Hemoptysis
Perspective
Large, contemporary series of patients with massive hemoptysis are lacking, and most causative data originate from small, often rural, studies in which tuberculosis (TB) and bronchiectasis are responsible for the majority of cases.1 In developed nations, cancer, cystic fibrosis, arteriovenous malformations, and postprocedural complications play more prominent roles. Pediatric hemoptysis is rare but can be caused by infection, congenital heart disease, cystic fibrosis, or bleeding from a preexisting tracheostomy.2
Pathophysiology
Bronchiectasis, a chronic necrotizing infection resulting in bronchial wall inflammation and dilation, is one of the most common causes of massive hemoptysis. As tissue destruction and remodeling occur, rupture of nearby bronchial vessels can result in bleeding. Bronchiectasis can complicate chronic airway obstruction, necrotizing pneumonia, TB, or cystic fibrosis. Broncholithiasis, the formation of calcified endobronchial lesions following a wide array of granulomatous infections, is an uncommon problem with a similar propensity to erode nearby vessels. Hemorrhage control often requires surgical intervention.3–5
Iatrogenic hemoptysis complicates 2 to 10% of all endobronchial procedures, especially percutaneous lung biopsies.6,7 Diffuse alveolar hemorrhage can be seen with autoimmune vasculitides such as Wegener’s granulomatosis, systemic lupus erythematosus, and Goodpasture’s syndrome. An uncommon cause of hemoptysis occurs when ectopic endometrial tissue within the lung results in monthly catamenial episodes of bleeding.8 Less common causes include pulmonary hereditary telangiectasias and hydatidiform infections. Any episode of hemoptysis can be exacerbated by coagulopathy and thrombocytopenia.
Diagnostic Approach
Rapid Assessment and Stabilization
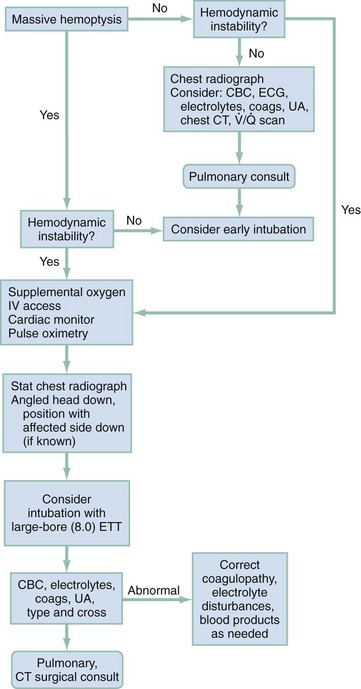
As a mitigating maneuver in patients with a known lateralizing source of bleeding, the “lung-down” position can be employed. For this position the patient is turned such that the bleeding lung is more dependent, promoting continued protection and ventilation of the unaffected lung and improved oxygenation. If intubation is required, a large diameter (8.0) endotracheal tube should be used to facilitate emergent fiberoptic bronchoscopy. In selected cases of confirmed left-sided bleeding, a single-lumen right-mainstem intubation often can be successfully performed through advancement of the tube in the neutral position or use of a 90-degree rotational technique, during which the tube is rotated 90 degrees in the direction of desired placement and advanced until resistance is met.9 Left-mainstem intubations are more difficult but may be attempted when the bleeding site is the right lung and simple lung-down positioning is not sufficient to stabilize the patient’s airway and oxygenation.
When these measures fail or the hemoptysis is life-threatening, anesthesia consultation is sought for consideration of placement of a double-lumen endotracheal tubes for lung isolation. The correct positioning of blindly placed double-lumen tubes is difficult and requires confirmation by auscultation and fiberoptic bronchoscopy, both of which have are severely impaired by massive hemoptysis. Complications of double-lumen tubes include unilateral and bilateral pneumothoraces, pneumomediastinum, carinal rupture, lobar collapse, and tube malposition.10
Pivotal Findings
Physical Examination
After a primary survey and stabilization, a targeted examination may suggest the location and cause of bleeding but does so in less than 50% of cases.11 Focal adventitious breath sounds may indicate pneumonia or pulmonary abscess. A new heart murmur, especially in a febrile patient, may reflect endocarditis causing septic pulmonary emboli. Symptoms and signs of deep venous thrombosis should suggest pulmonary embolism. Ecchymoses and petechiae can indicate coagulopathy and thrombocytopenia, respectively.
Ancillary Testing
Initial laboratory studies include a complete blood count, coagulation tests, and a type and screen or crossmatch. Renal function tests should be performed if vasculitis is suggested or contrast computed tomography (CT) is planned. Plain chest radiography screens for causes of hemoptysis (including infection and malignancy), although its sensitivity is poor. A prospective study of 184 consecutive patients with varying degrees of hemoptysis revealed that more than 40% of patients with a normal chest radiograph had positive findings on chest CT.12
In patients with massive hemoptysis, plain films may localize the site of hemorrhage in as many as 80% of patients; however, high-resolution multidetector CT of the chest is the principle diagnostic test for investigating both bronchial and nonbronchial causes of massive hemoptysis.13 A chest CT scan should be obtained in the high-risk patient (smokers, oncology patients) or in any patient with moderate to severe bleeding even if the initial chest radiograph is normal. CT localization of hemorrhage can expedite bronchoscopic evaluation and guide subsequent interventional procedures.
CT is diagnostically comparable to conventional angiography but less invasive and more rapidly available. Angiography is the first-line study when the cause of the hemoptysis is known (e.g., malignancy) and angiography-assisted embolization therapy is contemplated.14–16
Diagnostic Algorithm
Potential causes of hemoptysis vary and include systemic illnesses as well as pulmonary parenchymal diseases. Box 24-1 includes the most common causes.
Management
Since the advent of high-resolution CT, radiologic evaluation has had an integral role in the evaluation and treatment of patients with hemoptysis. The challenge to the emergency physician is to rapidly assess the need for airway control before radiographic evaluation and hemodynamic stabilization. Unless the initial chest radiograph is diagnostic or the patient is hemodynamically unstable, a chest CT should be obtained. Further management decisions should be guided by the CT results and made in conjunction with pulmonary and thoracic surgery consultants (Fig. 24-1).
Bronchoscopy
Chest CT is as diagnostically accurate as bronchoscopy in locating bleeding peripheral vessels not accessible by a flexible bronchoscope.17 Chest CT can be used to identify the site of bleeding to determine whether angiography is indicated. There may be little added benefit to bronchoscopy before interventional angiography if the bleeding source has already been accurately identified on CT.18
Interventional Angiography
Bronchial arterial embolization is an effective first-line therapy for massive hemoptysis and is the procedure of choice for patients either unable to tolerate surgery or in whom bronchoscopy has been unsuccessful. Hemostatic rates range from 91 to 98%, but as many as 20 to 50% of patients have early episodes of repeat bleeding. The risk of delayed bleeding may exist for up to 36 months.19–21 To guide therapy, initial localization of bleeding by bronchoscopy or CT is preferred. Rare complications include arterial perforation and dissection.
References
1. Reechaipichitkul, W, Latong, S. Etiology and treatment outcomes of massive hemoptysis. Southeast Asian J Trop Med Public Health. 2005;36:2.
2. Batra, PS, Holinger, LD. Etiology and management of pediatric hemoptysis. Arch Otolaryngol Head Neck Surg. 2001;127:377.
3. Minami, H, Sano, I, Matsuo, S. Broncholithiasis managed by surgical resection. Gen Thorac Cardiovasc Surg. 2007;55:138.
4. Reza, B, Ziaollah, H. Surgical management of TB broncholithiasis with hemoptysis: Experience with five operated cases. Ann Thorac Cardiovasc Surg. 2007;13:185.
5. Potaris, K, Miller, DL, Trastek, VF. Role of surgical resection in broncholithiasis. Ann Thorac Surg. 2000;70:248.
6. Wescott, JL. Percutaneous transthoracic needle biopsy. Radiology. 1988;169:593.
7. Cordasco, EM, Jr., Mehta, AC, Ahmad, M. Bronchoscopically induced bleeding: A summary of nine years’ Cleveland Clinic experience and review of the literature. Chest. 1991;100:1141.
8. Augoulea, A, Lambrinoudaki, I, Christodoulakos, G. Thoracic endometriosis syndrome. Respiration. 2008;75:113.
9. Bair, AE, Doherty, MJ, Harper, R, Albertson, TE. An evaluation of a blind rotational technique for selective mainstem intubation. Acad Emerg Med. 2004;11:1105.
10. Sucato, DJ, Girgis, M. Bilateral pneumothoraces, pneumomediastinum, pneumoperitoneum, pneumoretroperitoneum, and subcutaneous emphysema following intubation with a double-lumen endotracheal tube for thoracoscopic anterior spinal release and fusion in a patient with idiopathic scoliosis. J Spinal Disord Tech. 2002;15:133.
11. Haro Estarriol, M, et al. Utility of history, physical examination and radiography in the localization of bleeding in patients with hemoptysis. An Med Interna. 2002;19:289.
12. Tsoumakidai, M, et al. A prospective analysis of 184 cases of hemoptysis: Diagnostic impact of chest x-ray, computed tomography, bronchoscopy. Respiration. 2006;73:808.
13. Khalil, A, et al. Severe hemoptysis of pulmonary arterial origin: Signs and role of multidetector row CT angiography. Chest. 2008;133:212.
14. Remy-Jardin, M, et al. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: Comparison with conventional angiography. Radiology. 2004;233:741.
15. Yoon, YC, et al. Hemoptysis: Bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology. 2005;234:292.
16. Yoon, W, et al. Massive hemoptysis: Prediction of nonbronchial systemic arterial supply with chest CT. Radiology. 2003;227:232.
17. Revel, MP, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol. 2002;179:1217.
18. Hsiao, EI, Kirsch, CM, Kagawa, FT. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol. 2001;177:861.
19. Swanson, KL, et al. Bronchial artery embolization: Experience with 54 patients. Chest. 2001;121:789.
20. Osaki, S, et al. Prognosis of bronchial artery embolization in the management of hemoptysis. Respiration. 2000;67:412.
21. Poyanli, A, et al. Endovascular therapy in the management of moderate and massive haemoptysis. Br J Radiol. 2007;80:331.

 , ventilation-perfusion.
, ventilation-perfusion.


