Chapter 80 Hepatocellular carcinoma
Overview
Hepatocellular carcinoma (HCC) is the most common primary liver tumor. It currently represents the sixth most common cancer worldwide and is the third major case of cancer death. Its incidence presents marked geographic differences (Table 80.1; Ferlay et al, 2010). Asia and sub-Saharan Africa constitute high-risk areas with yearly rates exceeding 20 per 100,000 population. Southern Europe and Japan have intermediate rates, and rates are low in Northern Europe and North America (Bosch et al, 2004; El-Serag, 2004; Ferlay et al, 2010). Several registries have shown that the incidence figures have changed slightly during the past several years. Vaccination plans against hepatitis B virus (HBV) and improvement in health standards have decreased the incidence in some high-risk areas, such as Taiwan (Chang et al, 1997; see Chapter 64). The incidence is decreasing in Japan, where the risk of HCC is linked significantly to chronic infection with hepatitis C virus (HCV) that was acquired decades ago, when health care was less concerned by blood-borne viral disease. By contrast, HCC incidence is increasing in the United States (El-Serag & Mason, 1999) and in Northern Europe (Bosch et al, 2004), probably reflecting the different timing in the appearance of risk factors, mostly HCV infection (Tanaka et al, 2002; see Chapter 64). Another major risk factor for HCC is alcohol intake, and this also varies widely in different countries (Morgan et al, 2004). Aflatoxin B1 uptake (Colombo & Donato, 2005), cigarette smoking, and heavy alcohol consumption are independent risk factors and may have synergistic effects (Kuper et al, 2000; see Chapter 64). Finally, recent studies have suggested that coffee intake reduces the risk (Bravi et al, 2007).
Table 80.1 Incidence of Hepatocellular Carcinoma According to Geographic Area and Distribution of Risk Factors
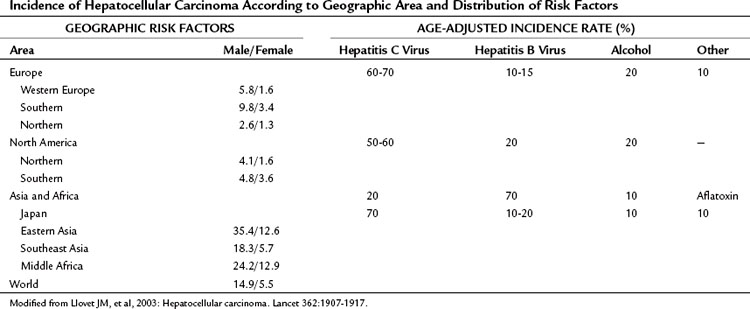
In all areas of the world, cirrhosis underlies HCC in almost 90% of the cases (Bruix et al, 2004; Fattovich et al, 2004). Cirrhosis is related to chronic HBV and HCV, which contribute to HCC development in 80% of cases. Alcohol consumption is a frequent cofactor, and surveys indicate that a significant proportion of HCC in cryptogenic cirrhosis is due to nonalcoholic fatty liver disease (Calle et al, 2003; Clark, 2006; El-Serag et al, 2001; Marrero et al, 2005; Regimbeau et al, 2004), a condition associated with diabetes or obesity (Day, 2002; Marchesini et al, 2003; see Chapter 65). Because of the obesity epidemic, this factor may gain greater relevance in the near future. Follow-up studies have disclosed that HCC development is now the most frequent cause of death in patients with cirrhosis (Benvegnu et al, 2004; Degos et al, 2000; Forner et al, 2009; Sangiovanni et al, 2006). Prevention, diagnosis, and treatment of this neoplasm are major areas of clinical and research activity.
The mean annual incidence of HCC in cirrhotic patients in the West is 3% to 4%, and this figure increases in parallel to liver function impairment (Benvegnu et al, 2004; Bolondi et al, 2001; Bruix & Sherman, 2011; Degos et al, 2000; Forner et al, 2009; Sangiovanni et al, 2006). Some specific characteristics have been associated with a higher risk. The most consistent are male sex; advanced age, this being a surrogate for the duration of the underlying liver disease; and increased α-fetoprotein (AFP) concentration (Bruix & Sherman, 2011). HBV and HCV coinfection imply a higher risk (Fattovich et al, 2004).
Data of patients coinfected with the human immunodeficiency virus (HIV) suggest that the incidence of HCC has increased in this population, liver function deteriorates more rapidly, and the HCC appears in younger ages and frequently in symptomatic, advanced stages (Brau et al, 2007) associated with poorer outcome (Puoti et al, 2004). In addition, several authors have reported higher potential of tumoral progression before and after liver transplantation in HIV/HCV-coinfected patients despite similar pathologic findings (Vibert et al, 2011). Further studies are needed to confirm whether HIV coinfection increases the HCC incidence, and if it is an independent prognostic factor. Irregular regeneration, high proliferative stage, and presence of dysplastic foci in biopsy samples also have been correlated with higher HCC incidence during follow-up, but the need for tissue sampling has prevented their robust validation and wide clinical acceptance (Bruix et al, 2004).
Risk Factors and Prevention
All types of cancer are the consequence of genetic changes that accumulate through time, ultimately conferring a survival advantage to cells with an abnormal phenotype. The relationship between chronic hepatitis and long-lasting liver damage with HCC development suggests that persistent inflammation is a key factor leading to cancer. Maintained injury and repair with high proliferation rate increase the risk for DNA mutation (Arbuthnot & Kew, 2001). In addition to genetic damage secondary to inflammation, HBV itself may have direct genetic or epigenetic effects, being able to integrate into the host genome (Brechot, 2004; Farazi & DePinho, 2006). This integration into the host genome is accompanied by rearrangement and increased mutagenesis that may affect crucial target genes (Farazi & DePinho, 2006; Ferber et al, 2003; Ryu, 2003; Villanueva et al, 2007). HCV is an RNA virus and does not integrate into host DNA (see Chapter 8C).
The most effective prevention of HCC is avoidance of viral infection or of toxin intake. Effective vaccination for HBV exists, and the vaccination plans in Taiwan have shown the efficacy of this action in diminishing cancer rates in children and adults (Chang et al, 1997). Proper controls in health care settings prevent HCV dissemination through blood transmission. Alcohol intake should be the target of health campaigns in the community; in addition, aflatoxin contamination of food can be prevented by avoiding grain storage in humid conditions. The epidemic of obesity with associated nonalcoholic steatohepatitis leading to HCC must be controlled by health education.
If viral infection is already present, it has been shown that a decrease in viral replication and subsequently of chronic hepatic damage could result in a reduced incidence of HCC in the long term (Liaw et al, 2004). Effective therapy is available for HBV and HCV, and prevention may be feasible for both agents; however, if chronic liver disease already has caused cirrhosis, it is unclear whether antiviral therapy has any preventive capacity, because cell damage and transformation may already be present (Bruno et al, 2007). Recent studies have shown that long-term interferon administration in does not reduce the risk of HCC in patients with cirrhosis (Bruix et al, 2011; Di Bisceglie et al, 2008). In patients with HBV infection, the genetic damage may occur in the absence of major liver insult, and this would explain the appearance of HCC in an otherwise healthy liver (Pollicino et al, 2004). This circumstance is more frequent in areas where viral transmission occurs early after birth or in childhood, stressing the benefits of HBV vaccination. Secondary prevention in patients with chronic liver disease requires the prior recognition of affected individuals, but currently no proof of the cost-effectiveness of population screening for viral liver disease has been offered, and this is not recommended (Bruix & Sherman, 2005; see Chapter 64).
Screening and Recall of High-Risk Individuals
A single randomized controlled trial performed in China recruited HBV patients to compare screening versus no screening; it showed the positive impact of screening on survival (Zhang & Yang, 1999). Unfortunately, such proof of efficacy is lacking in the West, and the establishment of surveillance for HCC in patients with cirrhosis is based on expert opinion (Bruix & Sherman, 2011; Bruix et al, 2001). The aim of screening is to detect HCC at an early stage, when the tumor is potentially curable (Bruix & Sherman, 2011; Forner et al, 2010).
Success of screening depends on several factors that include adequate ultrasound (US) performance, accurate diagnostic and staging criteria, and timely availability of curative treatments. If these requirements are not met, screening will never result in an increase of life expectancy. Even when accounting for the potential lead-time bias (i.e., patients apparently survive longer simply because their tumors were diagnosed earlier) and length-time bias (i.e., the interval between screenings misses the more aggressive tumors that will be detected when symptoms appear), it is assumed that screening results in more frequent detection of early stage HCC with more common application of effective treatment and improved outcome (Sangiovanni et al, 2004).
Effective treatment of HCC and long-term cure are feasible only with early detection; diagnosis at the symptomatic stage reflects an advanced stage and lack of effective treatment. Based on these arguments, the European Association for the Study of the Liver (EASL) panel of experts suggested abdominal US scanning and AFP measurements every 6 months for patients with liver cirrhosis who would be treated if diagnosed with HCC (Bruix et al, 2001). Because of the suboptimal accuracy of AFP (Marrero et al, 2009; Trevisani et al, 2001), the determination of this marker is no longer recommended, and screening is currently based on regular US (Bruix & Sherman, 2011). The screening recommendation excludes patients with severe associated conditions and those with advanced liver disease who are already candidates for transplantation. If this is not an option, screening for HCC aiming to detect early tumor will never be followed by treatment, and it makes no clinical sense.
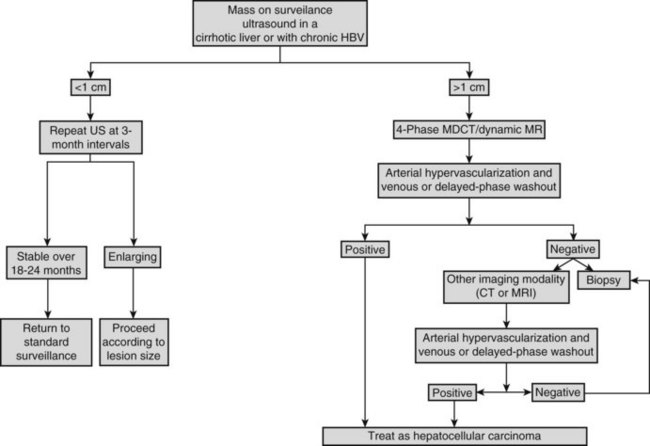
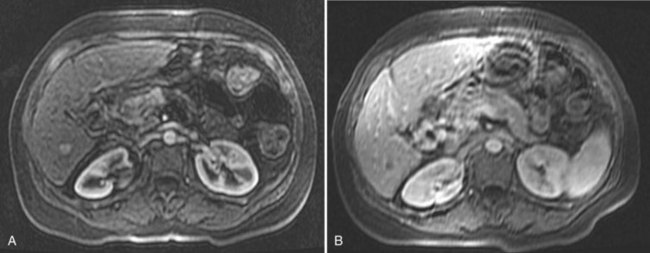
On detection of an abnormal finding, patients must be evaluated. Figure 80.1 depicts the recall strategy proposed by the American Association for the Study of Liver Diseases (AASLD) panel, and Box 80.1 summarizes proposed diagnostic criteria (Bruix & Sherman, 2011). Nodules measuring less than 1 cm in a cirrhotic liver may not correspond to a malignant focus in more than 60% of cases (Forner et al, 2008); even so, confident diagnosis is difficult with current diagnostic tools. Close follow-up to detect any increase in size is therefore recommended (Bruix & Sherman, 2005). Nodules in a cirrhotic liver that are larger than 1 cm can be diagnosed as HCC if the vascular profile on imaging techniques is characteristic of this neoplasm—that is, intense contrast uptake is seen in the arterial phase followed by contrast washout in the delayed venous phase (Bruix & Sherman, 2005; see Chapters 16 and 17). In a tumor larger than 1 cm in diameter, a single dynamic imaging technique can establish the diagnosis (e.g., computed tomography [CT] or magnetic resonance imaging [MRI]; Fig. 80.2). Contrast ultrasound is no longer recommended because it is not able to differentiate cholangiocarcinoma from HCC (Chen 2010; Vilana 2010). If the characteristic dynamic profile is not recognized by imaging techniques, it is recommended that the diagnosis be based on fine needle biopsy (Fig. 80.3; see Chapter 20). This is so even in the presence of increased AFP values, because cholangiocarcinoma and hepatic metastases may produce AFP (Bruix & Sherman, 2011; Rimola et al, 2009). It must be stressed that biopsy is not 100% sensitive, hence a negative result does not exclude HCC (Forner et al, 2008). Tumor staging is done through spiral CT and MRI (see Chapters 13, 16, and 17); MRI is the most sensitive to detect nodules smaller than 2 cm (Burrel et al, 2003). The technical advances in these techniques have made angiography unnecessary for diagnosis and staging (see Chapter 19). Other tumor markers, such as protein induced by vitamin K absence (Marrero et al, 2009), glypican-3 (Capurro et al, 2003), and AFP fractions (Marrero et al, 2009), have been proposed to be used alone or in combination with AFP, but their usefulness in routine clinical practice has not been established (Bruix & Sherman, 2011).
Box 80.1
Diagnostic Criteria for Hepatocellular Carcinoma
Noninvasive criteria (cirrhotic patients)
Focal lesion >2 cm: one imaging technique (e.g., dynamic computed tomography, magnetic resonance imaging) with arterial hypervascularization and venous washout
From Bruix J, Sherman M, 2011: Management of hepatocellular carcinoma: an update. Hepatology 53:1020-1022.
Staging and Prognostic Evaluation
Staging systems are crucial tools to stratify patients into different subgroups according to prognosis. Ideally, they should establish a link with treatment and predict outcome after therapy. A well-validated and internationally accepted staging system is lacking for HCC. In most tumors, the prognosis is related mainly to tumor stage at the time of diagnosis. The presence of underlying liver disease makes prognostic evaluation of patients with HCC more complex, because liver disease influences outcome and the possibility of treatment (see Chapter 2, Chapter 70A, Chapter 70B ). This must take into account tumor stage and liver function and explains why one-dimensional systems taking into account one of the two aspects have no power to predict outcome or to establish the best treatment (Forner et al, 2010). This is the case for the tumor-node-metastasis (TNM) system used by the American Joint Commission on Cancer (2002) and the Child-Turcotte-Pugh (CTP) classification (Pugh et al, 1973). Despite several modifications over time, the TNM system is still not valid, because it relies predominantly on pathologic findings. Combination with a rough assessment of the underlying liver status (cirrhosis versus no cirrhosis; Vauthey et al, 2002) offers some advantages, but it still lacks accuracy to predict prognosis in nonsurgical cases.
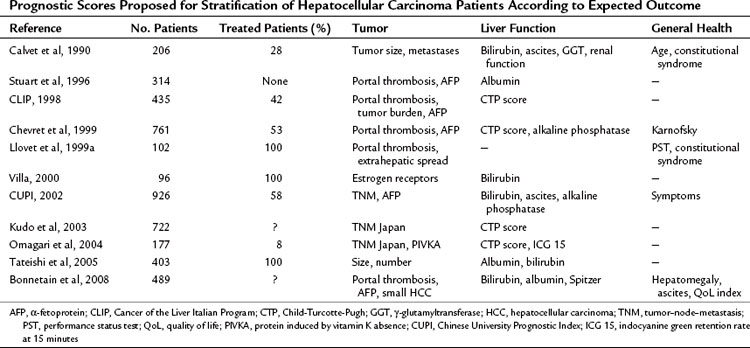
The Okuda classification is based on rough assessment of tumor burden, either on imaging or by surgery, and liver function. It stratifies patients into three groups with different outcomes (Okuda et al, 1985), but its main capacity is to identify end-stage patients. Several additional scoring systems have been proposed in Uganda (Primack et al, 1975), the United States (Stuart et al, 1996), Barcelona (Calvet et al, 1990), France (Bonnetain et al, 2008; Chevret et al, 1999), Italy (1998), Austria (Schoniger-Hekele et al, 2001), Hong Kong (Leung et al, 2002), and Japan (Kudo et al, 2003; Omagari et al, 2004; Tateishi et al, 2005; Table 80.2). The relevant prognostic parameters are not the same in all systems, and despite their capacity to stratify subjects, none of them has shown transportability; that is, strata of patients do not have the same survival in different settings. The major usefulness of these systems is the identification of end-stage patients with a poor prognosis, but none of these systems provides any link to treatment indication. This lack of reproducibility indicates major heterogeneity among the different studies, and no widely accepted universal scoring and staging system is currently available.
Table 80.2 Prognostic Scores Proposed for Stratification of Hepatocellular Carcinoma Patients According to Expected Outcome
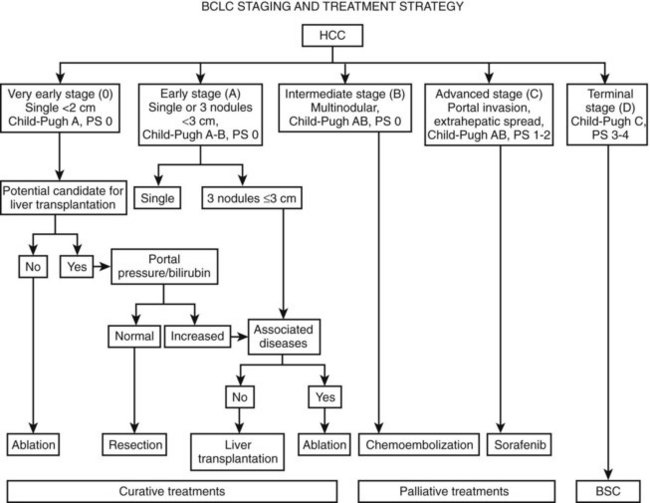
The Barcelona Clinic Liver Cancer (BCLC) Staging Strategy avoids the use of a single scoring system for all patients. Instead, it divides patients into four major groups: 1) very early/early, 2) intermediate, 3) advanced, and 4) end stage. It then develops a prognostic model within each group, taking into account the impact of treatment (Fig. 80.4; Forner et al, 2010, 2012; Llovet et al, 1999a). This model was constructed based on the results obtained in the setting of several cohort studies and randomized controlled trials. The proposal constitutes a regularly updated staging classification derived from the combination of the data of several independent prognostic studies in different disease stages and treatments. Because it links staging with treatment indication, it has become a widely used clinical tool for treatment decision making (Bruix & Sherman, 2011; Llovet et al, 2008b). It considers variables related to tumor stage, liver functional status, physical status, and cancer-related symptoms. Patients at an early stage present with solitary tumors (usually <5 cm) or two or three nodules (none >3 cm). Depending on the degree of liver function impairment, patients may benefit from curative therapies, such as resection, transplantation, or percutaneous ablation (see Chapter 83, Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D, Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E, Chapter 90F, Chapter 97D ). The 5-year survival ranges from 50% to 70%. The intermediate stage comprises patients with large or multifocal tumors with preserved liver function and no cancer-related symptoms or vascular invasion. The only treatment that has shown survival benefit is transarterial chemoembolization, which achieved a 50% survival at 3 years. Patients at an advanced stage have cancer-related symptoms, vascular invasion, or extrahepatic spread, and their median survival is less than 1 year. They may benefit from sorafenib treatment, the sole agent that has been confirmed to improve the survival of HCC patients (see Chapter 88). Finally, end-stage patients have major impairment of liver function or major cancer-related symptoms with severe deterioration of physical condition as reflected by a performance status (PS) greater than 2. Their short-term prognosis is poor, and they should receive palliative care (Forner et al, 2010).
Treatment Strategy
The selection of the best treatment in patients with HCC is the result of the evaluation of several factors, the most relevant among which are the status of the underlying liver and the tumor stage. Accurate definition of the status of the nontumor liver is crucial. The few patients in whom HCC appears in a noncirrhotic liver are good candidates for liver resection (see Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E, Chapter 90F ). Major lobectomies are well tolerated, and resection is the first-line option (Llovet et al, 2005). By contrast, in most patients, cirrhosis underlies HCC, and the degree of functional impairment may preclude safe surgery. In these patients, the first-line treatment should be liver transplantation (see Chapter 97D); if this is not feasible, these patients should be considered for percutaneous ablation through any of the available techniques: ethanol injection, radiofrequency, cryotherapy, or microwave (see Chapter 83, Chapter 84A, Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D ). These options are thought to offer potential long-term cure (Forner et al, 2010). The sole palliative approaches that have been shown to have a positive impact in survival are transarterial chemoembolization (TACE) (Forner 2012; Llovet & Bruix, 2003; see Chapter 83) and sorafenib administration (Cheng et al, 2009; Llovet et al, 2008a; see Chapter 88). Most of these approaches are discussed in other chapters; we briefly discuss the Barcelona strategy to select the best option for each patient (see Fig. 80.4).
Liver function is assessed initially through the CTP classification, and this is combined with a detailed evaluation of tumor extent (Forner et al, 2010; see Chapter 2, Chapter 70A, Chapter 70B ). This assessment has to search for the presence of daughter nodules, vascular invasion, extrahepatic disease, and portal vein thrombosis. Extrahepatic disease is ruled out by chest CT scan and bone scintigraphy. The general condition of the patient not only includes consideration of comorbid conditions that may increase perioperative or intervention-related morbidity and mortality but also the assessment of cancer-related symptoms as reflected by performance status (Sorensen et al, 1993). This provides an evaluation similar to the Karnofsky index (Schag et al, 1984) but simpler. Heavily affected patients (PS 3 to 4) belong in a terminal disease stage and should receive palliative treatment.
Liver resection (see Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E, Chapter 90F ) is the first-line treatment in patients with solitary tumors who do not present with underlying cirrhosis and in patients with HCC in well-compensated cirrhosis (Belghiti et al, 2002; Forner et al, 2010). This status cannot be assessed through the CTP classification. Only patients with normal bilirubin concentration and an absence of clinically relevant portal hypertension should be considered optimal candidates for resection (Llovet et al, 1999b). These patients tolerate the intervention without risk of hepatic decompensation, and survival at 5 years is approximately 70%. Hepatic vein catheterization is the most accurate tool to measure portal pressure, and a hepatic pressure gradient less than 10 mm Hg is the best cutoff value. Patients with portal hypertension are at high risk of liver decompensation and death after surgery, and their survival at 5 years drops to 50% (Ishizawa et al, 2008; Llovet et al, 1999b).
Liver transplant (see Chapter 97A, Chapter 97B, Chapter 97C, Chapter 97D, Chapter 97E ) is offered to patients who do not fit the optimal profile for resection, and the best candidates are those with solitary tumors less than 5 cm, or those with two or three nodules, each less than 3 cm (Mazzaferro et al, 1996). Vascular invasion and extrahepatic dissemination should be ruled out, and patients should not have general contraindications for liver transplantation. Percutaneous treatment is proposed if expected waiting time exceeds 6 months, and all patients also are proposed to enter the live donor program (Sala et al, 2004b; see Chapters 97D and 98B).
If patients are not candidates for surgery, they are offered percutaneous ablation (Forner et al, 2010). Radiofrequency ablation (RFA) is the main technique, but ethanol injection can be used in the presence of contraindications for RFA, such as tumor in a subcapsular location or in the vicinity of the gallbladder or heart. The best results of ablation are achieved in solitary tumors smaller than 2 cm, where these techniques may achieve complete necrosis, and recurrence rates are similar to resection in 90% of the cases (Livraghi et al, 2008; Sala et al, 2004a). Accordingly, ablation may compete with resection in patients with solitary nodules smaller than 2 cm and it has become the preferred treatment option in patients with HCC at a very early stage who are not potential candidates for liver transplantation (Forner et al, 2012).
Patients with more advanced disease (large or multifocal HCC) without portal vein invasion are candidates for TACE (see Chapter 83), if liver function is preserved, and they are still free of cancer-related symptoms. Response to this locoregional approach is associated with improved survival, and it is the sole locoregional palliative therapy that has a statistically significant impact on outcome at this stage of the disease (Llovet & Bruix, 2003; Llovet et al, 2002; Lo et al, 2002). If chemoembolization is not feasible, but patients still have preserved liver function (CTP class A or B) without major cancer-related symptoms, they should be considered for sorafenib administration (see Chapter 88). Current trials explore the combination of other molecular therapies with sorafenib and the usefulness of second-line alternatives. If patients are diagnosed at a terminal stage, identified by severe liver function deterioration (CTP class C), and the extent of the tumor prevents transplantation and has induced severe cancer-related symptoms (performance status >2), patients are offered palliative care.
Future Prospects
Major research in recent years has been devoted to allowing an earlier diagnosis of HCC and more accurate staging at the time of diagnosis. The improvements in treatment have had a minor impact, because most patients still are diagnosed at an advanced stage, when there is no option for long-term cure. Most of the effective treatments available today should be considered invasive, and it is hoped that active laboratory research translates into new instruments to predict the biology of the tumor and identify new treatment targets. As happened years ago in breast cancer (Perou et al, 2000; Somiari et al, 2003; van de Vijver et al, 2002), prostate cancer (Dhanasekaran et al, 2001; Grubb et al, 2003), lung cancer (Yanagisawa et al, 2003), non-Hodgkin B lymphoma (Alizadeh et al, 2000), and melanoma (Bittner et al, 2000), this is now a reality in HCC; additional molecular profiling is evolving, using gene or protein profiling (Boyault et al, 2007; Chiang et al, 2008; Hoshida et al, 2008) that will further affect how we face diagnosis, evaluation, and treatment of patients with liver cancer (Hoshida et al, 2010; Villanueva et al, 2010).
Alizadeh AA, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503-511.
Arbuthnot P, Kew M. Hepatitis B virus and hepatocellular carcinoma. Int J Exp Pathol. 2001;82:77-100.
Belghiti J, et al. Resection of hepatocellular carcinoma: a European experience on 328 cases. Hepatogastroenterology. 2002;49:41-46.
Benvegnu L, et al. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744-749.
Bittner M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536-540.
Bolondi L, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251-259.
Bonnetain F, et al. Quality of life as a prognostic factor of overall survival in patients with advanced hepatocellular carcinoma: results from two French clinical trials. Qual Life Res. 2008;17:831-843.
Bosch FX, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16.
Boyault S, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42-52.
Brau N, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007;47:527-537.
Bravi F, et al. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007;46:430-435.
Brechot C. Pathogenesis of hepatitis B virus–related hepatocellular carcinoma: old and new paradigms. Gastroenterology. 2004;127:S56-S61.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236.
Bruix J, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430.
Bruix J, et al. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215-219.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
Bruno S, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579-587.
Burrel M, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034-1042.
Calle EE, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638.
Calvet X, et al. Prognostic factors of hepatocellular carcinoma in the West: a multivariate analysis in 206 patients. Hepatology. 1990;12:753-760.
Capurro M, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89-97.
Chang MH, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859.
Chen LD, et al. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol. 2010;20:743-753.
Cheng AL, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
Chevret S, et al. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31:133-141.
Chiang DY, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779-6788.
Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40:S5-S10.
1998 A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755.
Colombo M, Donato MF. Prevention of hepatocellular carcinoma. Semin Liver Dis. 2005;25:155-161.
Day CP. Non-alcoholic steatohepatitis (NASH): where are we now and where are we going? Gut. 2002;50:585-588.
Degos F, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47:131-136.
Dhanasekaran SM, et al. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822-826.
Di Bisceglie AM, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429-2441.
El-Serag H. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27-S34.
El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750.
El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462-2467.
Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-687.
Fattovich G, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50.
Ferber MJ, et al. Integrations of the hepatitis B virus (HBV) and human papillomavirus (HPV) into the human telomerase reverse transcriptase (hTERT) gene in liver and cervical cancers. Oncogene. 2003;22:3813-3820.
Ferlay J, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
Forner A, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104.
Forner A, et al. [Diagnosis and treatment of hepatocellular carcinoma]. Med Clin (Barc). 2009;132:272-287.
Forner A, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74.
Forner A, et al. Hepatocelullar carcinoma. Lancet. 2012. In press
Greene FL, American Joint Commission on Cancer, 2002: Cancer Staging Handbook. Chicago, AJCC
Grubb RL, et al. Signal pathway profiling of prostate cancer using reverse phase protein arrays. Proteomics. 2003;3:2142-2146.
Hoshida Y, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995-2004.
Hoshida Y, et al. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin Liver Dis. 2010;30:35-51.
Ishizawa T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916.
Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215.
Kuper H, et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498-502.
Leung TW, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769.
Liaw YF, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531.
Livraghi T, et al. Sustained complete response and complications rate after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82-89.
Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429-442.
Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440.
Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181-200.
Llovet JM, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
Llovet JM, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711.
Lo CM, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171.
Marchesini G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923.
Marrero JA, et al. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218-224.
Marrero JA, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110-118.
Mazzaferro V, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699.
Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87-S96.
Okuda K, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment: study of 850 patients. Cancer. 1985;56:918-928.
Omagari K, et al. Preliminary analysis of a newly proposed prognostic scoring system (SLiDe score) for hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:805-811.
Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747-752.
Pollicino T, et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126:102-110.
Primack A, et al. A staging system for hepatocellular carcinoma: prognostic factors in Ugandan patients. Cancer. 1975;35:1357-1364.
Pugh RN, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649.
Puoti M, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS. 2004;18:2285-2293.
Regimbeau JM, et al. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10:S69-S73.
Rimola J, et al. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50:791-798.
Ryu WS. Molecular aspects of hepatitis B viral infection and the viral carcinogenesis. J Biochem Mol Biol. 2003;36:138-143.
Sala M, Varela M, Bruix J. Selection of candidates with HCC for transplantation in the MELD era. Liver Transpl. 2004;10:S4-S9.
Sala M, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352-1360.
Sangiovanni A, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005-1014.
Sangiovanni A, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310.
Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187-193.
Schöniger-Hekele M, et al. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut. 2001;48:103-109.
Somiari RI, et al. High-throughput proteomic analysis of human infiltrating ductal carcinoma of the breast. Proteomics. 2003;3:1863-1873.
Sorensen JB, et al. Performance status assessment in cancer patients: an inter-observer variability study. Br J Cancer. 1993;67:773-775.
Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217-2222.
Tanaka Y, et al. Inaugural article: a comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci U S A. 2002;99:15584-15589.
Tateishi R, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419-425.
Trevisani F, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575.
van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999-2009.
Vauthey JN, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527-1536.
Vibert E, et al. Liver transplantation for hepatocellular carcinoma: the impact of human immunodeficiency virus infection. Hepatology. 2011;53:475-482.
Vilana R, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51(6):2020-2029.
Villa E, et al. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32:233-238.
Villanueva A, et al. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55-76.
Villanueva A, et al. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis and therapy. Annu Rev Med. 2010;61:317-328.
Yanagisawa K, et al. Proteomic patterns of tumour subsets in non–small-cell lung cancer. Lancet. 2003;362:433-439.
Zhang B, Yang B. Combined alpha-fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6:108-110.