Chapter 83 Hepatic artery embolization and chemoembolization of liver tumors
Overview
Primary and secondary liver tumors cause tremendous neoplastic morbidity and mortality. Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most common cause of cancer-related death in the world (Bosch et al, 2005). The highest incidences of HCC are found in Eastern Asia and sub-Saharan Africa, where hepatitis B is endemic; however, the incidence of HCC has been increasing steadily in the United States and Western Europe, largely owing to increased prevalence of viral hepatitis C in the past 2 decades (El-Serag et al, 2003). The majority of metastatic cancers involve the liver at some point as disease progresses. Despite the presence of extrahepatic spread, many patients with liver involvement die as a consequence of local tumor growth and hepatic parenchymal destruction.
Basic Principles of Hepatic Arterial Embolotherapy
Blood Supply of Liver Tumors
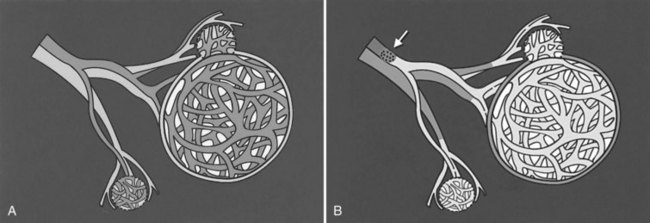
The basic physiologic principle that makes HAE feasible in patients with liver tumors is the dual blood supply to the liver (see Chapter 1B). The portal vein provides more than 75% of the blood flow to the normal hepatic parenchyma and is the primary trophic blood supply. Conversely, most of the blood supply (90% to 100%) to liver tumors comes from the hepatic artery (Breedis & Young, 1954), thus embolization of tumor-feeding arteries leads to selective ischemic damage of the tumor, while sparing the normal liver parenchyma, mainly supplied by the portal vein. Moreover, the pharmacokinetic advantage of locoregional drug administration enhances the theoretic benefit. For example, hepatic drug exposure has been estimated to be double for doxorubicin, 7 times greater for cisplatin, 8 times greater for mitomycin, 10 times greater for 5-fluorouracil, and up to 400 times higher for 5-fluorodeoxyuridine, when these were given through the hepatic artery rather than through the systemic veins (Ensminger & Gyves, 1983; see Chapter 86).
Hepatocarcinogenesis is a multistep process that causes gradual arterialization in blood supply to tumors (Pang & Poon, 2006), therefore the blood supply of liver tumors can be variable, according to the carcinogenetic stage of the tumors. For example, encapsulated nodular HCC is almost exclusively supplied by the hepatic artery, and well-differentiated HCC and the extracapsular infiltrating edge of advanced HCC can be supplied by the portal vein or both the portal vein and the hepatic artery (Goseki et al, 1995). Similar observations have been made in metastatic liver tumors; in its early stage, less than 200 µm is supplied almost exclusively by sinusoidal blood. As the tumor grows, its blood supply becomes progressively arterialized, but even in the advanced stage, most liver metastases still have a distinct portal blood supply (Strohmeyer et al, 1987); therefore early stage tumors and some fraction of advanced tumors may be resistant to hepatic arterial embolotherapy.
Chemoembolization
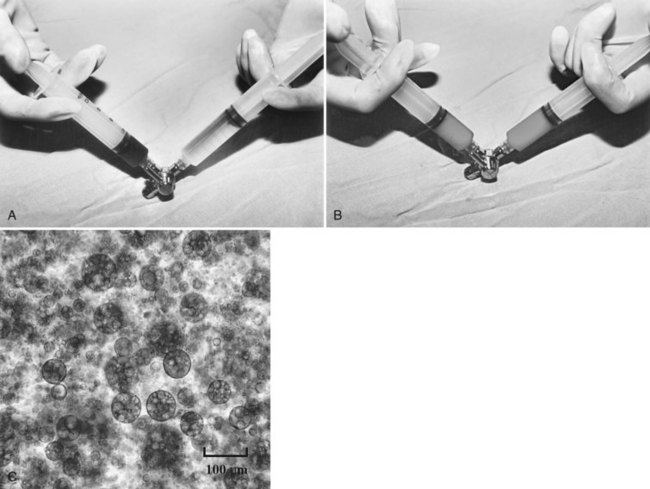
In the past 3 decades, many papers have been published describing a myriad different techniques for performing HAE; combined with intraarterial chemotherapy (chemoembolization), HAE has been paramount among them as the most widely used procedure. The goal of chemoembolization is to combine the effects of targeted tumor ischemia by embolization with intraarterial chemotherapy. To date, the most common sole-agent chemotherapeutic drug has been doxorubicin, followed by cisplatin, epirubicin, mitoxantrone, mitomycin C, and a chemical conjugate of a synthetic copolymer of styrene maleic acid and neocarcinostatin (SMANCS) (Marelli et al, 2007). The chemotherapeutic drug is dissolved in water or water-soluble contrast agent and is then mixed with iodized oil (Lipiodol; Laboratoire Guerbet, France) and administered as an emulsion (Fig. 83.1). When injected into the hepatic artery, iodized oil is trapped selectively in the tumor because of the hemodynamic difference between the tumor and normal liver parenchyma and presumably the absence of Kupffer cells in the tumor (Kan et al, 1993; Nakajo et al, 1988). Thus mixing iodized oil with a chemotherapeutic drug allows slow release of the chemotherapeutic drug over a period of 6 to 12 weeks (Raoul et al, 1992). In contrast, in the normal liver parenchyma, the iodized oil does not occlude the hepatic artery; rather it accumulates in the peripheral portal vein through arterioportal communications and subsequently passes through sinusoids into the systemic circulation (Chung et al, 1993). HAE after infusion of chemotherapeutic drugs increases drug dwelling time in the tumor by slowing the rate of efflux from the hepatic circulation. Furthermore, ischemic damage by embolization potentiates absorption of chemotherapeutic drugs by impeding the function of transmembrane pumps in tumor cells (Yu & Keeffe, 2003).
After infusion of the chemotherapeutic drug and iodized oil mixture, tumor-feeding peripheral hepatic arteries are embolized. Proximal arterial occlusion is not desirable, because it will not only induce development of intrahepatic and extrahepatic collateral vessels, it will also preclude repeat procedure. Thus it is important to select embolic materials of the proper size. The optimal size should be small enough to reach and occlude the capillaries to the tumor but bigger than the arteriovenous shunt and peribiliary plexus, to avoid the risk of pulmonary embolization and bile duct necrosis. To date, gelatin sponge particles have been the most frequently used agent, but polyvinyl alcohol (PVA) particle (Ramsey et al, 2002), absolute ethanol (Matsui et al, 1993), starch microspheres (Maluccio et al, 2008), cyanoacrylate (Berghammer et al, 1998), and even autologous blood clot (Gunji et al, 2002) have also been used.
Gelfoam is used as 500- to 1000-µm cubes, which only occludes the artery temporarily, with recanalization taking place within 2 weeks (Coldwell et al, 1994). It was proved not to cause serious hepatic damage in patients with good hepatic functional reserve (Kan et al, 1993; Sonomura et al, 1997); however, gelfoam powder should not be used, as it may cause biliary stenosis and biloma resulting from embolization and necrosis of small arteriolar branches, which may cause biliary damage (Makuuchi et al, 1985). PVA particles cause a permanent or semipermanent arterial occlusion and can achieve more distal obstruction because of their smaller size (50 to 250 µm in diameter; Coldwell et al, 1994). Autologous blood clot achieves the same temporary artery occlusion as a gelatin sponge. Because the clot is lysed faster after embolization, there might be less chance of arterial thrombosis after several sessions of chemoembolization (Gunji et al, 2002). In a recent comparative nonrandomized study, no difference in patient survival was found between chemoembolization using gelatin sponge particles and that using PVA particles (Brown et al, 2005).
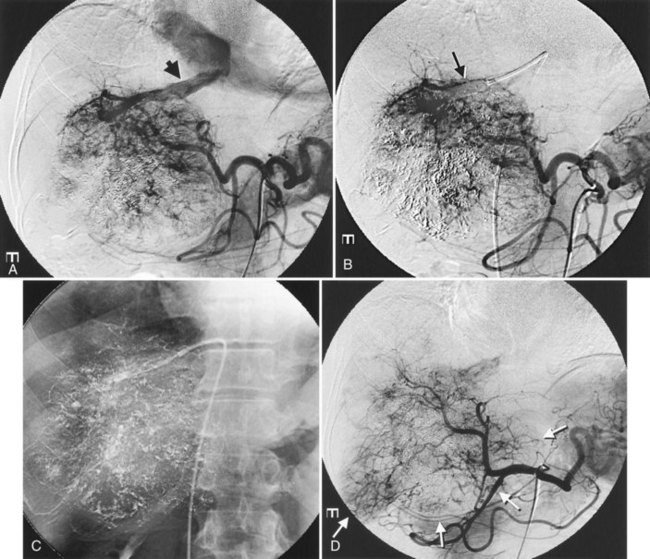
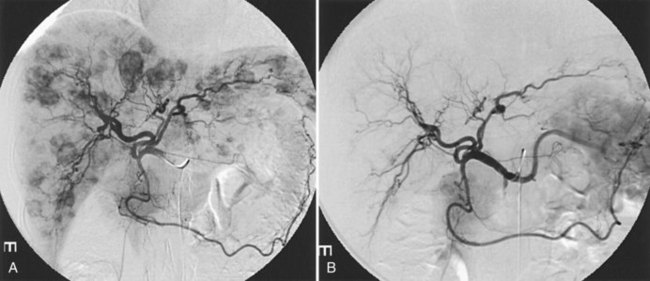
It is important to evaluate tumor-related arteriovenous shunt for safe procedures. In patients with HCC, angiographic incidence of shunting has been reported in 31.2%, shunting into the portal vein has been reported in 28.8%, and hepatic vein shunting has been reported in 2.4% (Ngan & Peh, 1997). The more liver parenchyma is occupied by the tumor, the more arteriovenous shunts exist; these are associated with poor prognosis (Granov et al, 1992) and are frequently problematic in chemoembolization of liver tumors (Izaki et al, 2004). Severe arterioportal shunt can cause hepatofugal portal flow, ascites, and variceal bleeding. In patients with a prominent arteriovenous shunt, embolization of the shunt is recommended prior to HAE (Huang et al, 2004). After embolization for massive arterioportal shunt, hepatofugal portal flow may convert to hepatopetal flow, and performance status and ascites may be improved (Izaki et al, 2004).
Patient Selection
Chemoembolization is indicated for the treatment of unresectable hypervascular liver tumors, which receive more blood supply from the hepatic artery than normal liver parenchyma, which makes the tumor suitable to HAE. The hemodynamic characteristics of a tumor may be documented on contrast-enhanced ultrasonography (Suzuki et al, 2003), computed tomography (CT) (Tajima et al, 2002b), and magnetic resonance imaging (MRI) (Levy, 2002).
The most common indication of chemoembolization is unresectable HCC. The determination of resectability of HCC should be based on either the extent of tumor involvement, underlying liver function, or a combination of these (see Chapters 2 and 90A). The majority of patients with HCC have underlying liver cirrhosis, which often requires a larger remnant liver after surgery to maintain hepatic function than in patients without underlying liver disease; therefore tumors that might be resectable in patients with normal liver parenchyma may not be resectable in patients with cirrhosis. Hepatic dysfunction also acts as a limitation in selecting patients for chemoembolization. Patients with poor hepatic function are not likely to tolerate extensive arterial embolization, because their livers are more dependent on arterial blood supply than normal liver, and moreover, patients with severe cirrhosis are more likely to die of their underlying liver disease than of HCC. Thus chemoembolization is typically recommended in patients with reasonably preserved liver function (Child-Turcotte-Pugh [CTP] class of A or B) and performance status (Eastern Cooperative Oncology Group [ECOG] 0 or 1). In general, patients with class C cirrhosis should not undergo chemoembolization as a primary treatment.
The extent of tumor is another important consideration in selecting patients for chemoembolization. Several methods have been proposed to weigh both hepatic function and the extent of tumor. In Okuda staging, patients are given points for presence of ascites, serum bilirubin and albumin levels, and the extent of tumor, and the patient is staged as I, II, or III (Okuda et al, 1985). The Cancer of the Liver Italian Program (CLIP) group proposed a more complex scoring system that includes the CTP score, tumor morphology and extension, presence of portal vein thrombosis, and serum α-fetoprotein (AFP) levels. The CLIP score was validated worldwide in multiple clinical trials and was proven to give more accurate prognostic information and greater survival predictive power than the Okuda staging system (CLIP, 2000; Ueno et al, 2001); however, those staging systems for predicting prognosis of patients with HCC did not indicate which patients would benefit from HAE. Recently, the Barcelona Clinic Liver Cancer (BCLC) staging system was developed to allow for the indication of the best therapy for each stage of HCC (Llovet et al, 1999a). In this staging system, chemoembolization is recommended as first-line therapy for intermediate stage HCC (Okuda staging 1 to 2, performance score 0, and large or multinodular disease) and some advanced-stage HCC (Okuda 1 to 2, performance score 1, no extrahepatic disease).
Spontaneous rupture of HCC is an indication for emergency HAE, regardless of underlying liver function (Kirikoshi et al, 2009). Even in patients with advanced liver cirrhosis, nodular HCC showing exophytic growth can be managed safely by selective embolization to prevent tumor rupture without diminishing liver function. Chemoembolization plays additional neoadjuvant roles as a downstaging therapy before resection or as a bridge therapy for patients awaiting liver transplantation (see Chapter 97D).
Although no absolute contraindications to HAE exist, patients with two or three major poor prognostic indicators should not be treated. Generally accepted relative contraindications are extensive tumor involvement of more than 50% to 75% of the liver and class C cirrhosis. Major portal vein invasion by the tumor has been considered another relative contraindication, but this can be safely and effectively managed by an adjustment of the embolization protocol to reduce the amount of chemoembolic agents and the extent of the embolization, especially in patients with a limited parenchymal tumor and adequate liver function (Chung et al, 1995). Recent evidence demonstrates that HAE can be safely performed, even in patients with main portal vein occlusion, if hepatopetal collateral flow is present (Lee et al, 1997; Tazawa et al, 2001; Georgiades et al, 2005). In addition, active gastrointestinal bleeding, refractory ascites, extrahepatic spread, hepatic encephalopathy, and biliary obstruction are also considered relative contraindications. Regardless of the liver tumors, anaphylactoid reaction to contrast media or renal insufficiency, uncorrectable coagulopathy, and severe peripheral vascular disease can preclude embolotherapy.
Procedure
Before any chemoembolization procedure, laboratory tests should be done that include a complete blood cell count, prothrombin time, creatinine levels, and liver function testing. The baseline serum levels of tumor markers should be measured before treatment to monitor changes after treatment. By means of cross-sectional imaging studies, the size and extent of the tumor, its growth pattern (expansile vs. replacing or infiltrating), and macroscopic angioinvasion into the hepatic or portal veins are evaluated. For successful chemoembolization, accurate segmental localization of the tumor is crucial, and cross-sectional images are useful for that purpose (Yoon et al, 2008). In addition, imaging studies of the chest, abdomen, and pelvis are recommended to assess comorbid disease and ensure the presence or absence of metastatic disease.
Patients are fasted overnight and hydrated with normal saline at a rate of 200 to 300 mL/h. Antiemetics are administered intravenously, and patients with contrast allergies receive oral prednisone 1 hour before the procedure. The use of prophylactic antibiotics is controversial except in patients with a bilioenteric anastomosis or biliary stent (Geschwind et al, 2002; Ong et al, 2004; Tarazov, 1995). After infiltration of local anesthetic, the Seldinger technique is used to gain access to the common femoral artery, and initial diagnostic visceral arteriography is performed to determine arterial anatomy to the liver and patency of the portal vein. For complete angiography, all hepatic arteries should be adequately opacified, and all feeding arteries of the tumor should be identified.
Anatomic variations of celiac trunk and hepatic arteries are commonly encountered. With the introduction of multidetector row CT, these anatomic variations can be predicted before the procedure by careful review of the arterial phase scan (Song et al, 2010). Most common hepatic artery variations are the right hepatic artery arising from the superior mesenteric artery and the left hepatic artery arising from the left gastric artery, thus celiac and superior mesenteric arteriography is mandatory to identify the variations. Frequently, additional views in different angles and magnifications and selective contrast injection with use of a microcatheter are necessary to identify small feeding arteries (Yoon et al, 2008). To avoid nontargeted embolization, it is important to recognize the origin of the cystic artery and the right gastric artery, presence of a large falciform artery, and the accessory left gastric artery originating from the left hepatic artery (Song et al, 2006).
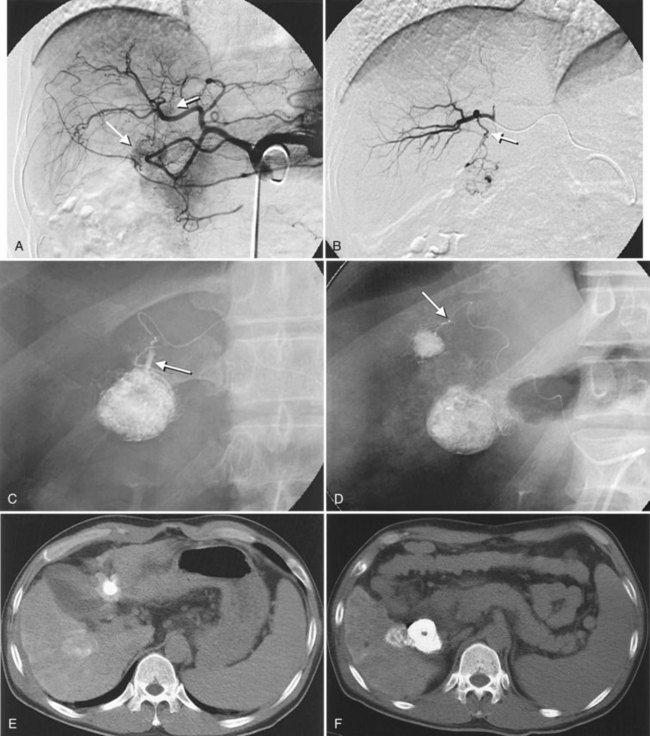
After the catheter is positioned appropriately, the mixture of iodized oil and chemotherapeutic drug is injected (Figs. 83.2 and 83.3). If there is a prominent arteriovenous shunt that precludes chemoembolic agents reaching the tumor vasculature, it is recommended first to embolize the shunt before injection of chemotherapeutic drugs (Fig. 83.4). The dose of iodized oil and chemotherapeutic agent depends on the size and vascularity of the tumor. The generally accepted upper limit of iodized oil is 15 mL. For a small tumor, enough iodized oil to saturate the entire tumor neovasculature could be administered. The end point for the mixture administration is stasis in tumor-feeding arteries or appearance of iodized oil in portal vein branches (see Fig. 83.3). If flow is a persistent in the tumor-feeding arteries after infusion of chemotherapeutic drug, HAE should be performed. Gelatin sponge particles are the embolic agents most frequently used, because they degrade in a few weeks, allowing repeat treatment. PVA particle is another commonly used embolic agent, which causes a more permanent arterial occlusion (Ramsey et al, 2002).
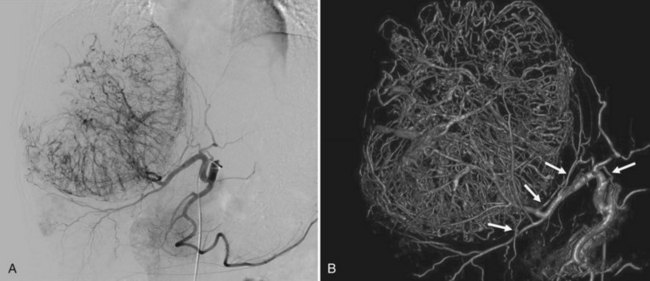
Embolization of extrahepatic collaterals supplying the tumors is crucial to achieve successful outcomes. When a tumor is adjacent to a hepatic bare area or suspensory ligaments, or it invades into an adjacent organ, selective arteriogram of possible extrahepatic arteries should be obtained. With recent advances in CT imaging, those collateral vessels can be detected in preprocedure imaging studies (Kim et al, 2005). Common extrahepatic collaterals include the inferior phrenic artery, omental artery, internal mammary artery, colic branch of superior mesenteric artery, adrenal artery, intercostal artery, renal capsular artery, and gastric arteries (Chung et al, 2006; Kim et al, 2005, 2007, 2009; Won et al, 2003; Figs. 83.5 and 83.6). When the hepatic artery and extrahepatic collaterals supply the tumor, additional chemoembolization of the extrahepatic collaterals can be tried to increase the therapeutic efficacy of chemoembolization. These extrahepatic collaterals can be used as an access route to the tumors in patients with hepatic artery occlusion.
Recently, the use of C-arm cone-beam CT has been increasing. It can provide information about a patient’s vascular anatomy, associated liver parenchyma, and the target lesion that constitutes a substantial improvement over conventional digital-subtraction angiography (DSA) and fluoroscopy (Carlson et al, 2001). C-arm cone-beam CT enables more selective catheterizations, which increases the amount of therapeutic agent delivered to the target area and decreases nontumorous liver parenchymal exposure to the agent (Meyer et al, 2007; Wallace et al, 2008; Fig. 83.7) In addition, confident identification of extrahepatic arteries supplying nontarget organs, such as supraduodenal or retroduodenal arteries, right gastric artery, phrenic artery, and falciform artery, is crucial to avoid nontarget embolization (Kim et al, 2009; Liu et al, 2005). When feeding arteries for a tumor are multiple, the portion of the tumor supplied by a catheterized feeding artery can be estimated, and the chemoembolic regimen can be proportioned accordingly. Iodized oil preferentially accumulated within tumors can be imaged without use of additional contrast media; thus completeness of therapy can be assessed immediately after treatment. If the oil has not accumulated in a portion of the tumor, the operator can search for hepatic or extrahepatic collateral tumor supply (Meyer et al, 2007; Wallace et al, 2007).
Intraarterial lidocaine and intravenous narcotic analgesics are given before the procedure to relieve pain during and after (Lee et al, 2001b). After the procedure, hydration is continued with normal saline. Narcotic analgesics, chlorpromazine, and acetaminophen are additionally supplied for 1 to 3 days as needed. Patients can be discharged once they have resumed adequate oral intake, and intravenous analgesics are no longer required.
Laboratory studies of liver function and serum levels of tumor markers should be repeated 2 to 4 weeks after the procedure. Multiphase contrast-enhanced CT or MRI is recommended 4 to 8 weeks after the procedure to evaluate the efficacy of the treatment and to detect local or remote tumor recurrences. If viable tumor is evident on imaging studies, serum levels of tumor markers are elevated, or both, patients return for another session of embolotherapy according to their hepatic functional recovery. No consensus has been reached on the ideal protocol for repeat procedures, but based on a retrospective comparison, survival rates and patient tolerance seemed to be higher when treatment was repeated, but only when progression of HCC was noted radiographically (Ernst et al, 1999).
Therapeutic Efficacy of Chemoembolization
Hepatocellular Carcinoma
Numerous studies have shown that chemoembolization induces a significant tumor necrosis without negative influence on liver function in patients with preserved liver function. The extent of tumor necrosis has been reported to range from 60% to 100%. With introduction of subsegmental chemoembolization using a microcatheter, the tumor necrosis rate was markedly improved. Matsui and colleagues (1993) reported complete necrosis in about 70% of 100 small hypervascular HCCs less than 4 cm in diameter treated by subsegmental chemoembolization; however, especially in large tumors, even though necrosis may appear macroscopically complete, histologic examination reveals viable neoplastic cells responsible for the high rate of recurrence after treatment. During follow-up the residual tumor nests recover their blood supply, and the tumor continues to grow. Additional treatments may be administered, either at specified intervals or as needed, but no data plainly favors either strategy (Bruix et al, 2004). Several studies have suggested that tumor characteristics lead to favorable response after chemoembolization—including small, encapsulated, expansile, hypervascular tumors—and long-term retention of iodized oil may be seen on CT (Itsubo et al, 2002; Kuroda et al, 1991; Nakamura et al, 1993). Pathologically, trabecular-type HCC showed more prominent necrosis than scirrhous, compact, or well-differentiated HCC (Jinno et al, 1992).
Several case-control and retrospective studies have shown a survival benefit in patients treated with chemoembolization compared with untreated patients or historic control subjects (Aoyama et al, 1992; Bismuth et al, 1992; Bronowicki et al, 1994; Rose et al, 1999). Bronowicki and colleagues (1994) performed a case-control study that stratifed 254 patients according to CTP classification and Okuda staging. The survival rates of the chemoembolization group were significantly higher (64%, 38%, 27%, and 27% at 1, 2, 3, and 4 years, respectively) than those of the conservative management group (18%, 6%, and 5% at 1, 2, and 3 years, respectively). The survival benefit from chemoembolization was not confirmed in early randomized, controlled trials (RCTs) performed in the 1990s (Camma et al, 2002; Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire, 1995; Pelletier et al, 1990). However, two newer RCTs, one from Barcelona (Llovet et al, 2002) and one from Hong Kong (Lo et al, 2002), reported a significant positive impact of chemoembolization on patient survival. This permitted a positive result in a cumulative meta-analysis of all RCTs published from 1978 to 2002; thus, according to the guidelines published by the American Association for Study of Liver Diseases (AASLD) (Bruix & Sherman, 2005) and the European Association for the Study of the Liver (EASL) (Bruix et al, 2001), chemoembolization is recommended as first-line noncurative therapy for nonsurgical patients with large or multifocal HCC who do not have vascular invasion or extrahepatic spread. The improvement in survival in treated patients ranges from 20% to 60% at 2 years (Bruix et al, 2004), but it is clear that the relevance of the improvement, as compared with the outcome if untreated, is largely dependent on the patients’ baseline characteristics regarding tumor stage, liver function, and general health status (Llovet et al, 2003). In a recent prospective cohort study that included 8510 patients with unresectable HCC, the median survival rate was 34 months, with an overall survival at 1, 3, 5, and 7 years of 82%, 47%, 26%, and 16%, respectively (Takayasu et al, 2006).
Chemoembolization as a neoadjuvant therapy in candidates for hepatic resection is controversial (see Chapter 90A). Several retrospective studies reported a possible survival benefit in patients treated with chemoembolization before resection of HCC when compared with resection alone (Gerunda et al, 2000; Zhang et al, 2000). Zhang and colleagues (2000) retrospectively analyzed postoperative outcomes of 1457 HCC patients treated with hepatectomy and concluded that effective preoperative chemoembolization can improve disease-free survival after hepatectomy. On the contrary, in a randomized trial by Zhou and colleagues (2009), preoperative chemoembolization did not improve surgical outcomes, and no significant difference was found between the preoperative chemoembolization group and the control group in disease-free survival rates and overall survival rates. Moreover, the preoperative chemoembolization group had a lower resection rate and longer operative time. Choi and colleagues (2007) studied 273 patients who underwent curative resection for HCC; 120 of them underwent preoperative chemoembolization. The 1-, 3-, and 5-year disease-free survival rates were 76.0%, 57.7%, and 51.3%, respectively, in the chemoembolization group and 70.9%, 53.8%, and 46.8%, respectively, in the nonchemoembolization group. Although a difference was noted between two groups, it was not significant. Chemoembolization has also been performed to reduce tumor volume (downstaging) before hepatic resection. Majno and colleagues (1997) showed that downstaging (42%) or complete necrosis (50%) was achieved in 49 patients.
Orthotopic liver transplantation (OLT) has been shown to be the best treatment for early-stage HCC (see Chapter 97D); however, because of a steadily increasing waiting time, a noteworthy proportion of patients are excluded from OLT because of tumor progression. Llovet and colleagues (1999b) reported a 23% dropout rate as a result of tumor progression in the first 6 months of waiting for OLT without any neoadjuvant therapy. Chemoembolization has been performed as a bridge therapy prior to liver transplantation, to inhibit tumor growth while a patient awaits an organ, but no controlled study is available for comparing patients who underwent OLT for HCC with and without neoadjuvant chemoembolization. Four noncomparative studies reported dropout rates in patients who underwent chemoembolization that ranged from 0% to 46%. Other trials used chemoembolization prior to transplantation to prevent tumor dissemination during surgery, which may result in better posttransplantation outcomes (Decaens et al, 2005; Stockland et al, 2007), although the role of chemoembolization for this purpose is still being debated. In a case-control study that included 100 patients who received pretransplant chemoembolization and 100 control patients, pretransplant chemoembolization does not influence posttransplantation survival or disease-free survival (Decaens et al, 2005). Another purpose of pretransplantation chemoembolization is to downstage advanced HCC and expand selection criteria for liver transplantation. Chapman and colleagues (2008) performed pretransplantation chemoembolization in 76 patients with advanced HCC (American Liver Tumor Study Group stage III/IV). Of these 76 patients, 17 (22.4%) were successfully downstaged to qualify for OLT under the Milan criteria. Importantly, patients who are successfully downstaged and transplanted have excellent midterm disease-free and overall survival, similar to stage II HCC. Similarly, Yao and colleagues (2008) showed that successful downstaging of HCC can be achieved in the majority of carefully selected patients, and it is associated with excellent posttransplantation outcome.
Neuroendocrine Tumors (See Chapter 81B)
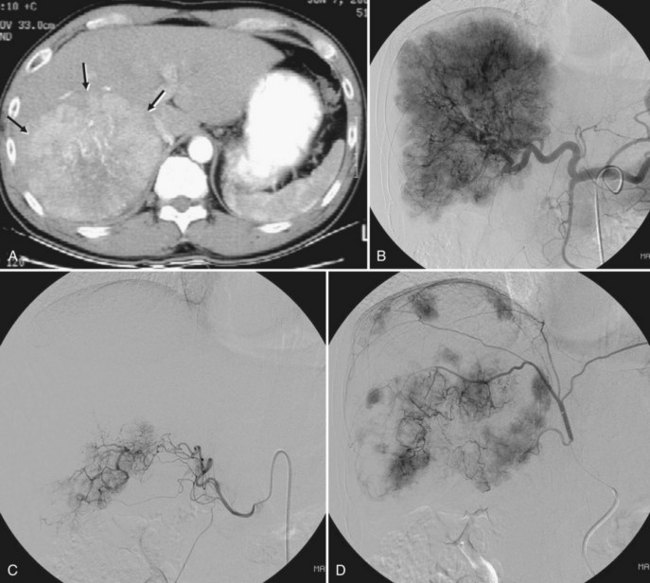
NETs are the most common secondary liver cancer, and they have also been managed with hepatic embolotherapy (Fig. 83.8). They are slow-growing tumors, frequently found when the disease has become metastatic. Curative surgery should always be considered as a first-line treatment, but less than 10% of patients have resectable tumors because of the diffuse nature of the disease. The development of liver metastasis causes synthesis and release of hormonal substances that lead to a constellation of symptoms, including rash, hypertension, diarrhea, and electrolyte disorders; these are collectively known as carcinoid syndrome. In the later stages of the disease, significant hepatomegaly by bulky metastatic tumors may cause progressive pain and dyspnea, and the primary goal of hepatic embolotherapy is to reduce liver tumor burden and palliate symptoms.
To date, partial or complete response after embolotherapy has ranged widely in reports, from 6.7% to 100%, and median survival has been reported from 13 to 80 months. Although many studies have reported median survival of more than 3 years, it is unclear whether embolotherapy significantly improves survival because of the indolent nature of the tumor; however, it is clear that outcomes are inversely related to the degree of liver replacement by tumor, and patients with carcinoid tumor have significantly prolonged survival compared with patients with islet cell tumor after embolization. Recently, Gupta and colleagues (2005) performed bland embolization or chemoembolization in 69 patients with carcinoid tumors and in 54 with pancreatic islet cell carcinomas. In patients with carcinoid tumors, bland embolization had a higher response rate than chemoembolization. In contrast, patients with islet cell carcinomas treated with chemoembolization had a prolonged overall survival time and improved response rate compared with those treated with bland embolization; this was not an unexpected result, because carcinoid tumors have a lower response rate to systemic chemotherapy than do islet cell tumors.
Sarcoma and Gastrointestinal Tumors (See Chapter 81C)
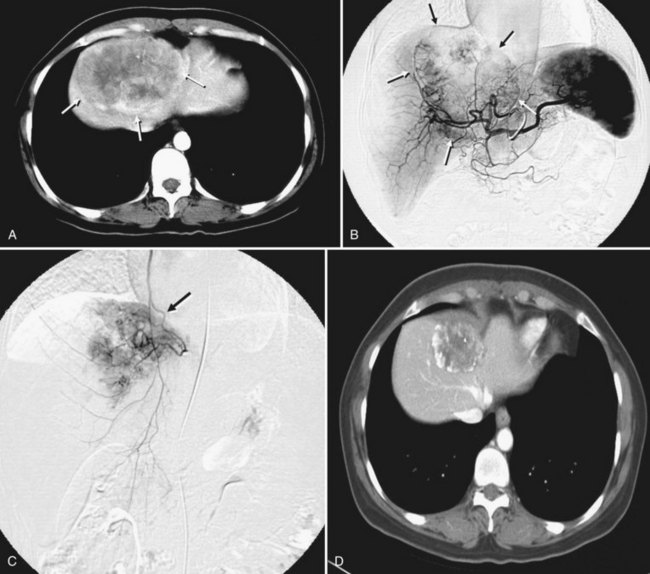
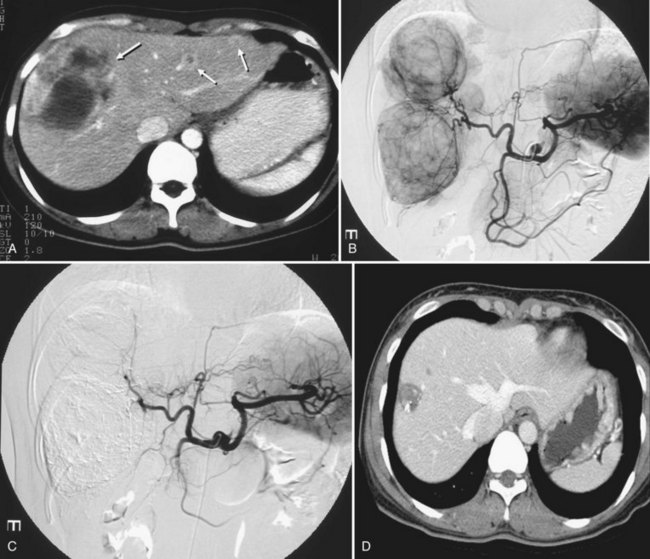
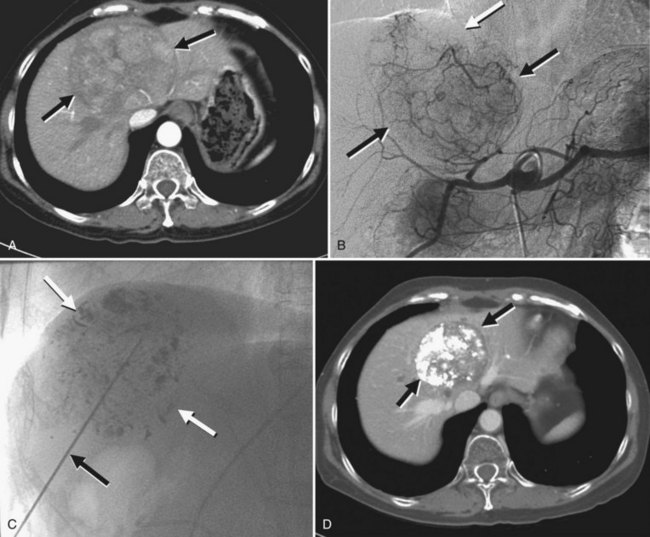
A small number of investigations have addressed hepatic embolotherapy for the treatment of sarcoma and GISTs. Most gastrointestinal soft-tissue neoplasms previously classified as leiomyomas, schwannomas, leiomyoblastomas, or leiomyosarcomas are today classified as GISTs on the basis of molecular and immunohistologic features. The liver is the most common site of metastasis from malignant GISTs. Surgical resection is the treatment of choice for a single metastasis, but the majority of patients with liver-dominant metastases have unresectable disease. Because this tumor is frequently hypervascular, hepatic embolotherapy has been an effective palliative treatment for unresectable liver metastases (Fig. 83.9). Mavligit and colleagues (1995) treated 15 patients with metastatic leiomyosarcoma with chemoembolization using PVA particle, cisplatin, and vinblastine, which produced a major tumor response—more than 50% regression—that lasted 8 to 31 months in 70% of patients. Similarly, in a small series by Rajan and colleagues (2001), partial response rate was 69% with a median survival of 13 months after chemoembolization. A more recent study that employed bland embolization demonstrated a radiologic response of 60% and a median survival of 24 months (Maluccio et al, 2006). A significant survival benefit was documented in patients with a radiologic response (median, 63 vs. 19 months). Because imatinib mesylate (Gleevec), a tyrosine kinase inhibitor, proved to be highly effective against GISTs (Demetri et al, 2002), hepatic embolotherapy is currently recommended for patients who failed therapy or became resistant to Gleevec.
Colorectal Metastases (See Chapter 81A)
Colon cancer is the second leading cause of cancer-related death in the Western world, and the liver is the commonest site of distant metastasis of colon cancer. Approximately 50% to 60% of patients with colorectal cancers will develop liver metastasis in their lifetime, and over half will die from their metastatic liver diseases. Surgical resection is the only potentially curative treatment for liver metastasis from colon cancer, but less than 30% of patients have resectable disease at initial evaluation (Fusai & Davidson, 2003; Meyers et al, 2003). Systemic chemotherapy remains the standard of care for this population.
Recent advances in chemotherapeutic drugs have resulted in improved survival, but overall median survival is still just more than 20 months. Bland hepatic artery embolization and chemoembolization have been used as a second-line therapy, when a liver tumor progresses during systemic chemotherapy. A limited number of series have documented that survival rates are significantly improved in selected patients with good performance status, no extrahepatic disease, and solitary lesions (Martinelli et al, 1994; Salman et al, 2002; Sanz-Altamira et al, 1997).
Preoperative chemoembolization has been proposed as a possible means of decreasing perioperative tumor dissemination and downstaging of tumor to allow resection, but only in a small number of patients. Vogl and colleagues (2003) suggested chemoembolization combined with ablation therapy as a viable treatment option. They performed sequential chemoembolization to reduce tumor size to 5 cm or less, and then treated tumors with laser-induced thermal ablation. This regimen resulted in a mean survival of more than 26 months, versus 12.8 months for patients treated by chemoembolization only.
Ocular Melanoma (See Chapter 81C)
Ocular melanoma is a rare but highly malignant disease with median survival of 2 to 6 months. Liver metastasis is found up to 40% of patients at initial evaluation, and a sole metastasis is found in more than 80% of patients. A few series used chemoembolization for local control of metastatic uveal melanoma (Feldman et al, 2004; Mavligit et al, 1988), and a retrospective study of 201 patients compared cisplatin-based chemoembolization with systemic chemotherapy and reported that chemoembolization produced a better response rate (36%) (Bedikian et al, 1995). These results were similar to those of a previous study that reported a response rate of 46% and a median survival of 11 months (Mavligit et al, 1988). Because most patients die of systemic disease, chemoembolization is unlikely to improve survival of patients.
Complications
Hepatic embolotherapy is associated with diverse complications. Procedure-related mortality rate is less than 5%, and the incidence of major complications has been reported to be less than 5%, including liver failure or infarction, liver abscess, biliary necrosis, and nontarget embolization (Chan et al, 2002; Ramsey et al, 2002; Song et al, 2001). Important predisposing factors for major complications are major portal vein obstruction, compromised hepatic functional reserve, biliary obstruction, previous biliary surgery, excessive use of iodized oil, and nonselective embolization (Chung et al, 1996). Recognition of predisposing factors before the procedure, selective embolization with an adequate amount of chemoembolic material, and careful postprocedure monitoring of patients is mandatory to prevent these complications.
Postembolization Syndrome
Postembolization syndrome (PES) occurs in approximately 80% of patients and consists of pain, fever, nausea, and vomiting. PES is more of a side effect than a complication of hepatic embolotherapy. The etiology of PES is not fully understood, but it is thought to be a type of tumor lysis syndrome. Abrupt tumor cell death by ischemic damage causes release of intracellular toxins into circulation, which may cause fever, nausea, and vomiting (Patel et al, 2000). Possible causes of pain are acute ischemia of liver parenchyma, distension of the liver capsule, and gallbladder ischemia secondary to inadvertent embolization of the cystic artery. Leung and colleagues (2001) reported that inadvertent gallbladder embolization and the dose of chemotherapeutic drug are associated with this syndrome. PES is treated with supportive management that includes antiemetics, analgesics, and antipyretics; it typically subsides after 1 to 3 days.
Liver Failure
The most serious complication of hepatic embolotherapy is hepatic insufficiency. In a prospective study that included 197 sessions of chemoembolization, acute liver failure developed in 20% of patients, and 3% of these cases were irreversible. Hyperbilirubinemia, prolonged prothrombin time, higher dose of chemotherapeutic drug, and advanced cirrhosis were associated with irreversible failure (Chan et al, 2002). In general, chemoembolization does not influence liver function in patients with class A or B cirrhosis, and most liver dysfunction that requires hospitalization develops in patients with class C disease (Shah et al, 1998).
Liver Abscess (See Chapter 66)
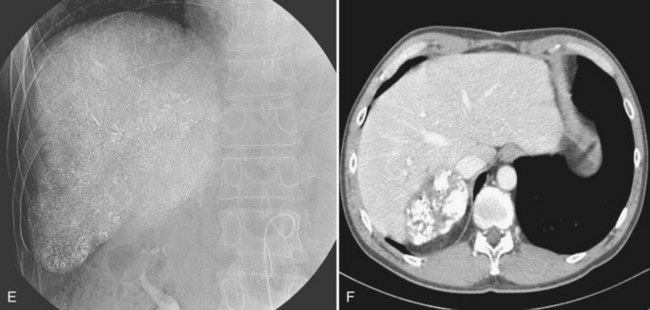
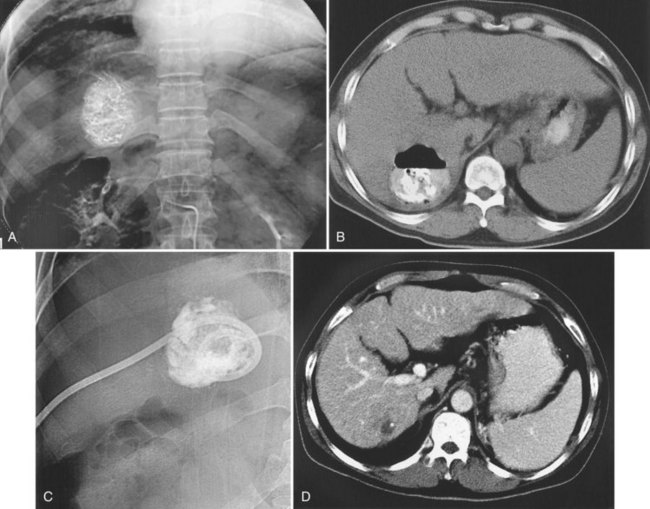
Liver abscess is a rare (0.5% to 2%) but serious complication of hepatic embolotherapy (Kim et al, 2001). In a study by Song and colleagues (2001), predisposing factors for liver abscess included portal vein obstruction, metastatic tumors, and biliary abnormalities. Prior bilioenteric bypass surgery or sphincter of Oddi dysfunction for any reason have been reported as the most important predisposing factors for intrahepatic abscess following embolotherapy (Kim et al, 2001; Ong et al, 2004). Biliary concentrated antibiotics, such as piperacillin, with bowel preparation before the procedure is recommended to prevent liver abscess in these patients (Geschwind et al, 2002), who typically have leukocytosis and spiking fever that occur 7 to 10 days after the procedure. Liver abscess can be managed with percutaneous drainage and parenteral antibiotics (Song et al, 2001; Fig. 83.10).
Bile Duct Injury (See Chapter 42A, Chapter 42B )
Intrahepatic and extrahepatic bile ducts may be damaged by hepatic arterial embolotherapy, because they are supplied by the peribiliary capillary plexus from the hepatic artery. This complication is relatively common (2% to 12.5%) and manifests as intrahepatic biloma, focal stricture of the common bile duct, or diffuse dilatation of intrahepatic bile duct (Kim et al, 2001; Kobayashi et al, 1993; Miyayama et al, 2010). The intrahepatic bile duct injury may cause obliteration of adjacent portal vein, liver parenchymal atrophy, and liver abscess (Yu et al, 2001). Sakamoto and colleagues (2003) reported that bile duct injury is more frequent in patients with metastatic tumor, noncirrhotic liver, and in those who underwent selective embolization of distal hepatic arteries. Most patients are asymptomatic, but when signs of biliary infection are present, parenteral antibiotics and percutaneous drainage are required.
Extrahepatic Nontargeted Embolization
Nontarget embolization is the most dreaded complication of hepatic embolotherapy, but it occurs infrequently if diagnostic angiogram has been carefully performed, and the appropriate technique of embolization has been followed. The gallbladder is the most common nontarget organ, because the cystic artery is often not opacified on arteriogram. Inadvertent embolization of the cystic artery causes prolonged PES with fever, pain, nausea, and vomiting. Most cases follow a self-limited clinical course, but more serious conditions have been reported, such as gallbladder perforation and gangrenous or emphysematous cholecystitis, in which cholecystectomy or percutaneous cholecystostomy is required (Tarazov et al, 2000).
Skin complications can develop after chemoembolization of extrahepatic collateral vessels, such as the internal mammary artery and intercostal artery (Lee et al, 2001a; Hama et al, 2000). Painful induration and discoloration are often observed, but transmural necrosis that requires skin graft is rare. A supraumbilical skin rash can develop, if the emulsion of iodized oil and chemotherapeutic drug flows into the falciform artery. To avoid this complication, when a well-developed falciform artery is identified on hepatic arteriogram, it should be embolized using coils before injection of chemoembolic agent into the left hepatic artery (Ueno et al, 1995).
Gastroduodenal ulcer can occur as a result of inadvertent embolization of the accessory left gastric arteries that arise from the left hepatic artery and the right gastric artery that arises from the proper or left hepatic arteries (Morante et al, 2006). If these gastric branches are not recognized before the procedure, and appropriate protective measures are not taken, gastric complications are unavoidable. Embolization of gastric branches with coil or balloon occlusion of the proper hepatic artery can redirect the blood flow.
Chemoembolization of the inferior phrenic artery frequently results in pulmonary complications, including iodized oil accumulation in the lung field, consolidation, and pleural effusion (Tajima et al, 2002a). Diaphragmatic weakness can also develop in about one third of patients as an ischemic complication (Lee et al, 2009; Shin et al, 2006). In the presence of an arteriovenous shunt, a potential risk of pulmonary embolism or infarction exists, because the iodized oil can pass through the shunt. The incidence of pulmonary oil embolization increases to 43% when a large amount of iodized oil is used for chemoembolization (Chung et al, 1993). Respiratory symptoms of cough, hemoptysis, and dyspnea developed in 2 to 5 days and completely cleared 10 to 28 days after chemoembolization in most cases, but serious complications such as respiratory arrest may occur. To prevent pulmonary oil embolism, it is recommended to use no more than 15 mL of iodized oil, administered superselectively, and to observe arteriovenous hepatic shunts carefully on initial arteriograms and during the procedure. An intracardiac right-to-left shunt via a patent oval foramen or intrapulmonary arteriovenous shunt can lead to cerebral Lipiodol embolism (Matsumoto et al, 2007).
Iatrogenic Vascular Injury
Iatrogenic arterial injury, such as dissection, may occur in the visceral arteries during hepatic embolotherapy. The two most common sites of dissection are the celiac and proper hepatic artery. Although iatrogenic dissection of the arteries heals spontaneously in most patients, it may result in complete obstruction or pseudoaneurysm formation (Jang et al, 2009; So et al, 2003; Yoon et al, 1995).
Combined Therapy with Other Treatment
Chemoembolization enhances the diffusion of ethanol within tumor tissue, causing more extensive tumor necrosis by PEI. Several trials compared this combination therapy with stand-alone PEI or chemoembolization in small HCC (Kamada et al, 2002; Koda et al, 2001) and large HCC (Allgaier et al, 1998; Bartolozzi et al, 1995; Yamamoto et al, 1997). Koda and colleagues (2001) compared the combined therapy versus PEI alone in 52 patients with HCC 2 cm or smaller. In their study, combined therapy improved local recurrence rates (8.7% and 19.3% at 1 and 3 years, respectively) and survival rates (100%, 80%, and 40% at 1, 3, and 5 years, respectively). Kamada and colleagues (2002) compared combined therapy versus chemoembolization alone in 69 patients with HCC 3 cm smaller. The 5-year survival rates were better in the combined-therapy group than in the group that received chemoembolization alone (50% vs. 22%). In a prospective comparative study of 132 patients with unresectable large HCC, median survival time was better in the chemoembolization-PEI group (25 months) compared with the PEI group (18 months), chemoembolization group (8 months), and conservatively treated group (2 months) (Allgaier et al, 1998).
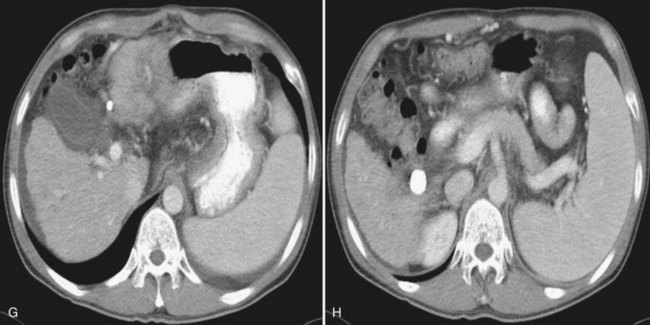
RFA is designed to destroy tumor by heating tissue to lethal temperatures, exceeding 60° C (see Chapter 85C). It has proved to be an effective treatment of small HCC, but tumor volume that can be effectively ablated is limited. HAE eliminates the heat-sink effect by blood flow, thus it induces a larger ablation area by RFA (Rossi et al, 2000; Fig. 83.11). Many reports have demonstrated this combination to be a useful therapeutic method to control large HCC. Rossi and colleagues (2000) performed RFA after bland embolization in 62 patients with HCC (3.5 to 8.5 cm) and observed a 1-year survival rate of 87% and a recurrence rate of 19%. Takaki and colleagues (2009) treated 20 patients with HCC larger than 5 cm with a chemoembolization-RFA combination and reported an encouraging 5-year survival rate of 41%. A recent retrospective study that included 104 patients with early stage HCC, defined as three or fewer tumors 3 cm or smaller each or a single tumor 5 cm or smaller, demonstrated that a chemoembolization-RFA combination resulted in a disease-free and overall survival similar to those achieved by surgical resection (Yamakado et al, 2008); however, the synergistic efficacy of chemoembolization with RFA has not been proven in RCTs. Recently, a prospective study did not find any survival benefit with the combined treatment compared with stand-alone RFA in HCC 3 cm or smaller (Shibata et al, 2009). Therefore, considering the increased cost and patient discomfort of additional chemoembolization, it may be reasonable to restrict this combination to large HCC or small lesions with infiltrative margins. Regarding the safety of this combination and the ability of patients to tolerate it, recent trials have demonstrated that chemoembolization and RFA can be safely performed, even in a single session (Kang et al, 2009), and it can improve quality of life scores better than chemoembolization alone (Wang et al, 2007).
Some investigators combined chemoembolization with laser thermal ablation and used chemoembolization to downsize a tumor that could be ablated with LITT. Zangos and colleagues (2007) performed repeated chemoembolization (mean, 3.5 sessions/patient) in 48 patients with HCC 50 to 80 mm in diameter to reach a favorable size for LITT, and then the patients were treated with MR-guided LITT. Repeated chemoembolization reduced the tumor size in 32 patients (66.7%), and subsequent LITT resulted in a median survival of 36 months after the first treatment. The same combination was used by Vogl and colleagues (2003), who also showed that chemoembolization can be used as a downsizing tool before LITT in 50.6% of patients with unresectable liver metastasis. Pacella and colleagues (2001) used LITT to reduce tumor volume and then performed chemoembolization in HCCs ranging from 3.5 to 9.6 cm in diameter. After combined treatment, complete response was achieved in 90% of the tumors with a local recurrence rate of 7% at 3 years.
The combination of chemoembolization and three-dimensional conformal radiotherapy (3D CRT) have involved three strategies (Hawkins & Dawson, 2006; see Chapter 84B). The most common approach is chemoembolization followed by radiotherapy (RT), in which RT is used to treat portal vein and inferior vena cava tumor thrombus to enhance the therapeutic effect by chemoembolization. From 40 Gy to 60 Gy over 5 to 6 weeks have been delivered safely to the macrovascular disease (Ishikura et al, 2002; Tazawa et al, 2001; Zeng et al, 2005). In a case-control study, 15 (34.1%) of 44 patients who underwent the combination therapy showed complete disappearance of venous tumor thrombi, and the 1-year survival rate was better in the combination group compared with the group that received chemoembolization alone (34.8% vs. 11.4%). A second approach involves RT sandwiched between chemoembolization, with RT used as a planned “consolidation” procedure to target residual viable tumor after chemoembolization (Hawkins & Dawson, 2006); in addition, the chemotherapeutic drugs retained in the tumor by chemoembolization may have a radiosensitizing effect (Seong et al, 2001). In the third approach, repetitive chemoembolization for large HCC is given until optimal response, followed by RT.
Tumor shrinkage after chemoembolization allows the use of smaller irradiation fields, which permits higher tumor doses and improves the normal liver tolerance (Guo et al, 2003). Despite the heterogeneity of disease, patients, and treatment techniques, the majority of studies have suggested a benefit of the combined therapy compared with stand-alone therapies in treatment of advanced HCC (Zeng, 2005; Guo et al, 2003; Meng et al, 2009). The introduction of molecular targeted therapies that attack pathways critical for cancer survival and progression has created a new hope against advanced HCC. Sorafenib, an oral multitarget tyrosine kinase inhibitor with antiangiogenic and antiproliferative actions, is the first systemic therapy to prolong the survival of patients with advanced HCC (Llovet et al, 2008; see Chapter 88). Currently underway are studies to investigate the effects of sorafenib as an adjuvant therapy after chemoembolization, to prevent microscopic tumor residues from reestablishing their blood supply and thus reduce tumor recurrence.
Emerging Techniques of Hepatic Arterial Embolotherapy
In bland embolization, because the therapeutic efficacy depends on terminal vessel blockade, the smallest particles commercially available have been used. Until the late 1990s, 50-µm PVA particles were the smallest particles available, when Embospheres (BioSphere Medical, Rockland, MA), a hydrophilic tris-acyl gelatin microsphere, become available in 100- to 700-µm particles. Despite the larger size, Embospheres have several ideal characteristics of an embolic agent: they are spherical and have a hydrophilic surface that prevents aggregation, which results in a lower rate of catheter occlusion and better penetration into the more distal small vessels (Rand et al, 2005). Recently, 40- to 120-µm Embospheres became available. Maluccio and colleagues (2008) performed bland embolization using PVA particle (50 µm) or microspheres (40 to 120 µm) in 322 patients with HCC and reported 1-, 2-, and 3-year overall survival rates of 66%, 46%, and 33%, respectively. In patients without extrahepatic disease or portal vein involvement by tumor, the overall 1-, 2-, and 3-year survival rates increased to 84%, 66%, and 51%, respectively. Subgroups of patients with solitary HCC who also underwent thermal ablation the day after bland embolization had similar survival results after surgical resection (Maluccio et al, 2005). The same authors reported results of bland embolization in metastatic NETs and metastatic sarcomas that parallel those reported by others using chemoembolization (Brown et al, 1999; Maluccio et al, 2006).
Drug-eluting beads (DEBs) are specifically designed PVA beads that release chemotherapeutic drugs over an extended period. DEBs consist of nondegradable hydrogel microspheres based on PVA with a smooth surface and a precisely calibrated size that ranges from 100 to 900 µm. They are characterized by a high content of negatively charged 2-acrylamido-2-methylpropanesulfonate (AMPS). The unsaturated AMPS monomer crosslinks the PVA backbone and allows the load and release of positively charged drugs, such as doxorubicin hydrochloride or irinotecan hydrochloride, by an increased number of negatively charged sulfonate moieties (Hong et al, 2006). DEBs can achieve higher doses of the chemotherapeutic drugs and can prolong contact time with cancer cells and thus enhance therapeutic efficacy and at the same time reduce toxicity because of minimum systemic exposure (Hong et al, 2006).
The DC Bead (Biopcompatibles, Surrey, UK) is a DEB that has proven favorable kinetics and clinical characteristics (Lewis et al 2006a, 2006b; Varela et al, 2007). Malagari and colleagues (2008) treated 62 patients with single, unresectable HCC 3 to 9 cm using DC Beads impregnated with doxorubicin. After three sessions of chemoembolization, a complete response was seen in 12.2%, and an objective response was seen in 80.7%, according to EASL criteria. Lammer and colleagues (2010) conducted a prospective randomized trial to compare conventional chemoembolization with chemoembolization using DC Beads. The DEB group showed higher rates of complete response, objective response, and disease control compared with the conventional chemoembolization group (27% vs. 22%, 52% vs. 44%, and 63% vs. 52%, respectively) determined by EASL criteria at 6 months. A subgroup with more advanced disease (CTP class B, ECOG 1, bilobar disease, and recurrent disease) showed a significant increase in objective response compared with conventional chemoembolization. Moreover, DC Beads were associated with improved tolerability, with a significant reduction in serious liver toxicity and a significantly lower rate of doxorubicin-related side effects. DEBs loaded with irinotecan and oxaliplatin for treatment for metastatic liver tumors are under investigation (Martin et al, 2009; Poggi et al, 2008).
Allgaier HP, et al. Survival benefit of patients with inoperable hepatocellular carcinoma treated by a combination of transarterial chemoembolization and percutaneous ethanol injection: a single-center analysis including 132 patients. Int J Cancer. 1998;79:601-605.
Aoyama K, et al. Evaluation of transcatheter arterial embolization with epirubicin-Lipiodol emulsion for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1992;31(Suppl):S55-S59.
Bartolozzi C, et al. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812-818.
Bedikian AY, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76:1665-1670.
Berghammer P, et al. Arterial hepatic embolization of unresectable hepatocellular carcinoma using a cyanoacrylate/Lipiodol mixture. Cardiovasc Intervent Radiol. 1998;21:214-218.
Bismuth H, et al. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg. 1992;163:387-394.
Bosch FX, et al. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:91-211.
Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969-977.
Bronowicki JP, et al. Transcatheter oily chemoembolization for hepatocellular carcinoma: a 4-year study of 127 French patients. Cancer. 1994;74:16-24.
Brown DB, et al. Hepatic arterial chemoembolization for hepatocellular carcinoma: comparison of survival rates with different embolic agents. J Vasc Interv Radiol. 2005;16:1661-1666.
Brown KT, et al. Particle embolization of hepatic neuroendocrine metastases for control of pain and hormonal symptoms. J Vasc Interv Radiol. 1999;10:397-403.
Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S179-S188.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236.
Bruix J, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430.
Camma C, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54.
Cancer of the Liver Italian Program (CLIP) Investigators. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;31:840-845.
Carlson SK, et al. Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology. 2001;219:515-520.
Chan AO, et al. A prospective study regarding the complications of transcatheter intraarterial Lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747-1752.
Chapman WC, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625.
Choi GH, et al. Is preoperative transarterial chemoembolization needed for a resectable hepatocellular carcinoma? World J Surg. 2007;31:2370-2377.
Chung JW, et al. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993;187:689-693.
Chung JW, et al. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165:315-321.
Chung JW, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996;198:33-40.
Chung JW, et al. Transcatheter arterial chemoembolization of hepatocellular carcinoma: prevalence and causative factors of extrahepatic collateral arteries in 479 patients. Korean J Radiol. 2006;7:257-266.
Coldwell DM, Stokes KR, Yakes WF. Embolotherapy: agents, clinical applications, and techniques. Radiographics. 1994;14:623-643.
Decaens T, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767-775.
Demetri GD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480.
El-Serag HB, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817-823.
Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983;10:176-182.
Ernst O, et al. Treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization: comparison of planned periodic chemoembolization and chemoembolization based on tumor response. AJR Am J Roentgenol. 1999;172:59-64.
Feldman ED, Pingpank JF, Alexander HRJr. Regional treatment options for patients with ocular melanoma metastatic to the liver. Ann Surg Oncol. 2004;11:290-297.
Fusai G, Davidson BR. Management of colorectal liver metastases. Colorectal Dis. 2003;5:2-23.
Georgiades CS, et al. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653-1659.
Gerunda GE, et al. Role of transarterial chemoembolization before liver resection for hepatocarcinoma. Liver Transpl. 2000;6:619-626.
Geschwind JF, et al. Influence of a new prophylactic antibiotic therapy on the incidence of liver abscesses after chemoembolization treatment of liver tumors. J Vasc Interv Radiol. 2002;13:1163-1166.
Goseki N, et al. Nourishment of hepatocellular carcinoma cells through the portal blood flow with and without transcatheter arterial embolization. Cancer. 1995;76:736-742.
Granov AM, Tarazov PG, Ryzhkov VK. Arterioportal fistulas (APFs) in liver tumors: prognosis in relation to treatment. HPB Surg. 1992;5:87-94.
Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. A comparison of Lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995;332:1256-1261.
Gunji T, et al. Long-term outcomes of transcatheter arterial chemoembolization with autologous blood clot for unresectable hepatocellular carcinoma. Int J Oncol. 2002;21:427-432.
Guo WJ, et al. Comparison between chemoembolization combined with radiotherapy and chemoembolization alone for large hepatocellular carcinoma. World J Gastroenterol. 2003;9:1697-1701.
Gupta S, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104:1590-1602.
Hama Y, Iwasaki Y, Kusano S. Supraumbilical skin rash after chemoembolization for hepatocellular carcinoma. Eur Radiol. 2000;10:1356.
Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer. 2006;106:1653-1663.
Hong K, et al. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12:2563-2567.
Huang MS, et al. Comparison of long-term effects between intra-arterially delivered ethanol and Gelfoam for the treatment of severe arterioportal shunt in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:825-829.
Ishikura S, et al. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189-193.
Itsubo M, et al. Subsegmental transcatheter arterial embolization for small hepatocellular carcinoma. Hepatogastroenterology. 2002;49:735-739.
Izaki K, et al. Transcatheter arterial embolization for advanced tumor thrombus with marked arterioportal or arteriovenous shunt complicating hepatocellular carcinoma. Radiat Med. 2004;22:155-162.
Jang ES, et al. A case of acute ischemic duodenal ulcer associated with superior mesenteric artery dissection after transarterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2009;32:367-370.
Jinno K, et al. Clinicopathological study on combination therapy consisting of arterial infusion of Lipiodol-dissolved SMANCS and transcatheter arterial embolization for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1992;31(Suppl):S7-S12.
Kamada K, et al. Combination of transcatheter arterial chemoembolization using cisplatin-lipiodol suspension and percutaneous ethanol injection for treatment of advanced small hepatocellular carcinoma. Am J Surg. 2002;184:284-290.
Kan Z, et al. Distribution and effect of iodized poppyseed oil in the liver after hepatic artery embolization: experimental study in several animal species. Radiology. 1993;186:861-866.
Kang SG, et al. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol. 2009;20:1570-1577.
Kim HC, et al. Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics. 2005;25(Suppl 1):S25-S39.
Kim HC, et al. Hepatocellular carcinoma with internal mammary artery supply: feasibility and efficacy of transarterial chemoembolization and factors affecting patient prognosis. J Vasc Interv Radiol. 2007;18:611-619.
Kim HC, et al. Transcatheter arterial chemoembolization for hepatocellular carcinoma: prospective assessment of the right inferior phrenic artery with C-arm CT. J Vasc Interv Radiol. 2009;20:888-895.
Kim HK, et al. Ischemic bile duct injury as a serious complication after transarterial chemoembolization in patients with hepatocellular carcinoma. J Clin Gastroenterol. 2001;32:423-427.
Kirikoshi H, et al. Outcomes and factors influencing survival in cirrhotic cases with spontaneous rupture of hepatocellular carcinoma: a multicenter study. BMC Gastroenterol. 2009;30:9-29.
Kobayashi S, et al. Postmortem survey of bile duct necrosis and biloma in hepatocellular carcinoma after transcatheter arterial chemoembolization therapy: relevance to microvascular damages of peribiliary capillary plexus. Am J Gastroenterol. 1993;88:1410-1415.
Koda M, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma: a randomized control study. Cancer. 2001;92:1516-1524.
Kuroda C, et al. Limitation of transcatheter arterial chemoembolization using iodized oil for small hepatocellular carcinoma: a study in resected cases. Cancer. 1991;67:81-86.
Lammer J, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V Study. Cardiovasc Intervent Radiol. 2010;33:41-52.
Lee DH, et al. Development of diaphragmatic weakness after transcatheter arterial chemoembolization of the right inferior phrenic artery: frequency and determinant factors. J Vasc Interv Radiol. 2009;20:484-489.
Lee HS, et al. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction: a prospective controlled study. Cancer. 1997;79:2087-2094.
Lee JH, et al. An ischemic skin lesion after chemoembolization of the right internal mammary artery in a patient with hepatocellular carcinoma. Yonsei Med J. 2001;42:137-141.
Lee SH, Hahn ST, Park SH. Intraarterial lidocaine administration for relief of pain resulting from transarterial chemoembolization of hepatocellular carcinoma: its effectiveness and optimal timing of administration. Cardiovasc Intervent Radiol. 2001;24:368-371.
Leung DA, et al. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321-326.
Levy AD. Malignant liver tumors. Clin Liver Dis. 2002;6:147-164.
Lewis AL, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:335-342.
Lewis AL, et al. Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J Vasc Interv Radiol. 2006;17:1335-1343.
Liu DM, et al. Angiographic considerations in patients undergoing liver-directed therapy. J Vasc Interv Radiol. 2005;16:911-935.
Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917.
Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440.
Llovet JM, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
Lo CM, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171.
Majno PE, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688-701.
Makuuchi M, et al. Bile duct necrosis: complication of transcatheter hepatic arterial embolization. Radiology. 1985;156:331-334.
Malagari K, et al. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31:269-280.
Maluccio M, et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interv Radiol. 2005;16:955-961.
Maluccio MA, et al. Treatment of metastatic sarcoma to the liver with bland embolization. Cancer. 2006;107:1617-1623.
Maluccio MA, et al. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2008;19:862-869.
Marelli L, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6-25.
Martin RC, et al. Transarterial chemoembolization of metastatic colorectal carcinoma with drug-eluting beads, irinotecan (DEBIRI): multi-institutional. registry. J Oncol. 2009;2009:539795. Epub Oct 29 2009
Martinelli DJ, et al. Utility of embolization or chemoembolization as second-line treatment in patients with advanced or recurrent colorectal carcinoma. Cancer. 1994;74:1706-1712.
Matsui O, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79-83.
Matsumoto K, et al. Cerebral lipiodol embolism: a complication of transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2007;30:512-514.
Mavligit GM, et al. Regression of ocular melanoma metastatic to the liver after hepatic arterial chemoembolization with cisplatin and polyvinyl sponge. JAMA. 1988;260:974-976.
Mavligit GM, et al. Gastrointestinal leiomyosarcoma metastatic to the liver. Durable tumor regression by hepatic chemoembolization infusion with cisplatin and vinblastine. Cancer. 1995;75:2083-2088.
Meng MB, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2009;92:184-194.
Meyer BC, et al. Contrast-enhanced abdominal angiographic CT for intra-abdominal tumor embolization: a new tool for vessel and soft tissue visualization. Cardiovasc Intervent Radiol. 2007;30:743-749.
Meyers MO, Sasson AR, Sigurdson ER. Locoregional strategies for colorectal hepatic metastases. Clin Colorectal Cancer. 2003;3:34-44.
Miyayama S, et al. Main bile duct stricture occurring after transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:1168-1179.
Morante A, et al. Massive gastric ulceration after transarterial chemoembolization for hepatocellular carcinoma. Gastrointest Endosc. 2006;63:718-720.
Nakajo M, et al. Biodistribution and in vivo kinetics of iodine-131 lipiodol infused via the hepatic artery of patients with hepatic cancer. J Nucl Med. 1988;29:1066-1077.
Nakamura H, et al. Response to transcatheter oily chemoembolization in hepatocellular carcinoma 3 cm or less: a study in 50 patients who underwent surgery. Hepatogastroenterology. 1993;40:6-9.
Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. 1997;52:36-40.
Okuda K, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment: study of 850 patients. Cancer. 1985;56:918-928.
Ong GY, et al. Liver abscess complicating transcatheter arterial embolization: a rare but serious complication: a retrospective study after 3878 procedures. Eur J Gastroenterol Hepatol. 2004;16:737-742.
Pacella CM, et al. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219:669-678.
Pang R, Poon RT. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006;242:151-167.
Patel NH, et al. Hepatic artery embolization: factors predisposing to postembolization pain and nausea. J Vasc Interv Radiol. 2000;11:453-460.
Pelletier G, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990;11:181-184.
Poggi G, et al. Transhepatic arterial chemoembolization with oxaliplatin-eluting microspheres (OEM-TACE) for unresectable hepatic tumors. Anticancer Res. 2008;28:3835-3842.
Rajan DK, et al. Sarcomas metastatic to the liver: response and survival after cisplatin, doxorubicin, mitomycin-C, Ethiodol, and polyvinyl alcohol chemoembolization. J Vasc Interv Radiol. 2001;12:187-193.
Ramsey DE, et al. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S211-S221.
Rand T, et al. Arterial embolization of unresectable hepatocellular carcinoma with use of microspheres, lipiodol, and cyanoacrylate. Cardiovasc Intervent Radiol. 2005;28:313-318.
Raoul JL, et al. Chemoembolization of hepatocellular carcinomas: a study of the biodistribution and pharmacokinetics of doxorubicin. Cancer. 1992;70:585-590.
Rose DM, et al. Transcatheter arterial chemoembolization as primary treatment for hepatocellular carcinoma. Am J Surg. 1999;177:405-410.
Rossi S, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119-126.
Sakamoto I, et al. Intrahepatic biloma formation (bile duct necrosis) after transcatheter arterial chemoembolization. AJR Am J Roentgenol. 2003;181:79-87.
Salman HS, et al. Randomized phase II trial of embolization therapy versus chemoembolization therapy in previously treated patients with colorectal carcinoma metastatic to the liver. Clin Colorectal Cancer. 2002;2:173-179.
Sanz-Altamira PM, et al. Selective chemoembolization in the management of hepatic metastases in refractory colorectal carcinoma: a phase II trial. Dis Colon Rectum. 1997;40:770-775.
Seong J, Kim SH, Suh CO. Enhancement of tumor radioresponse by combined chemotherapy in murine hepatocarcinoma. J Gastroenterol Hepatol. 2001;16:883-889.
Shah SR, et al. Tumour ablation and hepatic decompensation rates in multi-agent chemoembolization of hepatocellular carcinoma. QJM. 1998;91:821-828.
Shibata T, et al. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905-913.
Shin SW, et al. Diaphragmatic weakness after transcatheter arterial chemoembolization of inferior phrenic artery for treatment of hepatocellular carcinoma. Radiology. 2006;241:581-588.
So YH, Chung JW, Park JH. Balloon fenestration of iatrogenic celiac artery dissection. J Vasc Interv Radiol. 2003;14:493-496.
Song SY, et al. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors, and clinical outcome. J Vasc Interv Radiol. 2001;12:313-320.
Song SY, et al. Nonhepatic arteries originating from the hepatic arteries: angiographic analysis in 250 patients. J Vasc Interv Radiol. 2006;17:461-469.
Song SY, et al. Celiac axis and common hepatic artery variations in 5002 patients: systematic analysis with spiral CT and DSA. Radiology. 2010;255:278-288.
Sonomura T, et al. Dependency of tissue necrosis on gelatin sponge particle size after canine hepatic artery embolization. Cardiovasc Intervent Radiol. 1997;20:50-53.
Stockland AH, et al. Preoperative chemoembolization in patients with hepatocellular carcinoma undergoing liver transplantation: influence of emergent versus elective procedures on patient survival and tumor recurrence rate. Cardiovasc Intervent Radiol. 2007;30:888-893.
Strohmeyer T, Haugeberg G, Lierse W. Angioarchitecture and blood supply of micro- and macrometastases in human livers: an anatomic-pathological investigation using injection-techniques. J Hepatol. 1987;4:181-189.
Suzuki Y, et al. Clinical utility of sequential imaging of hepatocellular carcinoma by contrast-enhanced power Doppler ultrasonograpy. Eur J Radiol. 2003;48:214-219.
Tajima T, et al. Pulmonary complications after hepatic artery chemoembolization or infusion via the inferior phrenic artery for primary liver cancer. J Vasc Interv Radiol. 2002;13:893-900.
Tajima T, et al. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002;178:885-897.
Takaki H, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas larger than 5 cm. J Vasc Interv Radiol. 2009;20:217-224.
Takayasu K, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461-469.
Tarazov PG. Hepatic chemoembolization: are prophylactic antibiotics necessary? J Vasc Interv Radiol. 1995;6:658-659.
Tarazov PG, et al. Ischemic complications of transcatheter arterial chemoembolization in liver malignancies. Acta Radiol. 2000;41:156-160.
Tazawa J, et al. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660-665.
Ueno K, et al. Embolization of the hepatic falciform artery to prevent supraumbilical skin rash during transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1995;18:183-185.
Ueno S, et al. Discrimination value of the new Western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients: Cancer of the Liver Italian Program. Hepatology. 2001;34:529-534.
Varela M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481.
Vogl TJ, et al. Liver metastases: neoadjuvant downsizing with transarterial chemoembolization before laser-induced thermotherapy. Radiology. 2003;229:457-464.
Wallace MJ, et al. Impact of C-arm CT on hepatic arterial interventions for hepatic malignancies. J Vasc Interv Radiol. 2007;18:1500-1507.
Wallace MJ, et al. Three-dimensional C-arm cone-beam CT: applications in the interventional suite. J Vasc Interv Radiol. 2008;19:799-813.
Wang YB, et al. Quality of life after radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: comparison with transcatheter arterial chemoembolization alone. Qual Life Res. 2007;16:389-397.
Won JY, et al. Supplemental transcatheter arterial chemoembolization through a collateral omental artery: treatment for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2003;26:136-140.
Yamakado K, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008;247:260-266.
Yamamoto K, et al. Evaluation of combined therapy with chemoembolization and ethanol injection for advanced hepatocellular carcinoma. Semin Oncol. 1997;24(2 Suppl 6):S6-S50. S6-S55
Yao FY, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827.
Yoon CJ, et al. Hepatocellular carcinoma in the caudate lobe of the liver: angiographic analysis of tumor-feeding arteries according to subsegmental location. J Vasc Interv Radiol. 2008;19:1543-1550.
Yoon DY, et al. Iatrogenic dissection of the celiac artery and its branches during transcatheter arterial embolization for hepatocellular carcinoma: outcome in 40 patients. Cardiovasc Intervent Radiol. 1995;18:16-19.
Yu AS, Keeffe EB. Management of hepatocellular carcinoma. Rev Gastroenterol Disord. 2003;3:8-24.
Yu JS, et al. Bile duct injuries leading to portal vein obliteration after transcatheter arterial chemoembolization in the liver: CT findings and initial observations. Radiology. 2001;221:429-436.
Zangos S, et al. Large-sized hepatocellular carcinoma (HCC): a neoadjuvant treatment protocol with repetitive transarterial chemoembolization (TACE) before percutaneous MR-guided laser-induced thermotherapy (LITT). Eur Radiol. 2007;17:553-563.
Zeng ZC, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432-443.
Zhang Z, et al. The effect of preoperative transcatheter hepatic arterial chemoembolization on disease-free survival after hepatectomy for hepatocellular carcinoma. Cancer. 2000;89:2606-2612.
Zhou WP, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195-202.