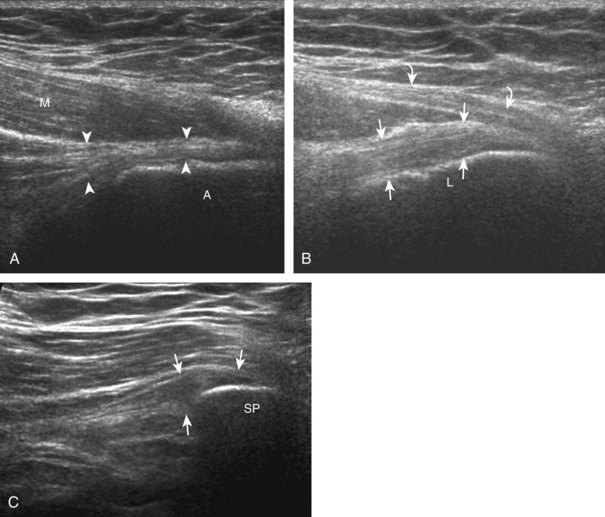
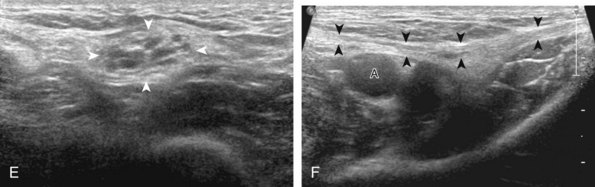
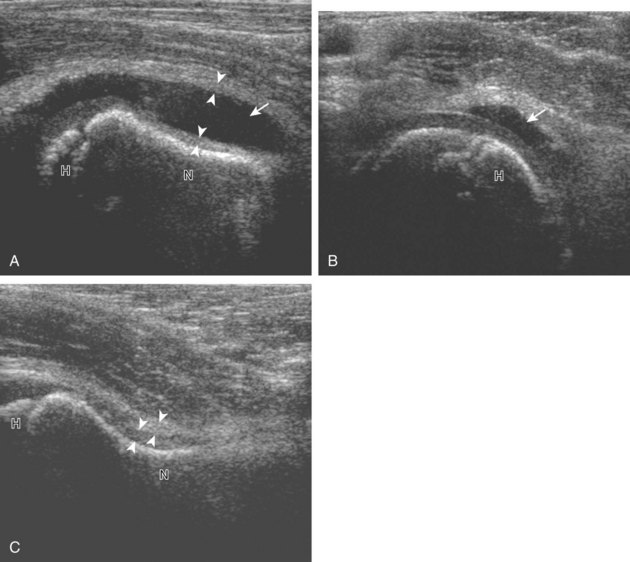
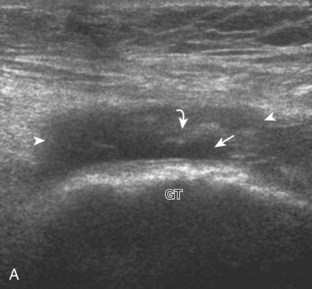
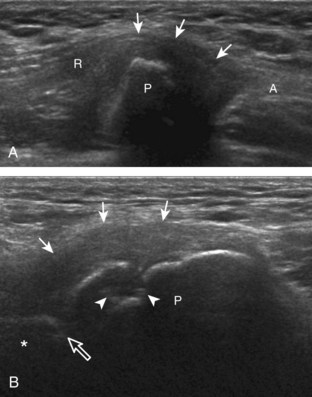
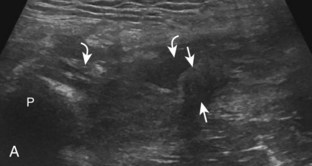
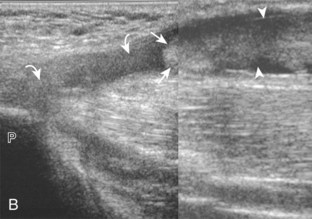
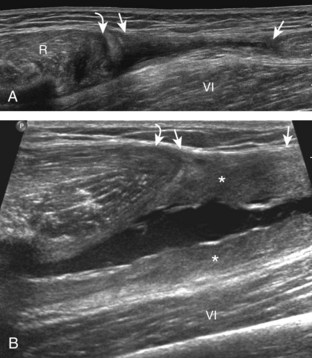
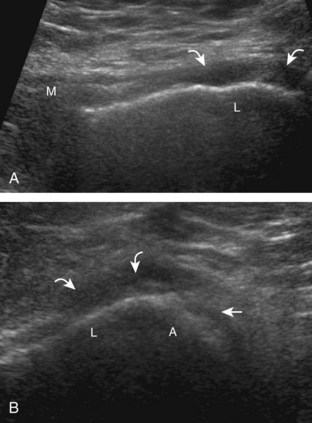
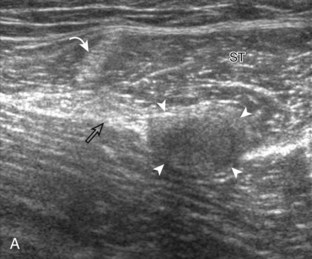
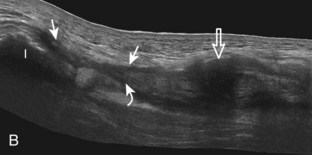
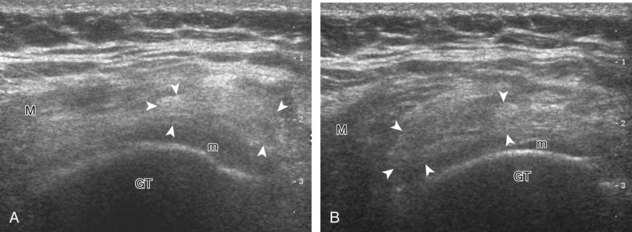
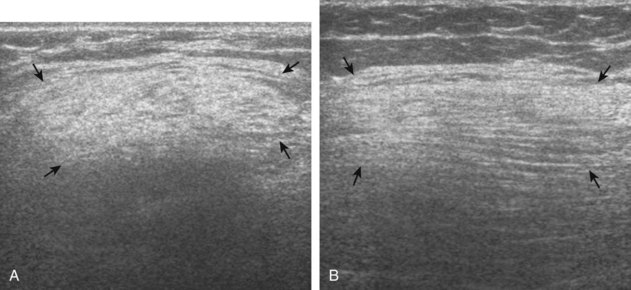
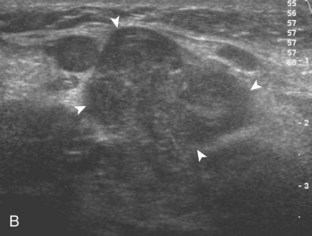
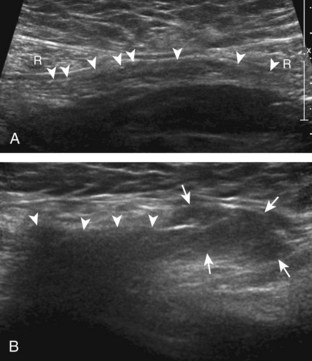
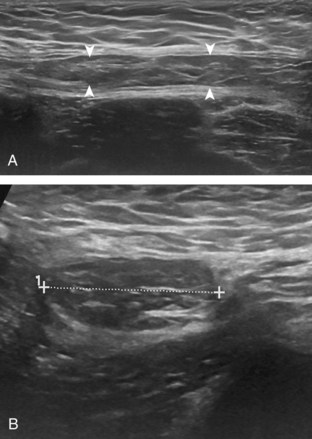
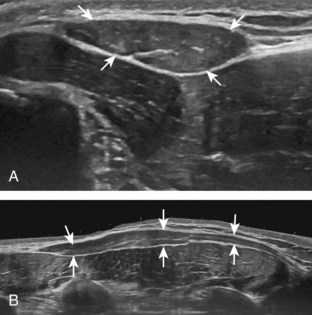
To evaluate the soft tissues over the greater trochanter, bone landmarks are essential (Fig. 6-10). The patient rolls toward the opposite hip to access the posterolateral region of the hip and the transducer is placed over the lateral hip (Fig. 6-11A). To locate the greater trochanter, one begins in short axis to the femur as described earlier. With movement of the transducer cephalad, the bony protuberance of the greater trochanter is identified laterally. The key landmark is the apex of the greater trochanter between the anterior and lateral facets (see Fig. 6-11B).2 Posterior to the lateral facet is the rounded posterior facet of the greater trochanter. The gluteus minimus tendon is identified over the anterior facet, the distal gluteus medius over the lateral facet, and the gluteus maximus over the posterior facet. To confirm that the apex between the lateral and anterior facets is correctly identified, the soft tissues superficial to the gluteus medius and minimus should be evaluated. Superficial to the gluteus medius tendon over the lateral facet one should identify the iliotibial tract, a hyperechoic band of tissue, which is a continuation of the fascial layers that envelop the gluteus maximus posteriorly and the tensor fascia latae anteriorly (see Fig. 6-10). Superficial to the gluteus minimus tendon over the anterior facet is seen the hypoechoic muscle of the gluteus medius and iliotibial tract. Each greater trochanter facet should be evaluated separately in short (see Fig. 6-11) and long axis (Fig. 6-12); the transducer should be positioned so that the cortex of each individual facet is perpendicular to the sound beam to eliminate anisotropy of each overlying tendon (see Fig. 6-11B and C). Evaluation includes assessment for the subgluteus minimus bursa, subgluteus medius bursa, and trochanteric (subgluteus maximus) bursa, which are located between each tendon and their respective greater trochanter facet.2 Because the trochanteric bursa is located between the gluteus maximus and posterior facet, it is essential to position the transducer posteriorly so as not to overlook bursal distention. When distended, the trochanteric bursa may extend laterally between the gluteus medius tendon and overlying iliotibial tract. For evaluation of the gluteus minimus tendon in long axis, the transducer is first positioned over the anterior facet in short axis as described previously and turned 90 degrees (see Fig. 6-12A). The same technique is used over the lateral facet to evaluate the gluteus medius tendon in long axis (see Fig. 6-12B). Because the gluteus medius tendon is attached to two facets (lateral and superoposterior), the transducer should be moved cephalad and posterior to visualize the full extent of the gluteus medius tendon attachment (see Fig. 6-12C).
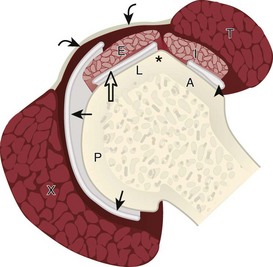
FIGURE 6-10 Greater trochanter anatomy.
(Modified from an illustration by Carolyn Nowak, Ann Arbor, Mich. http://www.carolyncnowak.com/MedTech.html.)
Hip Evaluation: Posterior
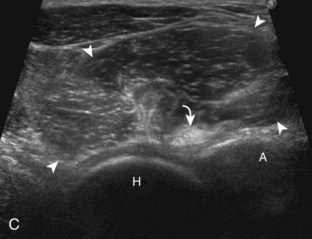
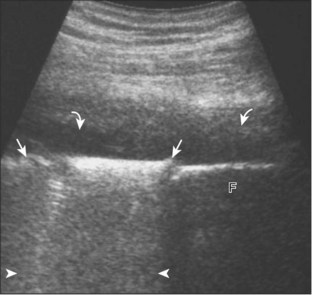
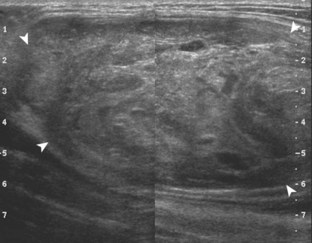
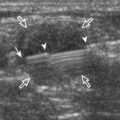
Evaluation of the posterior hip and pelvis is not typically considered part of a routine hip evaluation but rather is guided by patient history and symptoms. Structures of interest include the sacroiliac joints, piriformis, superior gemellus, obturator internus, inferior gemellus, and quadriceps femoris. Evaluation can begin with the sacroiliac joint by first positioning the transducer in midline over the sacrum (Fig. 6-13) and then moving the transducer laterally to visualize the posterior sacral foramina and more laterally to view the sacroiliac joint (Video 6-4)![]() .14 The posterior sacral foramina are differentiated from the sacroiliac joint by their more medial location as well as the characteristic focal disruptions in the cortex when scanning superior to inferior, which is in contrast to the more lateral and linear disruption of the sacroiliac joint. The superior aspect of the sacroiliac joint is widened at the fibrocartilage or ligamentous articulation (see Fig. 6-13A), whereas the more inferior true synovial articulation is narrow (see Fig. 6-13B).
.14 The posterior sacral foramina are differentiated from the sacroiliac joint by their more medial location as well as the characteristic focal disruptions in the cortex when scanning superior to inferior, which is in contrast to the more lateral and linear disruption of the sacroiliac joint. The superior aspect of the sacroiliac joint is widened at the fibrocartilage or ligamentous articulation (see Fig. 6-13A), whereas the more inferior true synovial articulation is narrow (see Fig. 6-13B).
To identify the piriformis, a curvilinear transducer with a frequency of less than 10 MHz is essential given the required depth of penetration. The transducer is first positioned in the transverse plane over the sacroiliac joint, as described previously, and then moved inferior into the greater sciatic foramen and angled inferiorly and laterally toward the greater trochanter to identify the piriformis in long axis (see Fig. 6-13C).15,16 The muscle belly will be located medial to the ilium, while the tendon will be seen directly over the ilium extending to the greater trochanter. Passive hip rotation will assist in its identification because of its movement (Videos 6-5 and 6-6)![]() .
.
To identify the quadratus femoris, obturators, and gemelli, examination can begin in the transverse plane at the level of the hamstring origin (Fig. 6-14A). Deep to the sciatic nerve between the ischium and proximal femur is located the quadratus femoris and obturator externus. More cephalad (see Fig. 6-14B), the inferior gemellus muscle is seen, which has a slightly different course compared with the quadratus femoris as it extends deep to its lateral insertion on the medial aspect of the greater trochanter. In their short axis, from cephalad to caudal, the superior gemellus, obturator internus, inferior gemellus, and quadratus femoris are identified deep to the sciatic nerve (see Fig. 6-14C).
Inguinal Region Evaluation
Sonographic evaluation of the inguinal region for hernias may incorporate evaluation of the anterior abdominal wall for abnormalities.8 Evaluation is begun in the transverse plane over the mid-abdomen below the umbilicus with the patient supine. At this location, the linea alba is seen as a hyperechoic fascial layer between the rectus abdominis muscles. The transducer is then moved to the lateral margin of a rectus abdominis muscle. As the transducer is moved inferior in the transverse plane, the inferior epigastric artery can be identified beneath the rectus abdominis (Fig. 6-15A, online). It is here at the lateral margin of the rectus abdominis that spigelian hernias are seen, between the rectus abdominis muscle and lateral abdominal musculature. More inferiorly, the site where the inferior epigastric artery joins the external iliac artery is a very important landmark; just lateral and superior to this location is the deep inguinal ring (see Fig. 6-15B, online). Hernias that originate lateral to the inferior epigastric artery at the deep inguinal ring and extend superficially and medially within the inguinal canal are indirect inguinal hernias. Hernias that originate medial to the inferior epigastric origin in the Hesselbach triangle and move in an anterior direction are direct hernias.8 At the deep inguinal ring, the transducer is then angled toward the pubis, parallel and just superior to the inguinal ligament, and long axis to the inguinal canal (see Fig. 6-15C, online). In male patients, the serpiginous and mixed-echogenicity spermatic cord can be identified (see Fig. 6-15D and E, online). In this location, the patient is asked to tighten the stomach or perform the Valsalva maneuver (forced expiration against a closed airway) to evaluate for transient herniation of intra-abdominal structures or tissue; the patient can be asked to blow against the back of the hand and puff the cheeks outward. This maneuver is also repeated with the transducer more medial, at the pubis, to evaluate for direct hernias. It is also important to image the Hesselbach triangle at its medial and superior aspects both in long and short axis to the inguinal canal for complete evaluation because the cephalocaudal extent of this triangle is greatest medially. Evaluation for inguinal hernias should also be completed in the sagittal plane. For example, when imaging the inguinal canal and spermatic cord (in males) in short axis, an indirect inguinal hernia will be seen moving in and out of the ultrasound plane displacing the spermatic cord. Similar to the transverse plane, a direct hernia will appear as focal abnormal anterior movement. After returning the transducer long axis to the inguinal ligament, the transducer is moved distally over the common femoral artery just beyond the inguinal ligament to evaluate for femoral hernias. Although the causes of “sports hernia” are debated, evaluation for hip or groin pain in the athlete should include the pubis symphyseal region, the hip joint, and the labrum (see earlier), in addition to evaluation for inguinal region hernias.17
Thigh Evaluation: Anterior
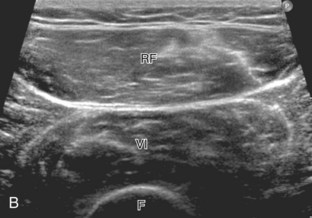
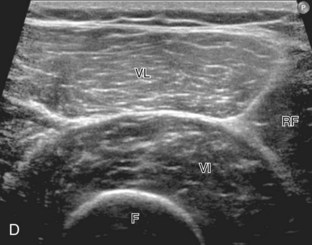
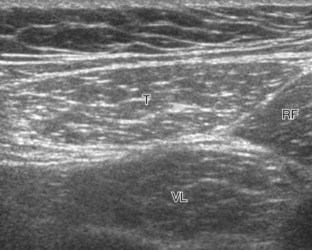
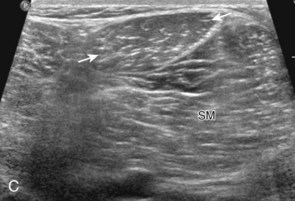
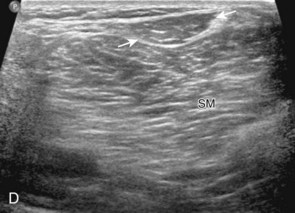
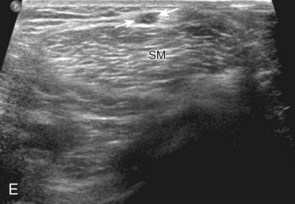
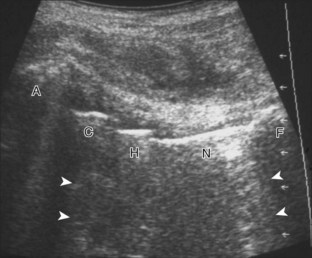
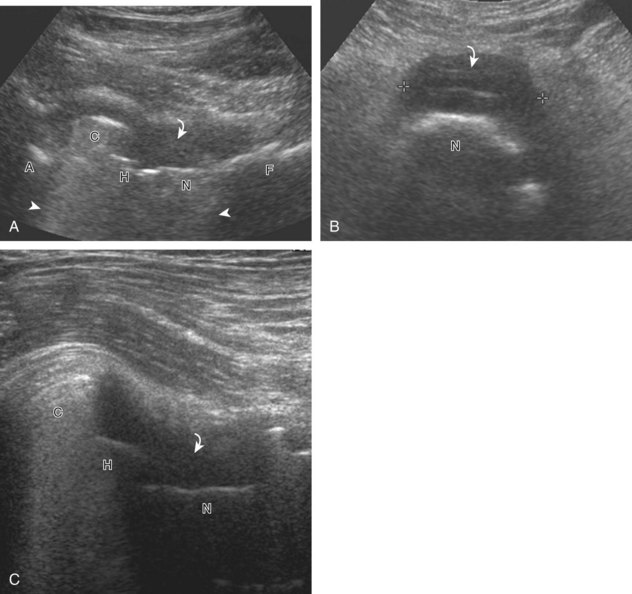
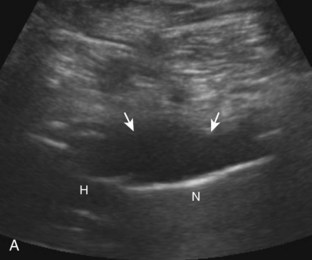
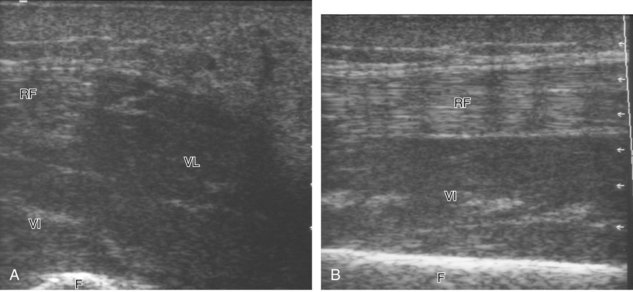
Structures of interest anteriorly in the thigh include the four muscles that make up the quadriceps femoris. Examination is begun in the transverse plane over the mid-anterior thigh, where the four individual muscles can be identified (Fig. 6-16A) (Videos 6-7 and 6-8)![]() . Directly below the transducer and most superficial is the rectus femoris muscle (see Fig. 6-16B). Deep to this and immediately adjacent to the femur is the vastus intermedius. Lateral to these two structures is the vastus lateralis (see Fig. 6-16C and D), and medial is the vastus medialis (see Fig. 6-16E and F). Muscle at ultrasound is predominantly hypoechoic, although interspersed hyperechoic septa are identified. The quadriceps femoris is then evaluated in long axis (Fig. 6-17). As one moves the transducer distally, the rectus femoris tapers to a tendon, followed by the vastus musculature, which forms the trilaminar quadriceps tendon that inserts on the superior pole of the patella. The superficial layer of the distal quadriceps tendon is made up of the rectus femoris, the middle layer is composed of both the vastus medialis and lateralis tendons, and the deep layer is made up of the vastus intermedius tendon. Some quadriceps tendon fibers continue over the patella (termed the prepatellar quadriceps continuation) to attach to the tibial tuberosity by means of the patellar tendon.18 The distal tapering appearance of the rectus femoris is best appreciated in long axis in the sagittal plane. The individual muscles of the quadriceps can then be evaluated more proximally. As described earlier, the rectus femoris tendon proximally originates at the ilium (see Fig. 6-6), where its direct head originates from the anterior inferior iliac spine and the indirect or reflected head originates at the lateral aspect. In the thigh, the direct head flattens superficially, the indirect head continues within the central region of the rectus femoris, and more distally a posterior aponeurosis forms.4 The adjacent tensor fasciae latae is seen lateral to the rectus femoris muscle (Fig. 6-18); the fascia of the tensor fascia latae continues laterally as the iliotibial tract (see Fig. 6-10).
. Directly below the transducer and most superficial is the rectus femoris muscle (see Fig. 6-16B). Deep to this and immediately adjacent to the femur is the vastus intermedius. Lateral to these two structures is the vastus lateralis (see Fig. 6-16C and D), and medial is the vastus medialis (see Fig. 6-16E and F). Muscle at ultrasound is predominantly hypoechoic, although interspersed hyperechoic septa are identified. The quadriceps femoris is then evaluated in long axis (Fig. 6-17). As one moves the transducer distally, the rectus femoris tapers to a tendon, followed by the vastus musculature, which forms the trilaminar quadriceps tendon that inserts on the superior pole of the patella. The superficial layer of the distal quadriceps tendon is made up of the rectus femoris, the middle layer is composed of both the vastus medialis and lateralis tendons, and the deep layer is made up of the vastus intermedius tendon. Some quadriceps tendon fibers continue over the patella (termed the prepatellar quadriceps continuation) to attach to the tibial tuberosity by means of the patellar tendon.18 The distal tapering appearance of the rectus femoris is best appreciated in long axis in the sagittal plane. The individual muscles of the quadriceps can then be evaluated more proximally. As described earlier, the rectus femoris tendon proximally originates at the ilium (see Fig. 6-6), where its direct head originates from the anterior inferior iliac spine and the indirect or reflected head originates at the lateral aspect. In the thigh, the direct head flattens superficially, the indirect head continues within the central region of the rectus femoris, and more distally a posterior aponeurosis forms.4 The adjacent tensor fasciae latae is seen lateral to the rectus femoris muscle (Fig. 6-18); the fascia of the tensor fascia latae continues laterally as the iliotibial tract (see Fig. 6-10).
Thigh Evaluation: Medial
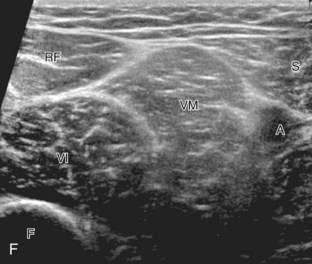
Structures of interest in the medial thigh include the femoral nerve, artery, and vein and the sartorius, gracilis, and adductor musculature. Ultrasound examination is begun similar to the anterior thigh for orientation, with initial identification of the rectus femoris muscle. The transducer is then moved cephalad into the medial upper thigh (see Fig. 6-16E). The femoral artery is identified at the medial aspect of the rectus femoris and vastus medialis muscles and is a very helpful landmark (Fig. 6-19A). Directly superficial to the femoral artery is the sartorius muscle. Medial and posterior to these structures are the adductor muscles (see Fig. 6-19B). The most anterior is the adductor longus muscle, next posterior is the adductor brevis muscle, and most posterior and largest is the adductor magnus muscle. Between these respective muscles are located the anterior and posterior branches of the obturator nerve. Superficial and medial to the adductor muscles is the gracilis muscle, just below the subcutaneous tissues (see Fig. 6-19C). For each of these medial thigh muscles, the proximal to distal extents can be visualized in short axis. The transducer can also be turned in long axis over each muscle to visualize the proximal origins and distal attachments (see Fig. 6-19D).
Thigh Evaluation: Posterior
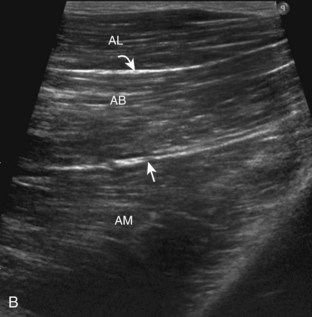
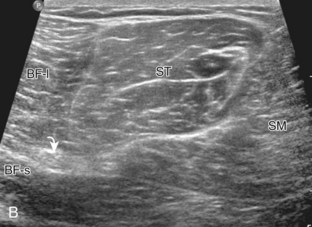
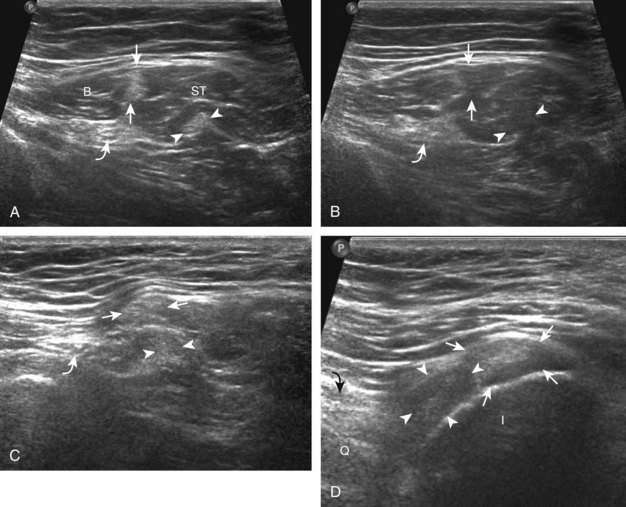
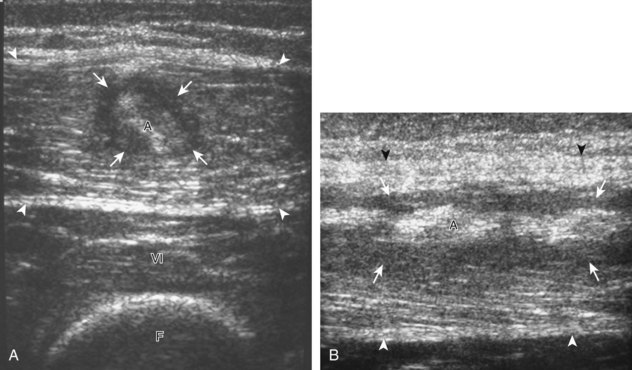
Structures of interest in the posterior thigh include the semimembranosus, the semitendinosus, the biceps femoris, and the sciatic nerve. Ultrasound evaluation can begin in the transverse plane at the level of the mid-thigh, or more proximally at the horizontal gluteal crease or ischial tuberosity. At the level of the mid-posterior thigh (Fig. 6-20A), three distinct muscles can be identified medial to lateral, which are the semimembranosus, semitendinosus, and biceps femoris muscles (see Fig. 6-20B). The short head of the biceps femoris can be identified deep to the long head at the femoral cortex at the level of the mid-femur. When the transducer is moved in the transverse plane distally toward the knee, the semitendinosus becomes a thin tendon and moves directly superficial to the semimembranosus muscle (see Fig. 6-20B to D). This is an additional finding that aids the identification of the posterior thigh muscles. In the mid-thigh, the honeycomb appearance of the sciatic nerve can be identified between the biceps femoris muscle and the semitendinosus muscle (see Fig. 6-20B).
As the transducer is moved cephalad in the transverse plane toward the ischium, the semimembranosus tendon and aponeurosis move anterior or deep to the conjoined biceps femoris long head and semitendinosus tendons (or conjoint tendon) and semitendinosus muscle belly (Fig. 6-21A). At this location, the conjoint tendon, the semimembranosus tendon, and the sciatic nerve are in the arrangement of a triangle, with the semimembranosus and sciatic nerve forming the base of the triangle and the conjoint tendon the more superficial apex. Toggling the transducer to eliminate anisotropy is helpful to visualize the tendons as hyperechoic (see Fig. 6-21B). As the transducer is moved in short axis more cephalad toward the ischial tuberosity, the semimembranosus tendon moves lateral and crosses under or deep to the conjoint tendon (see Fig. 6-21C). At the ischial tuberosity, the conjoint tendon originates superficially, whereas the semimembranosus origin is relatively lateral and deep (see Fig. 6-21C). In long axis (Fig. 6-22A), the conjoint tendon is visualized directly superficial to the semimembranosus tendon (see Fig. 6-22B). At the ischial tuberosity, the conjoint tendon originates in a superficial location (see Fig. 6-22C). To visualize the semimembranosus tendon, the transducer is moved slightly lateral to the conjoint tendon and angled toward midline (see Fig. 6-22D). The sciatic nerve is also identified and should not be mistaken for tendon (see Fig. 6-22E).
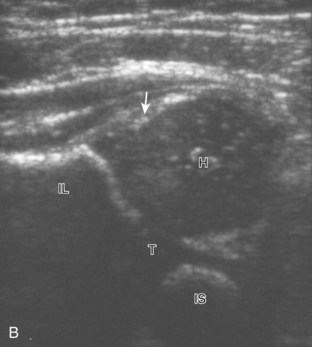
Hip Evaluation for Dysplasia in a Child
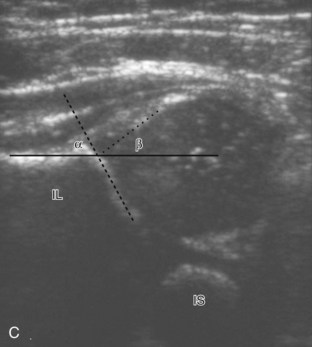
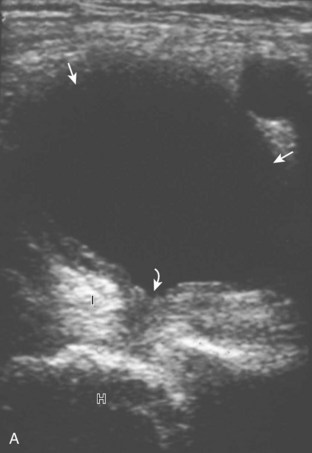
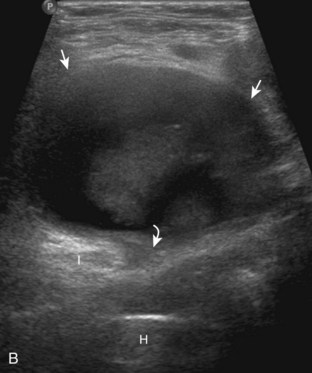
There are several opinions with regard to the ultrasound technique for hip dysplasia. Whereas one method favors the position of the femoral head and measurements, another emphasizes dynamic evaluation of position and stability using the Ortolani and Barlow maneuvers. Regardless, a minimal examination should include coronal neutral or coronal flexion positions (with optional stress and measurements) and a transverse flexion position with and without stress.19 An ultrasound protocol for hip dysplasia may be divided into several steps. The first is a coronal view with the hip in neutral position, slightly flexed (Fig. 6-23A, online). The resulting image is likened to an egg on a spoon, in which a line drawn from the flat ilium covers at least 50% of the head and an acetabular α angle is greater than 60 degrees (see Fig. 6-23B and C, online). The α angle measures the angle between the lateral ilium (baseline) and the acetabular roof line, whereas the β angle measures the angle between the lateral ilium baseline and a line drawn through the hyperechoic labral tip from the lateral acetabulum (inclination line).20 The ossified acetabulum and proximal femur are hyperechoic with shadowing, and the unossified femoral head and triradiate cartilage of the acetabulum appear speckled and hypoechoic. The second position is in the coronal plane with the hip flexed (Fig. 6-24A, online). In this position, in addition to assessment of the femoral head position, the transducer is moved posteriorly over the triradiate cartilage, and posteriorly directed stress is applied to evaluate for posterior subluxation of the femoral head (see Fig. 6-24B, online). In the third position, the hip remains flexed, and the transducer is turned to the transverse plane (Fig. 6-25A, online). In this position, dynamic hip adduction with posteriorly directed stress (the Barlow test) (see Fig. 6-25B, online) evaluates for hip subluxation, and hip abduction with anteriorly directed stress (the Ortolani test) evaluates for relocation if there is subluxation or dislocation of the hip.
Joint and Bursal Abnormalities
Joint Effusion and Synovial Hypertrophy
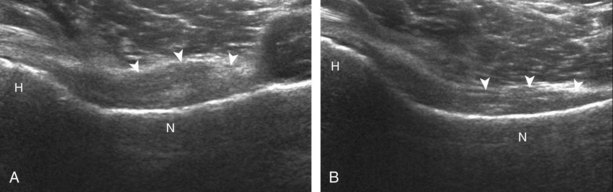
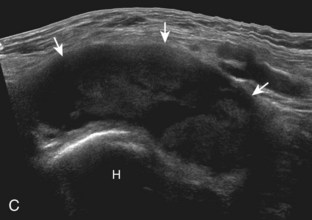
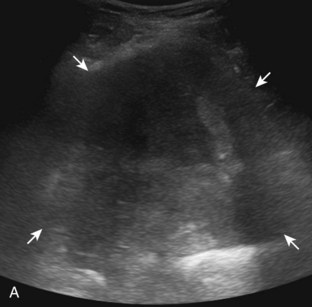
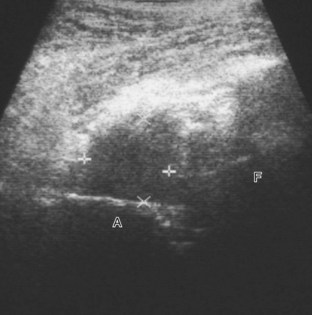
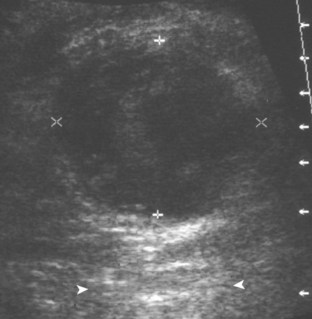
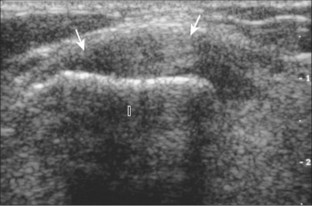
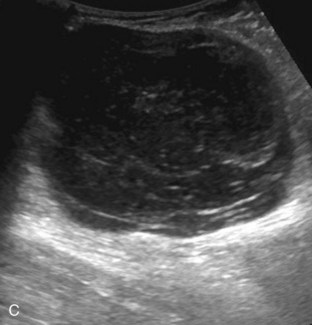
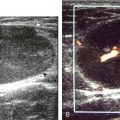
The diagnosis of a hip joint effusion relies on distention of the anterior joint recess when imaged long axis to the femoral neck (Fig. 6-26). The criterion for abnormal joint distention in a child is 2 mm of separation of the anterior and posterior capsule layers (Fig. 6-27).1 In the adult, total capsular distention of 7 mm (measured from the femoral neck surface to the outer margin of the capsule, to include both anterior and posterior layers) or 1 mm of asymmetry compared with the contralateral asymptomatic hip has been shown to indicate joint distention,21 although a 5-mm threshold has also been used (Fig. 6-28) (Video 6-9)![]() .22 Regardless, when the femoral neck and anterior capsule are imaged perpendicular to the sound beam, even small amounts of joint fluid can be seen separating the anterior capsule layers. Leg extension and abduction may also improve visualization of a hip joint effusion. In addition, a convex or bulging surface of the anterior joint recess suggests abnormal distention.1 Internal rotation of the leg may cause bulging of the normal joint capsule, which should not be misinterpreted as effusion (Fig. 6-29) (Video 6-10)
.22 Regardless, when the femoral neck and anterior capsule are imaged perpendicular to the sound beam, even small amounts of joint fluid can be seen separating the anterior capsule layers. Leg extension and abduction may also improve visualization of a hip joint effusion. In addition, a convex or bulging surface of the anterior joint recess suggests abnormal distention.1 Internal rotation of the leg may cause bulging of the normal joint capsule, which should not be misinterpreted as effusion (Fig. 6-29) (Video 6-10)![]() .1 Uncommonly, joint effusion may extend superficially through a defect in the hip joint capsule within a pseudodiverticulum of the synovial membrane (Fig. 6-30).1
.1 Uncommonly, joint effusion may extend superficially through a defect in the hip joint capsule within a pseudodiverticulum of the synovial membrane (Fig. 6-30).1
It is important to be familiar with the appearance of the normal anterior hip joint recess, which may appear hyperechoic (if imaged perpendicular) or hypoechoic (if imaged obliquely or in large patients) with a thickness of less than 4 to 6 mm, owing to the normal capsular reflection (see Figs. 6-2 and 6-3). This appearance should not be misinterpreted as joint effusion or synovial hypertrophy. In fact, it has been shown that in children with toxic hip synovitis, synovial thickening is not visible at ultrasound (see Fig. 6-27).1 Joint recess distention from an effusion may range from anechoic (if simple fluid) to hyperechoic (if synovial hypertrophy or complex fluid from hemorrhage or infection). Neither joint recess echogenicity nor flow on color or power Doppler imaging can distinguish between aseptic and septic effusion; diagnostic ultrasound-guided percutaneous aspiration should be considered if there is concern for infection.23 In addition, it may be difficult to appreciate a small joint effusion in patients with increased soft tissues superficial to the hip and in those with a large body habitus.24 In this situation, percutaneous aspiration should be considered regardless of ultrasound findings if there is clinical concern for infection. A large body habitus may cause anechoic fluid to appear artifactually hypoechoic or isoechoic, even with lower-frequency transducers and tissue harmonic imaging.
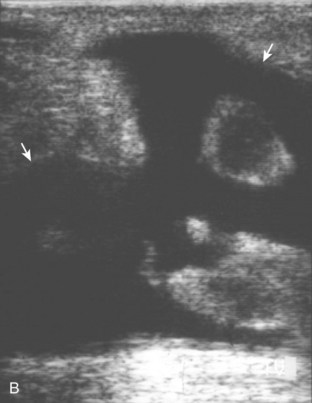
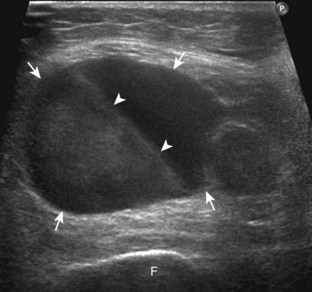
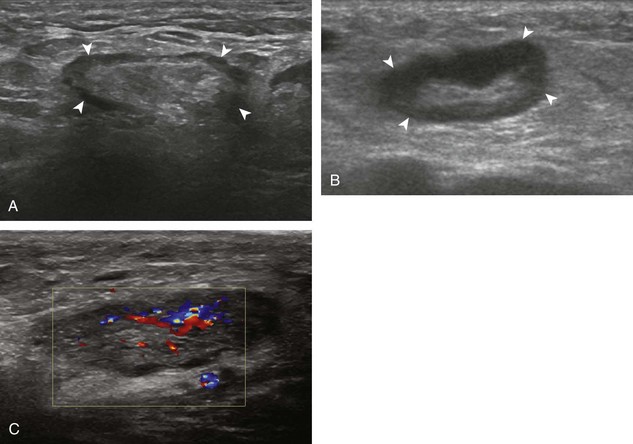
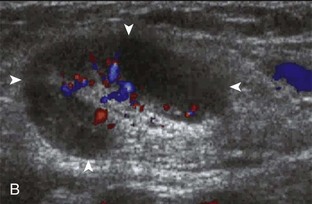
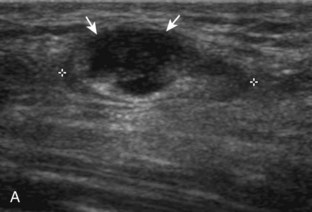
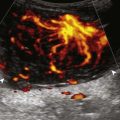
Causes of hip effusion include reactive fluid, trauma, infection, and hemorrhage. Hypoechoic, isoechoic, or hyperechoic distention of the hip joint recess can be caused by either complex fluid (see Fig. 6-30) or synovial hypertrophy (Fig. 6-31). In the latter condition, lack of compressibility or redistribution and positive flow on color or power Doppler imaging suggests synovial hypertrophy. Causes of synovitis include infection and inflammatory arthritis (Fig. 6-32). Other synovial proliferative disorders such as pigmented villonodular synovitis can appear similar, as can synovial osteochondromatosis (although the latter may show hyperechoic calcific foci). Intra-articular bodies appear as hyperechoic foci with possible posterior acoustic shadowing within the joint recess.
Labrum and Proximal Femur Abnormalities
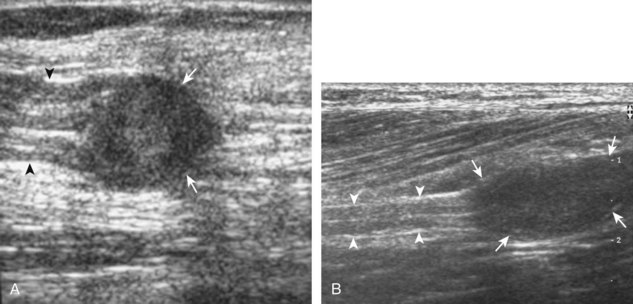
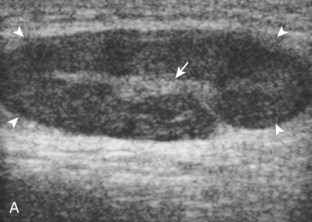
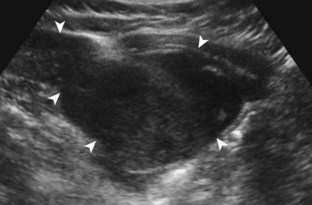
Other intra-articular structures visible by ultrasound include the hypoechoic hyaline cartilage that covers the femoral head and the hyperechoic triangle-shaped fibrocartilage acetabular labrum. A labrum tear may appear as a defined hypoechoic or anechoic cleft, which is more conspicuous when there is adjacent joint fluid (Fig. 6-33). The presence of a hypoechoic or anechoic paralabral cyst is also an indicator of underlying hip labrum tear (Fig. 6-34). The accuracy of ultrasound in the diagnosis of hip labrum tear is variable because limitations exist given the depth of and limited access to the labrum.25,26 Chondrocalcinosis, which may be seen with pseudogout, will create punctate reflective echoes within the labrum (Fig. 6-35).
Ultrasound is very sensitive to cortical irregularity, and correlation with radiography is essential. A step-off deformity of the femoral neck can indicate a fracture.27 An osteophyte at the femoral neck indicates osteoarthritis (Fig. 6-36). Cortical irregularity or bone protuberance of the anterosuperior femoral head-neck junction can be seen in cam-type femoroacetabular impingement.28–30 Dynamic imaging with hip flexion and internal rotation may show direct contact between the labral tear and femoral cortical irregularity, which supports the diagnosis (Fig. 6-37) (Videos 6-11 and 6-12)![]() . Treatment of femoroacetabular impingement includes osteoplasty, which will appear as a cortical defect at the femoral head-neck junction (Fig. 6-38).
. Treatment of femoroacetabular impingement includes osteoplasty, which will appear as a cortical defect at the femoral head-neck junction (Fig. 6-38).
Bursal Abnormalities
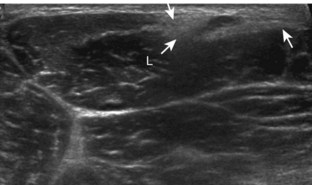
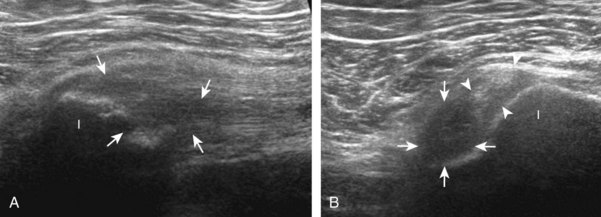
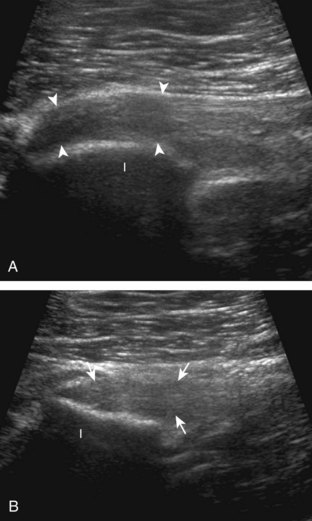
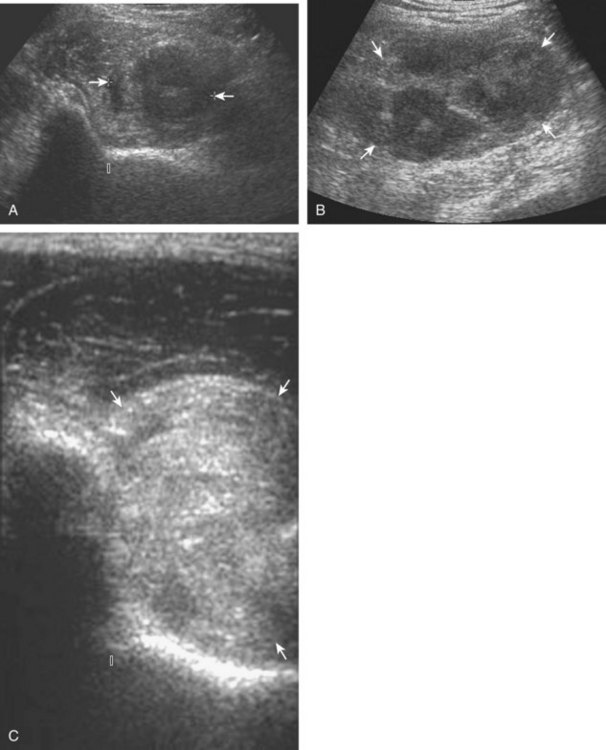
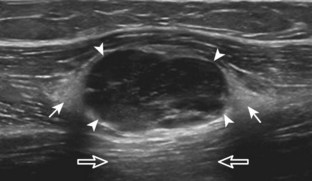
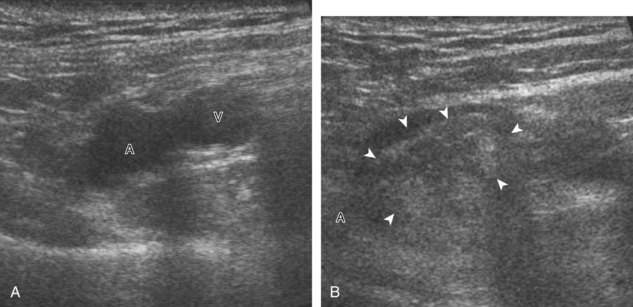
There are several bursae that can be found about the hip. The iliopsoas bursa is located anterior to the hip joint.31 When distended, it is seen medial to the iliopsoas tendon but may extend anterior and wrap anterolateral to the tendon, or extend lateral between the iliopsoas tendon and the acetabulum.32 The iliopsoas bursa communicates with the hip joint in up to 15% of the population, and its distention is often related to hip joint pathology.6 Possible communication between the iliopsoas bursa and the hip joint can be visualized in the transverse plane at the level of the femoral head immediately medial to the iliopsoas tendon (Fig. 6-39A). The iliopsoas bursa may be distended with simple fluid, complex fluid (see Fig. 6-39B and C), or synovial hypertrophy, which may range from anechoic to hyperechoic. Similar to joint recess distention, lack of compressibility and the presence of flow on color or power Doppler imaging suggest synovial hypertrophy. An abnormally distended bursa may extend into the abdomen and should not be confused for an intra-abdominal or psoas abscess.33 In addition, distention of the bursa does not imply inflammation or true bursitis; the presence of pain with transducer pressure, increased flow on color or power Doppler imaging, and distention out of proportion to hip joint recess distention suggest true inflammation and bursitis.
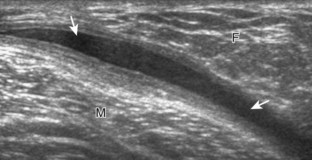
The greater trochanteric region is also evaluated for abnormal bursal distention.2 The trochanteric (or subgluteus maximus) bursa originates between the gluteus maximus and posterior facet of the greater trochanter but may extend laterally between the gluteus medius tendon and overlying iliotibial tract (Fig. 6-40). It is important to completely evaluate the posterior facet of the greater trochanter so as not to overlook bursal distention. Similar to other bursae, distention of the trochanteric bursa can be from simple fluid, complex fluid, or synovial hypertrophy (Fig. 6-41) (Video 6-13)![]() . As described earlier, the subgluteus minimus bursa (Fig. 6-42) and subgluteus medius bursa are located between the greater trochanter and their respective tendons. In the setting of greater trochanteric pain syndrome, identification of a distended and inflamed bursa is not common.34 Gluteus minimus and medius tendon abnormalities are found more often and may be associated with bursal abnormalities.35 Uncommonly, an obturator externus bursa may be seen at the medial aspect of the lesser trochanter of the femur7 or an ischial (ischiogluteal) bursa (Fig. 6-43) superficial to the ischial tuberosity.36
. As described earlier, the subgluteus minimus bursa (Fig. 6-42) and subgluteus medius bursa are located between the greater trochanter and their respective tendons. In the setting of greater trochanteric pain syndrome, identification of a distended and inflamed bursa is not common.34 Gluteus minimus and medius tendon abnormalities are found more often and may be associated with bursal abnormalities.35 Uncommonly, an obturator externus bursa may be seen at the medial aspect of the lesser trochanter of the femur7 or an ischial (ischiogluteal) bursa (Fig. 6-43) superficial to the ischial tuberosity.36
Postsurgical Hip
In evaluation of the postsurgical hip, it is important first to understand the normal sonographic appearances. With regard to hip replacement, the femoral head and proximal femur are typically replaced with material composed of metal or ceramic, with a plastic, metal, or ceramic acetabular cup. At sonography, these components demonstrate a hyperechoic surface and possible posterior reverberation (with a metal surface) (Fig. 6-44).37 When imaging the proximal femur in long axis to the femoral neck, one will see the echogenic and shadowing proximal femur disrupted by the echogenic surface contours of the arthroplasty. The posterior reverberation artifact of the arthroplasty is contrasted by the posterior acoustic shadowing of the native femur. The echogenic edge of the acetabular cup is also seen; the adjacent native acetabulum more proximally produces posterior acoustic shadowing. Hypoechogenicity superficial to the neck of the prosthesis and up to 6 mm superficial to the native femur at the prosthesis-bone junction has been described in asymptomatic patients after total hip arthroplasty.38
A hip joint effusion appears as a hypoechoic or anechoic layer over the femoral neck of the prosthesis (Fig. 6-45).24 The margins of the effusion may be ill defined if the hip joint capsule has been resected because the fluid will then be outlined by a pseudocapsule. Identification of a small joint effusion may be difficult because of a patient’s large body habitus, an issue compounded by the possible hypoechoic postsurgical changes.24 One should consider percutaneous aspiration when there is high clinical concern for infection, regardless of the sonographic findings. A large joint effusion can become quite prominent, especially in infection, in which complex fluid commonly extends beyond the joint into the surrounding soft tissues (Fig. 6-46).37 Pseudocapsule distention greater than 3.2 mm over the native femur immediately adjacent to the neck of the prosthesis suggests a septic joint.37 It is also important to evaluate the soft tissues anterior to the femoral neck before attempting percutaneous aspiration using fluoroscopy. The latter is recommended to avoid the potential contamination of a sterile joint by passing a needle through an overlying soft tissue infection. In addition, if no fluid is present at joint aspiration attempt, lavage and re-aspiration are recommended to exclude infection.
Other causes of joint effusion after arthroplasty include prosthesis loosening and particle disease, the latter representing inflammatory reaction to breakdown of the prosthesis components that may cause osteolysis and joint distention. An adverse periprosthetic soft tissue reaction associated with metal-on-metal hip arthroplasties has been termed pseudotumor and can appear solid or cystic with ultrasound (Fig. 6-47).39 After hip replacement, it is also important to image any symptomatic area, which may reveal bursal abnormality40 (Fig. 6-48) and infection (Fig. 6-49) (Video 6-14)![]() . The gluteus tendons should also be evaluated for abnormality after arthroplasty, especially if an arthroplasty is placed using a direct lateral or modified anterolateral approach.41 Another cause of symptoms includes iliopsoas impingement from the anterior aspect of the femoral component42 or acetabular cup43 (Fig. 6-50) of a hip arthroplasty. Acetabular liner displacement may also be detected.44
. The gluteus tendons should also be evaluated for abnormality after arthroplasty, especially if an arthroplasty is placed using a direct lateral or modified anterolateral approach.41 Another cause of symptoms includes iliopsoas impingement from the anterior aspect of the femoral component42 or acetabular cup43 (Fig. 6-50) of a hip arthroplasty. Acetabular liner displacement may also be detected.44
Regardless of the type of surgery, an incision site is a common location for pathology such as infection, hematoma (Fig. 6-51A), and seroma (see Fig. 6-51B). Heterotopic ossification may also be seen (see Fig. 6-51C). Another surgical procedure of the hip involves complete femoral head and neck resection after infection (Girdlestone procedure) (Fig. 6-52, online). Post-surgical changes can be seen at the femoral head-neck junction after osteoplasty for treatment of femoroacetabular impingement (see Fig. 6-38).
Tendon and Muscle Abnormalities
Tendon and Muscle Injury
Similar to other tendons in the body, a degenerative condition of tendons called tendinosis or tendinopathy is characterized by hypoechoic swelling of the affected tendon.5,45,46 These terms are used instead of tendinitis because there are no significant acute inflammatory cells in this situation but rather mucoid degeneration and possible interstitial tearing. Chronic tendinopathy at a tendon attachment may produce marked cortical irregularity of the adjacent bone. Partial-thickness tendon tears are characterized by more defined hypoechoic or anechoic clefts within the involved tendon, but without the complete tendon disruption and retraction that are characteristic of full-thickness tears. In this latter condition, the tendon is torn and retracted with interposed heterogeneous but predominantly hypoechoic hemorrhage. Muscles that cross two joints are prone to tears at the musculotendinous junction; chronic injuries occur at the entheses, and direct impact injuries involve the muscle belly.
With regard to the adductor musculature and the pubic symphyseal region, tendinosis and partial-thickness tears commonly involve the adductor longus origin at the pubis. This finding may be associated with abnormality of the common aponeurosis between the rectus abdominis and the adductor longus tendons superficial to the pubis, a finding described with sports-related hernia (Fig. 6-53).13,47 Full-thickness tears are characterized by tendon retraction and interposed hemorrhage (Fig. 6-54). Distal to the adductor origin, a muscle strain from a stretch injury (Fig. 6-55) or a hematoma from direct impact injury may be found (Fig. 6-56). At the adductor insertion onto the posteromedial femur, chronic repetitive stress injury has been termed thigh splints or adductor insertion avulsion syndrome.48 In this condition, an irregular bone surface can indicate periostitis and possible stress fracture and is typically the site of point tenderness with transducer pressure (Fig. 6-57).
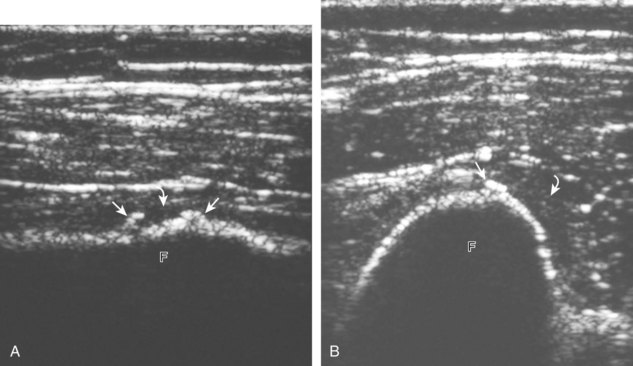
FIGURE 6-57 Thigh splints (adductor insertion avulsion syndrome).
(From Weaver JS, Jacobson JA, Jamadar DA, et al: Sonographic findings of adductor insertion avulsion syndrome with magnetic resonance imaging correlation. J Ultrasound Med 22:403–407, 2003. Reproduced with permission from the American Institute of Ultrasound in Medicine.)
With regard to the rectus femoris, injuries may involve its origin at the anterior inferior iliac spine, where complete tears of the direct and indirect heads result in a full-thickness tear and retraction (Fig. 6-58). Injury can also occur at the central myotendinous aponeurosis, which will appear as abnormal hypoechogenicity surrounding the indirect head within the muscle belly (Fig. 6-59).4 Partial tear can appear as hypoechoic fiber disruption (Fig. 6-60).46 More distally, the posterior aponeurosis may be injured with resulting hematoma (Fig. 6-61A and B), which may later appear as hyperechoic scar (see Fig. 6-61C). A complete tear of the distal rectus femoris is characterized by muscle retraction and may be associated with anechoic fluid (Fig. 6-62). It is not uncommon for patients to present later with a palpable pseudomass, which represents the retracted muscle and tendon. A direct impact injury may cause an intramuscular hematoma (Fig. 6-63). Distal quadriceps tendon tears are discussed in Chapter 7.
The gluteus minimus and medius tendons may also be abnormal at their greater trochanter insertion, ranging from tendinosis (Fig. 6-64) to tendon tear (Fig. 6-65).49 As described earlier, patients with greater trochanteric pain syndrome are much more likely to have gluteal tendon abnormalities rather than an isolated true bursitis as the cause of symptoms.34 The reported sensitivity of ultrasound in the diagnosis of gluteal tendons tears ranges from 79% to 100%.50 Gluteus medius tendon tears are more common than gluteus minimus tendon tears, and often a bursal abnormality is associated with the tendon tear.35 Identification of the characteristic bone contours of the greater trochanter is essential for orientation and accurate localization of tendon and soft tissue abnormalities (see Fig. 6-10).2
With regard to the hamstrings, chronic injury produces tendinosis, possible partial-thickness tear, and bone irregularity of the ischium.5,51 Injury may selectively involve the semimembranosus tendon origin at the lateral surface of the ischial tuberosity (Fig. 6-66) or the conjoint tendon of the biceps femoris long head and semitendinosus at the superficial surface (Fig. 6-67). Isolated tear of one of the two tendons may also be possible (Fig. 6-68). Complete tear of the hamstring tendon origin is characterized by absence of tendon fibers and retraction (Fig. 6-69). Chronic injuries may be associated with hyperechoic scar formation and possible pseudotumor appearance at physical examination as a result of muscle retraction (Fig. 6-70).
Other muscle and tendon injuries can also involve the sartorius (Fig. 6-71) and proximal tensor fascia latae (Fig. 6-72). Spontaneous muscle hemorrhage has been described with the iliopsoas in the setting of hemophilia and in patients who are anticoagulated (Fig. 6-73). One must be aware of this condition because the often mixed-echogenicity mass-like swelling of the iliopsoas may simulate soft tissue tumor (Video 6-15)![]() .
.
Snapping Hip Syndrome
An abnormal snapping with hip movement has been termed snapping hip syndrome, which can be divided into intra-articular and extra-articular causes. Intra-articular causes relate to joint processes, such as intra-articular bodies or prior trauma. Extra-articular causes can occur medially and laterally.52 The medial variety is the result of snapping of the iliopsoas tendon at the level of the anterior aspect of the acetabulum near a bony protuberance called the iliopectineal eminence (Fig. 6-74) (Videos 6-16 and 6-17)![]() . To diagnose this specific condition, the patient is asked to reproduce the snapping sensation, or a flexed, abducted, and externally rotated hip (frog-leg position) is straightened during sonographic visualization. Normally, the psoas major tendon moves laterally, and the iliacus muscle fibers move as well, in a smooth clockwise rotation (for the right hip; counterclockwise for the left).53 In the abnormal condition (although asymptomatic in up to 40%), there is abrupt movement and snapping of the psoas major tendon against the superior pubic ramus, which is felt through the transducer.53 The actual cause of the snap is temporary entrapment of the medial muscle fibers of the iliacus between the psoas major tendon and the superior pubic ramus.53 A bifid iliopsoas tendon has also been described as a cause of snapping.54 The lateral variety of external snapping hip syndrome can result from snapping the gluteus maximus tendon (Video 6-18)
. To diagnose this specific condition, the patient is asked to reproduce the snapping sensation, or a flexed, abducted, and externally rotated hip (frog-leg position) is straightened during sonographic visualization. Normally, the psoas major tendon moves laterally, and the iliacus muscle fibers move as well, in a smooth clockwise rotation (for the right hip; counterclockwise for the left).53 In the abnormal condition (although asymptomatic in up to 40%), there is abrupt movement and snapping of the psoas major tendon against the superior pubic ramus, which is felt through the transducer.53 The actual cause of the snap is temporary entrapment of the medial muscle fibers of the iliacus between the psoas major tendon and the superior pubic ramus.53 A bifid iliopsoas tendon has also been described as a cause of snapping.54 The lateral variety of external snapping hip syndrome can result from snapping the gluteus maximus tendon (Video 6-18)![]() or iliotibial tract over the greater trochanter (Fig. 6-75) (Video 6-19)
or iliotibial tract over the greater trochanter (Fig. 6-75) (Video 6-19)![]() .55,56 Again, the patient is asked to reproduce the symptom, and many times the patient has to stand and shift weight on the affected limb, or the patient may lie on the contralateral hip to allow hip flexion and extension. Ultrasound transverse over the greater trochanter shows abnormal abrupt snapping of the involved tendon or muscle, which is often thickened, over the greater trochanter.55
.55,56 Again, the patient is asked to reproduce the symptom, and many times the patient has to stand and shift weight on the affected limb, or the patient may lie on the contralateral hip to allow hip flexion and extension. Ultrasound transverse over the greater trochanter shows abnormal abrupt snapping of the involved tendon or muscle, which is often thickened, over the greater trochanter.55
Calcific Tendinosis
Although more common in the rotator cuff, calcium hydroxyapatite deposition may occur in a number of tendons around the hip, including the gluteus maximus, gluteus medius (Fig. 6-76), and rectus femoris (Fig. 6-77) tendons. In this situation, the hyperechoic focus may show posterior acoustic shadowing, and the involved tendon may be abnormally hypoechoic with increased flow on color or power Doppler imaging. Ultrasound-guided percutaneous lavage and aspiration may be used for treatment.
Diabetic Muscle Infarction
In evaluation of a painful or swollen thigh, it is important to consider a condition called diabetic muscle infarction. Common to the thigh and calf, diabetic muscle infarction occurs in patients with longstanding diabetes, and it may be bilateral. The cause is not completely known, but a possible consideration is vascular occlusive disease. At ultrasound, the involved musculature is hypoechoic and swollen, although muscle fibers are still identified (Fig. 6-78).57 This is an important finding to help exclude a soft tissue abscess. Subfascial fluid may also be another finding in diabetic muscle infarction. Because the differential diagnosis includes early infection, correlation with laboratory values and the patient’s signs and symptoms is important; follow-up ultrasound examination may be considered.
Pseudohypertrophy of the Tensor Fasciae Latae
The most common effects of chronic denervation are muscle atrophy and fatty replacement. Uncommonly, fatty infiltration of the involved muscle may cause muscle enlargement or pseudohypertrophy. This situation may involve the tensor fasciae latae muscle in the upper thigh, although involvement of other muscles of the lower extremity have been described.58 At ultrasound, the muscle is enlarged, with increased echogenicity and sound beam attenuation resulting from the fatty infiltration (Fig. 6-79). The finding is usually asymmetrical, and it has been described in older individuals in the setting of chronic nerve impingement and partial denervation related to lumbar spine degenerative disease. Chronic peripheral neuropathy may also be a cause, and pseudohypertrophy has been described with muscular dystrophies.58 It is important to be familiar with this entity because it may present clinically as a soft tissue mass.
Peripheral Nerve Abnormalities
Several peripheral nerve abnormalities are specific to the hip and thigh. One nerve entrapment condition specific to the hip involves the lateral femoral cutaneous nerve. As in other entrapment conditions, the involved nerve may demonstrate hypoechoic swelling at the site of entrapment or injury and is symptomatic with transducer pressure. The lateral femoral cutaneous nerve is susceptible to injury because of its course and variations in its location as it exits the pelvis at the inguinal ligament, sartorius, or iliac crest.9,12
Another peripheral nerve abnormality is nerve transection, which is characterized by nerve discontinuity, a swollen and hypoechoic terminal neuroma at the transection site, and possible retraction if completely disrupted (Fig. 6-80) (Video 6-20)![]() .59 Neuroma formation is an expected finding after nerve transection as part of attempted nerve regeneration. After knee amputation, sonographic evaluation for neuromas is very useful. Ultrasound can diagnose the neuromas and can also identify which neuroma is the cause of patients’ symptoms through application of transducer pressure over each neuroma.
.59 Neuroma formation is an expected finding after nerve transection as part of attempted nerve regeneration. After knee amputation, sonographic evaluation for neuromas is very useful. Ultrasound can diagnose the neuromas and can also identify which neuroma is the cause of patients’ symptoms through application of transducer pressure over each neuroma.
Miscellaneous Conditions
Morel-Lavallée Lesion
This post-traumatic condition or Morel-Lavallée lesion is described at the thigh and proximal hip and is characterized by a fluid collection between the subcutaneous fat and the adjacent fascia as a result of a closed degloving injury. At ultrasound, the resulting fluid collection, usually anechoic or hypoechoic, can be heterogeneous when acute but becomes more homogeneous and flat in shape when chronic, and it is located at the interface between the superficial fat and the underlying fascial tissues (Fig. 6-81).60
Inguinal Lymph Node
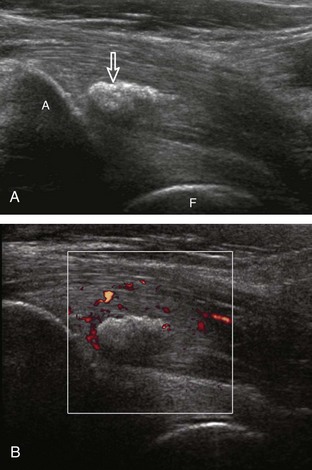
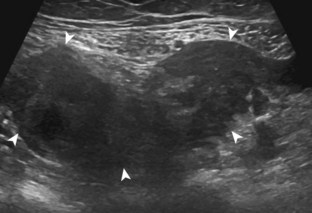
With regard to inguinal lymph nodes, normally, a hyperechoic hilum with uniform hypoechoic cortex and hilar pattern of blood flow (if present) are demonstrated (Fig. 6-82). An inguinal lymph node is considered abnormal if its short axis is greater than 1.5 cm61 or if there is eccentric thickening of the hypoechoic cortex.62 An enlarged lymph node may be benign or malignant, although lymph node size is not a reliable criterion in this differentiation. Benign enlargement may be inflammatory or reactive, and typically the hyperechoic hilum remains present (Fig. 6-83) (Video 6-21)![]() . In contrast, a malignant lymph node is characterized by a round shape, absence or narrowing of the echogenic hilum, thickening of the cortex, and a mixed or peripheral blood flow pattern (Fig. 6-84).62–64 Many times, percutaneous biopsy is required to determine the cause of lymph node enlargement.
. In contrast, a malignant lymph node is characterized by a round shape, absence or narrowing of the echogenic hilum, thickening of the cortex, and a mixed or peripheral blood flow pattern (Fig. 6-84).62–64 Many times, percutaneous biopsy is required to determine the cause of lymph node enlargement.
Other Soft Tissue Masses
Most soft tissue masses are nonspecific in the thigh and hip region; however, some soft tissue tumors commonly involve the thigh. One benign soft tissue tumor, an intramuscular myxoma, which has a uniformly heterogeneous but overall hypoechoic appearance (Fig. 6-85), may simulate a complex cyst because it is well defined and oval, often with increased through-transmission.65 A hyperechoic rim and hyperechoic cap have been described with intramuscular myxomas related to adjacent fatty atrophy and fatty infiltration.65
With regard to malignancy, the thigh is a common site for malignant sarcomas (Fig. 6-86), although other primary malignant tumors (Fig. 6-87) and soft tissue metastases (Fig. 6-88) may occur. Such tumors are generally hypoechoic relative to the adjacent tissues; however, heterogeneity is common with larger and more aggressive tumors, especially when they are associated with necrosis and hemorrhage. In addition, many solid soft tissue tumors demonstrate increased through-transmission. Although increased flow on color or power Doppler imaging is more common with malignant tumors, such findings may also be seen with benign conditions. After surgery, ultrasound is effective in evaluating for soft tissue sarcoma recurrence (Fig. 6-89).66,67 In evaluation of a palpable soft tissue mass, it is important to exclude disorders that may produce a pseudomass appearance, such as pseudohypertrophy of the tensor fasciae latae and chronic retracted tendon or muscle tears, as described earlier.
Hernias
The key to successful evaluation for inguinal region hernias lies in sonographic technique, knowledge of anatomy, and identification of key sonographic landmarks.8 With the Valsalva maneuver (forced expiration against a closed airway) and possibly scanning with the patient upright, one looks for abnormal movement of intra-abdominal contents from one space to another at a key anatomic location. For example, a spigelian hernia occurs at the lateral margin of the rectus abdominis (Fig. 6-90, online) (Video 6-22)![]() . Most commonly, hyperechoic fat is visualized in the hernia and, less commonly, the bowel. It is important to ascertain whether hernias are transient, reducible, or incarcerated.
. Most commonly, hyperechoic fat is visualized in the hernia and, less commonly, the bowel. It is important to ascertain whether hernias are transient, reducible, or incarcerated.
An indirect inguinal hernia begins at the deep inguinal ring, at the lateral aspect of the external iliac artery just proximal to the origin of the inferior epigastric artery. Intra-abdominal contents enter into the inguinal canal, beginning at the deep inguinal ring, and move superficially and then medially toward the pubic symphysis parallel to the skin surface (Fig. 6-91, online) (Videos 6-23 through 6-25)![]() . It is important to evaluate for indirect hernia both long axis and short axis to the inguinal canal; limiting evaluation to long axis may cause one to overlook a hernia that is not included in the imaging plane. This pitfall is avoided with short axis imaging, in which the hernia can be visualized within the inguinal canal adjacent to the spermatic cord or round ligament. An indirect hernia may extend to the external inguinal ring (Fig. 6-92, online) (Video 6-26
. It is important to evaluate for indirect hernia both long axis and short axis to the inguinal canal; limiting evaluation to long axis may cause one to overlook a hernia that is not included in the imaging plane. This pitfall is avoided with short axis imaging, in which the hernia can be visualized within the inguinal canal adjacent to the spermatic cord or round ligament. An indirect hernia may extend to the external inguinal ring (Fig. 6-92, online) (Video 6-26![]() ) and into the scrotum in males.
) and into the scrotum in males.
In contrast to the location of an indirect inguinal hernia, a direct inguinal hernia originates medial to the external iliac vessels and moves toward the transducer with the Valsalva maneuver (Fig. 6-93, online) (Videos 6-27 and 6-28)![]() . It is essential to evaluate for direct hernia both in the sagittal and transverse planes over the Hesselbach triangle. If one limits evaluation to the transverse plane, it is possible to misinterpret the normal abdominal contents moving from a superior to inferior direction into the imaging plane, which can simulate a direct hernia. Sagittal imaging will differentiate normal movement of abdominal contents inferiorly from a true direct hernia, in which abdominal contents move in a direct anterior direction (see Fig. 6-93C and D, online). Posterior deficiency of the posterior wall of the inguinal canal, described as a potential finding with a sports-related hernia, appears as anterior convex bulging of the inguinal canal posterior wall near the superficial inguinal ring during straining or the Valsalva maneuver.17
. It is essential to evaluate for direct hernia both in the sagittal and transverse planes over the Hesselbach triangle. If one limits evaluation to the transverse plane, it is possible to misinterpret the normal abdominal contents moving from a superior to inferior direction into the imaging plane, which can simulate a direct hernia. Sagittal imaging will differentiate normal movement of abdominal contents inferiorly from a true direct hernia, in which abdominal contents move in a direct anterior direction (see Fig. 6-93C and D, online). Posterior deficiency of the posterior wall of the inguinal canal, described as a potential finding with a sports-related hernia, appears as anterior convex bulging of the inguinal canal posterior wall near the superficial inguinal ring during straining or the Valsalva maneuver.17
Another type of hernia seen below the level of the inguinal ligament is a femoral hernia, in which intra-abdominal contents appear adjacent to the femoral vein with the Valsalva maneuver (Fig. 6-94, online) (Videos 6-29 and 6-30)![]() . A ventral hernia occurs in midline through diastasis of the rectus abdominis (Fig. 6-95, online) or through an incision. After surgical repair of a hernia, mesh may be seen either as a linear area of speckled echoes (Fig. 6-96A, online) (Video 6-31)
. A ventral hernia occurs in midline through diastasis of the rectus abdominis (Fig. 6-95, online) or through an incision. After surgical repair of a hernia, mesh may be seen either as a linear area of speckled echoes (Fig. 6-96A, online) (Video 6-31)![]() or a continuous echogenic area with posterior shadowing (see Fig. 6-96B, online).68 A recurrent indirect hernia will show abnormal movement of abdominal contents into the inguinal canal (see Fig. 6-96B, online) (Video 6-32)
or a continuous echogenic area with posterior shadowing (see Fig. 6-96B, online).68 A recurrent indirect hernia will show abnormal movement of abdominal contents into the inguinal canal (see Fig. 6-96B, online) (Video 6-32)![]() . It is also important to consider other causes of a clinically suspected hernia, such as lipoma of the spermatic cord (Fig. 6-97, online) (Video 6-33)
. It is also important to consider other causes of a clinically suspected hernia, such as lipoma of the spermatic cord (Fig. 6-97, online) (Video 6-33)![]() , abdominal wall mass (Fig. 6-98, online), and intra-abdominal malignancy (Fig. 6-99, online).
, abdominal wall mass (Fig. 6-98, online), and intra-abdominal malignancy (Fig. 6-99, online).
Developmental Dysplasia of the Hip
One screening protocol using ultrasound to detect hip dysplasia depends on the clinical examination.19 With abnormal clinical examination findings during the Barlow and Ortolani maneuvers, ultrasound is performed in patients younger than 2 weeks of age if the hip is unstable or at 4 to 6 weeks of age if there is a stable click. Minor physiologic laxity may disappear during the first month of life without treatment. With a normal clinical examination, ultrasound examination is performed at 4 to 6 weeks if there are risk factors for dysplasia, such as family history, breech presentation, and postural deformity, to name a few. With normal clinical examination and no risk factors present, no ultrasound examination is performed.
As described in the earlier section on ultrasound examination technique, diagnostic criteria for hip dysplasia rely on dynamic evaluation with optional measurements. Findings of hip dysplasia include subluxation or dislocation of the femoral head during the dynamic maneuvers with applied stress (Fig. 6-100, online). On the coronal image with the hip neutral or slightly flexed, an α angle is normally greater than 60 degrees. When the α angle is less than 50 degrees, the β angle becomes important and is greater than 77 degrees with femoral head subluxation and dislocation.20 The presence of interposed echogenic fibroadipose tissue between the femoral head and the acetabulum is also noted because this is associated with hip dysplasia. Ultrasound also enables one to evaluate for proximal femoral focal deficiency and to assess for variable degrees of femoral aplasia, including the unossified femoral head cartilage.
1 Robben SG, Lequin MH, Diepstraten AF, et al. Anterior joint capsule of the normal hip and in children with transient synovitis: US study with anatomic and histologic correlation. Radiology. 1999;210:499–507.
2 Pfirrmann CW, Chung CB, Theumann NH, et al. Greater trochanter of the hip: attachment of the abductor mechanism and a complex of three bursae—MR imaging and MR bursography in cadavers and MR imaging in asymptomatic volunteers. Radiology. 2001;221:469–477.
3 Hasselman CT, Best TM, Hughes CT, et al. An explanation for various rectus femoris strain injuries using previously undescribed muscle architecture. Am J Sports Med. 1995;23:493–499.
4 Bianchi S, Martinoli C, Waser NP, et al. Central aponeurosis tears of the rectus femoris: sonographic findings. Skeletal Radiol. 2002;31:581–586.
5 Linklater JM, Hamilton B, Carmichael J, et al. Hamstring injuries: anatomy, imaging, and intervention. Semin Musculoskelet Radiol. 2010;14:131–161.
6 Blankenbaker DG, Tuite MJ. Iliopsoas musculotendinous unit. Semin Musculoskelet Radiol. 2008;12:13–27.
7 Robinson P, White LM, Agur A, et al. Obturator externus bursa: anatomic origin and MR imaging features of pathologic involvement. Radiology. 2003;228:230–234.
8 Jamadar DA, Jacobson JA, Morag Y, et al. Sonography of inguinal region hernias. AJR Am J Roentgenol. 2006;187:185–190.
9 Aszmann OC, Dellon ES, Dellon AL. Anatomical course of the lateral femoral cutaneous nerve and its susceptibility to compression and injury. Plast Reconstr Surg. 1997;100:600–604.
10 Damarey B, Demondion X, Boutry N, et al. Sonographic assessment of the lateral femoral cutaneous nerve. J Clin Ultrasound. 2009;37:89–95.
11 Bodner G, Bernathova M, Galiano K, et al. Ultrasound of the lateral femoral cutaneous nerve: normal findings in a cadaver and in volunteers. Reg Anesth Pain Med. 2009;34:265–268.
12 Tagliafico A, Serafini G, Lacelli F, et al. Ultrasound-guided treatment of meralgia paresthetica (lateral femoral cutaneous neuropathy): technical description and results of treatment in 20 consecutive patients. J Ultrasound Med. 2011;30:1341–1346.
13 Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247:797–807.
14 Klauser A, De Zordo T, Feuchtner G, et al. Feasibility of ultrasound-guided sacroiliac joint injection considering sonoanatomic landmarks at two different levels in cadavers and patients. Arthritis Rheum. 2008;59:1618–1624.
15 Finnoff JT, Hurdle MF, Smith J. Accuracy of ultrasound-guided versus fluoroscopically guided contrast-controlled piriformis injections: a cadaveric study. J Ultrasound Med. 2008;27:1157–1163.
16 Smith J, Hurdle MF, Locketz AJ, et al. Ultrasound-guided piriformis injection: technique description and verification. Arch Phys Med Rehabil. 2006;87:1664–1667.
17 Garvey JF, Read JW, Turner A. Sportsman hernia: what can we do? Hernia. 2010;14:17–25.
18 Wangwinyuvirat M, Dirim B, Pastore D, et al. Prepatellar quadriceps continuation: MRI of cadavers with gross anatomic and histologic correlation. AJR Am J Roentgenol. 2009;192:W111–W116.
19 Harcke HT. Screening newborns for developmental dysplasia of the hip: the role of sonography. AJR Am J Roentgenol. 1994;162:395–397.
20 Graf R. Fundamentals of sonographic diagnosis of infant hip dysplasia. J Pediatr Orthop. 1984;4:735–740.
21 Koski JM, Anttila PJ, Isomaki HA. Ultrasonography of the adult hip joint. Scand J Rheumatol. 1989;18:113–117.
22 Moss SG, Schweitzer ME, Jacobson JA, et al. Hip joint fluid: detection and distribution at MR imaging and US with cadaveric correlation. Radiology. 1998;208:43–48.
23 Strouse PJ, DiPietro MA, Adler RS. Pediatric hip effusions: evaluation with power Doppler sonography. Radiology. 1998;206:731–735.
24 Weybright PN, Jacobson JA, Murry KH, et al. Limited effectiveness of sonography in revealing hip joint effusion: preliminary results in 21 adult patients with native and postoperative hips. AJR Am J Roentgenol. 2003;181:215–218.
25 Troelsen A, Jacobsen S, Bolvig L, et al. Ultrasound versus magnetic resonance arthrography in acetabular labral tear diagnostics: a prospective comparison in 20 dysplastic hips. Acta Radiol. 2007;48:1004–1010.
26 Troelsen A, Mechlenburg I, Gelineck J, et al. What is the role of clinical tests and ultrasound in acetabular labral tear diagnostics? Acta Orthop. 2009;80:314–318.
27 Safran O, Goldman V, Applbaum Y, et al. Posttraumatic painful hip: sonography as a screening test for occult hip fractures. J Ultrasound Med. 2009;28:1447–1452.
28 Buck FM, Hodler J, Zanetti M, et al. Ultrasound for the evaluation of femoroacetabular impingement of the cam type: diagnostic performance of qualitative criteria and alpha angle measurements. Eur Radiol. 2011;21:167–175.
29 Leunig M, Beck M, Kalhor M, et al. Fibrocystic changes at anterosuperior femoral neck: prevalence in hips with femoroacetabular impingement. Radiology. 2005;236:237–246.
30 Pfirrmann CW, Mengiardi B, Dora C, et al. Cam and pincer femoroacetabular impingement: characteristic MR arthrographic findings in 50 patients. Radiology. 2006;240:778–785.
31 Wunderbaldinger P, Bremer C, Schellenberger E, et al. Imaging features of iliopsoas bursitis. Eur Radiol. 2002;12:409–415.
32 Bianchi S, Martinoli C, Keller A, et al. Giant iliopsoas bursitis: sonographic findings with magnetic resonance correlations. J Clin Ultrasound. 2002;30:437–441.
33 Pritchard RS, Shah HR, Nelson CL, et al. MR and CT appearance of iliopsoas bursal distention secondary to diseased hips. J Comput Assist Tomogr. 1990;14:797–800.
34 Silva F, Adams T, Feinstein J, et al. Trochanteric bursitis: refuting the myth of inflammation. J Clin Rheumatol. 2008;14:82–86.
35 Fearon AM, Scarvell JM, Cook JL, et al. Does ultrasound correlate with surgical or histologic findings in greater trochanteric pain syndrome? A pilot study. Clin Orthop Relat Res. 2010;468:1838–1844.
36 Kim SM, Shin MJ, Kim KS, et al. Imaging features of ischial bursitis with an emphasis on ultrasonography. Skeletal Radiol. 2002;31:631–636.
37 van Holsbeeck MT, Eyler WR, Sherman LS, et al. Detection of infection in loosened hip prostheses: efficacy of sonography. AJR Am J Roentgenol. 1994;163:381–384.
38 Hoefnagels EM, Obradov M, Reijnierse M, et al. Sonography after total hip replacement: reproducibility and normal values in 47 clinically uncomplicated cases. Acta Orthop. 2007;78:81–85.
39 Ostlere S. How to image metal-on-metal prostheses and their complications. AJR Am J Roentgenol. 2011;197:558–567.
40 Steinbach LS, Schneider R, Goldman AB, et al. Bursae and abscess cavities communicating with the hip: diagnosis using arthrography and CT. Radiology. 1985;156:303–307.
41 Garcia FL, Picado CH, Nogueira-Barbosa MH. Sonographic evaluation of the abductor mechanism after total hip arthroplasty. J Ultrasound Med. 2010;29:465–471.
42 Brew CJ, Stockley I, Grainger AJ, et al. Iliopsoas tendonitis caused by overhang of a collared femoral prosthesis. J Arthroplasty. 2011;26:504. e17–19
43 Wank R, Miller TT, Shapiro JF. Sonographically guided injection of anesthetic for iliopsoas tendinopathy after total hip arthroplasty. J Clin Ultrasound. 2004;32:354–357.
44 Jin W, Chun YS, Park SY, et al. Sonographic detection of acetabular polyethylene liner dissociation in total hip arthroplasty. J Ultrasound Med. 2009;28:1597–1600.
45 Davis KW. Imaging of the hamstrings. Semin Musculoskelet Radiol. 2008;12:28–41.
46 Douis H, Gillett M, James SL. Imaging in the diagnosis, prognostication, and management of lower limb muscle injury. Semin Musculoskelet Radiol. 2011;15:27–41.
47 Robinson P, Bhat V, English B. Imaging in the assessment and management of athletic pubalgia. Semin Musculoskelet Radiol. 2011;15:14–26.
48 Weaver JS, Jacobson JA, Jamadar DA, et al. Sonographic findings of adductor insertion avulsion syndrome with magnetic resonance imaging correlation. J Ultrasound Med. 2003;22:403–407.
49 Kong A, Van der Vliet A, Zadow S. MRI and US of gluteal tendinopathy in greater trochanteric pain syndrome. Eur Radiol. 2007;17:1772–1783.
50 Westacott DJ, Minns JI, Foguet P. The diagnostic accuracy of magnetic resonance imaging and ultrasonography in gluteal tendon tears: a systematic review. Hip Int. 2011;21:637–645.
51 Koulouris G, Connell D. Hamstring muscle complex: an imaging review. Radiographics. 2005;25:571–586.
52 Pelsser V, Cardinal E, Hobden R, et al. Extraarticular snapping hip: sonographic findings. AJR Am J Roentgenol. 2001;176:67–73.
53 Guillin R, Cardinal E, Bureau NJ. Sonographic anatomy and dynamic study of the normal iliopsoas musculotendinous junction. Eur Radiol. 2009;19:995–1001.
54 Deslandes M, Guillin R, Cardinal E, et al. The snapping iliopsoas tendon: new mechanisms using dynamic sonography. AJR Am J Roentgenol. 2008;190:576–581.
55 Choi YS, Lee SM, Song BY, et al. Dynamic sonography of external snapping hip syndrome. J Ultrasound Med. 2002;21:753–758.
56 Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182–188.
57 Delaney-Sathy LO, Fessell DP, Jacobson JA, et al. Sonography of diabetic muscle infarction with MR imaging, CT, and pathologic correlation. AJR Am J Roentgenol. 2000;174:165–169.
58 Petersilge CA, Pathria MN, Gentili A, et al. Denervation hypertrophy of muscle: MR features. J Comput Assist Tomogr. 1995;19:596–600.
59 Stuart RM, Koh ES, Breidahl WH. Sonography of peripheral nerve pathology. AJR Am J Roentgenol. 2004;182:123–129.
60 Neal C, Jacobson JA, Brandon C, et al. Sonography of Morel-Lavallee lesions. J Ultrasound Med. 2008;27:1077–1081.
61 Grey AC, Carrington BM, Hulse PA, et al. Magnetic resonance appearance of normal inguinal nodes. Clin Radiol. 2000;55:124–130.
62 Vassallo P, Wernecke K, Roos N, et al. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology. 1992;183:215–220.
63 Krishna RP, Sistla SC, Smile R, et al. Sonography: an underutilized diagnostic tool in the assessment of metastatic groin nodes. J Clin Ultrasound. 2008;36:212–217.
64 Wu CH, Shih JC, Chang YL, et al. Two-dimensional and three-dimensional power Doppler sonographic classification of vascular patterns in cervical lymphadenopathies. J Ultrasound Med. 1998;17:459–464.
65 Girish G, Jamadar DA, Landry D, et al. Sonography of intramuscular myxomas: the bright rim and bright cap signs. J Ultrasound Med. 2006;25:865–869.
66 Arya S, Nagarkatti DG, Dudhat SB, et al. Soft tissue sarcomas: ultrasonographic evaluation of local recurrences. Clin Radiol. 2000;55:193–197.
67 Choi H, Varma DG, Fornage BD, et al. Soft-tissue sarcoma: MR imaging vs sonography for detection of local recurrence after surgery. AJR Am J Roentgenol. 1991;157:353–358.
68 Jamadar DA, Jacobson JA, Girish G, et al. Abdominal wall hernia mesh repair: sonography of mesh and common complications. J Ultrasound Med. 2008;27:907–917.