Hereditary angioedema
Management strategy
Therapy for HAE is dependent on three considerations:
 Relief of acute angioedema, especially preservation of the airway
Relief of acute angioedema, especially preservation of the airway
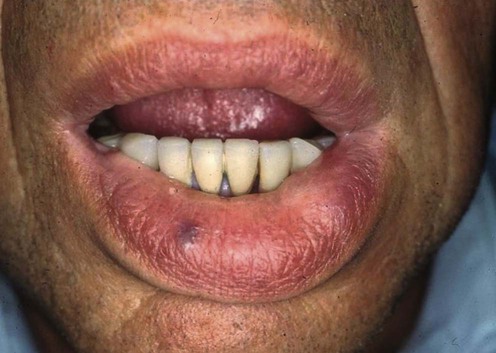
 Prevention of relapse due to dental and surgical interventions
Prevention of relapse due to dental and surgical interventions
Prevention of relapse due to dental and surgical interventions
Patients with HAE should all wear a MedicAlert disk stating the diagnosis and emergency treatment.
Acute Angioedema
First-line therapies
Second-line therapies
Long-term prophylaxis of hereditary angioedema
First-line therapies
Second-line therapies





 C1-INH concentrate
C1-INH concentrate Fresh frozen plasma
Fresh frozen plasma Icatibant
Icatibant Ecallantide
Ecallantide Danazol
Danazol Stanozolol
Stanozolol Tranexamic acid
Tranexamic acid Epsilon-aminocaproic acid
Epsilon-aminocaproic acid C1-INH concentrate
C1-INH concentrate Fresh frozen plasma
Fresh frozen plasma Danazol
Danazol C1-INH concentrate
C1-INH concentrate