Chapter 14 Heart Transplantation
The first human heart transplant was performed in 1967 by Christian Barnard in South Africa. After this landmark operation there was a burst of activity, and 101 such operations were performed by 54 teams in 22 countries within a year. As a result of acute graft failure and rejection, however, outcomes were poor, and patients rarely survived longer than 1 year. Over the next decade very few heart transplant operations were performed. As with lung transplantation, it was not until the introduction of cyclosporine in the early 1980s that outcomes improved and heart transplantation became a viable treatment option for patients with end-stage heart failure.1
Current survival rates after heart transplantation are 90% at 3 months, 87% at 1 year, 78% at 3 years, and 70% at 5 years.2 These figures are substantially better than those observed after lung transplantation (see Chapter 13). It is important to note that quality of life is significantly improved by heart transplantation, with most patients being able to resume a relatively normal life, including return to work, leisure activities, and study.3
The number of patients on the waiting list for heart transplantation in the United States is slightly over 3000,4 with approximately 2000 undergoing the procedure in the United States each year (4000 worldwide).5 The waiting time for heart transplantation has risen considerably in the past few years. US Registry data has shown that in 2003, 49% of patients waited more than 2 years for a transplant, up from 23% in 1994.2 In the year to July 2004, 494 patients died while awaiting heart transplantation,2 about 16% of the patients on the waiting list.
In this chapter, the preoperative evaluation and perioperative management of heart transplant recipients are described, with particular emphasis on early postoperative hemodynamic stabilization. Heterotopic heart transplantation, in which a donor heart is used as support for the native heart, is rarely performed now and is not discussed. Heart-lung transplantation is also infrequently performed; it is discussed briefly in Chapter 13.
SELECTION CRITERIA
Recipient Criteria and Preoperative Assessment
Patients undergoing heart transplantation must have end-stage cardiac disease with limited life expectancy. Management options other than transplantation should be fully explored. These include optimization of medical therapy; coronary artery bypass graft surgery for patients with suitable coronary anatomy and reversible ischemia on viability testing; mitral valve repair for patients with severe mitral regurgitation; insertion of an implantable defibrillator for those with recurrent arrhythmias; and biventricular pacing for patients with prolonged intraventricular conduction. In addition, because of the shortage of suitable donors, patients with end-stage heart failure may be offered a ventricular-assist device as “destination” therapy (see Chapter 22).
The two main pathologies that give rise to end-stage cardiac failure necessitating heart transplantation are ischemic cardiomyopathy and the nonischemic cardiomyopathies (dilated, hypertrophic, restrictive), which make up about 45% of cases each.5 The remaining cases include valvular heart disease, congenital heart disease, and primary heart transplant failure. Inclusion and exclusion criteria for recipients of heart transplantation are outlined in Table 14-1.6
Table 14-1 Recipient Selection Criteria for Heart Transplantation
| Severe end-stage heart failure with NYHA functional class III-IV |
| VO2 max <14 ml/kg/min* |
| Age <65 years |
| Normal function or reversible renal/hepatic dysfunction |
| Absence of active infection |
| Compliance with medication |
| Psychological stability with social support |
| Absence of malignancy |
| Absence of significant coexisting systemic conditions |
| Abstinence from alcohol and recreational drug use |
| Cross-match compatibility |
| Absence of significant peripheral or cerebrovascular disease |
| Pulmonary vascular resistance <5 Wood units |
| Nonobese |
* Mancini DM, Eisen H, Kussmaul W, et al: Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 83:778-786, 1991. NYHA, New York Heart Association; VO2 max, maximal exercise.
(Modified from Costanzo MR, Augustine S, Bourge R, et al: Selection and treatment of candidates for heart transplantation: a statement for health professionals from the Committee on Heart Failure and Cardiac Transplantation of the Council on Clinical Cardiology, American Heart Association. Circulation 92:3539-3612, 1995.)
The assessment of a patient’s suitability for heart transplantation includes a right heart catheter study (see Chapter 5). This is a critical investigation because the presence of elevated pulmonary vascular resistance (>5 Wood units) contraindicates heart transplantation. A resistance level above 3 Wood units indicates an increased risk for postoperative right ventricular dysfunction.
Patients undergoing heart transplantation in the United States are typically extremely unwell: 70% are hospitalized; 25% require some form of mechanical cardiac support (ventricular-assist device or intraaortic balloon pump); and almost half are undergoing intravenous inotropic infusions.5 For patients with decompensated heart failure who are receiving inotropic drugs, the insertion of a ventricular-assist device prior to transplantation (i.e., as a bridge to transplantation rather than as destination therapy) may improve outcome after surgery.7
Donor Criteria and Management
As well as recipient criteria, donor criteria are vital in the decision to proceed with transplantation. An important criterion is the absence or low probability of coronary artery disease in the donor heart. For this reason, the upper donor age limit in most transplant units is 55 to 60 years.8 An echocardiogram is performed to assess valvular and ventricular function. The presence of segmental wall motion abnormalities is suggestive of myocardial ischemia or infarction and precludes the use of the organ. In some centers, coronary angiography is performed in potential donors who have risk factors for coronary artery disease.
The requirement for high-dose inotropic support in a donor is a relative contraindication to use of the heart for transplantation, particularly if it is associated with echocardiographic evidence of impaired ventricular function. The neuroendocrine failure that accompanies brain death is associated with marked vasodilation (see Chapter 38), and it usual for there to be some requirement for a vasopressor. The effects of neuroendocrine failure may be ameliorated by employing hormonal therapy (vasopressin, cortisol, triiodothyronine, insulin-glucose) in the donor. This regime may increase the number and quality of organs available for use in heart transplantation.
Ideally, the donor heart’s ischemic time should be less than 6 hours. In one study, an ischemic time greater than 4 hours was associated with increased rates of early mortality.9 In an attempt to extend the safe ischemic time, continuous perfusion systems (as opposed to standard cold storage) have been developed. These systems are complicated and have not been widely adopted.
Recipient-Donor Matching
INTRAOPERATIVE MANAGEMENT
Surgical access is made by means of a standard midline sternotomy. Cardiopulmonary bypass (CPB) is established by employing bicaval cannulation (see Chapter 9). Myocardial protection of the native heart is not required, but perfusion of the donor coronary arteries with cardioplegia solution is commonly performed prior to implantation, particularly if the ischemic time is prolonged. The conventional heart transplant operation involves four suture lines: the aortic, pulmonary artery, and left and right atrial anastomoses. Recently, the single right atrial anastomosis has been modified to include separate anastomoses of the superior and inferior vena cavae. This modification reduces the incidence of atrial arrhythmias and, by minimizing distortion of the tricuspid valve, decreases the incidence of tricuspid regurgitation.11 Separate pulmonary venous anastomoses are also performed in some centers in an attempt to reduce the incidence of left atrial thrombus formation. Together, these modifications to the atrial anastomoses are known as total heart transplant.
In many situations, heart transplantation is a reoperation, and it is commonly performed in patients who have been treated with warfarin for anticoagulation. Thus, postoperative bleeding is common. Aprotinin has been shown to reduce bleeding significantly during reoperative heart transplantation12; its use has now become routine in most centers for all heart transplant procedures.
ROUTINE POSTOPERATIVE CARE
Patients who have had uncomplicated intraoperative courses, are on low-level inotropic support, and are not bleeding excessively can be awakened and extubated in the usual manner (see Chapter 17). Chest drains are removed the morning after surgery according to standard criteria.
Some degree of graft dysfunction is common following heart transplantation, with the right ventricle usually being affected more than the left (see subsequent material). Slightly higher filling pressures (e.g., central venous pressure of 12 to 15 mmHg and pulmonary artery wedge pressures of 14 to 18 mmHg) are usually required for the first few days. However, overfilling of the heart can induce or exacerbate tricuspid regurgitation and should be avoided. Patients usually benefit from moderate tachycardia, which may be achieved with pacing at 90 to 110 beats/min. Low-dose inotropic support for a few days is advisable, even in patients who are relatively stable. Traditionally, isoproterenol has been used for this purpose because of its pulmonary vasodilatory and chronotropic effects. However, important metabolic side effects occur with this agent, and they limit its utility (see Chapter 3). Milrinone or dobutamine in combination with pacing is an alternative to isoproterenol.
Prevention of Infection
The combination of end-stage cardiac disease, major surgery, and immunosuppression increases the risk for developing nosocomial infections following heart transplantation. Protective isolation (see Chapter 35) is not required, but ideally, transplant recipients are nursed in a single room. Thorough hand washing is mandatory prior to all patient contact. The likelihood of infection is minimized by early extubation and early removal of intravascular catheters and drains.
Glucose intolerance is common in the early postoperative period because of the effects of corticosteroids, calcineurine inhibitors, and β agonists. Given the numerous adverse effects of hyperglycemia (see Chapter 36), particularly with respect to wound healing and infection, blood glucose levels should be tightly controlled, ideally kept between 80 and 110 mg/dl (4.5 to 6.6 mmol/l).13 In patients who have negative levels of cytomegalovirus (CMV) antibodies, all blood transfused should be leukocyte depleted or, alternatively, a white-cell filter should be used.
Immunosuppression
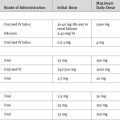
Immunosuppression regimens for heart transplant recipients are essentially the same as those for recipients of lung transplants (see Chapter 13). Standard immunosuppression consists of triple therapy: a calcineurine inhibitor, an antiproliferative agent, and a corticosteroid. The most commonly used regime consists of cyclosporine, mycophenolate mofetil, and prednisone.5 Induction therapy is used in less than 50% of patients.5
Other Medication
In addition to its antihypertensive effect, diltiazem has two additional benefits for heart transplant recipients. First, it retards the development of transplant coronary disease,14 and second, it inhibits the metabolism of calcineurine inhibitors (see Chapter 13); diltiazem has a dose-sparing effect on these drugs. Pravastatin, as well as having beneficial effects on lipid profiles, has been shown to improve 1-year survival rates and to decrease the incidence of transplant coronary disease.15 Limited data on other statins are less compelling. Aspirin is indicated for the prevention of the complications of coronary artery disease.
EARLY COMPLICATIONS
Primary Graft Failure
The major cause of early mortality (fewer than 5 days after heart transplantation) is primary graft failure.5 Although this description includes left and right ventricular dysfunction, the latter is much more common. The graft may have suffered significant myocardial stunning due to prolonged ischemic arrest and inadequate myocardial protection. The graft will have come from a donor with normal pulmonary vascular resistance, whereas pulmonary vascular resistance is often chronically elevated in the recipient as a consequence of chronic heart failure. Added to this is the acute increase in pulmonary vascular resistance that occurs perioperatively due to the effects of CPB, lung atelectasis, acidosis, hypoxemia, and raised intrathoracic pressure. These problems are compounded if a small donor heart is used in a relatively large recipient. Diastolic dysfunction is a problem for both ventricles, but it is particularly poorly tolerated by the right ventricle because right ventricular performance is highly dependent on preload.
The treatment of right ventricular failure is described in detail in Chapter 20. If the hemodynamic state continues to deteriorate despite maximal conventional therapy, mechanical support by means of a right ventricular assist device16,17 or extracorporeal membrane oxygenation (ECMO) should be considered. ECMO may be superior to a right ventricular assist device in this situation,18 and it is certainly preferred if there is concomitant respiratory failure. Left ventricular dysfunction also may occur as a manifestation of primary graft failure. In severe cases, support with a biventricular assist device or ECMO is indicated.
Hyperacute Rejection
Hyperacute rejection is an uncommon cause of primary graft failure that results from preformed antibodies directed against ABO or HLA antigens. The likelihood of the presence of anti-HLA antibodies is increased by previous exposure to blood-product transfusions (particularly platelets), mechanical support, pregnancy, and previous transplantation. The risk for forming antibodies is reduced with the use of leukocyte-depleted transfusions, and this should be mandatory for all potential transplant recipients. The development of hyperacute rejection is greatly reduced, but not eliminated, by performing a donor-recipient cross-match prior to transplantation. In patients with a high percentage of panel-reactive antibodies (<10%), perioperative plasmapheresis combined with immunoglobulin therapy may be used to reduce the incidence and severity of antibody-mediated (and cell-mediated) rejection. These interventions have allowed for transplantation between donors and recipients with positive cross-matches.19 Hyperacute rejection is manifested as severe biventricular failure that, in the absence of immediate retransplantation, is usually fatal.
Tricuspid Regurgitation
Tricuspid regurgitation may occur early after heart transplantation and is typically caused by right ventricular dysfunction or distortion of the tricuspid valve by the right atrial anastomosis. Tricuspid regurgitation that develops weeks or months later may be caused by graft dysfunction resulting from rejection or by damage to a valve leaflet or a subvalvular apparatus during endomyocardial biopsy. In one study, mild, moderate, and severe tricuspid regurgitation were identified intraoperatively by TEE in 9%, 10%, and 0.8% of heart transplant recipients, respectively.20 In this study, the presence of even mild tricuspid regurgitation was associated with increased perioperative mortality rates. If moderate or severe tricuspid regurgitation is identified at the time of transplant surgery, immediate tricuspid repair may be considered. Medical treatment of tricuspid regurgitation involves inotropic support and careful control of intravascular volume by fluid restriction and diuretics.
Bleeding
Bleeding is a common postoperative problem. Coagulation tests (aPTT, PT, fibrinogen, platelet count, thromboelastography) should be obtained in all heart transplant recipients on arrival in the intensive care unit and should be repeated after the administration of blood products. Hypothermia should be corrected but hyperthermia must be avoided. For actively bleeding patients, aprotinin may be continued into the early postoperative period, depending on the results of thromboelastography. For patients who received warfarin up until the time of the transplant, vitamin K (5 to 10 mg daily for 1 to 3 days) may be given if the prothrombin time is elevated. An algorithm for blood component therapy is provided in Figure 17-1.
Renal Failure and Fluid Overload
Renal replacement therapy is required in 10% to 15% of heart transplant recipients during the early postoperative period.21,22 Preoperative renal dysfunction is strongly predictive of the development of postoperative renal dysfunction and is associated with an adverse outcome.21 In one study, a preoperative creatinine clearance of less than 50 ml/min was associated with a twofold increase in the 30-day mortality rate.9 Because of the risk of exacerbating tricuspid regurgitation by fluid overload, early institution of renal replacement therapy for oliguric renal failure is appropriate. Calcineurine inhibitors should be reduced or withheld during the period of renal failure and another agent such as sirolimus substituted.
Gastrointestinal Complications
Diarrhea, nausea, and vomiting can occur as side effects of immunosuppressant therapy, particularly of mycophenolate or, less commonly, secondary to infection by CMV. Serious gastrointestinal complications such as cholecystitis, pancreatitis, gastrointestinal bleeding, and mesenteric ischemia are more common after heart transplantation than after other types of cardiac surgery.23,24 This may reflect the debilitated preoperative state and complicated postoperative course of many heart transplant recipients. Nonspecific signs, such as abdominal distension, abdominal pain, and intolerance of enteral feeds must be thoroughly investigated because they may represent serious underlying illness.
LATER COMPLICATIONS
Complications that occur after the first 5 days are considered to be later complications. From the first week to 1 year after transplantation, acute rejection and infection are the most important postoperative problems. After this period, chronic rejection and malignancy become more important (Fig. 14-1).25

Figure 14.1 Hazard function curves for specific causes of death following heart transplantation.
(Redrawn, with permission, from Kirklin JK, Naftel DC, Bourge RC, et al: Evolving in risk profiles and causes of death after heart transplantation: a ten-year multi-institutional study. J Thorac Cardiovasc Surg 125:881-890, 2003.)
Infection
Infective complications that occur after heart transplantation are similar to those that occur after lung transplantation (see Chapter 13). In the first month the most common infections are bacterial, often by nosocomial organisms such as Pseudomonas aeruginosa. Viral and fungal infections are usually encountered a little later, 1 to 2 months after surgery, whereas protozoal infections by organisms such as Giardia species usually have a peak occurrence several months after transplantation.
Systemic Embolism
Left atrial thrombosis occurs in up to 50% of patients who have undergone heart transplantation by means of conventional surgical techniques, with systemic embolism occurring in 6% to 14%.26,27 The incidence of left atrial thrombosis is markedly reduced when the “total transplant” surgical technique is used.27 If thrombus is identified, anticoagulation with warfarin is indicated.
Transplant Coronary Disease Caused by Chronic Rejection
Chronic rejection is manifested as an atypical, aggressive form of coronary artery disease. In contrast to conventional atherosclerosis, transplant coronary disease is associated with intimal hyperplasia, is commonly concentric and diffuse, and in many cases involves small branch arteries.28
From 1 to 5 years after transplantation, coronary disease is the leading cause of death, with an incidence on coronary angiography of 50% at 5 years.25,29 Intravascular ultrasound studies show an incidence of subclinical disease of up to 75% at 1 year.30 Coronary disease may also be detected as wall motion abnormalities on surveillance echocardiography or stress imaging. Initially, transplant coronary disease is usually clinically silent, although a small number of patients develop angina, presumably due to partial reinnervation of the graft. Eventually patients develop congestive cardiac failure.
Transplant coronary disease is poorly responsive to percutaneous coronary intervention and bypass graft surgery. These procedures provide only short-term benefit and are considered palliative.31 Prognosis is poor; 1-year survival after the first ischemic event is less than 20%.32 Recently, some hope has been provided by the observation that sirolimus retards the development of coronary artery vasculopathy.33,34
Malignancy
Beyond 5 years after transplantation, malignancy surpasses vasculopathy as the major cause of death, with an incidence of 24% at 7 years (see Fig 14-1).25 The vast majority of cancers are of the skin, which occur in 18% of patients at 7 years.5 The remainder are predominantly solid organ neoplasms or lymphoproliferative disorders. These malignancies can often be ameliorated by a reduction in immunosuppression. Transplant recipients are strongly advised to protect their skin against sun exposure and refrain from smoking.
1 Shiba N, Chan MC, Kwok BW, et al. Analysis of survivors more than 10 years after heart transplantation in the cyclosporine era: Stanford experience. J Heart Lung Transplant. 2004;23:155-164.
2 Scientific Registry of Transplant Recipients: www.ustransplant.org.
3 Grady KL, Jalowiec A, White-Williams C. Improvement in quality of life in patients with heart failure who undergo transplantation. J Heart Lung Transplant. 1996;15:749-757.
4 The Organ Procurement and Transplantation Network: www.optn.org.
5 Taylor DO, Edwards LB, Boucek MM, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report —2004. J Heart Lung Transplant. 2004;23:796-803.
6 Costanzo MR, Augustine S, Bourge R, et al. Selection and treatment of candidates for heart transplantation: a statement for health professionals from the Committee on Heart Failure and Cardiac Transplantation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1995;92:3593-3612.
7 Bank AJ, Mir SH, Nguyen DQ, et al. Effects of left ventricular assist devices on outcomes in patients undergoing heart transplantation. Ann Thorac Surg. 2000;69:1369-1374.
8 Zaroff JG, Rosengard BR, Armstrong WF, et al. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations. Circulation. 2002;106:836-841.
9 Ostermann ME, Nelson SR, Rogers CA, et al. Pre-existing renal failure doubles 30-day mortality after heart transplantation. J Heart Lung Transplant. 2004;23:1231-1237.
10 Renlund DG, Taylor DO, Kfoury AG, et al. New UNOS rules: historical background and implications for transplantation management: United Network for Organ Sharing. J Heart Lung Transplant. 1999;18:1065-1070.
11 el Gamel A, Yonan NA, Grant S, et al. Orthotopic cardiac transplantation: a comparison of standard and bicaval Wythenshawe techniques. J Thorac Cardiovasc Surg. 1995;109:721-729.
12 Prendergast TW, Furukawa S, Beyer AJ3rd, et al. Defining the role of aprotinin in heart transplantation. Ann Thorac Surg. 1996;62:670-674.
13 van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
14 Schroeder JS, Gao SZ, Alderman EL, et al. A preliminary study of diltiazem in the prevention of coronary artery disease in heart-transplant recipients. N Engl J Med. 1993;328:164-170.
15 Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621-627.
16 Chen JM, Levin HR, Rose EA, et al. Experience with right ventricular assist devices for perioperative right-sided circulatory failure. Ann Thorac Surg. 1996;61:305-310.
17 Moazami N, Pasque MK, Moon MR, et al. Mechanical support for isolated right ventricular failure in patients after cardiotomy. J Heart Lung Transplant. 2004;23:1371-1375.
18 Taghavi S, Zuckermann A, Ankersmit J, et al. Extracorporeal membrane oxygenation is superior to right ventricular assist device for acute right ventricular failure after heart transplantation. Ann Thorac Surg. 2004;78:1644-1649.
19 Pisani BA, Mullen GM, Malinowska K, et al. Plasmapheresis with intravenous immunoglobulin G is effective in patients with elevated panel reactive antibody prior to cardiac transplantation. J Heart Lung Transplant. 1999;18:701-706.
20 Anderson CA, Shernan SK, Leacche M, et al. Severity of intraoperative tricuspid regurgitation predicts poor late survival following cardiac transplantation. Ann Thorac Surg. 2004;78:1635-1642.
21 Stevens LM, El-Hamamsy I, Leblanc M, et al. Continuous renal replacement therapy after heart transplantation. Can J Cardiol. 2004;20:619-623.
22 Ouseph R, Brier ME, Jacobs AA, et al. Continuous venovenous hemofiltration and hemodialysis after orthotopic heart transplantation. Am J Kidney Dis. 1998;32:290-294.
23 Augustine SM, Yeo CJ, Buchman TG, et al. Gastrointestinal complications in heart and in heart-lung transplant patients. J Heart Lung Transplant. 1991;10:547-555.
24 Sharma S, Reddy V, Ott G, et al. Gastrointestinal complications after orthotopic cardiac transplantation. Eur J Cardiothorac Surg. 1996;10:616-620.
25 Kirklin JK, Naftel DC, Bourge RC, et al. Evolving trends in risk profiles and causes of death after heart transplantation: a ten-year multi-institutional study. J Thorac Cardiovasc Surg. 2003;125:881-890.
26 Derumeaux G, Mouton-Schleifer D, Soyer R, et al. High incidence of left atrial thrombus detected by transoesophageal echocardiography in heart transplant recipients. Eur Heart J. 1995;16:120-125.
27 Bouchart F, Derumeaux G, Mouton-Schleifer D, et al. Conventional and total orthotopic cardiac transplantation: a comparative clinical and echocardiographical study. Eur J Cardiothorac Surg. 1997;12:555-559.
28 Billingham ME. Histopathology of graft coronary disease. J Heart Lung Transplant. 1992;11:S38-S44.
29 Gao SZ, Schroeder JS, Alderman EL, et al. Prevalence of accelerated coronary artery disease in heart transplant survivors: comparison of cyclosporine and azathioprine regimens. Circulation. 1998;80:III100-III105.
30 Rickenbacher PR, Pinto FJ, Chenzbraun A, et al. Incidence and severity of transplant coronary artery disease early and up to 15 years after transplantation as detected by intravascular ultrasound. J Am Coll Cardiol. 1995;25:171-177.
31 Halle AA3rd, DiSciascio G, Massin EK, et al. Coronary angioplasty, atherectomy and bypass surgery in cardiac transplant recipients. J Am Coll Cardiol. 1995;26:120-128.
32 Keogh AM, Valantine HA, Hunt SA, et al. Impact of proximal or midvessel discrete coronary artery stenoses on survival after heart transplantation. J Heart Lung Transplant. 1992;11:892-901.
33 Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847-858.
34 Keogh A, Richardson M, Ruygrok P, et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110:2694-2700.
35 Mancini DM, Eisen H, Kussmaul W, et al. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778-786.