Chapter 49 Hearing Prosthetics
Surgical Techniques
Hearing Loss
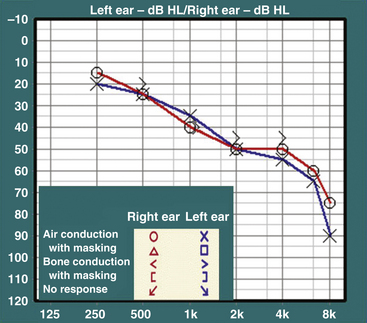
Hearing loss is usually evaluated through audiologic testing. A typical audiogram has two parts: pure tone audiometry and speech audiometry. In pure tone audiometry, air- and bone-conduction thresholds are tested at different frequencies (Fig. 49-1). In conductive hearing loss, the disparity between air- and bone-conduction thresholds results in an air–bone gap. In sensorineural hearing loss, both air- and bone-conduction thresholds are elevated. In speech audiometry, the ability to perceive and discriminate words and sentences is tested. The speech reception threshold is a measure in decibels (dB) of the speech perception threshold, whereas the speech discrimination score (SDS) is a measure as a percentage of words from a standardized list presented at suprathreshold levels that are recognized and repeated by the patient.
Bone-Anchored Osseointegrated Implants
Osseointegrated implants were first introduced into clinical practice in Scandinavia in the late 1960s, ostensibly for intraoral rehabilitation.1 Since then, osseointegrated implants have gained widespread acceptance in the fields of dental, oral/maxillofacial, craniofacial, and orthopedic surgery. Application of this technology to bone-anchored hearing devices represents a refinement of conventional bone-conduction hearing aids. In bone-conduction hearing aids, sound is transmitted through vibration of the skull to the cochlea. However, the utility of conventional bone-conduction devices is now considered limited due to patient discomfort and limited sound fidelity secondary to soft tissue attenuation. Coupling the bone vibrator to an osseointegrated implant, as originally performed by Tjellström and his team at the Institute of Applied Biotechnology in Sweden in 1977, averts many of these limitations of conventional bone conducting devices.2
The success of this technology relies on two basic principles: the creation of a permanent percutaneous connection and the placement of an osseointegrated titanium abutment upon which a transducer is coupled. The cutaneous-implant interface as originally conceived by Brånemark3 is based on biologic principles observed throughout nature. Teeth and nails (as well as talons, tusks, or claws as found in other species) all interface with a thin, firmly attached cutaneous or mucosal border with little to no hair. This tissue architecture limits tissue mobility and preserves stability of tissue planes while inhibiting the penetration of microbes and subsequent inflammation or infection.4,5 By mimicking these attributes in the skull, the surgeon creates a permanent cutaneous-implant border that the patient may easily maintain.
Advances in metallic biomaterials facilitate the creation of permanent, well-tolerated implant fixtures upon which the transducer is placed. Titanium is the most notable among several materials that have found clinical application in anchoring dental prostheses. This is because of its ability to create a corrosion-resistant oxide layer on the surface of the implant that confers osseointegration potential.6,7 Because the implant may be worn for several decades or longer, the toxicity and carcinogenicity of the oxide coating takes on particular importance.8 Reports have shown that titanium is superior to stainless steel, lacking steel’s high potential for corrosion or the toxicity of its components.9,10 To date, pure titanium appears free of the adverse sequelae seen with other metals and thus continues to represent an ideal implant material.7
Currently, the most widely used osseointegrated hearing device is the Baha implant (Cochlear Corporation, Englewood, CO), shown in Fig. 49-2. The Baha consists of a pure titanium implant and a sound processor. The processor couples directly to the titanium implant via a skin penetrating abutment, utilizing a force-fit, plastic coupling.
Indications
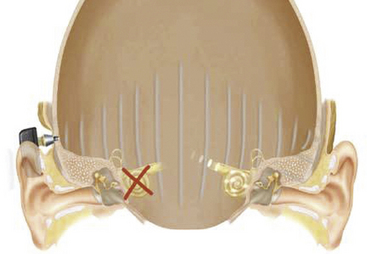
Baha implants were first developed for patients with conductive hearing loss and chronically draining ears, those with discomfort from the sound levels required from a traditional hearing aid, and patients unable to tolerate a hearing aid because of a large mastoid bowl or meatoplasty following chronic ear surgery. Patients with aural atresia are also candidates for Baha implants. Patients who have undergone external auditory canal closure following extensive skull base surgery with preserved inner ear function are also not amenable to traditional hearing aids. This group of patients may benefit from a device that offers bone-anchored hearing.11 Baha implants are also approved for patients with single-sided deafness, for example, due to the effects of a tumor in the cerebellopontine angle or its treatment. In this instance, sound is delivered to the skull on the side lacking sensorineural function and transmitted by bone conduction to the normal contralateral ear, where it is perceived (Fig. 49-3).
Surgical Technique
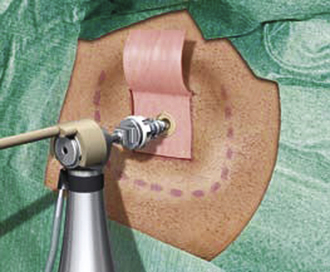
The postauricular area is cleaned and shaved, and the site of the abutment placement is marked. The abutment is typically placed 50 to 55 mm posterior to the ear canal along the temporal line. A dermatome is used to raise an anteriorly based skin flap. The underlying and surrounding soft tissues are removed, leaving the periosteum intact. A small circle of the periosteum (about 6 mm2) is removed, exposing the underlying bony cortex. A 4-mm hole is drilled at the center of the exposed bony cortex. A countersink is then used to enlarge the hole. The abutment is slowly threaded into the previously prepared hole using the abutment inserter (Fig. 49-4). The skin flap is reflected back to its original position, with a central opening to accommodate the abutment. The edges of the skin flap are then sutured to the surrounding periosteum and skin. The abutment is left undisturbed for 3 to 4 months, which allows osseointegration to occur. Once the abutment is osseointegrated, it is ready for coupling to the sound processor.
Complications
In a study looking at postoperative complications of Baha implant in 149 patients, House and Kutz12 found that the most common complication is skin growth over the abutment (7.4%). This is managed by either steroid application or skin flap revision with removal of the underlying scar tissues. In some cases, a split-thickness skin graft may be needed. Other complications include implant extrusion (3.4%), wound infection (1.3%), and flap necrosis (0.7%). Implant extrusion is more likely to occur in radiated bone and in the pediatric population. Pretreatment with hyperbaric oxygen is suggested in radiated patients. The delay to use is extended in this population and in children by 2 to 3 months to allow for optimal osseointegration.
Outcomes
In one of the largest series to date studying the Baha implant, Håkansson et al.13 reported results from 147 patients over 10 years. Patients were divided into three groups based on their pure tone average (PTA) bone-conduction thresholds: 0 to 45 dB, 46 to 60 dB, and more than 60 dB hearing level. The authors noted a strong relationship between PTA and successful rehabilitation. In the group with the best cochlear reserve (PTA of up to 45 dB), 89% of patients said their hearing was subjectively improved by the implant, while 8% felt their hearing was worse. Conversely, in the groups with progressively less cochlear function (the 46 to 60 dB and the more than 60 dB groups), 61% and 22% of patients reported subjective hearing improvement, respectively. Furthermore, SDSs improved on average from 14% unaided and 67% with a traditional hearing aid to 81% with the Baha. This number increased to 85% if people with a sensorineural loss greater than the 60 dB hearing level were excluded and to 89% if subjects with a PTA worse than 45 dB were excluded. Based on these results, the authors recommended that to be in consideration for a “high success rate” with the Baha, patients should have a PTA by bone conduction that is less than the 45 dB hearing level, though improvements in hearing should still be expected for a PTA of up to 60 dB.
Lustig et al.14 performed a review of experience with the Baha in the United States. The most common indications for implantation included chronic otitis media and external auditory canal stenosis and/or aural atresia. Patients who had undergone skull base surgery and had complete closure of the external auditory canal were also included. Overall, each patient had an average improvement of 32 ± 19 dB with the use of the Baha. Complications were limited to local infection and inflammation at the implant site in 3 of 40 patients and failure to osseointegrate in 1 patient. Patient response to the implant was uniformly satisfactory.
In addition to implantation for purely conductive or mixed hearing losses, emerging data indicate the value of Baha amplification for patients with unilateral profound sensorineural hearing loss. The Baha on the deafened ear effectively expanded the sound field for the patient and improved the patient’s speech understanding in noise, much like a CROS hearing aid or transcranial CROS system.15,16 However, in contrast to CROS, the Baha does not require the placement of an ear mold in the better-hearing ear. As the better-hearing ear functions normally, the acoustic “head shadow” can be used to isolate sounds incident to the deafened side but heard in the better ear through transcranial bone conduction from the Baha. This avoids the potential discomfort and perceptual costs of wearing an ear mold on the better-hearing ear. Preliminary results show subjective improvement in both sound quality and speech understanding in noise.17
Cochlear Implants
Cochlear implants are neural prostheses that convey sound information to the auditory cortex via electrical stimulation of the auditory nerve, bypassing the dysfunctional cochlea in individuals with bilateral severe to profound sensorineural deafness. A typical cochlear implant consists of an external component, which includes the microphone and the speech processor, and an internal component, which includes the receiver-stimulator and the stimulating electrodes. The speech processor is battery powered and is housed in a behind-the-ear unit similar to a hearing aid or in a “body” style encasement worn at the waist, carried in a pocket, or otherwise harnessed to the body. A microphone captures acoustic input and delivers it to the speech processor that, in turn, converts it into electrical signals. An external antenna magnetically retained behind the ear transmits the encoded signals across the scalp via radiofrequency to the antenna of the internal device. The signals are then sorted tonotopically and are delivered to different auditory nerve fibers along the cochlear spiral. An example of a cochlear implant is shown in Fig. 49-5.
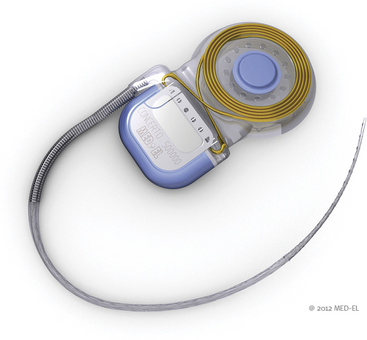
FIGURE 49-5 Cochlear implant. The electrode array and the ground lead are connected to the internal processor.
(Courtesy of Med El.)
Indications
Severe to profound impairment of cochlear function in both ears and anatomic preservation of the auditory nerve in the implanted ear are requirements for cochlear implantation. Currently, criteria vary somewhat but generally include an upper threshold of 40% to 50% speech discrimination with a hearing aid for the poorer hearing ear, with up to 60% in the better-hearing ear, and PTA hearing loss (the average threshold for 500, 1000, and 2000 Hz) of 70 dB or greater in both ears. As experience with cochlear implants grows, outcomes have continued to improve and candidacy criteria based on speech discrimination have continued to evolve toward higher levels of function over the past 25 years.18,19 Mean recognition scores for words in isolation after implantation now far exceed the 40% level, and individuals with some preserved speech-recognition ability preoperatively often score substantially higher postoperatively than prior to implantation.20,21 Residual hearing as reflected in aided speech-recognition levels is an important predictor of implant success.22,23 When combined with duration of deafness, preoperative scores on tests of sentence recognition provide a predictive composite that accounts for approximately 80% of the variance in postoperative word recognition.19
Preservation of auditory nerve integrity should be strongly considered in all neurofibromatosis type 2 (NF2) cases—whether or not the contralateral ear has already demonstrated hearing loss—because it provides an opportunity for future benefit from prosthetic hearing at the cochlea. If the contralateral tumor ear continues to benefit from a hearing aid but is undergoing measurable decline in function, early implantation of the resected side provides an opportunity for the patient to transfer to electrical hearing as acoustic hearing diminishes. Lustig et al. reported on audiologic results in seven patients with NF2 who were implanted following surgical resection with nerve preservation or stereotactic radiation of vestibular schwannomas.24 Whereas hearing acuity and awareness of environmental sound was achieved in all cases, the variability in speech understanding exceeded what is typical for cochlear implantation for cochlear disease. Three patients acquired open-set speech perception in auditory-only testing conditions. In most cases, even when speech understanding was poor, lipreading was enhanced by improved sound awareness, as was sound localization in cases with some residual hearing in the contralateral ear. Trotter and Briggs25 observed favorable communication results in three NF2 patients with cochlear implants in ears whose tumors were treated with stereotactic radiation. Subtotal removal of vestibular schwannoma in two patients and stereotactic radiation therapy in a third were associated with high open-set function in all patients.26 Neff et al. reported sustained open- and closed-set speech perception benefit during an average of 7.9 years.27 They emphasized the utility of promontory electrical stimulation as a predictor of favorable outcome.
Comprehensive assessment of candidacy is essential to minimize risks and realize benefits of cochlear implantation. To ensure complete assessment of candidacy, clinicians should consider the many factors likely to affect performance with a cochlear implant, including audiologic, medical, surgical, developmental, cognitive, and psychosocial factors. Candidates should understand that the cochlear implant is a communication tool and is not a cure for deafness, because expectations largely shape postoperative satisfaction with any form of auditory rehabilitation.28
Candidacy should be considered in the context of current functional status and likely outcome with and without cochlear implantation. Patient age, etiology of hearing loss, unaided and aided hearing, duration of deafness, and circumstances of social support surrounding the candidate carry predictive value. Environments that enrich and promote spoken language are likely to exert a favorable influence over use of the device and contribute to maximal benefit from it. Tyler and Summerfield29 observed evidence of the influence of auditory plasticity in adults with postlingual hearing loss. They found that speech perception ability and the duration of profound/total deafness before implantation were significantly correlated with postimplantation hearing outcome. Performance improved over time after implantation. For adult patients, the level of performance measured shortly after implantation was about half the level measured eventually. Performance tended to reach an asymptote after approximately 3 years of implant use. Such observations suggest that an established pathway for auditory processing is present even in profound sensorineural hearing loss and that refined processing develops over time. Although there are additional negative correlations between duration of deafness and performance,20,22,23,29 such correlations do not apply to every case. Even a prolonged period of deafness does not rule out prospects for speech understanding with a cochlear implant, provided that basic foundations of communicating through audition (e.g., prior hearing aid use, use of lipreading, and production of speech) are in place.
Consideration should be given to conditions for which a patient may need future assessment with magnetic resonance imaging (MRI). Implantation of a magnet in the internal device may be contraindicated in these patients. A nonmagnetic modification of commercially available devices is available for patients whose medical or neurologic condition mandates future MRI studies.30 However, Baumgartner et al.31 found that MRI applied to cochlear implant patients using different devices with indwelling magnets did not cause implant malfunction or patient injury when imaged at 1 tesla (T). Our own experience (unpublished) suggests that MRI with a 1.5-T magnet poses no significant threats if the device is immobilized using externally applied molding material and is firmly bound.
Imaging Studies
High-resolution computed tomography (CT) scans of the temporal bone define surgical anatomy and provide information about cochlear abnormalities that can aid the surgeon in surgical planning and patient counseling. Temporal bone CT scans should be obtained and reviewed for evaluation of temporal bone anatomy, with particular attention paid to mastoid pneumatization, ossicular anatomy, position of great vessels, position of the facial nerve, caliber of the internal auditory canal, and labyrinthine anatomy.32 Scans are examined for evidence of cochlear malformation and ossification, enlarged vestibular aqueduct, and other inner ear and skull base anomalies that can affect implant surgery. CT findings of cochlear patency generally correlate with surgical findings,33 but significant discrepancies can occur as a result of volume averaging.34,35
MRI may be a useful adjunct to CT for assessment of implant candidacy.36–38 Whereas CT is the procedure of choice for detailing bony anatomy, MRI is ideal for imaging soft tissues such as the membranous labyrinth, nerves in the internal auditory canal, and soft tissue within a cochlea en route to cochlear ossification after meningitis. High-resolution T2-weighted MRI is especially helpful for determining cochlear patency (by revealing the presence or absence of fluid within the scalae) and the presence or absence of nerves within the internal auditory canal.
Surgical Technique
A simple mastoidectomy is performed, preserving a slight overhang at the superior and posterior cortical margins. This provides protection for the connecting leads. The facial recess is opened to maximize visualization of the incudostapedial joint and cochlear promontory after adequate thinning of the bony canal wall, opening of the antrum, and removal of adequate air cells to enable systematic exposure of the horizontal semicircular canal, fossa incudus, and chordafacial angle. Before insertion of the electrode array, a well is created behind the mastoid to accommodate the receiver-stimulator portion of the internal device.
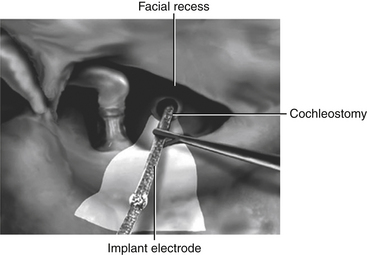
Thinning of the bone anterior to the vertical segment of the facial nerve maximizes visualization of the round window niche via the facial recess. The round window niche should be exposed, and the round window membrane should be identified.39 Inspection of the posterior promontory allows for identification of the round window niche if it is bridged with bone; the niche is never more than 2 mm from the inferior margin of the oval window. A cochleostomy is created in the scala tympani, either directly through the round window membrane or indirectly through the promontory anteroinferior to the center of the round window niche. The preferred approach is debatable, but the priority should be to access the scala tympani without inducing direct injury to the basilar membrane yet while providing adequate space for unimpeded insertion of the electrode array.40 This array is advanced under direct visualization along a trajectory tangential to the basal turn of the scala tympani (Fig. 49-6). Resistance to array insertion suggests the risk of buckling of the carrier. Because buckling of the implant can injure the spiral ligament, basilar membrane, or cochlear nerve endings, aggressive insertion is avoided. Full insertion of the array within the basal turn of the cochlea requires an insertion depth of 25 to 30 mm, depending on array length. After insertion of the array, the cochleostomy should be sealed gently with a small piece of fascia. The connecting lead should be stabilized within the facial recess to reduce the likelihood of the array extruding from the cochlea. Drilling a notch in the bridge of bone that defines the superior end of the facial recess provides a convenient slot in which the array lead can be stabilized. Once the electrode array is in place, the receiver-stimulator is stabilized with sutures to the bony cortex or by a tight-fit pocket subjacent to the pericranium and deep fascia of the temporalis muscle. As the incision is closed, the implanted device is covered completely with the scalp. Initial activation of the cochlear implant typically takes place 3 to 6 weeks after implantation to allow sufficient time for healing of the surgical incision and resolution of the accompanying edema.
Complications
The risks for cochlear implantation include the risks inherent in extended mastoid surgery and those associated with the implanted device. Cohen et al.41 characterized implant-related complications as major if they required revision surgery and minor if they resolved with minimal or no treatment. A survey of 459 cochlear implant operations reported 55 major (12%) and 32 minor (7%) complications. Webb et al.,42 reporting on their experience with 153 patients, found 13.7% major complications and 13.7% minor complications. Hoffman and Cohen43 noted that in later follow-up, 220 (8%) major and 119 (4.3%) minor complications occurred among 2751 implantations. Direct comparisons of complication rates between reports fail to include information on duration of device use, and studies vary in length and frequency of follow-up. Notwithstanding these limitations, longitudinal tracking indicates a substantial reduction in the incidence of major complications in the past 10 years.43
Although much less prevalent, device failure as a result of loss of electrical function in the internal device is of considerably greater concern. An internal device failure typically presents as either immediate cessation of hearing or intermittent hearing loss associated with reduced quality of sound and a period of diminishing function over days to weeks. Reports of painful stimulation have been noted but are rare. A review of experiences at Johns Hopkins University reveals a 1.3% annual risk of revision surgery, including reimplantation, in adults.44 Device failure (65%) was the most common indication of reimplantation, of which about one third presented with complete cessation of device function and two thirds experienced less dramatic declines in function. Other indications included infection (12%), electrode extrusion (15%), and facial nerve stimulation (8%). Cochlear implants have maintained a historical reliability of 99% at 1 year. Reported trends suggest that device reliability has improved over the past 30 years.45 For example, the failure rate for the third-generation Cochlear Corporation (Lane Cove, New South Wales, Australia) device released in 2001 (0.3% per year) is approximately one third of that associated with the company’s first-generation device (0.8% per year) used from 1985 to 1998.
The risk of bacterial infection of an implanted device producing labyrinthitis or meningitis and associated reactive fibrosis and destruction of neural elements appears to be low. Franz et al.46 inoculated the bullae of implanted cats with streptococci to evaluate the risk of infection spreading from the middle ear into the implanted cochlea. Despite resulting inflammation of the round window niche, cochlear inflammation was absent. This finding was attributed to a seal that had formed around the electrode at the entrance to either the round window or the cochleostomy, thus forming a protective barrier against implant-surface colonization and bacterial biofilm.47
In 2002, an increase in the number of postimplantation meningitis cases was noted anecdotally. Initial reports suggested a higher risk of meningitis in patients implanted with a particular device design—especially when placed with an intracochlear shim (electrode positioner)—and the manufacturer ultimately recalled unimplanted devices utilizing the positioner. The level of risk did not suggest the need for positioner removal unless repeated infections occurred. Since then, continued studies have revealed a higher risk for the disease in patients with all cochlear implants compared to the general population. Children appear to be particularly affected: of the 52 cases originally reported by the U.S. Food and Drug Administration, 33 (63%) were under the age of 7.48
Reefhuis et al.49 conducted a study of 4264 children implanted between 1997 and 2002 and found 29 cases of bacterial meningitis in 26 children. This rate of meningitis (caused by Streptococcus pneumoniae) was 30 times the incidence in the general population. While the use of a positioner increased the likelihood of contracting meningitis, even children implanted without a positioner had rates of meningitis 16 times higher than the general population. Case-control analysis found the following risk factors: a history of placement of a ventriculoperitoneal cerebrospinal fluid (CSF) shunt, a history of otitis media prior to implantation, the presence of CSF leakage alone or inner ear malformations with CSF leakage, the use of a positioner, incomplete insertion of the electrode, signs of middle ear inflammation at the time of implantation, and exposure to smoking in the household. While the incidence of meningitis decreased considerably after the perioperative period, cases still appeared even 2 years after implantation.
The design of the study conducted by Reefhuis et al.,47 as noted by the authors, did not permit the comparison of meningitis risk between deaf children with and without a cochlear implant. That is, there is no comprehensive epidemiologic analysis of the risk of meningitis in deaf children in general. It is biologically plausible that deafness itself poses a greater risk of meningitis given the strong association of deafness with skull base anomalies that predispose individuals to the disease. It is also possible that any surgical implant placed in or near the skull base of toddlers and young children (e.g., CSF shunts) carries a prolonged risk of meningitis.
Outcomes
Clinical observations in adult patients with current processors indicate that for patients with implant experience beyond 6 months, mean scores on word recognition approximate 40%, with a range of 0% to 100%.22 Results achieved with the most recently developed speech processing strategies reveal mean scores above 75% correct on words-in-sentence testing, again with a range of scores from 0% to 100%.
Bilateral Cochlear Implantation
Tyler et al.50 noted that bilateral cochlear implantation generally enables head shadow effects in discerning speech in noise. However, only some patients benefit from summation and squelch effects. Most adult bilateral recipients have shown improved horizontal plane localization. Similarly, Litovsky et al.51 and Schoen et al.52 have observed general trends toward additional benefit with the use of a second implant. However, different binaural benefits were obtained in different subjects, and these studies document cases wherein no additional benefit with bilateral implantation was achieved.
Although the “era of bilateral implantation” has been introduced, there are few controlled trials to date. Preliminary results show promise in enabling the use of the head shadow, an expanded sound field, and some sound localization ability to the majority of bilateral recipients.53,54 Moreover, some observed benefits are attributable to improved summation and squelch. These findings have demonstrated that the brain can integrate electrical stimulation from the two ears. However, systems that enable integrated, bilateral sound field processing have not yet been introduced clinically, possibly limiting the potential benefits of bilateral implantation.
Auditory Brain Stem Implants
Patients with bilateral hearing loss due to bilateral vestibular schwannomas (e.g., in NF2) experience devastation of their spoken communication abilities. In such patients, auditory nerve damage due to tumor progression or tumor removal may render cochlear implantation ineffective. Instead, electrical hearing can be achieved with an electrode device placed on the cochlear nucleus of the pontomedullary brain stem. The first single-channel auditory brain stem implant (ABI) was placed by House and Hitselberger.55 Since then, significant technical advancement and modifications have taken place. The current ABI device (Fig. 49-7) consists of an electrode array with 21 channels (Cochlear Corporation, Englewood, CO). Newer designs employing multitined penetrating microelectrodes may permit access to deeper and more discrete populations of cochlear nucleus neurons and may offer improved spectral and dynamic range.56
Indications
More recently in Europe, ABIs have been placed in non-NF2 patients.57–58 These patients have conditions that preclude cochlear implant placement: severe cochlear malformations, cochlear nerve dysplasia/hypoplasia, cranial nerve VIII avulsion, or cochlear ossification.
Surgical Technique
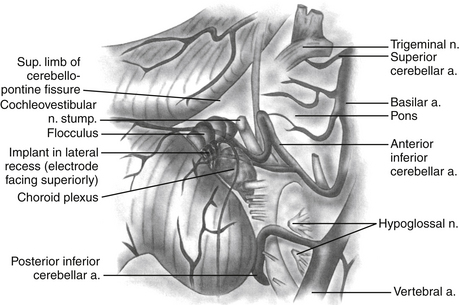
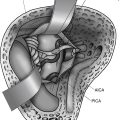
ABIs can be placed via the translabyrinthine or the retrosigmoid approaches, often in the same setting as tumor resection. The details of these two surgical approaches are discussed elsewhere in the text. A well is created in the bony cortex posterior to mastoidectomy cavity or the retrosigmoid craniotomy site to allow for placement of the receiver-stimulator, similar to cochlear implantation. Once the receiver-stimulator is secured in place, the cochlear nucleus is located. The anatomic landmarks for locating the cochlear nucleus include the choroids plexus, which marks the entrance to the foramen of Luschka (lateral opening of the fourth ventricle) and lateral recess.55 Cranial nerves V and VII through X and the anterior inferior cerebellar artery are identified and used to locate the area in which the cochlear nuclei reside in the superolateral aspect of the floor of the fourth ventricle (Fig. 49-8). Anatomic landmarks may be obscured by choroids plexus and displaced by effects of tumor growth. The electrode array is placed on the surface of the brain stem overlying the ventral cochlear nuclei, in the anterosuperolateral aspect of the lateral recess. Placement is adjusted based on intraoperative auditory brain stem response waveforms elicited by stimulation via the implant, and then the implant is stabilized. The surgical site is then closed as in translabyrinthine and retrosigmoid approaches for tumor resection, taking every precaution to prevent CSF leakage.
Outcomes
The ABI is usually activated within 4 to 6 weeks after surgery. Otto et al. reviewed the ABI experience at the House Ear Institute.59 This study included 61 patients who underwent placement of an ABI. In this cohort, 6 patients report no useful auditory sensation after the ABI. Patients with the ABI scored well above chance for closed-set speech tests (Monosyllable Trochee Spondee, or MTS, test and Northwestern University Children’s Perception of Speech test), but significant open-set word recognition was rare. Otto et al. found that for most patients with an ABI, the benefit in communication occurs when sound is used in conjunction with lipreading. There were 2 patients who experienced CSF leakage that resolved with pressure dressing and lumbar drain placement.
In a study by Nevison et al., the results of an ABI in 27 patients were reviewed.60 All but 1 patient received useful auditory sensation after implantation. In the study, 16 patients were tested using a closed-set MTS word test. Of the 16 patients, 15 scored close to 100% in the auditory–visual condition. In the auditory-only condition, scores were lower but were well above chance. Seventeen patients underwent open-set sentence-recognition tests. Eight patients scored at or greater than 50% in auditory–visual condition. One patient even scored 68% in the auditory-only condition. Two patients experienced facial nerve stimulation, which resulted in nonuse. One patient experienced a gradual increase in epileptic seizures after ABI. No CSF leak was reported in this study.
As mentioned earlier, ABI has been placed more recently in non-NF2 patients with deafness, which precludes cochlear implant placement. In a study comparing the ABI hearing outcomes in NF2 patients versus non-NF2 patients, Colletti et al. found that the average open-set speech-recognition score for non-NF2 patients was 59%, whereas the average score for NF2 patients was 10%.58 Colletti et al. postulated that the poorer performance seen in NF2 patients is secondary to damage to the cochlear nucleus by tumor; thus, the central auditory system never receives sufficient information from the auditory periphery.
Brackmann D.E., Hitselberger W.E., Nelson R.A., et al. Auditory brainstem implant: I. Issues in surgical implantation. Otolaryngol Head Neck Surg. 1993;108:624-633.
Cohen N.L., Hoffman R.A., Stroschein M. Medical or surgical complications related to the nucleus multichannel cochlear implant. Ann Otol Rhinol Laryngol Suppl. 1988;135:8-13.
Colletti V., Shannon R., Carner M., et al. Outcomes in nontumor adults fitted with the auditory brainstem implant: 10 years’ experience. Otol Neurotol. 2009;30(5):614-618.
Friedland D.R., Venick H.S., Niparko J.K. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otol Neurotol. 2003;24(4):582-589.
Håkansson B., Liden G., Tjellström A., et al. Ten years of experience with the Swedish bone-anchored hearing system. Ann Otol Rhinol Laryngol Suppl. 1990;151:1-16.
House J.W., Kutz J.W.Jr. Bone-anchored hearing aids: incidence and management of postoperative complications. Otol Neurotol. 2007;28(2):213-217.
Lustig L.R., Arts H.A., Brackmann D.E., et al. Hearing rehabilitation using the BAHA bone-anchored hearing aid: results in 40 patients. Otol Neurotol. 2001;22(3):328-334.
Lustig L., Niparko J. Osseointegrated implants in otology. Adv Otolaryngol Head Neck Surg. 1999;13:105-126.
Lustig L.R., Yeagle J., Driscoll C.L.W., et al. Cochlear implantation in patients with neurofibromatosis type 2 and bilateral vestibular schwannoma. Otol Neurotol. 2006;27:512-518.
NIH Consensus Conference. Cochlear implants in adults and children. JAMA. 1995;274(24):1955-1961.
NIH Consensus Development Statement. Cochlear Implants. NIH Consensus Statement. 1988;7(2):1-25.
Niparko J.K., Cox K.M., Lustig L.R. Comparison of the bone anchored hearing aid implantable hearing device with contralateral routing of offside signal amplification in the rehabilitation of unilateral deafness. Otol Neurotol. 2003;24(1):73-78.
Otto S.R., Brackmann D.E., Hitselberger W.E., et al. Multichannel auditory brainstem implant: update on performance in 61 patients. J Neurosurg. 2002;96(6):1063-1071.
Rauschecker J.P., Shannon R.V. Sending sound to the brain. Science. 2002;295(5557):1025-1029.
Reefhuis J., Honein M.A., Whitney C.G., et al. Risk of bacterial meningitis in children with cochlear implants. N Engl J Med. 2003;349:435-445.
Rivas A., Marlowe A.L., Chinnici J.E., et al. Revision cochlear implant surgery in adults: indications and results. Otol Neurotol. 2008;29(5):639-648.
Rubinstein J.T., Parkinson W.S., Tyler R.S., et al. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol. 1999;20(4):445-452.
Tjellström A., Lindstrom J., Hallen O., et al. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol. 1981;4:304-310.
Waltzman S.B., Fisher S.G., Niparko J.K., et al. Predictors of postoperative performance with cochlear implants. Ann Otol Rhinol Laryngol Suppl. 1995;165:15-18.
Webb R.L., Lehnhardt E., Clark G.M., et al. Surgical complications with the cochlear multiple-channel intracochlear implant: experience at Hannover and Melbourne. Ann Otol Rhinol Laryngol. 1991;100(2):131-136.
1. Brånemark P., Breine U., Lindstrom J., et al. Intra-osseous anchorage of dental prostheses I: Experimental studies. Scand J Plast Reconstr Surg. 1969;3:81-100.
2. Tjellström A., Lindstrom J., Hallen O., et al. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol. 1981;4:304-310.
3. Brånemark P., Labrektsson T. Titanium implants permanently penetrating human skin. Scand J Plast Reconstr Surg. 1982;16:17-21.
4. Adell R., Lekholm U., Rockler B., et al. Marginal tissue reactions as osseointegrated titanium fixtures: I. A three year longitudinal prospective study. Int J Oral Surg. 1986;15:53-61.
5. Lekholm U., Adell R., Lindhe J., et al. Marginal tissue reactions at osseointegrated titanium fixtures. (II) A cross-sectional retrospective study. Int J Oral Maxillofac Surg. 1986;15(1):53-61.
6. Zarb G. Impact of Osseointegration on prosthodontics. In: Laney W., Tolman D. Tissue Integration in Oral, Orthopedic and Maxillofacial Reconstruction. Carol Stream, IL: Quintessence Publishing, 1992.
7. Johansson C.B. On tissue reactions to metal implants. Göteborg: Biomaterials/Handicap Research, University of Göteborg; 1991. Ph.D. Thesis
8. Eriksson E., Brånemark P. Osseointegration from the perspective of the plastic surgeon. Plast Reconstr Surg. 1994;93:626-637.
9. von Ludinghausen M., Meister P., Probst J. Metallosis after osteosynthesis. Pathol Eur. 1970;5(3):307-314.
10. Pazzaglia U.E., Minoia C., Ceciliani L., et al. Metal determination in organic fluids of patients with stainless steel hip arthroplasty. Acta Orthop Scand. 1983;54:574-579.
11. Lustig L., Niparko J. Osseointegrated implants in otology. Adv Otolaryngol Head Neck Surg. 1999;13:105-126.
12. House J.W., Kutz J.W.Jr. Bone-anchored hearing aids: incidence and management of postoperative complications. Otol Neurotol. 2007;28(2):213-217.
13. Håkansson B., Liden G., Tjellström A., et al. Ten years of experience with the Swedish bone-anchored hearing system. Ann Otol Rhinol Laryngol Suppl. 1990;151:1-16.
14. Lustig L.R., Arts H.A., Brackmann D.E., et al. Hearing rehabilitation using the BAHA bone-anchored hearing aid: results in 40 patients. Otol Neurotol. 2001;22(3):328-334.
15. Vaneecloo F.M., Ruzza I., Hanson J.N., et al. The monaural pseudo-stereophonic hearing aid (BAHA) in unilateral total deafness: a study of 29 patients. Rev Laryngol Otol Rhinol (Bord). 2001;122(5):343-350.
16. Niparko J.K., Cox K.M., Lustig L.R. Comparison of the bone anchored hearing aid implantable hearing device with contralateral routing of offside signal amplification in the rehabilitation of unilateral deafness. Otol Neurotol. 2003;24(1):73-78.
17. Wazen J.J., Spitzer J.B., Ghossaini S.N., et al. Transcranial contralateral cochlear stimulation in unilateral deafness. Otolaryngol Head Neck Surg. 2003;129(3):248-254.
18. NIH consensus conference. Cochlear implants in adults and children. JAMA. 1995;274(24):1955-1961.
19. NIH Consensus Development Statement. Cochlear Implants. NIH Consensus Statement. 1988;7(2):1-25.
20. Waltzman S.B., Fisher S.G., Niparko J.K., et al. Predictors of postoperative performance with cochlear implants. Ann Otol Rhinol Laryngol Suppl. 1995;165:15-18.
21. Tyler R.S., Moore B.C., Kuk F.K. Performance of some of the better cochlear-implant patients. J Speech Hear Res. 1989;32(4):887-911.
22. Rubinstein J.T., Parkinson W.S., Tyler R.S., et al. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol. 1999;20(4):445-452.
23. Friedland D.R., Venick H.S., Niparko J.K. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otol Neurotol. 2003;24(4):582-589.
24. Lustig L.R., Yeagle J., Driscoll C.L.W., et al. Cochlear implantation in patients with neurofibromatosis type 2 and bilateral vestibular schwannoma. Otol Neurotol. 2006;27:512-518.
25. Trotter M.I., Briggs R.J. Cochlear implantation in neurofibromatosis type 2 after radiation therapy. Otol Neurotol. 2010;31(2):216-219.
26. Huy P.T., Kania R., Frachet B., et al. Auditory rehabilitation with cochlear implantation in patients with neurofibromatosis type 2. Acta Otolaryngologica. 2009;129:971-975.
27. Neff B.A., Wiet R.M., Lasak J.M., et al. Cochlear implantation in the neurofibromatosis type 2 patient: long-term follow-up. Laryngoscope. 2007;117:1069-1072.
28. Ross M., Levitt H. Consumer satisfaction is not enough: hearing aids are still about hearing. Sem Hear. 1997;18(1):7-11.
29. Tyler R.S., Summerfield A.Q. Cochlear implantation: relationships with research on auditory deprivation and acclimatization. Ear Hear. 1996;17(3 suppl):38S-50S.
30. Heller J.W., Brackmann D.E., Tucci D.L., et al. Evaluation of MRI compatibility of the modified nucleus multichannel auditory brainstem and cochlear implants. Am J Otol. 1996;17(5):724-729.
31. Baumgartner W.D., Youssefzadeh S., Hamzavi J., et al. Clinical application of magnetic resonance imaging in 30 cochlear implant patients. Otol Neurotol. 2001;22:818-822.
32. Woolley A.L., Oser A.B., Lusk R.P., et al. Pre-operative temporal bone computed tomography scan and its use in evaluating the pediatric cochlear implant candidate. Laryngoscope. 1997;108:1100-1106.
33. Langman A.W., Quigley S.M. Accuracy of high-resolution computed tomography in cochlear implantation. Otolaryngol Head Neck Surg. 1996;114(1):38-43.
34. Wiet R.J., Pyle G.M., O’Connor C.A., et al. Computed tomography: how accurate a predictor for cochlear implantation? Laryngoscope. 1990;100(7):687-692.
35. Frau G.N., Luxford W.M., Lo W.W., et al. High-resolution computed tomography in evaluation of cochlear patency in implant candidates: a comparison with surgical findings. J Laryngol Otol. 1994;108(9):743-748.
36. Harnsberger H.R., Dart D.J., Parkin J.L., et al. Cochlear implant candidates: assessment with CT and MR imaging. Radiology. 1987;164(1):53-57.
37. Casselman J.W., Kuhweide R., Deimling M., et al. Constructive interference in steady state-3DFT MR imaging of the inner ear and cerebellopontine angle. Am J Neuroradiol. 1993;14:47-57.
38. Bettman R., Beek E., Van Olphen A., et al. MRI versus CT in assessment of cochlear patency in cochlear implant candidates. Acta Otolaryngol. 2004;124(5):577-581.
39. Proctor B., Bollobas B., Niparko J.K. Anatomy of the round window niche. Ann Otol Rhinol Laryngol. 1986;95(5 Pt 1):444-446.
40. Adunka O., Gstoettner W., Hambek M., et al. Preservation of basal inner ear structures in cochlear implantation. ORL J Otorhinolaryngol Relat Spec. 2004;66(6):306-312.
41. Cohen N.L., Hoffman R.A., Stroschein M. Medical or surgical complications related to the nucleus multichannel cochlear implant. Ann Otol Rhinol Laryngol Suppl. 1988;135:8-13.
42. Webb R.L., Lehnhardt E., Clark G.M., et al. Surgical complications with the cochlear multiple-channel intracochlear implant: experience at Hannover and Melbourne. Ann Otol Rhinol Laryngol. 1991;100(2):131-136.
43. Hoffman R.A., Cohen N.L. Surgical pitfalls in cochlear implantation. Laryngoscope. 1993;103:741.
44. Rivas A., Marlowe A.L., Chinnici J.E., et al. Revision cochlear implant surgery in adults: indications and results. Otol Neurotol. 2008;29(5):639-648.
45. Cochlear Americas. Nucleus Report, 2004 Nov/Dec Available http://www.cochlearamericas.com/professional/PDFs/N30791_Nucleus_Report_AQM.pdf Accessed Aug 7, 2005
46. Franz B.K., Clark G.M., Bloom D.M. Effect of experimentally induced otitis media on cochlear implants. Ann Otol Rhinol Laryngol. 1987;96(2 Pt 1):174-177.
47. Antonelli P.J., Lee J.C., Burne R.A. Bacterial biofilms may contribute to persistent cochlear implant infection. Otol Neurotol. 2004;25(6):953-957.
48. U.S. Food and Drug Administration. Risk of bacterial meningitis in children with cochlear implants, July 24, 2002 http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm064526.htm
49. Reefhuis J., Honein M.A., Whitney C.G., et al. Risk of bacterial meningitis in children with cochlear implants. N Engl J Med. 2003;349:435-445.
50. Tyler R.S., Dunn C.C., Witt S.A., et al. Residual speech perception and cochlear implant performance in postlingually deafened adults. Ear Hear. 2003;24(6):539-544.
51. Litovsky R.Y., Parkinson A., Arcaroli J., et al. Bilateral cochlear implants in adults and children. Arch Otolaryngol Head Neck Surg. 2004;130(5):648-655.
52. Schoen F., Mueller J., Helms J., et al. Sound localization and sensitivity to interaural cues in bilateral users of the Med-El Combi 40/40+ cochlear implant system. Otol Neurotol. 2005;26(3):429-437.
53. Laszig R., Aschendorff A., Stecker M., et al. Benefits of bilateral electrical stimulation with the nucleus cochlear implant in adults: 6-month postoperative results. Otol Neurotol. 2004;25(6):958-968.
54. Schleich P., Nopp P., D’Haese P. Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBI 40/40+ cochlear implant. Ear Hear. 2004;25(3):197-204.
55. Brackmann D.E., Hitselberger W.E., Nelson R.A., et al. Auditory brainstem implant: I. Issues in surgical implantation. Otolaryngol Head Neck Surg. 1993;108:624-633.
56. Rauschecker J.P., Shannon R.V. Sending sound to the brain. Science. 2002;295(5557):1025-1029.
57. Colletti V., Shannon R.V. Open set speech perception with auditory brainstem implant? Laryngoscope. 2005;115(11):1974-1978.
58. Colletti V., Shannon R., Carner M., et al. Outcomes in nontumor adults fitted with the auditory brainstem implant: 10 years’ experience. Otol Neurotol. 2009;30(5):614-618.
59. Otto S.R., Brackmann D.E., Hitselberger W.E., et al. Multichannel auditory brainstem implant: update on performance in 61 patients. J Neurosurg. 2002;96(6):1063-1071.
60. Nevison B., Laszig R., Sollmann W.P., et al. Results from a European clinical investigation of the nucleus multichannel auditory brainstem implant. Ear Hear. 2002;23(3):170-183.