Chapter 7 Herbals and Natural Products
| Abbreviations | |
|---|---|
| DHEA | Dehydroepiandrosterone |
| DNA | Deoxyribonucleic acid |
| DSHEA | Dietary Supplement Health and Education Act |
| FDA | Food and Drug Administration |
| HIV | Human immunodeficiency virus |
| MAOI | Monoamine oxidase inhibitors |
| NE | Norepinephrine |
Therapeutic Overview
Since prehistoric times, humans have used plants as medicines. Of the 520 new prescription drugs approved between 1983 and 1994, 39% were natural products or were derived from plants or animals, with 60% to 80% of antimicrobials and anticancer drugs obtained from such products. Over millions of years, plants have developed the capacity to synthesize a diverse array of chemicals, which attract or repel other organisms, serve as photocollectors or protectants, and respond to environmental challenges. For example, phytochemicals can assist plants in resisting pathogens, make them unpalatable, aid in collecting light energy, protect plants from photo-oxidation, or help dissipate excess light energy as heat. With the advent of modern scientific medicine, phytochemicals have been refined, or altered, to produce a share of the modern pharmacopoeia. Despite the increasing availability of many potent and selective drugs, there remains an increasing interest in folk remedies, including herbal medicines.
acids, vitamins, minerals), often referred to a nutraceuticals, a term coined in 1989 that refers to any substance considered “a food or part of a food that provides medical or health benefits, including the prevention and/or treatment of a disease.”
The Dietary Supplement Health and Education Act (DSHEA), passed by the United States Congress in 1994, defines a dietary supplement as a product that is intended to supplement the diet and contains a vitamin, mineral, amino acid, herb, or other botanical product intended for ingestion in the form of a capsule, powder, or extract. Dietary supplement products must bear an ingredient label that includes the name and quantity of each ingredient or the total quantity of all ingredients (excluding inert ingredients) in a blend. Labeling of products containing herbal or botanical ingredients must state the part of the plant from which the ingredient is derived. Botanicals may be obtained in many formulations listed in Box 7-1.
BOX 7–1 Major Botanical Preparations
Federal regulations provide for the use of various types of statements on the label of dietary supplements, but claims cannot be made about the use of a dietary supplement to diagnose, prevent, mitigate, treat, or cure a specific disease without sufficient clinical evidence. For example, a product may not carry the claim “cures diabetes” or “treats cancer,” unless that claim is supported by clear evidence. Some health claims can be made, if the product has been so approved. For example, the claim that calcium may reduce the risk of osteoporosis has been approved by the Food and Drug Administration (FDA). Products can make claims about classical nutrient deficiency diseases, provided the statements disclose the prevalence of the disease in the United States. In addition, manufacturers may describe the effects of a supplement on “structure or function” of the body or the “well-being” achieved by consuming the dietary ingredient. To use these claims, manufacturers must have substantiation that the statements are truthful and not misleading.
Mechanisms of Action
Like any other drug or chemical, the components of herbal medicines are presumed to exert their effects on physiological or biological systems. One major difference is that botanicals contain large numbers of chemicals, which may interact synergistically or antagonistically. Some remedies consist of mixtures of several herbs, so that the number of chemicals in a single preparation can reach into the hundreds or thousands. The most commonly used botanicals are shown in Box 7-2.
Oxidation of deoxyribonucleic acid (DNA), proteins, carbohydrates, and lipids by reactive oxygen species has been implicated in normal aging and a number of different diseases, including arthritis, cancer, and Alzheimer’s disease. Oxidative stress occurs when there is an imbalance between free radical generation (by the action of reactive oxygen species) and endogenous antioxidants in cells and tissues. Reactive oxygen species are produced by some toxins, ultraviolet light, normal biochemical pathways (e.g., nitric oxide synthase), and pathological events in cells (e.g., free radicals that escaped from the mitochondrial complex). Endogenous antioxidants include reduced glutathione and the enzymes superoxide dismutase, catalase, and glutathione peroxidase. Many plants contain antioxidants (Box 7-3), including several typically used as food; some vitamins also have antioxidant activity. Although there is little clinical evidence that supplementation with dietary antioxidants ameliorates or prevents any disease, there is a wealth of scientific evidence for the free radical scavenging ability of many plant-derived antioxidants; thus interest in this mechanism of action remains strong.
Some herbals are thought to act by modulation of immune function (Box 7-4). This modulation can be indirect, via antioxidant effects, or direct, via effects on immune cells. In general, herbal remedies are thought to enhance immune function by removing toxins from the body (a common concept in herbalism).
The herbal approach to immunomodulation is holistic and focuses on boosting liver function and cleansing the blood. In the herbal philosophy, detoxification by the liver has a crucial role in health and in regulating immune function. Again, there is a dearth of clinical evidence for the efficacy of any herbal medicine in altering the course of disease, but some evidence in animal models and cell cultures suggests possible effects on immune function.
Many plants contain compounds that are used or abused for their psychoactive qualities, usually for sedative, stimulant, or analgesic purposes. These include coffee (caffeine), tobacco (nicotine), coca (cocaine), opium poppy (opiates), marijuana (cannabinoids), and peyote (mescaline). Ethnobotanical studies of shamanism in native populations have revealed many other hallucinogenic plants. A variety of herbal products are commonly used for sedative, stimulant, analgesic, and antiemetic effects (Table 7-1).
TABLE 7–1 Herbs Proposed to Act on the Central Nervous System
| Sedatives | Stimulants |
|---|---|
| Kava | Lobelia |
| Valerian | Coffee |
| Skull cap | Tea |
| Passion flower | Tobacco |
| Lavender | Ginseng |
| Antiemetics | Analgesics |
| Black horehound | Cayenne |
| Lemon balm | White willow bark |
| Cayenne | Feverfew |
| Clove | Jamaican dogwood |
| Dill | St. John’s wort |
| Lavender | Ginseng |
| Meadowsweet | Corydalis (Corydalis yanhusuo) |
| Ginger |
Some herbs contain compounds that mimic or block the actions of hormones, notably estrogen. Currently used products include highly concentrated extracts of phytochemicals, synthetic derivatives, and even steroids like dehydroepiandrosterone (DHEA) and androstenedione, which are classified as dietary supplements because they are produced from plant precursor sterols (Box 7-5).
Other botanicals have been proposed to modify hormonal balance in men. Saw palmetto contains steroid-like compounds that may antagonize the actions of testosterone and is suggested for treatment of benign prostatic hyperplasia and prostate cancer. Tribulus and Tongkat Ali are thought to enhance testosterone production through stimulation of luteinizing hormone production. Yohimbe contains yohimbine, an antagonist at α2-adrenergic receptors known to increase norepinephrine (NE) release by blocking inhibitory presynaptic autoreceptors, thus enhancing sympathetic activity (see Chapter 11). Synthetic yohimbine is regulated as a drug and prescribed for erectile dysfunction, whereas yohimbe bark is sold as a dietary supplement. Pygeum may interfere with testosterone production by inhibition of 5-α-reductase and aromatase and is used for treating benign prostatic hyperplasia. DHEA is a naturally occurring adrenal hormone that is a precursor of estrogen and testosterone. Levels of DHEA decline with aging, so it is often used as a supplement to restore those levels toward more “youthful” values.
There are several approaches to herbal therapy of cancer (Box 7-6). Some herbal products have been suggested to prevent cancer by stimulating the immune system or by their antioxidant effects. Others are thought to act by direct toxic effects on neoplastic cells; for example, by inhibition of topoisomerases, inhibition of polyamine synthesis, or stimulation of apoptosis pathways. Other postulated mechanisms include blockage of angiogenesis (e.g., shark cartilage) and reversal of multidrug resistance pumps (e.g., flavonoids).
Several potent conventional cancer treatments (see Chapter 54) are derived from plants and other natural products. These include taxol from Pacific yew and the vinca alkaloids (vincristine, vinblastine) from Madagascar periwinkle.
Ergogenics are substances that increase energy production, use, or recovery. Many products claim to give athletes a competitive edge through an ergogenic effect (see Box 7-6). Surveys have shown that 75% of college athletes and 100% of body builders take supplements for this purpose. A handful of supplements on the market have been shown to be effective in high-quality clinical studies.
Pharmacokinetics
In most cases it is not possible to determine the pharmacokinetics of herbal products because of their complex nature. When the active components are unknown, it is difficult to select the key components to follow in a pharmacokinetic study.
An important issue that needs additional investigation is the interaction of herbal products with other drugs. Drug-herb interactions can occur at the level of absorption, distribution, metabolism, or excretion (see Chapter 2), but metabolic interactions have received the most attention. These interactions go in both directions, with drugs either interfering with or enhancing the effects of herbs, and herbs or other supplements either interfering with or enhancing the effects (and side effects) of drugs.
Drugs such as cholestyramine, colestipol, and sucralfate may bind to certain herbs, forming an insoluble complex and decreasing absorption of both substances. Absorption of herbs may also be adversely affected by drugs that change the pH of the stomach. Antacids, H2-histamine receptor antagonists, and proton pump inhibitors (see Chapter 18) such as cimetidine and omeprazole are used to neutralize, decrease, or inhibit secretion of stomach acid for treatment of ulcers or gastroesophageal reflux. With decreased stomach acid, herbs may not be broken down properly, leading to poor absorption in the intestines. Drugs that affect gastrointestinal motility may also affect herb absorption. Slower motility would mean the herbs stay in the intestines longer, thus increasing absorption. Metoclopramide and cisapride can increase gastrointestinal motility and decrease absorption of herbs. Haloperidol and some opiate narcotics decrease gastrointestinal motility and may increase absorption of herbs.
Many herbs induce the cytochrome P450 system (see Chapter 2), although the specific herbal components that induce P450s may be different from those responsible for therapeutic efficacy. Induction of P450s leads to increased metabolism of other concomitantly administered drugs that are metabolized through the same pathways.
Conversely, drugs that inhibit cytochrome P450s can increase the accumulation of herbs. Examples of drugs that inhibit liver metabolism include, but are not limited to, cimetidine, erythromycin, ethanol, and antifungal drugs such as fluconazole, itraconazole, and ketoconazole (see Chapter 50).
Relationship of Mechanisms of Action to Clinical Response
The complex nature of herbal medicines creates a major challenge to determining their mechanisms of action. The level of clinical evidence to support the efficacy of these agents is minimal at best; in only a few cases is there strong clinical evidence for efficacy (Box 7-7).
BOX 7–7 Levels of Clinical Evidence for Efficacy of Dietary Supplements (From Strongest to Weakest)
Pharmacovigilance: Side Effects, Clinical Problems, and Toxicity
Provisions in DSHEA state that the manufacturer is responsible for ensuring that its dietary supplement products are safe before they are marketed. Unlike drug products that must be proven safe and effective for their intended use before marketing (see Chapter 4), there are no provisions in the law for the FDA to “approve” dietary supplements for safety or effectiveness before they reach the consumer. Also, unlike manufacturers and distributors of drugs, those of dietary supplements are not currently required by law to record, investigate, or forward to government agencies any reports they receive of injuries or illnesses that may be related to the use of their products. Under DSHEA, once the product is marketed, the FDA has the responsibility for showing that a dietary supplement is “unsafe” before it can take action to restrict its use or remove it from the marketplace.
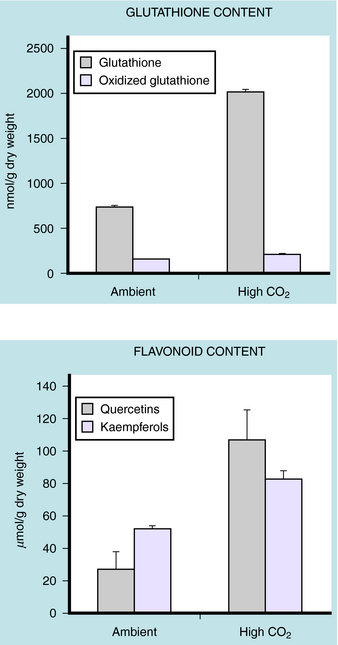
Finally, herbs are natural products, and the chemistry of the plant is determined by growing conditions, including seed stock. This can lead to considerable variation in the chemical composition of any given batch of herbs (Fig. 7-1). There are no “standards” or methods of certification that are accepted across the industry. When the active ingredients are not known, it is obviously impossible to standardize preparations to achieve a reproducible pharmacological effect. Not only does this make the clinical use of herbs difficult, but it is also a major impediment to research in this area.
New Horizons
Centuries of use have created the impression that herbal remedies are both safe and effective. The challenge to science is to provide controlled, clinical evidence for these claims. In a typically reductionist fashion, Western science has approached the study of herbs via a drug development model. Once an effect has been established, bioassay-directed fractionation of the plant extract can lead to isolation of a single active chemical. However, it is also of interest to investigate the more holistic philosophy, which requires development of research strategies for the study of highly complex systems. These might include complex mixtures of herbs, or a complex, individually tailored treatment plan using several different herbs at different times in conjunction with dietary changes. Research is also needed to determine potential mechanisms of action and to more rationally guide design of clinical trials. The concept of detoxification, with consequent increases in well-being and ability to ward off disease, is central to herbal medicine but has not been well studied by Western scientists. Safety concerns about herbal medicines require well-designed toxicological and pharmacokinetic studies and may lead to more restrictive approval and marketing requirements and more stringent monitoring of adverse effects (Box 7-8). Herbal medicine is practiced by several types of alternative medicine providers, including doctors of traditional Chinese medicine, naturopaths, homeopaths, and Ayurvedic physicians (the traditional medicine of India). An expanding trend in health care is the concept of complementary medicine, where such therapies are combined with conventional Western approaches. More research is needed to determine the advantages (or disadvantages) of these combined, integrated approaches, because this trend is being driven by patient demand as well as evidence for improvements in health care and outcomes.
BOX 7–8 Important Considerations in Using Herbals and Natural Products
Information on herbal medicine from the National Library of Medicine available online at: http://www.nlm.nih.gov/medlineplus/herbalmedicine.html.
National Center for Complementary and Alternative Medicine available online at: http://www.nccam.nih.gov.
Searchable herb database from Memorial Sloan Kettering Cancer Center available online at: http://www.mskcc.org/mskcc/html/11570.cfm.