Hereditary Disorders of The Skeleton
Endocrinologists can encounter a great diversity of heritable disorders of the skeleton.1–4 Some are clinical curiosities; some are lethal. Some cause focal bony abnormalities; some feature generalized disturbances of skeletal growth, modeling (shaping), and remodeling (turnover).3 A few are associated with overt derangements in mineral homeostasis. Cumulatively, the number of affected people is substantial.1 Each of these entities is important because all harbor clues concerning specific genes and their products that influence skeletal biology. Furthermore, as individual conditions become understood molecularly, patients are being referred increasingly to endocrinologists.
Sclerosing Bone Disorders
Increased skeletal mass is caused by many rare, often heritable osteochondrodysplasias,1,4 and by a variety of endocrine, metabolic, dietary, hematologic, infectious, and neoplastic diseases (Table 12-1). Osteosclerosis and hyperostosis refer to trabecular versus cortical bone thickening, respectively. A radiographic skeletal survey of the genetic disorders shows where and sometimes how bone mass is increased, and often provides sufficient clues for diagnosis.3,4
Osteopetrosis
Osteopetrosis (OPT; “marble bone disease”) was reported first in 1904 by Albers-Schönberg.5 Traditionally, two principal forms are discussed6: the autosomal recessive, infantile (malignant) type that is typically fatal during the first decade of life if untreated,7 and the autosomal dominant, adult (benign) type with distinctly less severe complications.8 Especially rare “intermediate” forms of OPT present during childhood, when the prognosis is poorly understood.9
Now, the gene defects that cause nearly all cases of OPT are known, greatly improving upon imprecise clinical nosologies.10 Although there are different types of OPT, all true forms result from failure of osteoclasts to resorb skeletal tissue.6 Consequently, the manifestations are largely predictable. Accumulation of primary spongiosa (calcified cartilage deposited during endochondral bone formation) represents the histopathologic hallmark.11 It is understandable, however, that the term osteopetrosis persists generically for radiodense skeletons, but should henceforth be applied with precision based on pathogenesis because therapies for genuine OPTs may be inappropriate or harmful for other sclerosing bone disorders.8
Clinical Features
Infantile OPT manifests in babies.7 There is failure to thrive. Cranial foramina do not widen, often compressing auditory, oculomotor, facial, and optic nerves. Blindness can result from retinal degeneration or raised intracranial pressure.12 Some patients develop hydrocephalus or sleep apnea. Nasal stuffiness due to underdeveloped sinuses occurs early. Dentition is delayed. Recurrent infections and spontaneous bruising and bleeding are common and are due to myelophthisis from excessive bone tissue, osteoclasts, and fibrosis crowding marrow spaces. Extramedullary hematopoiesis with hypersplenism and hemolysis may exacerbate already severe anemia. A large head, frontal bossing, “adenoid” appearance, nystagmus, hepatosplenomegaly, short stature, and genu valgum are noted. Bones are fragile. Untreated patients succumb, usually during the first decade of life, to pneumonia, severe anemia, hemorrhage, or sepsis.6,7
Intermediate OPT causes macrocephaly and short stature, sometimes with cranial nerve palsies, ankylosed teeth leading to osteomyelitis of the jaw, recurrent fractures, and mild or occasionally moderately severe anemia.9
Autosomal dominant “benign” OPT (Albers-Schönberg disease) features brittle long bones and fractures within the axial and the appendicular skeleton, and sometimes compromised vision and hearing, facial nerve palsy, mandibular osteomyelitis,13 psychomotor delay, carpal tunnel syndrome, slipped capital femoral epiphysis, or osteoarthritis.14 Some affected individuals are asymptomatic,8 and rare carriers show no radiographic findings.15
Carbonic anhydrase II (CA II) deficiency features OPT with renal tubular acidosis (RTA) and cerebral calcification.16 Severity varies among affected families.17 In infancy or early childhood, fractures, failure to thrive, developmental delay, or short stature manifest. Mental subnormality is common. Compression of the optic nerves and dental malocclusion are further complications. Metabolic acidosis presents as early as birth. Both proximal and distal RTA have been described,18 although distal (type I) RTA seems better documented and may explain any hypotonia, apathy, and muscle weakness. Periodic hypokalemic paralysis can occur. Life expectancy is uncertain, with the oldest published cases reported in young adults.19
Neuronal storage disease with OPT features severe skeletal manifestations accompanying epilepsy and neurodegenerative disease.20 Transient infantile OPT of unknown cause inexplicably resolves after the first months of life. OPT, lymphedema, anhidrotic ectodermal dysplasia, and immunodeficiency (OL-EDA-ID) is an X-linked recessive condition of boys.21
Radiographic Findings
A generalized, symmetrical increase in bone mass is the principal radiographic finding in OPT.22 Cortical and trabecular bone is thickened. In severe disease, all components of skeletal development are disrupted: bone growth, modeling, and remodeling. Furthermore, rachitic changes in growth plates may occur.23 The skull, especially the base, is thickened and dense, and the paranasal and mastoid sinuses are underpneumatized. Vertebrae may show a “bone-in-bone” (endobone) configuration.22
In CA II deficiency, skeletal radiographs typically are abnormal at diagnosis, although findings can be subtle at birth. Remarkably, the osteosclerosis and defective skeletal modeling then diminish over years.16 Cerebral calcification appears on computed tomography (CT) between ages 2 and 5 years and increases during childhood, affecting gray matter of the cortex and basal ganglia.17
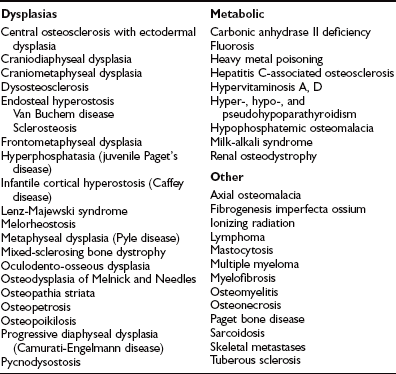
In Albers-Schönberg disease, abnormalities appear during childhood. An especially dense skull base, a “rugger-jersey” spine, and enigmatic alternating sclerotic and lucent bands in the metaphyses of major long bones are characteristic (Fig. 12-1). Metaphyses are wide and may have a club shape or “Erlenmeyer flask” appearance.8 Rarely, distal phalanges in the hands are eroded (more common in pycnodysostosis). Pathologic (“chalk-stick”) fractures occur in major long bones.22
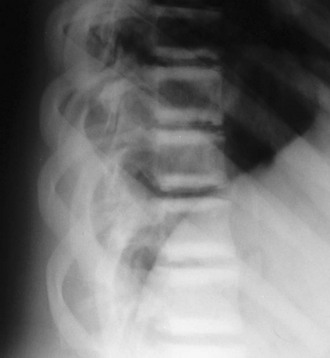
FIGURE 12-1 Osteopetrosis. This teenage girl with Albers-Schönberg disease has a “rugger-jersey” spine typical of adult (“benign”) osteopetrosis. The vertebral end plates are markedly thickened.
Skeletal scintigraphy reveals fractures and osteomyelitis.24 Magnetic resonance imaging (MRI) helps assess patients undergoing bone marrow transplantation, because engraftment restores medullary spaces.25
Laboratory Findings
In infantile OPT, serum calcium concentrations depend largely upon gastrointestinal calcium absorption, because of the impaired bone resorption.26 Secondary hyperparathyroidism with elevated serum levels of calcitriol is common.26 Hypocalcemia can occur, especially with achlorhydria, and causes rickets.23
In adult OPT, biochemical indices of mineral homeostasis usually are unremarkable, although serum PTH levels can be increased.27
Serum acid phosphatase and the brain isoenzyme of creatine kinase often are elevated and seem to originate from the dysfunctional osteoclasts.28
Histopathologic Findings
Failure of osteoclast action provides a pathognomonic finding in OPT6—primary spongiosa synthesized during endochondral bone formation persists as “islands” of calcified cartilage encased within trabecular bone. Osteoclast numbers are increased, normal, or, rarely, decreased. In infantile OPT, these cells are usually abundant.29 Their nuclei are especially numerous, but the “ruffled borders” and “clear zones” that characterize functioning osteoclasts are absent.30 Fibrosis often crowds marrow spaces. Adult OPT shows increased amounts of osteoid, and osteoclasts can be few and may lack ruffled borders, or they can be especially numerous and large.31
Cause and Pathogenesis
Most patients with OPT have diminished osteoclast-mediated acidification at sites of bone resorption due to defects in CA II, the α3 subunit of the vacuolar proton pump, or chloride channel 7 (CLCN7).32 Heterozygous loss-of-function mutation within CLCN7 causes Albers-Schönberg disease.33 Homozygous or compound heterozygous CLCN7 mutations lead to severe or intermediate OPT.33 Malignant OPT usually is due to deactivating mutations in the gene TCIRG1 (ATP6i), which encodes the α3 subunit of the vacuolar proton pump.34 Defects in the “gray-lethal” and OSTM1 genes cause especially severe OPT.35 OL-EDA-ID represents disruption of an essential modulator of NF-κB.21 Recently, loss-of-function mutations within the genes that encode the receptor activator of nuclear factor-κB (RANK) or its ligand (RANKL) were discovered in especially rare forms of autosomal recessive OPT.36,37
Ultimately, impaired skeletal resorption in OPT causes both myelophthisis and bone fragility resulting from the presence of fewer collagen fibrils interconnecting osteons.11
Treatment
Because the cause and pathogenesis, pattern of inheritance, and prognosis for the various forms of OPT can differ, a precise diagnosis is crucial before therapy is attempted. For example, infants or young children with CA II deficiency can have radiographic features of malignant OPT, yet sequential studies may show gradual resolution of bony sclerosis.6 Until recently, the patient’s family and investigation into the severity and progression of the disorder were the principal considerations. Now, diagnosis has been advanced greatly by mutation analysis.38
Bone Marrow Transplantation: Bone marrow transplantation from HLA-identical donors has improved remarkably some patients with infantile OPT.32 However, this procedure is not always appropriate6 (e.g., RANKL deficiency)36 because the pathogenetic defect must be corrected by entry of donor cells into the osteoclast lineage.32
Because severely crowded medullary spaces appear less likely to engraft, early intervention is best.39 Use of marrow from HLA-nonidentical donors warrants continued study. Purified progenitor cells in blood from HLA-haploidentical parents have been useful.39a Marked hypercalcemia can occur as osteoclast function begins.40
Dietary and Medical Therapy: Some success has been reported when a calcium-deficient diet is given. Conversely, calcium supplementation may be necessary for symptomatic hypocalcemia or rickets.23 Large oral doses of calcitriol together with dietary calcium restriction (to prevent absorptive hypercalciuria/hypercalcemia) sometimes improves infantile OPT.41 Calcitriol may stimulate defective osteoclasts, but resistance can occur.41 Long-term infusion of PTH helped one infant,42 perhaps by enhancing calcitriol synthesis. Diminished leukocyte production of superoxide serves as the basis for recombinant human interferon-γ-1b treatment for severely affected children.41
High-dose glucocorticoid treatment stabilizes pancytopenia and hepatomegaly. One case report describes inexplicable reversal of malignant OPT after prednisone therapy alone.43 Prednisone and a low-calcium/high-phosphate diet may be effective.44
In CA II deficiency, the RTA has been treated with bicarbonate supplementation, but the long-term impact is unknown. Bone marrow transplantation corrects the OPT and slows cerebral calcification of CA II deficiency, but does not alter the RTA.45
Supportive Therapy: Hyperbaric oxygenation can be important for osteomyelitis of the jaw.6 Surgical decompression of the optic and facial nerves may benefit some patients. Joint replacement can be helpful.46 Internal fixation may be necessary for femoral fractures.47
Early prenatal diagnosis of OPT by ultrasound generally has been unsuccessful. Conventional radiographs occasionally reveal malignant OPT late in pregnancy. However, mutation analysis is now available in commercial laboratories and can be used to detect OPT in utero.38
Pycnodysostosis
Pycnodysostosis was discovered in 1962.48 Most reports have come from the United States or Europe, but its prevalence seems greatest in Japan.49 Parental consanguinity with autosomal recessive inheritance explains ≈30% of cases. In 1996, loss-of-function mutation of the gene that encodes cathepsin K was identified.50
Clinical Features
Pycnodysostosis is diagnosed during infancy or early childhood because of disproportionate short stature and a relatively large cranium with fronto-occipital prominence, small facies and chin, beaked nose, high-arched palate, obtuse mandibular angle, dental malocclusion with retained deciduous teeth, proptosis, and bluish sclera.51 The anterior fontanel and cranial sutures remain open. Mental retardation affects ≈10% of cases.51 Hands are small and square and fingers are short and clubbed from acro-osteolysis or aplasia of terminal phalanges. Pectus excavatum may occur. Recurrent fractures typically involve the lower limbs and cause genu valgum deformity, although patients usually walk independently. Adult height ranges from 4 ft 3 in to 4 ft 11 in. Recurrent respiratory infections and right heart failure from upper airway obstruction caused by micrognathia trouble some patients.
Radiographic Findings
Pycnodysostosis shares many features with OPT.22 Both cause generalized osteosclerosis and recurrent fractures. The osteosclerosis first appears in childhood, is uniform, and increases with age. The calvarium and the skull base are sclerotic, and orbital ridges are dense. Although long bones have narrow medullary canals, the striking modeling defects of OPT do not occur. Endobones and radiodense striations are absent.22 Other distinguishing findings in pycnodysostosis include delayed closure of cranial sutures and fontanels (prominently the anterior), obtuse mandibular angle, wormian bones, gracile clavicles that are hypoplastic laterally, and hypoplasia or aplasia of the distal phalanges and ribs.52 Hypoplasia of facial bones, sinuses, and terminal phalanges is characteristic. Vertebral bodies are dense with anterior and posterior concavities, but transverse processes are uninvolved. Lumbosacral spondylolisthesis is not uncommon, and lack of segmentation of the atlas and axis may be noted.22
Laboratory Findings
Anemia is not a concern. Serum calcium and inorganic phosphate levels and alkaline phosphatase activity usually are normal. Cortical bone appears unremarkable except for diminished osteoblastic and osteoclastic activity.53 Abnormal inclusions have been described in chondrocytes. Electron microscopy suggests that degradation of collagen is defective.53
Cause and Pathogenesis
Deactivating mutations in the gene that encodes cathepsin K cause pycnodysostosis.50 Cathepsin K is a lysosomal cysteine protease that is highly expressed in osteoclasts.54 Hence, impaired collagen degradation is a fundamental defect. The rate of bone accretion and the size of the exchangeable calcium pool seem reduced. Bone remodeling and therefore quality are compromised.55 Accordingly, pycnodysostosis can be thought of as a form of OPT.
Additionally, killing activity and interleukin-1 secretion by circulating monocytes is compromised.56 Virus-like inclusions have been reported in osteoclasts.57 Defective growth hormone secretion and low serum insulin-like growth factor 1 levels have been described.58
Treatment
No medical therapy is recognized. Bone marrow transplantation has not been reported.
The orthopedic challenges have been reviewed briefly.59 Long bone fractures typically are transverse and heal at a satisfactory rate, but delayed union and massive formation of callus can occur. Intramedullary fixation of long bones is formidable because of their hardness.
Extraction of teeth is difficult, and mandibular fracture has occurred.51 Osteomyelitis of the mandible may require antibiotic and surgical treatment.
Progressive Diaphyseal Dysplasia (Camurati-Engelmann Disease)
Progressive diaphyseal dysplasia (PDD) is an autosomal dominant disorder that affects all races. The condition was described by Cockayne in 1920.60 Camurati discovered its heritable nature.61 Engelmann characterized the severe, typical form in 1929.61 In 2001, mutations that are activating defects were identified in the gene that encodes transforming growth factor (TGF)-β1.62
Characteristically in PDD, painful hyperostosis occurs gradually on both periosteal and endosteal surfaces of long bones.22 However, the clinical and radiographic expression is quite variable.63 In severe cases, osteosclerosis is widespread, including the skull and axial skeleton. Some carriers have no radiographic changes, but bone scintigraphy is abnormal.
Clinical Presentation
PDD typically presents during childhood with limping or a broad-based and waddling gait, leg pain, muscle wasting, and diminished subcutaneous fat in the extremities mimicking muscular dystrophy.64 However, severely affected patients also have a characteristic body habitus that includes an enlarged head with prominent forehead, proptosis, and thin limbs with thickened bones. Cranial nerve palsies can develop when the skull is affected. Puberty sometimes is delayed. Raised intracranial pressure may occur. Palpable bony thickening, skeletal tenderness, and sometimes hepatosplenomegaly are present, as well as Raynaud’s phenomenon and other findings suggestive of vasculitis.65 Radiologic studies typically show progressive disease, but the course is variable and symptom remission sometimes occurs during adult life.
Radiologic Features
Hyperostosis of major long bone diaphyses, the principal finding, represents proliferation of new bone on both periosteal and endosteal surfaces.22 Shafts of long bones gradually widen and develop irregular surfaces. Sclerosis is fairly symmetrical and spreads to involve metaphyses, but the epiphyses are characteristically spared (Fig. 12-2). Tibias and femurs most often are involved, less frequently the radii, ulnae, humeri, and, occasionally, the short tubular bones. Clavicles, scapulae, and the pelvis also may become thickened. Age of onset, rate of progression, and degree of bony involvement are highly variable. With mild disease, scintigraphic abnormalities may be confined to the lower limbs. Maturation of the new bone increases the hyperostosis. However, in severely affected children, some skeletal areas may appear osteopenic.
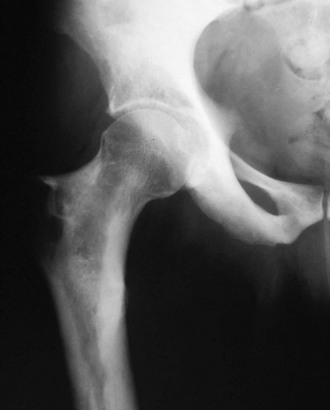
FIGURE 12-2 Progressive diaphyseal dysplasia. This man with Camurati-Engelmann disease has irregular hyperostosis (cortical thickening) of the proximal femur that characteristically does not extend to the end of the long bone.
Clinical, radiographic, and scintigraphic findings are generally concordant.66 Occasionally, however, bone scans are unremarkable despite marked radiographic changes. This seems to reflect advanced but quiescent disease. Increased radioisotope accumulation with few radiographic alterations can represent early skeletal involvement.
Laboratory Findings
Routine biochemical parameters of bone and mineral homeostasis typically are normal, although serum alkaline phosphatase levels can be elevated. Mild hypocalcemia and significant hypocalciuria may occur in severe disease, likely reflecting positive calcium balance.67 Mild anemia and leukopenia and an elevated erythrocyte sedimentation rate seem to reflect the poorly characterized systemic disturbances.65
Histopathology shows new bone formation along diaphyses. Disorganized woven bone undergoes maturation and then incorporation into the cortex. Electron microscopy of muscle has revealed myopathic changes and vascular abnormalities.64
Cause and Pathogenesis
The clinical and laboratory features of severe PDD and its responsiveness to glucocorticoid treatment indicate an inflammatory connective tissue disease.65 Now, the disorder is known to involve mutations in a specific region of the gene that encodes TGF-β1. Consequently, a “latency-associated peptide” encoded by this gene remains bound to TGF-β1, keeping this enhancer of bone formation active.62 Aberrant differentiation of precursor cells to osteoblasts has been discussed as a pathogenetic mechanism.68
Treatment
PDD is a chronic and somewhat unpredictable disorder. Symptoms may remit during adolescence or adult life. Glucocorticoid therapy (typically small doses of prednisone on alternate days) can relieve bone pain and improve histologic abnormalities in bone.69 Bisphosphonate therapy may increase skeletal pain.70
Endosteal Hyperostosis
In 1955, van Buchem et al.71 described hyperostosis corticalis generalisata. Subsequently, additional forms of endosteal hyperostosis were characterized.72 The hallmark of these disorders is thickening of cortical bone primarily on endosteal surfaces.22
Van Buchem disease is an autosomal recessive condition71 that is considerably rarer than the number of case reports might suggest.73 The principal clinical feature is progressive asymmetrical enlargement of the jaw during puberty. The mandible becomes markedly thickened with a wide angle, but no prognathism is noted. Dental malocclusion is uncommon. Affected individuals may be symptom free, but cranial sclerosis also occurs and recurrent facial nerve palsy, deafness, and optic atrophy from narrowing of cranial foramina are common and can develop during infancy. Long bones may hurt with applied pressure but are not fragile.71 Endosteal cortical thickening leads to homogenously dense diaphyses with narrowed medullary canals. However, long bones are shaped properly. Osteosclerosis also affects the skull base, facial bones, vertebrae, pelvis, and ribs.22 Serum alkaline phosphatase from bone may be increased, but calcium and inorganic phosphate levels are unremarkable.
Sclerosteosis, like van Buchem disease, is an autosomal recessive disorder that occurs primarily in people of Dutch ancestry.72 However, sclerosteosis differs from van Buchem disease in that patients are excessively tall and have syndactyly.72 At birth, only fused fingers may be noted.73a Syndactyly reflects cutaneous or bony fusion of the middle and index fingers. During early childhood, skeletal overgrowth involves especially the skull and causes facial disfigurement. Progressive bone thickening widens the jaw, resulting in prognathism.74 Patients become tall and heavy. Deafness and facial palsy are prominent problems. A small skull vault may increase intracranial pressure, causing headaches and compressing the brain stem.75 Intelligence is normal. Life expectancy can be shortened.76 Long bones become widened as cortices thicken. Vertebral pedicles, ribs, and the pelvis may become dense. Fusion of ossicles and narrowing of the internal auditory canals and cochlear aqueducts may occur.72 Enhanced osteoblast activity with failure of osteoclasts to compensate explains the dense bone of sclerosteosis.75 No abnormality of calcium homeostasis or of pituitary function has been documented.77 No specific medical treatment is available. Surgical correction of syndactyly is difficult if there is bony fusion. Management of the neurologic dysfunction has been reviewed.75
Deactivating mutations in the gene that encodes sclerostin (SOST) cause sclerosteosis,78,79 whereas van Buchem disease results from a 52-kb deletion that diminishes a downstream enhancer of SOST.80 Sclerostin binds to LRP5/6, antagonizes canonical Wnt signaling,81 and promotes the apoptosis of osteoblasts.82 Accordingly, sclerostin deficiency in sclerosteosis and van Buchem disease enhances bone formation.
An autosomal dominant (Worth) type83 of endosteal hyperostosis is relatively benign and was rediscovered recently as the “high bone mass phenotype.”84 Some affected individuals have torus palatineus.85 Certain domain-specific, gain-of-function mutations of LRP5 encoding low-density lipoprotein receptor–related protein 5 cause this form of increased skeletal mass.86 LRP5 activation may decrease the biosynthesis of systemic serotonin, leading to enhanced bone formation.87 Despite excesses of good quality bone, the condition is not always benign, as cranial nerve palsies, skeletal pain, and oropharyngeal exostoses may occur.88
Osteopoikilosis
Osteopoikilosis (spotted bones) is usually a radiographic curiosity transmitted as an autosomal dominant trait with a high degree of penetrance.89 However, joint contractions and limb length inequality can occur, especially with accompanying radiographic changes of melorheostosis. When connective tissue nevi called dermatofibrosis lenticularis disseminata are present, this is the Buschke-Ollendorff syndrome.90 The osteopoikilosis is asymptomatic, but if misunderstood can lead to studies for metastatic disease, etc.91 Hence, family members at risk should be counseled. Radiographs show numerous small foci of usually round or oval osteosclerosis22 involving the ends of the short tubular bones, the metaepiphyseal regions of the long bones, and the tarsal, carpal, and pelvic bones (Fig. 12-3). These are thickened trabeculae or islands of cortical bone and do not change appearance over decades. Radionuclide accumulation is not increased on bone scanning.91
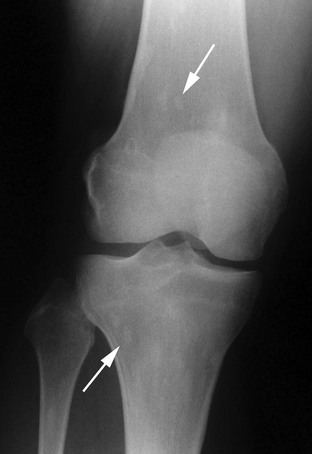
FIGURE 12-3 Osteopoikilosis. Faint, round or oval areas of osteosclerosis (arrows) are present in the metaphases of the distal femur and proximal tibia.
The nevi usually appear before puberty, involve the lower trunk or extremities, and are small asymptomatic papules or yellow or white disks or plaques, deep nodules, or streaks.90 They represent excessive, unusually broad, and markedly branched elastin fibers in the dermis.90
Heterozygous deactivating mutations in the LEMD3 gene cause osteopoikilosis and the Buschke-Ollendorff syndrome.92 LEMD3 is an inner nuclear membrane protein that antagonizes TGF-β1 and bone morphogenetic protein signaling.
Osteopathia Striata
Osteopathia striata features linear striations at the ends of long bones and in the ileum22 and is a curiosity if the skeletal findings occur alone as an autosomal dominant trait. However, osteopathia striata also occurs in clinically important syndromes. These include osteopathia striata with cranial sclerosis93 due to WTX mutations,94 where cranial nerve palsies are common.93 Osteopathia striata with focal dermal hypoplasia (Goltz syndrome) is a serious X-linked dominant disorder featuring widespread linear areas of dermal hypoplasia through which adipose tissue herniates, together with bony defects in the limbs.95
Gracile striations affect trabecular bone, especially the metaepiphyses of major long bones and the periphery of the iliac bones.22 Lesions are unchanged for years. Radionuclide accumulation is not increased during bone scanning.91
Pachydermoperiostosis
Pachydermoperiostosis (hypertrophic osteoarthropathy: primary or idiopathic) causes clubbing of the digits, hyperhidrosis, and thickening of the skin, especially involving the face and forehead (cutis verticis gyrata). Clubbing, periostitis, and pachydermia constitute the classic triad of features. However, some patients have just one or two of these findings. Radiographs reveal periosteal new bone formation, especially in the distal limbs. Autosomal dominant inheritance with variable expression is established,96 but autosomal recessive transmission also seems to occur.97
Blacks seem to be affected more often than whites, and men more severely than women. Presentation is typically during adolescence, but variable.96,97 Symptoms emerge over a decade, but then can abate.98 Progressive enlargement of the hands and feet may cause a “paw-like” appearance. Palms may be wet. Arthralgias of the elbows, wrists, knees, and ankles are common. Acro-osteolysis has been reported. Stiffness of the appendicular and axial skeleton can develop. Compression of cranial or spinal nerves has been described. Cutaneous changes include coarsening, thickening, furrowing, pitting, and oiliness of especially the scalp and face, with some affected individuals described as “acromegalic.” Fatigue is common. Myelophthisic anemia with extramedullary hematopoiesis may occur.
Radiologic Features
Severe periostitis thickens the distal portions of tubular bones—especially the radius, ulna, tibia, and fibula. Clubbing is obvious, and acro-osteolysis can occur. Ankylosis of joints, especially in the hands and in the feet, may trouble older patients.22 The principal diagnostic challenge is secondary hypertrophic osteoarthropathy (pulmonary or otherwise), but this presents a smooth, undulating appearance.99 In pachydermoperiostosis, periosteal proliferation is more exuberant and irregular and often involves epiphyses. Bone scanning in either condition reveals symmetrical, diffuse, regular uptake along the cortical margins of long bones, especially in the legs, causing a “double-stripe” sign.
Laboratory Findings
Periosteal new bone roughens the surface of cortical bone.100 The new osseous tissue undergoes cancellous compaction, rendering it difficult to distinguish from the original cortex on histopathologic examination.100 Osteopenia of trabeculae reflects quiescent bone formation.22 Mild cellular hyperplasia and thickening of subsynovial blood vessels occur near synovial membranes.101,102
Cause and Pathogenesis
In 2008, the cause of pachydermoperiostosis was discovered to be mutations in the gene that encodes 15-hydroxyprostaglandin dehydrogenase, the principal enzyme of prostaglandin degradation.103
Treatment
No specific medical treatment for pachydermoperiostosis is known, but recent identification of the gene defect will now allow targeting of prostaglandin excess. Painful synovial effusions may respond to nonsteroidal anti-inflammatory drugs.103 Contractures or neurovascular compression may require surgical intervention.
Osteoporoses
Osteogenesis imperfecta (OI), sometimes called brittle bone disease, is the most common heritable disorder of connective tissue.2 The nosology for OI devised by Sillence104 in 1981, according to clinical features and apparent modes of inheritance, has been the framework for prognostication and a foundation for biochemical/molecular studies. However, DNA-based findings subsequently have provided critical insights concerning genetic transmission patterns, especially for the severe forms, by revealing that autosomal dominant inheritance explains nearly all patients with OI.105 Also, the clinical heterogeneity of OI is now better understood because a great number of mutations are recognized within the two large genes that encode the protein strands (the pro-α1 and pro-α2 chains) that combine to form the type 1 collagen heterotrimer.105 All major clinical forms of OI represent quantitative and often qualitative abnormalities of this most abundant protein in bone.2 The clinical hallmark is osteoporosis that leads to recurrent fractures and skeletal deformity.106 However, type I collagen also occurs in teeth, skin, ligaments, sclerae, and elsewhere, and many patients with OI have dental disease caused by defective formation of dentin (dentinogenesis imperfecta) and abnormalities of other tissues that contain this fibrillar protein.4 It is understandable that the severity of OI is extremely variable and ranges from stillbirth to perhaps lifelong absence of symptoms.
Clinical Presentation
The differential diagnosis for OI in infants and children includes idiopathic juvenile osteoporosis, Cushing’s syndrome, homocystinuria, congenital indifference to pain, and child abuse. However, OI usually features distinctive signs and symptoms that allow for a correct diagnosis based on the patient’s medical history, physical features, and radiographic findings.105,106 A positive family history is especially helpful, but many patients represent heterozygous, sporadic mutation.105 Affected individuals can manifest ligamentous laxity with joint hypermobility, diaphoresis, susceptibility to bruises, fragile and discolored teeth, and hearing loss (≈50% of those <30 years, and nearly all who are older).107 Deafness typically has a conductive or mixed pathogenesis but sometimes results from sensorineural defects.107 Scleral discoloration ranges from a blue or gray tint that may be subtle or striking. Severe OI is characterized by a high-pitched voice, short stature, scoliosis, herniae, a disproportionately large head compared with body size, a triangular face, and chest deformity. Mitral valve “clicks” are not uncommon, but cardiac disease is unusual. Patients generally have normal intelligence. However, variable severity of OI can occur even within families.106
Type I OI features sclerae with bluish discoloration (especially apparent during childhood), relatively mild osteopenia with infrequent fractures (deformity is uncommon or slight), and deafness that manifests during early adult life. Approximately one third of cases represent new mutations. Elderly women with this type of OI can be mistaken as having postmenopausal osteoporosis alone. Type I OI has been subclassified into I-A and I-B disease depending on the absence or (more rarely) the presence, respectively, of dentinogenesis imperfecta.105
Type IV OI frequently explains multigenerational disease.105 The sclerae are unremarkable, but skeletal deformity, dental disease, and hearing loss are typical.
Additional, rarer forms of OI have been reported, including severe autosomal recessive types now understood at the gene level to involve enzymatic defects in collagen cross-linking.108
Radiographic Features
Characteristic x-ray findings manifest in patients with severe OI,22 including generalized osteopenia, modeling defects featuring gracile long bones, and deformities from recurrent fractures. Modeling abnormalities reflect impaired periosteal bone formation that retards circumferential widening of bones and their cortices. Multiple, recurrent fractures distort vertebrae as well as long bones.22 In some severely affected infants, however, micromelia occurs where major long bones appear wide. Wormian bones of significant number and size in the skull represent a common, but not pathognomonic, feature of OI.109 Excessive pneumatization of the frontal and mastoid sinuses and platybasia that can progress to basilar impression are common in severely affected patients.109 Osteoarthritis is a frequent problem for ambulatory adults with deformity.22
Radiographic abnormalities may worsen markedly during growth—a feature that helps to define progressively deforming, type III OI. Here, a characteristic is “popcorn” calcification. This finding appears in childhood as an acquired defect in the metaphyses of major long bones110 and is believed to reflect traumatic fragmentation of growth plate cartilage. The complication impairs long-bone growth and contributes importantly to short stature, but then appears to resolve when endochondral cartilage becomes fully mineralized at skeletal maturity and is replaced by bone. When fractures occur in OI, they often heal at normal rates. Occasionally, exuberant callus has been mistaken for skeletal malignancy.
Laboratory Findings
Routine biochemical parameters of bone and mineral metabolism usually are unremarkable in OI, although hypercalciuria is common in severely affected children.111 Fortunately, their renal function is intact.111 Elevations in serum and urinary markers of bone turnover occur in severely affected patients.
Bone histology reflects the abnormal skeletal matrix, especially in severely affected patients. Polarized light microscopy often shows an abundance of disorganized (woven) bone, or abnormally thin collagen bundles in lamellar osseous tissue. Numerous osteocytes are found in the cortical bone of some patients.112 This feature seems to reflect decreased amounts of bone produced by individual osteoblasts, yet many cells that are active simultaneously. Hence, the overall rate of skeletal turnover can be rapid, as assessed by in vivo tetracycline labeling before biopsy.113
Cause and Pathogenesis
The biochemical hallmark of OI is low levels of type I collagen synthesis that can be detected through the use of skin fibroblasts in culture.105 Often, the collagen itself is also defective. Various types of heterozygous mutation occur within the pro-α1 and pro-α2 type I collagen genes.105 The large size and complex nature of type I collagen are such that most OI families have unique (“private”) mutations in either of these two genes.105
Treatment
Promising results are reported increasingly for bisphosphonate treatment, especially in growing children, but few trials have been blinded or placebo controlled.114 The role of bisphosphonates or teriparatide is less certain or unknown, respectively, in adults with OI. Improving mouse models for OI provide new ways to test potential therapies. Patient management requires expert orthopedic, rehabilitative, and dental care. Rodding of long bones and bracing of the lower limbs have enabled some severely affected OI children to walk. Stapes surgery has been used for hearing loss.115 The current management of OI has been reviewed.116
National support groups (e.g., Osteogenesis Imperfecta Foundation, Inc.) are important sources of comfort and lay language information for patients with OI and their families. Genetic counseling for OI is now based on gene mutation analysis. Although especially rare patients with severe OI represent autosomal recessive disease,108 most sporadic cases result from new dominant mutations or reflect germline mosaicism for defects in the genes that encode type I collagen. The recurrence risk for type II OI is now estimated to be 5% to 10%117 based on germline mosaicism. Some mildly affected individuals are mosaic and have had a severely affected child.
Prenatal diagnosis of severe OI is possible through ultrasound examination at 14 to 18 weeks’ gestation.117 Biochemical and especially mutation analysis is proving increasingly important.117
Disorders of RANK/OPG/RANKL/NF-κB Signaling
Several extremely rare hereditary disorders of the skeleton feature osteoporosis from accelerated bone remodeling (and sometimes foci of osteolytic disease) due to disturbances of the receptor activator of nuclear factor-κB (RANK), osteoprotegerin (OPG), RANK ligand (RANKL), NF-κB signaling pathway.118 Discovery of this principal regulatory system for osteoclast formation and action began with identification of new ligands and receptors in the tumor necrosis factor (TNF) superfamily, and then characterization of knockout and transgenic mouse models.119 However, a full appreciation of this pathway in humans came from revelation of the genetic bases for several exceptional mendelian skeletal disorders. As is discussed below, the first represented constitutive activation of RANK, the second, deficiency of OPG.120
In the year 2000, heterozygous, gain-of-function, 18-bp and 27-bp tandem duplications in the gene that encodes RANK (TNFRSF11A) were discovered to cause familial expansile osteolysis (FEO) and early-onset Paget’s disease of bone in Japan,121 respectively. Soon after, a similar 15-bp duplication in RANK explained expansile skeletal hyperphosphatasia.122 In 2002, homozygous selective deletion of the gene that encodes OPG (TNFRSF11B) was discovered in Navajo patients with juvenile Paget’s disease (JPD).123
Before these particular disorders are discussed, a brief review of Paget’s disease of bone (PDB) is in order to appreciate how such rare conditions have taught us about this second most common metabolic bone disease that is increasingly appreciated to involve a heritable predisposition.118
Paget’s Disease of Bone
PDB is common in the United States (i.e., prevalence at least 1%, and perhaps as much as 2%)124 and features focally increased skeletal remodeling within axial or appendicular bones in adults.125 Initially, a “wave” of osteoclast-mediated osteolysis moves slowly but relentlessly through a bone, and then is followed by disorganized skeletal repair leading to bony expansion, as well as to osteosclerosis and hyperostosis. Pagetic bone is unsound and can cause pain, fracture, and deformity. Deafness of multifactorial pathogenesis is common,126 and dental problems include loosening and migration of teeth.127 Rarely, malignant transformation of pagetic lesions to osteosarcoma or chondrosarcoma occurs.125
Although the precise cause of PDB remains unknown, evidence is accumulating for paramyxovirus acting in osteoclasts and their precursor cells within PDB lesions. Here, the marrow microenvironment is especially osteoclastogenic, together with measles virus proteins, transcripts, and inclusion bodies.128 However, increasing evidence also indicates that PDB can be heritable. Its prevalence in first-degree relatives is now appreciated to be 12% to 40%, representing a sevenfold enhanced risk.129 In fact, despite its classic focal appearance, PDB can manifest generalized acceleration in skeletal remodeling, which has been attributed previously to elevated circulating PTH levels.130
Clinical characterization of FEO with its similarity to PDB (see later) and obvious autosomal dominant inheritance resurrected interest in a possible genetic basis (or predisposition) for PDB.131 Now, such predisposition is understood for patients with PDB to be genetically heterogeneous.132 In 2001, PDB was mapped to chromosome 5 in French-Canadian families, leading to discovery in 2002 of a heterozygous loss-of-function mutation in the gene that encodes sequestosome (SQSTM1).133 Additional SQSTM1 defects then were identified worldwide in a large number of familial and some sporadic PDB cases.134 Soon after, the rare autosomal dominant syndrome called inclusion body myopathy with early-onset PDB and frontotemporal dementia was shown to involve mutations in the gene that encodes valosin containing protein.135 SQSTM1 and valosin containing protein both seem to participate in the intracellular process of ubiquination.136
Familial Expansile Osteolysis
FEO is a remarkably instructive, autosomal dominant disorder.132 Patients can manifest deafness early in life followed by focal, lytic expansion of one or more major appendicular bones, causing pain, fracture, deformity, and sometimes osteosarcoma. Osteolytic defects in FEO are especially common in the lower extremities, and a tibia most often is involved.137 As in PDB, these lesions usually start at or near the end of a long bone and then slowly advance.136,138 Deafness presents at as young as age 4 years, but more commonly in the second decade, and ultimately conductive deafness becomes of mixed type.139 The long process of the incus disappears or becomes fibrous tissue. Loosening and pain and/or fracture of adult teeth can occur.139 In FEO dentition, root resorption is extensive and the size of pulp chambers and root canals is reduced.139 However, the most remarkable finding is “idiopathic external resorption” that destroys teeth.140
Skeletal lesions in FEO initially have the clinical, radiographic, and histopathologic appearance of PDB in its early osteolytic phase,125 but affected bones eventually instead become expanded, shell-like, and fat-filled rather than exhibiting the osteosclerosis and hyperostosis of advanced PDB.141 Furthermore, generalized osteopenia and a coarse trabecular pattern are present in adult life, suggesting that FEO is a systemic bone disease.138 Elevation in serum alkaline phosphatase activity and other biochemical markers of bone remodeling in FEO is explained in part by this diffuse acceleration in skeletal turnover.120 Light microscopy of FEO lesions early on shows filigree-like trabeculae of woven bone, abundant osteoclasts and osteoblasts lining trabeculae, giant osteoclasts with bizarre shapes and numerous nuclei, and fibrous marrow.139 On electron microscopy, microcylindrical nuclear inclusion bodies with ultrastructure similar to paramyxoviridae can resemble PDB.139,141 However, “mosaic bone,” the hallmark of advanced PDB, is rare—perhaps reflecting the extreme rates of bone remodeling. Instead, woven bone seems to be deposited rapidly and does not mature or remodel into cortical bone.138 Generally, little radiographic osteosclerosis occurs unless antiresorptive pharmaceuticals are administered.138 Intermediate-stage disease, however, features scanty skeletal matrix with abundant fibrous tissue and vascularity. Advanced disease shows almost total loss of cortical and trabecular bone, as well as fat-occupied medullary spaces.131,139
The molecular defect causing FEO was discovered in 2000 through a candidate gene approach.121 In three kindreds, an in-frame, 18-bp tandem duplication was identified in exon 1 of the TNFRSF11A gene encoding RANK. Transfection studies suggested increased NF-κB activity due to extension of the RANK signal peptide trapping this receptor intracellularly and causing constitutive activation.121
Treatment
In FEO, perhaps skeletal injury explains in part the focal nature of the bone lesions.138 Microscopic or macroscopic fracture in FEO might initiate skeletal repair that becomes deranged because excessive RANK effect enhances osteoclast numbers and activity. Similar changes may be noted in other disorders of RANK excess and enhanced RANK/OPG/RANKL/NF-κB signaling (see later). Once osteolysis runs its course, expanded bone becomes fat-filled, perhaps because the mesenchymal stem cell pool has differentiated excessively to adipocytes.141 It is understandable that advanced expansile lesions then are unresponsive to otherwise effective antiresorptive therapy.138
Osteoprotegerin Deficiency
Juvenile Paget’s disease (JPD), also called idiopathic or hereditary hyperphosphatasia, usually is diagnosed in infants or young children.142 Occasionally, the disease presents later in childhood. A relatively mild form of JPD is associated with mental retardation.142 In contrast to the autosomal dominant RANK activation disorders, all forms of JPD are considered to be autosomal recessive traits.132
JPD affects the entire skeleton.143 This has prompted objection to the disorder being called a type of PDB; however, JPD and the RANK disorders seem increasingly to share features with PDB.
JPD causes bone pain, fracture, and deformity.144 Premature loss of teeth and deafness are also typical. Radiographs show marked undertubulation of long bones with thin cortices (Fig. 12-4).145 Rapid skeletal remodeling, confirmed by histopathologic findings, leads to substantially elevated biochemical markers of bone turnover.123,142 In fact, the mosaic pattern of bone characteristic of advanced PDB is not found in JPD or the disorders of RANK excess.
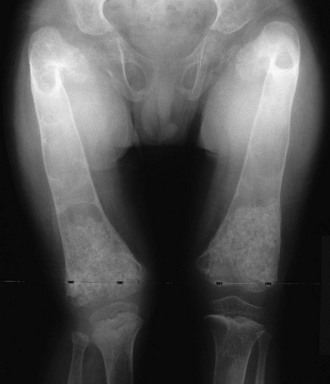
FIGURE 12-4 Osteoprotegerin deficiency. The femurs of this boy with juvenile Paget’s disease are widened, and thin cortices and irregular ossifications are evident. Marked angular deformity is present proximally.
Mild JPD features fewer fractures and less bony deformity, but radiographs show diffuse, acquired hyperostosis and osteosclerosis associated with biochemical and histologic evidence of accelerated skeletal turnover.123,142
Discovery in JPD of OPG deficiency from loss-of-function mutations in TNFRSF11B provided both a cause and a mechanism for this osteopathy.123 OPG normally is released into the marrow space from preosteoblasts and osteoblasts, and it acts as a decoy receptor for RANKL.119 Thus, OPG deficiency engenders high levels of RANKL activity, which markedly accelerate osteoclastogenesis and bone turnover.119
Observations in JPD also complement findings in the TNFRSF11B knockout mouse model, which suggest a further role for OPG in preventing vascular calcification. In these mice, aorta and renal artery mineralization is found through histopathologic methods.146 Although no calcifications are observed on radiographs or CT scans in patients with JPD, the literature describing JPD includes “calcifying arteriopathy” on histopathologic analysis.147 Furthermore, striking changes consistent with pseudoxanthoma elasticum, including granular and coarse deposits of calcium in the membranes and intima of the muscular arteries and arterioles, were reported in all autopsy tissues from a young man with JPD.148 Additionally, patients with OPG deficiency develop blindness from a retinopathy that seems to derive from microvascular calcification.149
Antiresorptive treatment using calcitonin or bisphosphonates has been beneficial for JPD.144,149 Recombinant OPG has been effective for two affected adult siblings.150 Anti-RANKL antibody could offer another therapeutic approach for the skeletal disease, but it might not slow vascular calcification.
References
1. Online Mendelian Inheritance in Man, OMIM (TM). McKusick-Nathans Institute of Genetic Medicine. http://www.ncbi.nlm.nih.gov/omim/..
2. Royce PM, Steinmann B, eds. Connective tissue and its heritable disorders, ed 2, New York: Wiley-Liss, 2002.
3. Castriota-Scanderbeg, A, Dallapiccola, B. Abnormal skeletal phenotypes: from simple signs to complex diagnoses. New York: Springer; 2005.
4. Rimoin, DL, Connor, JM, Pyeritz, RE, et al. Emery and Rimoin’s principles and practice of medical genetics, ed 5. Philadelphia: Churchill Livingstone; 2007.
5. Albers-Schönberg, H. Rontgenbilder einer seltenen, Knochenerkrankung. Meunch Med Wochenschr. 1904;51:365.
6. Whyte, MP. Osteopetrosis. In: Royce PM, Steinmann B, eds. Connective tissue and its heritable disorders. ed 2. New York: Wiley-Liss; 2002:789–807.
7. Loria-Cortes, R, Quesada-Calvo, E, Cordero-Chaverri, E. Osteopetrosis in children: a report of 26 cases. J Pediatr. 1977;91:43–47.
8. Johnston, CC, Jr., Lavy, N, Lord, T, et al. Osteopetrosis: a clinical, genetic, metabolic, and morphologic study of the dominantly inherited, benign form. Medicine (Baltimore). 1968;47:149–167.
9. Kahler, SG, Burns, JA, Aylsworth, AS. A mild autosomal recessive form of osteopetrosis. Am J Med Genet. 1984;17:451–464.
10. Del Fattore, A, Cappariello, A, Teti, A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42:19–29.
11. Marks, SC, Jr. Osteopetrosis: multiple pathways for the interception of osteoclast function. Appl Pathol. 1987;5:172–183.
12. Vanier, V, Miller, R, Carson, BS. Bilateral visual improvement after unilateral optic canal decompression and cranial vault expansion in a patient with osteopetrosis, narrowed optic canals, and increased intracranial pressure. J Neurol Neurosurg Psychiatry. 2000;69:405–406.
13. Waguespack, SG, Koller, DL, White, KE, et al. Chloride channel 7 (ClCN7) gene mutations and autosomal dominant osteopetrosis, type II. J Bone Miner Res. 2003;18:1513–1518.
14. Benichou, OD, Lareo, JD, De Verenjoul, MC. Type II autosomal dominant osteopetrosis (Albers-Schönberg disease): clinical and radiological manifestations in 42 patients. Bone. 2000;26:87–93.
15. Campos-Xavier, AB, Casanova, JL, Doumaz, Y, et al. Intrafamilial phenotypic variability of osteopetrosis due to chloride channel 7 (CLCN7) mutations. Am J Med Genet A. 2005;133:216–218.
16. Whyte, MP, Murphy, WA, Fallon, MD, et al. Osteopetrosis, renal tubular acidosis and basal ganglia calcification in three sisters. Am J Med. 1980;69:64–74.
17. Sly, WS, Whyte, MP, Sundaram, V, et al. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med. 1985;313:139–145.
18. Sly, WS, Whyte, MP, Krupin, T, et al. Positive renal response to acetazolamide in carbonic anhydrase II-deficient patients. Pediatr Res. 1985;19:1033–1036.
19. Awad, M, Al-Ashwal, AA, Sakati, N, et al. Long-term follow up of carbonic anhydrase II deficiency syndrome. Saudi Med J. 2002;23:25–29.
20. Jagadha, V, Halliday, WC, Becker, LE, et al. The association of infantile osteopetrosis and neuronal storage disease in two brothers. Acta Neuropathol (Berl). 1988;75:233–240.
21. Dupuis-Girod, S, Corradini, N, Hadj-Rabia, S, et al. Osteopetrosis, lymphedema, anhidrotic ectodermal dysplasia, and immunodeficiency in a boy and incontinentia pigmenti in his mother. Pediatrics. 2002;109:1–6.
22. Resnick, D, Niwayama, G. Diagnosis of bone and joint disorders, ed 4. Philadelphia: WB Saunders; 2002.
23. Di Rocco, M, Buoncompagni, A, Loy, A, et al. Osteopetrorickets: case report. Eur J Paediatr Neurol. 2000;159:579–581.
24. Park, H-M, Lambertus, J. Skeletal and reticuloendothelial imaging in osteopetrosis: case report. J Nucl Med. 1977;18:1091–1095.
25. Rao, VM, Dalinka, MK, Mitchell, DG, et al. Osteopetrosis: MR characteristics at 1.5 T. Radiology. 1986;161:217–220.
26. Cournot, G, Trubert-Thil, CL, Petrovic, M, et al. Mineral metabolism in infants with malignant osteopetrosis: heterogeneity in plasma 1,25- dihydroxyvitamin D levels and bone histology. J Bone Miner Res. 1992;7:1–10.
27. Bollerslev, J. Autosomal dominant osteopetrosis: bone metabolism and epidemiological, clinical and hormonal aspects. Endocr Rev. 1989;10:45–67.
28. Whyte, MP, Chines, A, Silva, DP, Jr., et al. Creatine kinase brain isoenzyme (BB-CK) presence in serum distinguishes osteopetrosis among the sclerosing bone disorders. J Bone Miner Res. 1996;11:1438–1443.
29. Flanagan, AM, Massey, HM, Wilson, C, et al. Macrophage colony-stimulating factor and receptor activator NF-κB ligand fail to rescue osteoclast-poor human malignant infantile osteopetrosis in vitro. Bone. 2002;30:85–90.
30. Helfrich, MH, Aronson, DC, Everts, V, et al. Morphologic features of bone in human osteopetrosis. Bone. 1991;12:411–419.
31. Bollerslev, J, Steiniche, T, Melsen, F, et al. Structural and histomorphometric studies of iliac crest trabecular and cortical bone in autosomal dominant osteopetrosis: a study of two radiological types. Bone. 1986;10:19–24.
32. Tolar, J, Teitelbaum, SL, Orchard, PJ. Osteopetrosis. N Engl J Med. 2004;351:2839–2849.
33. Campos-Xavier, AB, Saraiva, JM, Ribeiro, LM, et al. Chloride channel 7 (CLCN7) gene mutations in intermediate autosomal recessive osteopetrosis. Hum Genet. 2003;112:186–189.
34. Taranta, A, Migliaccio, S, Recchia, I, et al. Genotype-phenotype relationship in human ATP6i-dependent autosomal recessive osteopetrosis. Am J Pathol. 2003;162:57–68.
35. Ramirez, A, Faupel, J, Goebel, I, et al. Identification of a novel mutation in the coding region of the grey-lethal gene OSTM1 in human malignant infantile osteopetrosis. Hum Mutat. 2004;23:471–476.
36. Sobacchi, C, Frattini, A, Guerrini, MM, et al. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet. 2007;39:960–962.
37. Guerrini, MM, Sobacchi, C, Cassani, B, et al. Human osteoclast-poor osteopetrosis with hypogamma-globulinemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet. 2008;83:64–76.
38. Segovia-Silvestre, T, Neutzsky-Wulff, AV, Sorensen, MG, et al. Advances in osteoclast biology resulting from the study of osteopetrotic mutations. Hum Genet. 2009;124:561–577.
39a. Driessen, GJ, Gerritsen, EJ, Fischer, A, et al. Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone Marrow Transplant. 2003;32:657–663.
39. Tsuji, Y, Ito, S, Isoda, T, et al. Successful nonmyeloablative cord blood transplantation for an infant with malignant infantile osteopetrosis. J Pediatr Hematol Oncol. 2005;27:495–498.
40. Rawlinson, PS, Green, RH, Coggins, AM, et al. Malignant osteopetrosis: hypercalcaemia after bone marrow transplantation. Arch Dis Child. 1991;66:638–639.
41. Key, LL, Rodriguiz, RN, Willi, SM, et al. Recombinant human interferon gamma therapy for osteopetrosis. N Engl J Med. 1995;332:1594–1599.
42. Glorieux, FH, Pettifor, JM, Marie, PJ, et al. Induction of bone resorption by parathyroid hormone in congenital malignant osteopetrosis. Metab Bone Dis Relat Res. 1981;3:143–150.
43. Iacobini, M, Migliaccio, S, Roggini, M, et al. Case report: apparent cure of a newborn with malignant osteopetrosis using prednisone therapy. J Bone Miner Res. 2001;16:2356–2360.
44. Dorantes, LM, Mejia, AM, Dorantes, S. Juvenile osteopetrosis: effects of blood and bone of prednisone and low calcium, high phosphate diet. Arch Dis Child. 1986;61:666–670.
45. McMahon, C, Will, A, Hu, P, et al. Bone marrow transplantation corrects osteopetrosis in the carbonic anhydrase II deficiency syndrome. Blood. 2001;97:1947–1950.
46. Gwynne Jones, DP, Hodgson, BF, Hung, NA. Bilateral, uncemented total hip arthroplasty in osteopetrosis. J Bone Joint Surg Br. 2004;86:276–278.
47. Chhabra, A, Westerlund, LE, Kline, AJ, et al. Management of proximal femoral shaft fractures in osteopetrosis: a case series using internal fixation. Orthopedics. 2005;28:587–592.
48. Maroteaux, P, Lamy, M. La pycnodysostose. Presse Med. 1962;70:999–1002.
49. Sugiura, Y, Yamada, Y, Koh, J. Pycnodysostosis in Japan: report of six cases and a review of Japanese literature. Birth Defects. 1974;10:78–98.
50. Gelb, BD, Brömme, D, Desnick, RJ. 2001 Pycnodysostosis: cathepsin K deficiency. In: Scriver CR, Beaudet AL, Sly WS, eds. The metabolic and molecular bases of inherited disease. ed 8. New York: McGraw-Hill Book Company; 2001:3453–3468.
51. Wolpowitz, A, Matisson, A. A comparative study of pycnodysostosis, cleidocranial dysostosis, osteopetrosis and acro-osteolysis. S Afr Med J. 1974;48:1011–1118.
52. Soto, TJ, Mautalen, CA, Hojman, D, et al. Pycnodysostosis, metabolic and histologic studies. Birth Defects. 1969;5:109–115.
53. Everts, V, Aronson, DC, Beertsen, W. Phagocytosis of bone collagen by osteoclasts in two cases of pycnodysostosis. Calcif Tissue Int. 1985;37:25–31.
54. Motyckova, G, Fisher, DE. Pycnodysostosis: role and regulation of cathepsin K in osteoclast function and human disease. Curr Mol Med. 2002;2:407–421.
55. Fratzl-Zelman, N, Valenta, A, Roschger, P, et al. Decreased bone turnover and deterioration of bone structure in two cases of pycnodysostosis. J Clin Endocrinol Metab. 2004;89:1538–1547.
56. Karkabi, S, Reis, ND, Linn, S, et al. Pyknodysostosis: imaging and laboratory observations. Calcif Tissue Int. 1993;53:170–173.
57. Beneton, MNC, Harris, S, Kanis, JA. Paramyxovirus-like inclusions in two cases of pycnodysostosis. Bone. 1987;8:211–217.
58. Soliman, AT, Rajab, A, Al Salmi, I, et al. Defective growth hormone secretion in children with pycnodysostosis and improved linear growth after growth hormone treatment. Arch Dis Child. 1996;75:242–244.
59. Edelson, JG, Obad, S, Geiger, R, et al. Pycnodysostosis: orthopedic aspects, with a description of 14 new cases. Clin Orthop. 1992;280:263–276.
60. Cockayne, EA. A case for diagnosis. Proc R Soc Med. 1920;13:132–136.
61. Engelmann, G. Ein fall von osteopathia hyperostotica (sclerotisans) multiplex infantilis. Fortschr Geb Roentgen. 1929;39:1101–1106.
62. Saito, T, Kinoshita, A, Yoshiura, KI, et al. Domain-specific mutations of a transforming growth factor (TGF)-β1 latency-associated peptide cause Camurati-Engelmann disease because of the formation of a constitutively active form of TGF-β1. J Biol Chem. 2001;276:11469–11472.
63. Wallace, SE, Lachman, RS, Mekikian, PB, et al. Marked phenotypic variability in progressive diaphyseal dysplasia (Camurati-Engelmann disease): report of a four-generation pedigree, identification of a mutation in TGFβ1, and review. Am J Med Genet A. 2004;129:235–247.
64. Naveh, Y, Ludatshcer, R, Alon, U, et al. Muscle involvement in progressive diaphyseal dysplasia. Pediatrics. 1985;76:944–949.
65. Crisp, AJ, Brenton, DP. Engelmann’s disease of bone: a systemic disorder? Ann Rheum Dis. 1982;41:183–188.
66. Kumar, B, Murphy, WA, Whyte, MP. Progressive diaphyseal dysplasia (Englemann’s disease): scintigraphic-radiologic-clinical correlations. Radiology. 1981;140:87–92.
67. Smith, R, Walton, RJ, Corner, BD, et al. Clinical and biochemical studies in Engelmann’s disease (progressive diaphyseal dysplasia). Q J Med. 1977;46:273–294.
68. Labat, ML, Bringuier, AF, Seebold, C, et al. Monocytic origin of fibroblasts: spontaneous transformation of blood monocytes into neo-fibroblastic structures in osteomyelosclerosis and Engelmann’s disease. Biomed Pharmacother. 1991;45:289–299.
69. Naveh, Y, Alon, U, Kaftori, JK, et al. Progressive diaphyseal dysplasia: evaluation of corticosteroid therapy. Pediatrics. 1985;75:321–323.
70. Inaoka, T, Shuke, N, Sato, J, et al. Scintigraphic evaluation of pamidronate and corticosteroid therapy in a patient with progressive diaphyseal dysplasia (Camurati-Engelmann disease). Clin Nucl Med. 2001;26:680–682.
71. Van Buchem, FSP, Prick, JJG, Jaspar, HHJ. Hyperostosis corticalis generalisata familiaris (van Buchem’s disease). Amsterdam: Excerpta; 1976.
72. Beighton, P, Barnard, A, Hamersma, H, et al. The syndromic status of sclerostenosis and van Buchem disease. Clin Genet. 1984;25:175–181.
73. Eastman, JR, Bixler, D. Generalized cortical hyperostosis (van Buchem disease): nosologic considerations. Radiology. 1977;125:297–304.
73a. Beighton, P, Durr, L, Hamersma, H. The clinical features of sclerostenosis: a review of the manifestations in twenty-five affected individuals. Ann Intern Med. 1976;84:393–397.
74. Beighton, P, Cremin, BJ, Hamersma, H. The radiology of sclerostenosis. Br J Radiol. 1976;49:934–939.
75. Stein, SA, Witkop, C, Hill, S, et al. Sclerostenosis, neurogenetic and pathophysiologic analysis of an American kinship. Neurology. 1983;33:267–277.
76. Hamersma, H, Gardner, J, Beighton, P. The natural history of sclerostenosis. Clin Genet. 2003;63:192–197.
77. Epstein, S, Hamersma, H, Beighton, P. Endocrine function in sclerostenosis. S Afr Med J. 1979;55:1105–1110.
78. Brunkow, ME, Gardner, JC, Van Ness, J, et al. Bone dysplasia sclerostenosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589.
79. Kim, CA, Honjo, R, Bertola, D, et al. A known SOST gene mutation causes sclerosteosis in a familial and an isolated case from Brazilian origin. Genet Test. 2008;12:475–479.
80. Balemans, W, Patel, N, Ebeling, M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97.
81. Li, X, Zhang, Y, Kang, H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887.
82. Sutherland, MK, Geoghegan, JC, Yu, C, et al. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone. 2004;35:828–835.
83. Perez-Vicente, JA, Rodriguez de Castro, E, Lafuente, J, et al. Autosomal dominant endosteal hyperostosis: report of a Spanish family with neurological involvement. Clin Genet. 1987;31:161–169.
84. Whyte, MP. Searching for gene defects that cause high bone mass (editorial). Am J Hum Genet. 1997;60:1309–1311.
85. Boyden, LM, Mao, J, Belsky, J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;345:1513–1521.
86. Van Wesenbeeck, L, Cleiren, E, Gram, J, et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet. 2003;72:763–771.
87. Yadav, VK, Ryu, JH, Suda, N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837.
88. Rickels, MR, Zhang, X, Mumm, S. Oropharyngeal skeletal disease accompanying high bone mass and novel LRP5 mutation. J Bone Miner Res. 2005;20:878–885.
89. Berlin, R, Hedensio, B, Lilja, B, et al. Osteopoikilosis: a clinical and genetic study. Acta Med Scand. 1967;18:305–314.
90. Uitto, J, Santa Cruz, DJ, Starcher, BC, et al. Biochemical and ultrastructural demonstration of elastin accumulation in the skin of the Buschke-Ollendorff syndrome. J Invest Dermatol. 1981;76:284–287.
91. Whyte, MP, Murphy, WA, Seigel, BA. 99m Tc-pyrophosphate bone imaging in osteopoikilosis, osteopathia striata, and melorheostosis. Radiology. 1978;127:439–443.
92. Hellemans, J, Preobrazhenska, O, Willaert, A, et al. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nat Genet. 2004;36:1213–1218.
93. Rabinow, M, Unger, F. Syndrome of osteopathia striata, macrocephaly, and cranial sclerosis. Am J Dis Child. 1984;138:821–823.
94. Jenkins, ZA, van Kogelenberg, M, Morgan, T, et al. Germline mutations in WTX cause a sclerosing skeletal dysplasia but do not predispose to tumorigenesis. Nat Genet. 2008;41:95–100.
95. Clements, SE, Wessagowit, V, Lai-Cheong, JE, et al. Focal dermal hypoplasia resulting from a new nonsense mutation, p.E300X, in the PORCN gene. J Dermatol Sci. 2008;49:39–42.
96. Rimoin, DL. Pachydermoperiostosis (idiopathic clubbing and periostosis): genetic and physiologic considerations. N Engl J Med. 1965;272:923–931.
97. Matucci-Cerinic, M, Lott, T, Jajic, IVO, et al. The clinical spectrum of pachydermoperiostosis (primary hypertrophic osteoarthropathy). Medicine. 1991;79:208–214.
98. Herman, MA, Massaro, D, Katz, S. Pachydermoperiostosis: clinical spectrum. Arch Intern Med. 1965;116:919–923.
99. Ali, A, Tetalman, M, Fordham, EW. Distribution of hypertrophic pulmonary osteoarthropathy. Am J Roentgenol. 1980;134:771–780.
100. Vogl, A, Goldfischer, S. Pachydermoperiostosis: primary or idiopathic hypertrophic osteoarthropathy. Am J Med. 1962;33:166–187.
101. Lauter, SA, Vasey, FB, Huttner, I, et al. Pachydermoperiostosis: studies on the synovium. J Rheumatol. 1978;5:85–95.
102. Cooper, RG, Freemont, AJ, Riley, M, et al. Bone abnormalities and severe arthritis in pachydermoperiostosis. Ann Rheum Dis. 1992;51:416–419.
103. Uppal, S, Diggle, CP, Carr, IM, et al. Mutations in 15-hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat Genet. 2008;40:789–793.
104. Sillence, D. Osteogenesis imperfecta: an expanding panorama of variants. Clin Orthop. 1981;159:11–25.
105. Byers, PH. Disorders of collagen biosynthesis and structure. In Striver CR, Beaudet AL, Sly WS, et al, eds.: The metabolic and molecular bases of inherited disease, ed 8, New York: McGraw-Hill, 2001.
106. Albright, JA, Millar, EA. Osteogenesis imperfecta [symposium]. Clin Orthop Relat Res. 1981;159:1–156.
107. Pedersen, U. Hearing loss in patients with osteogenesis imperfecta. Scand Audiol. 1984;13:67–74.
108. Baldridge, D, Schwarze, U, Morello, R, et al. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat. 2008;29:1435–1442.
109. Cremin, B, Goodman, H, Prax, M, et al. Wormian bones in osteogenesis imperfecta and other disorders. Skeletal Radiol. 1982;8:35–38.
110. Goldman, AB, Davidson, D, Pavlor, H, et al. Popcorn calcifications: a prognostic sign in osteogenesis imperfecta. Radiology. 1980;136:351–358.
111. Chines, A, Boniface, A, McAlister, W, et al. Hypercalciuria in osteogenesis imperfecta: a follow-up study to assess renal effects. Bone. 1995;16:333–339.
112. Falvo, KA, Bullough, PG. Osteogenesis imperfecta: a histometric analysis. J Bone Joint Surg Am. 1973;55:275–286.
113. Baron, R, Gertner, JM, Lang, R, et al. Increased bone turnover with decreased bone formation by osteoblasts in children with osteogenesis imperfecta tarda. Pediatr Res. 1983;17:204–207.
114. Glorieux, HI, Bishop, NJ, Plotkin, H, et al. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–952.
115. Kuurila, K, Pynnönen, S, Grénman, R. Stapes surgery in osteogenesis imperfecta in Finland. Ann Otol Rhinol Laryngol. 2004;113:187–193.
116. Zeitlin, L, Fassier, F, Glorieux, FH. Modern approach to children with osteogenesis imperfecta. J Pediatr Orthop B. 2003;12:77–87.
117. Pepin, M, Atkinson, M, Starman, BJ, et al. Strategies and outcomes of prenatal diagnosis for osteogenesis imperfecta: a review of biochemical and molecular studies completed in 129 pregnancies. Prenat Diagn. 1997;17:559–570.
118. Whyte, MP. Paget’s disease of bone and genetic disorders of RANKL/OPG/RANK/NF-κB signaling. Ann NY Acad Sci. 2006;1068:143–164.
119. Martin, TJ. Paracrine regulation of osteoclast formation and activity: milestones in discovery. J Musculoskel Neuron Interact. 2004;4:243–253.
120. Whyte, MP, Mumm, S. Heritable disorders of the RANKL/OPG/RANK signaling pathway. J Musculoskel Neuron Interact. 2004;4:254–267.
121. Hughes, AE, Ralston, SH, Marken, J, et al. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet. 2000;24:45–48.
122. Whyte, MP, Hughes, AE. Expansile skeletal hyperphosphatasia is caused by a 15-base pair tandem duplication in TNFRSF11A encoding RANK and is allelic to familial expansile osteolysis. J Bone Miner Res. 2002;17:26–29.
123. Whyte, MP, Obrecht, SE, Finnegan, PM, et al. Osteoprotegerin deficiency and juvenile Paget’s disease. N Engl J Med. 2002;347:174–184.
124. Altman, RD, Bloch, DA, Hochberg, MC, et al. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461–465.
125. Kanis, JA. Pathophysiology and treatment of Paget’s disease of bone, ed 2. Malden, MA: Blackwell Science; 1998.
126. Nager, GT. Paget’s disease of the temporal bone. Ann Otol Rhinol Laryngol. 1975;84:1–32.
127. Smith, BJ, Eveson, JW. Paget’s disease of bone with particular reference to dentistry. J Oral Pathol. 1981;10:233–247.
128. Roodman, GD, Windle, JJ. Paget disease of bone. J Clin Invest. 2005;115:200–208.
129. Morales-Piga, AA, Rey-Rey, JS, Corres-Gonzalez, J, et al. Frequency and characteristics of familial aggregation of Paget’s disease of bone. J Bone Miner Res. 1995;10:663–670.
130. Meunier, PJ, Coindre, J, Edouard, CM, et al. Bone histomorphometry in Paget’s disease: quantitative and dynamic analysis of Paget’s disease and non-pagetic bone tissue. Arthritis Rheum. 1980;23:1095–1103.
131. Singer, FR, Leach, RJ. Genetics of Paget’s disease of bone. In: Econs MJ, ed. The genetics of osteoporosis and metabolic bone disease. Totowa, NJ: Humana Press; 2000:309–318.
132. McKusick VA: Mendelian inheritance in man: catalogs of human genes and genetic disorders, ed 12 (1998). Johns Hopkins University Press, Baltimore and Online Mendelian inheritance in man (2000). OMIM (TM) McKusick-Nathans institute for genetic Medicine, Johns Hopkins University, Baltimore and National Center for Biotechnology Information, National Library of Medicine, Bethesda, MD.
133. Laurin, N, Brown, JP, Morissette, J, et al. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. 2002;70:1582–1588.
134. Good, DA, Busfield, F, Fletcher, BH, et al. Identification of SQSTM1 mutations in familial Paget’s disease in Australian pedigrees. Bone. 2004;35:277–282.
135. Watts, GDJ, Wymer, J, Kovach, MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;10:1–5.
136. Asai, T, Tomita, Y, Nakatsuka, S, et al. VCP (p97) regulates NfκB signaling pathway, which is important for metastasis of osteosarcoma cell line. Jpn J Cancer Res. 2002;93:296–304.
137. Hughes, AE, Barr, J. Familial expansile osteolysis: a genetic model of Paget’s disease. In: Sharpe PT, ed. The molecular biology of Paget’s disease. Heidelberg: RG Landes; 1996:179–199.
138. Whyte, MP, Reinus, WR, Podgornik, MN, et al. Familial expansile osteolysis (excessive RANK effect) in a 5-generation American kindred. Medicine (Baltimore). 2002;81:101–121.
139. Wallace, RG, Barr, RJ, Osterberg, PH, et al. Familial expansile osteolysis. Clin Orthop. 1989;248:265–277.
140. Mitchell, CA, Kennedy, JG, Wallace, RG. Dental abnormalities associated with familial expansile osteolysis: a clinical and radiographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1990;70:301–307.
141. Dickson, GR, Shirodria, PV, Kanis, JA, et al. Familial expansile osteolysis: a morphological, histomorphometric and serological study. Bone. 1991;12:331–338.
142. Golob, DS, McAlister, WH, Mills, BG, et al. Juvenile Paget disease: life-long features of a mildly affected young woman. J Bone Miner Res. 1996;11:132–142.
143. Caffey, J. Familial hyperphosphatasemia with ateliosis and hypermetabolism of growing membranous bone: review of the clinical, radiographic and chemical features. Bull Hosp Joint Dis. 1972;33:81–110.
144. Cassinelli, HR, Mautalen, CA, Heinrich, JJ, et al. Familial idiopathic hyperphosphatasia (FIH): response to long-term treatment with pamidronate (APD). Bone Miner. 1992;19:175–184.
145. Resnick, D. Diagnosis of bone and joint disorders, ed 3. Philadelphia: WB Saunders; 1995.
146. Bucay, N, Narosi, I, Dunstan, CR, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:260–268.
147. Silve, C, Grosse, B, Tau, C, et al. Response to parathyroid hormone and 1,25-dihydroxyvitamin D3 of bone-derived cells isolated from normal children and children with abnormalities in skeletal development. J Clin Endocrinol Metab. 1986;62:583–590.
148. Mitsudo, SM. Chronic idiopathic hyperphosphatasia associated with pseudoxanthoma elasticum. J Bone Joint Surg Am. 1971;53:303–314.
149. Whyte, MP, Singhellakis, P, Petersen, MB, et al. Juvenile Paget’s disease: the second reported, oldest patient is homozygous for the TNFRSF11B “Balkan” mutation (966_969delTGACinsCTT) which elevates circulating immunoreactive osteoprotegerin levels. J Bone Miner Res. 2007;22:938–946.
150. Cundy, T, Davidson, J, Rutland, MD, et al. Recombinant osteoprotegerin for juvenile Paget’s disease. N Engl J Med. 2005;353:918–923.