Hand
Innervation of Muscles, Skin, and Joints of the Hand
Interaction of the Extrinsic and Intrinsic Muscles of the Fingers
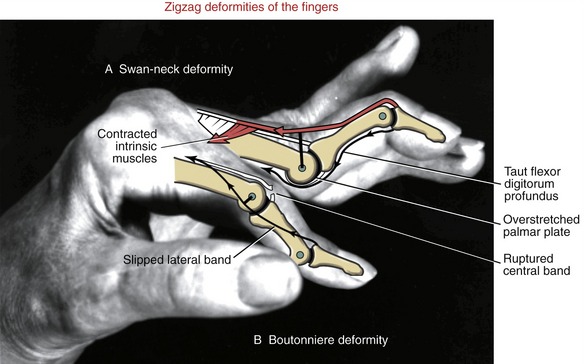
JOINT DEFORMITIES TYPICALLY CAUSED BY RHEUMATOID ARTHRITIS
Similar to the eye, the hand serves as a very important sensory organ for the perception of one’s surroundings (Figure 8-1). The hand is also a primary effector organ for our most complex motor behaviors, and the hand helps to express emotions through gesture, touch, music, and art.
Because of the hand’s enormous biomechanical complexity, its function involves a disproportionately large region of the cortex of the brain (Figure 8-2). Diseases or injuries affecting the hand often create a disproportionate disability. A hand totally incapacitated by rheumatoid arthritis, stroke, or nerve or bone injury, for instance, can dramatically reduce the function of the entire upper limb. This chapter describes the kinesiologic principles behind many of the musculoskeletal impairments of the hand frequently encountered in medical and rehabilitation settings. These principles often serve as the basis for treatment.
TERMINOLOGY
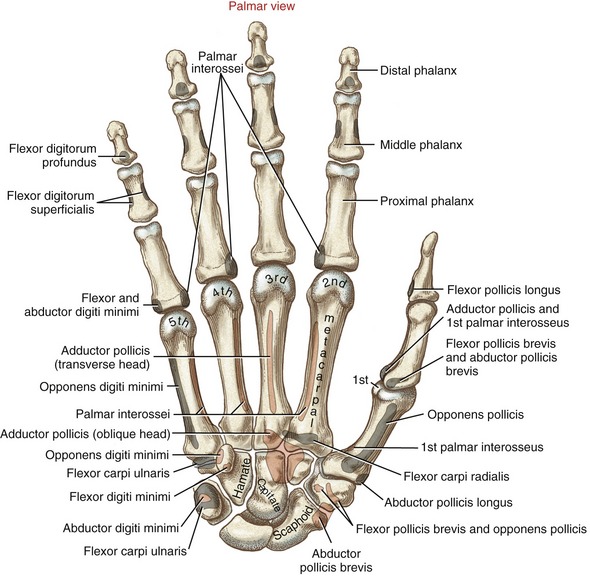
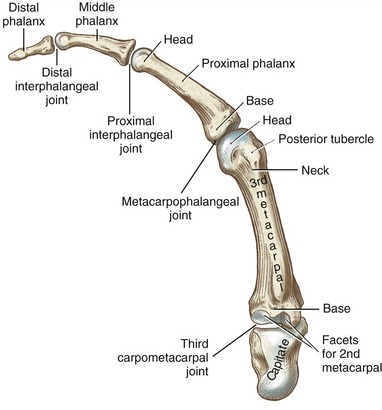
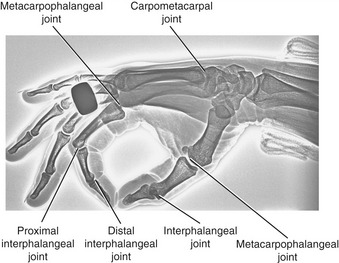
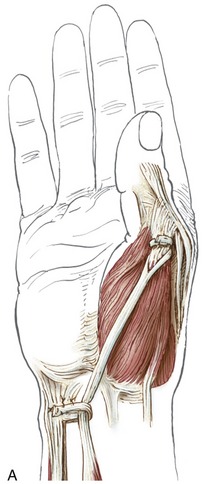
The wrist, or carpus, has eight carpal bones. The hand has five metacarpals, often referred to collectively as the “metacarpus.” Each of the five digits contains a set of phalanges. The digits are designated numerically from one to five, or as the thumb and the index, middle, ring, and small (little) fingers (Figure 8-3, A). A ray describes one metacarpal bone and its associated phalanges.
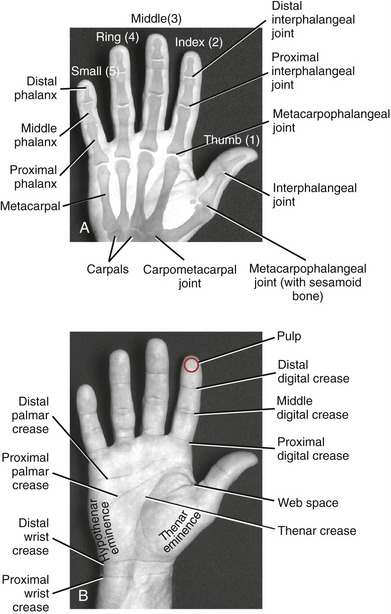
FIGURE 8-3. A palmar view of the basic anatomy of the hand. A, Major bones and joints. B, External landmarks.
The articulations between the proximal end of the metacarpals and the distal row of carpal bones form the carpometacarpal (CMC) joints (see Figure 8-3, A). The articulations between the metacarpals and the proximal phalanges form the metacarpophalangeal (MCP) joints. Each finger has two interphalangeal joints: a proximal interphalangeal (PIP) and a distal interphalangeal (DIP) joint. The thumb has only two phalanges and therefore only one interphalangeal (IP) joint.
Figure 8-3, B shows several features of the external anatomy of the hand. Note the palmar creases, or lines, that exist in the skin of the palm. They function as dermal “hinges,” marking where the skin folds on itself during movement, and to increase palmar skin adherence for enhancing the security of grasp. The location of the creases also serves as a useful clinical reference for the underlying anatomy. On the palmar (anterior) side of the wrist are the proximal and distal wrist creases. Of clinical interest is the fact that the distal wrist crease marks the location of the proximal margin of the underlying transverse carpal ligament. The thenar crease is formed by the folding of the dermis as the thumb is moved across the palm. The proximal digital creases are located distal to the actual joint line of the MCP joints. The distal and middle digital creases are superficial to the DIP and PIP joints, respectively.
OSTEOLOGY
Each metacarpal has similar anatomic characteristics (Figures 8-4 and 8-5). The first metacarpal (the thumb) is the shortest and stoutest; the second is usually the longest, and the length of the remaining three bones decreases from the radial to ulnar (medial) direction.
Each metacarpal has an elongated shaft with articular surfaces at each end (Figure 8-6). The palmar surface of the shaft is slightly concave longitudinally to accommodate many muscles and tendons in this region. Its proximal end, or base, articulates with one or more of the carpal bones. The bases of the second through the fifth metacarpals possess small facets for articulation with adjacent metacarpal bases.
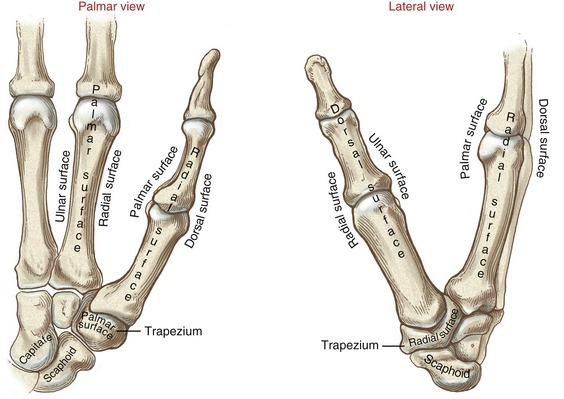
With the hand at rest in the anatomic position, the thumb’s metacarpal is oriented in a different plane than the other digits. The second through the fifth metacarpals are aligned generally side by side, with their palmar surfaces facing anteriorly. The position of the thumb’s metacarpal, however, is rotated almost 90 degrees medially (i.e., internally), relative to the other digits (see Figure 8-3). Rotation places the very sensitive palmar surface of the thumb toward the midline of the hand. Optimum prehension depends on the thumb flexing in a plane that intersects, versus parallels, the plane of the flexing fingers. In addition, the thumb’s metacarpal is positioned well anterior, or palmar, to the other metacarpals (Figure 7-14). This position of the first metacarpal and trapezium is strongly influenced by the palmar projection of the distal pole of the scaphoid.
The medially rotated thumb requires unique terminology to describe its movement as well as position. In the anatomic position the dorsal surface of the bones of the thumb (i.e., the surface where the thumbnail resides) faces laterally (Figure 8-7). The palmar surface therefore faces medially, the radial surface anteriorly, and the ulnar surface posteriorly. The terminology to describe the surfaces of the carpal bones and all other digital bones is standard: a palmar surface faces anteriorly, a radial surface faces laterally, and so forth.
Phalanges
The hand has 14 phalanges (from the Greek root phalanx, a line of soldiers). The phalanges within each finger are referred to as proximal, middle, and distal (see Figure 8-3, A). The thumb has only a proximal and a distal phalanx.
Except for differences in sizes, all phalanges within a particular digit have similar morphology (see Figure 8-5). The proximal and middle phalanges of each finger have a concave base, shaft, and convex head. As in the metacarpals, their palmar surfaces are slightly concave longitudinally. The distal phalanx of each digit has a concave base. At its distal end is a rounded tuberosity that anchors the fleshy pulp of soft tissue to the bony tip of each digit.
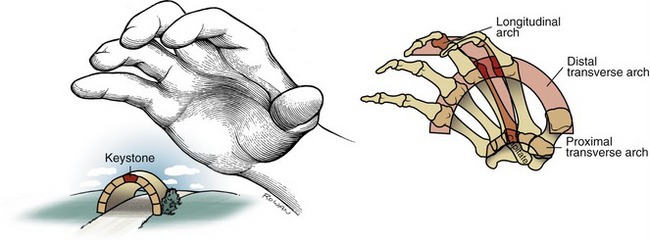
Arches of the Hand
Observe the natural concavity of the palmar surface of your relaxed hand. Control of this concavity allows the human hand to securely hold and manipulate objects of many and varied shapes and sizes. This palmar concavity is supported by three integrated arch systems: two transverse and one longitudinal (Figure 8-8). The proximal transverse arch is formed by the distal row of carpal bones. This is a static, rigid arch that forms the carpal tunnel (see Chapter 7). Like most arches in buildings and bridges, the arches of the hand are supported by a central keystone structure. The capitate bone is the keystone of the proximal transverse arch, reinforced by multiple contacts with other bones, and strong intercarpal ligaments.
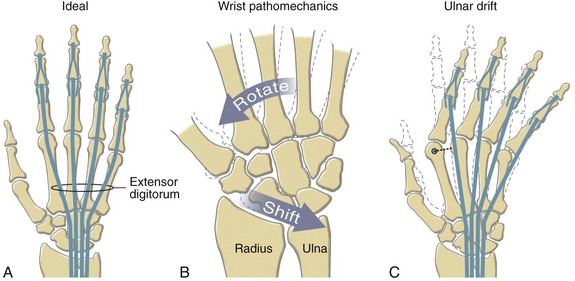
As depicted in Figure 8-8, all three arches of the hand are mechanically interlinked. Both transverse arches are joined together by a “rigid tie-beam” provided by the second and third metacarpals.27 In the healthy hand, this mechanical linkage reinforces the entire arch system. In the hand with joint disease, however, a structural failure at any arch may weaken another. A classic example is the destruction of the MCP joints from severe rheumatoid arthritis. This topic will be revisited at the end of this chapter.
ARTHROLOGY
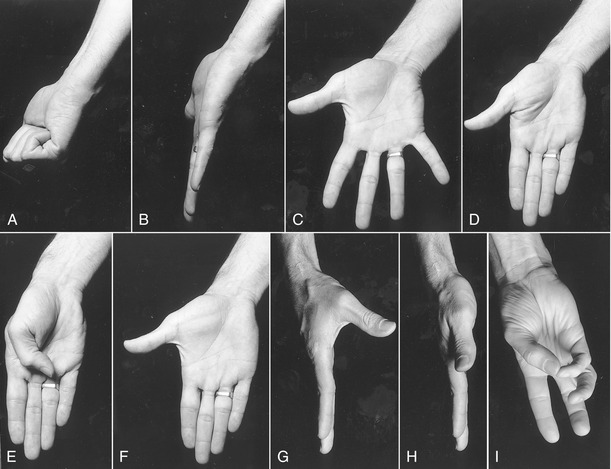
Before progressing to the study of the structure and function of the joints, the terminology that describes the movement of the digits must be defined. The following descriptions assume that a particular movement starts from the anatomic position, with the elbow extended, forearm fully supinated, and wrist in a neutral position. Movement of the fingers is described in the standard fashion using the cardinal planes of the body: flexion and extension occur in the sagittal plane, and abduction and adduction occur in the frontal plane (Figure 8-9, A to D). The middle finger is the reference digit for naming abduction and adduction. The side-to-side movement of the middle finger is called radial and ulnar deviation.
Because the entire thumb is rotated almost 90 degrees in relation to the fingers, the terminology used to describe thumb movement is different from that for the fingers (see Figure 8-9, E to I). Flexion is the movement of the palmar surface of the thumb in the frontal plane across the palm. Extension returns the thumb back toward its anatomic position. Abduction is the forward movement of the thumb away from the palm in a near sagittal plane. Adduction returns the thumb to the plane of the hand. (Although not used in this text, other terms frequently used to describe the movements of the thumb include ulnar adduction for flexion, radial abduction for extension, and palmar abduction for abduction.) Opposition is a special term describing the movement of the thumb across the palm, making direct contact with the tip of any of the fingers. Reposition is a movement from full opposition back to the anatomic position. This special terminology used to define the movement of the thumb serves as the basis for the naming of the muscles that act on the thumb (e.g., the opponens pollicis, extensor pollicis longus, and adductor pollicis).
Carpometacarpal Joints
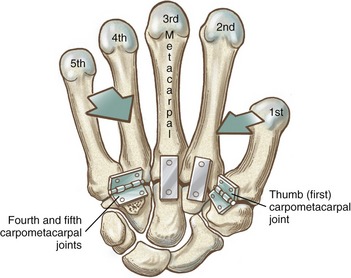
Figure 8-10 shows a mechanical illustration of the relative mobility at the CMC joints. The joints of the second and third digits are rigidly joined to the distal carpus, forming a stable central pillar throughout the hand. In contrast, the more peripheral CMC joints form mobile radial and ulnar borders, which are capable of folding around the hand’s central pillar. The function of the CMC joints allows the concavity of the palm to fit around many objects. This feature is one of the most impressive functions of the human hand. Cylindric objects, for example, can fit snugly into the palm, with the index and middle digits positioned to reinforce grasp. Without this ability, the dexterity of the hand is reduced to a primitive hingelike grasping motion.
SECOND THROUGH FIFTH CARPOMETACARPAL JOINTS
General Features and Ligamentous Support: The second CMC joint is formed through the articulation between the enlarged base of the second metacarpal and the distal surface of the trapezoid, and to a lesser extent the capitate and trapezium (see Figures 8-4 and 8-5). The third CMC joint is formed primarily by the articulation between the base of the third metacarpal and the distal surface of the capitate. The fourth CMC joint consists of the articulation between the base of the fourth metacarpal and the distal surface of the hamate and to lesser extent the capitate.70 The fifth CMC joint consists of the articulation between the base of the fifth metacarpal and the distal surface of the hamate only. (The hamate accepts both the fourth and fifth metacarpals, similar to the manner in which the cuboid bone of the foot accepts both the fourth and fifth metatarsals.) The bases of the second through fifth metacarpals have small facets for attachments to one another through intermetacarpal joints. These joints help stabilize the bases of the second through fifth metacarpals, thereby reinforcing the carpometacarpal joints.
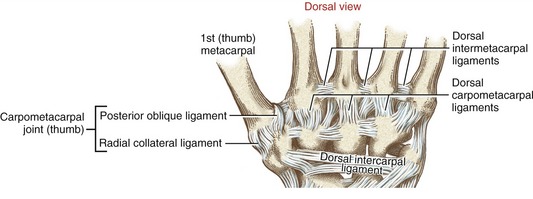
All CMC joints of the fingers are surrounded by articular capsules and strengthened by multiple dorsal, palmar, and interosseous ligaments.70 The dorsal ligaments are particularly well developed (Figure 8-11).
Joint Structure and Kinematics: The CMC joints of the second and third digits are difficult to classify, ranging from planar to complex saddle joints (Figure 8-12).101 Their jagged interlocking articular surfaces, coupled with strong ligaments, permit very little movement. As mentioned earlier, stability at these joints forms the central pillar of the hand. The inherent stability of these radial-central metacarpals also provides a very firm attachment for several key muscles, including the extensor carpi radialis longus and brevis, the flexor carpi radialis, and the adductor pollicis.
The slightly convex bases of the fourth and fifth metacarpals articulate with a slightly concave articular surface formed by the hamate. These two ulnar CMC joints contribute a subtle but important element of mobility to the hand. As depicted in Figure 8-10, the fourth and fifth CMC joints allow the ulnar border of the hand to fold toward the center of the hand, thereby deepening the palmar concavity. This mobility—often referred to as a “cupping” motion—occurs primarily by flexion and “internal” rotation of the ulnar metacarpals toward the middle digit. Measurements of maximal passive mobility on cadaver hands have shown that, on average, the fourth CMC joint flexes and extends about 20 degrees and rotates internally about 27 degrees.21 The fifth CMC joint (when tested with the fourth CMC joint firmly constrained) flexes and extends about 28 degrees and rotates internally 22 degrees. The range of flexion and extension of the fifth CMC joint increases to an average of 44 degrees when the closely positioned fourth CMC joint is unconstrained and free to move. This research demonstrates the strong mechanical link between the kinematics of the fourth and fifth CMC joints. This point should be considered when evaluating and treating limitations of motion in this region of the hand.
The greater relative mobility allowed at the ulnar CMC joints is evidenced by the movement of the fourth and fifth metacarpal heads while clenching a fist (Figure 8-13). The increased mobility of the fourth and fifth CMC joints improves the effectiveness of grasp, as well as enhancing the functional interaction with the opposable thumb. The irregular and varied shapes of these CMC joint surfaces prohibit standard roll-and-slide arthrokinematic descriptions.
CARPOMETACARPAL JOINT OF THE THUMB
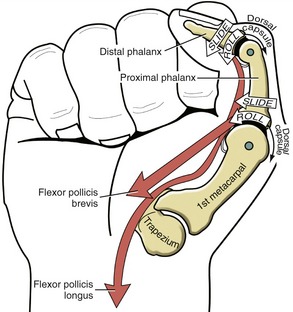
The CMC joint of the thumb is located at the base of the first ray, between the metacarpal and the trapezium (see Figure 8-7). This joint is by far the most complex of the CMC joints, enabling extensive movements of the thumb. Its unique saddle shape allows the thumb to fully oppose, thereby easily contacting the tips of the other digits. Through this action, the thumb is able to encircle objects held within the palm. Opposition greatly enhances the dexterity of human prehension.
Capsule and Ligaments of the Thumb Carpometacarpal Joint: The capsule at the CMC joint of the thumb is naturally loose to accommodate a large range of motion. The capsule, however, is strengthened by the action of ligaments and by the forces produced by the overriding musculature.
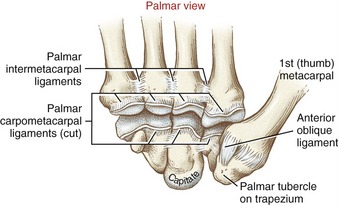
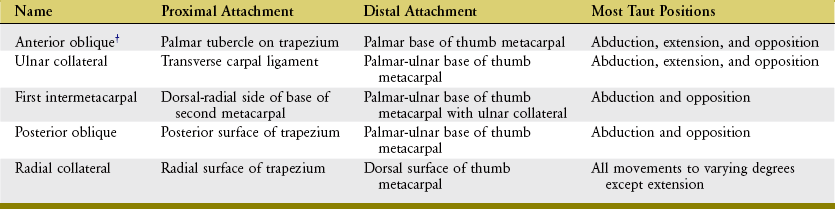
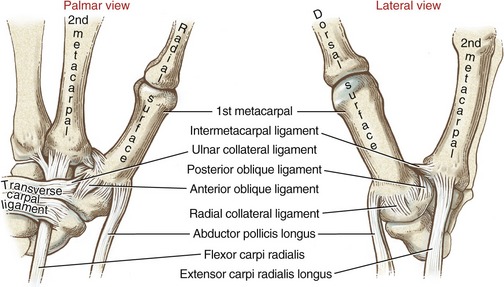
Many names have been used to describe the ligaments at the CMC joint of the thumb.6,20,37,80 The number of named, distinct ligaments reported to cross the base of the thumb ranges from three to perhaps as many as seven.72 This text focuses on five capsular ligaments, each adding an important element of stability to the CMC joint (Figure 8-14). As a set, the ligaments help control the extent and direction of joint motion, maintain joint alignment, and dissipate forces produced by activated muscle.76 Table 8-1 summarizes the major attachments of these ligaments and the motions that pull or wind them taut. In general, extension, abduction, and opposition of the thumb elongate most of the ligaments. Although all five ligaments listed in Table 8-1 are important stabilizers of the thumb’s CMC joint, the anterior oblique ligament warrants distinction.6,44,83 Rupture of this ligament secondary to severe arthritis or trauma often results in a radial dislocation of the joint, forming a characteristic “hump” at the base of the thumb.76
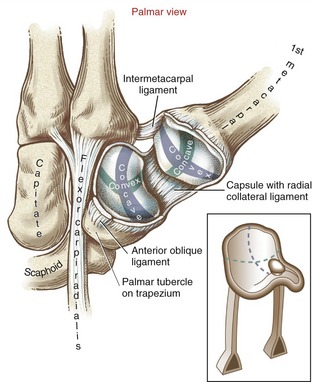
Saddle Joint Structure: The CMC joint of the thumb is the classic saddle joint of the body (Figure 8-15). The characteristic feature of a saddle joint is that each articular surface is convex in one dimension and concave in the other. The longitudinal diameter of the articular surface of the trapezium is generally concave from a palmar-to-dorsal direction. This surface is analogous to the front-to-rear contour of a horse’s saddle. The transverse diameter on the articular surface of the trapezium is generally convex in a medial-to-lateral direction—a shape analogous to the side-to-side contour of a horse’s saddle. The contour of the proximal articular surface of the thumb metacarpal has the reciprocal shape of that described for the trapezium (see Figure 8-15). The longitudinal diameter along the articular surface of the metacarpal is convex in a palmar-to-dorsal direction; its transverse diameter is concave in a medial-to-lateral direction.
Kinematics: The motions at the CMC joint occur primarily in two degrees of freedom. Abduction and adduction occur generally in the sagittal plane, and flexion and extension occur generally in the frontal plane. The axis of rotation for each plane of movement passes through the convex member of the articulation.38
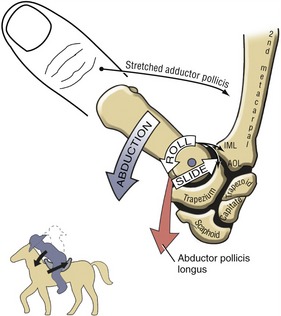
Abduction and Adduction at the Thumb Carpometacarpal Joint: In the position of adduction of the CMC joint, the thumb lies within the plane of the hand. Maximum abduction, in contrast, positions the thumb metacarpal about 45 degrees anterior to the plane of the palm. Full abduction opens the web space of the thumb, forming a wide concave curvature useful for grasping large objects.
The arthrokinematics of abduction and adduction are based on the convex articular surface of the thumb metacarpal moving on the fixed concave (longitudinal) diameter of the trapezium (review Figure 8-15). During abduction, the convex articular surface of the metacarpal rolls palmarly and slides dorsally on the concave surface of the trapezium (Figure 8-16). Full abduction at the CMC joint elongates the adductor pollicis muscle and most ligaments at the CMC joint. The arthrokinematics of adduction occur in the reverse order from those described for abduction.
Flexion and Extension at the Thumb Carpometacarpal Joint: Actively performing flexion and extension of the CMC joint of the thumb is associated with varying amounts of axial rotation of the metacarpal. During flexion, the metacarpal rotates medially (i.e., toward the third digit); during extension, the metacarpal rotates laterally (i.e., away from the third digit). The “automatic” axial rotation is apparent by the change in orientation of the nail of the thumb between full extension and full flexion. This rotation is not considered a third degree of freedom because it cannot be executed independently of the other motions.
In the anatomic position the CMC joint can be extended an additional 10 to 15 degrees.15 From full extension the thumb metacarpal flexes across the palm about 45 to 50 degrees.
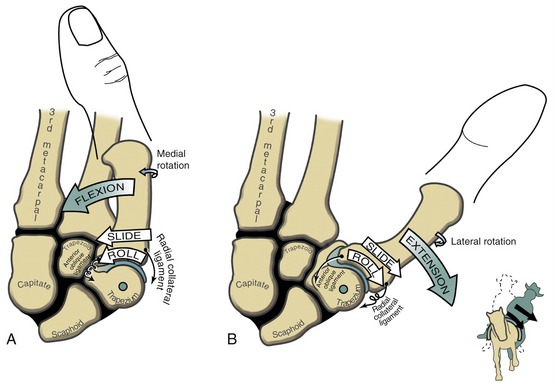
The arthrokinematics of flexion and extension at the CMC joint are based on the concave articular surface of the metacarpal moving across the convex (transverse) diameter on the trapezium (review Figure 8-15). During flexion, the concave surface of the metacarpal rolls and slides in an ulnar (medial) direction (Figure 8-17, A).38 A shallow groove in the transverse diameter of the trapezium helps guide the slight medial rotation of the metacarpal. Full flexion elongates tissues such as the radial collateral ligament.115
During extension of the CMC joint, the concave metacarpal rolls and slides in a lateral (radial) direction across the transverse diameter of the joint (see Figure 8-17, B). The groove on the articular surface of the trapezium guides the metacarpal into slight lateral rotation.15,51 Full extension stretches ligaments situated on the ulnar side of the joint, such as the anterior oblique ligament. Table 8-2 shows a summary of the kinematics for flexion-extension and abduction-adduction at the CMC joint of the thumb.
Opposition of the Thumb Carpometacarpal Joint: The ability to deliberately and precisely oppose the thumb to the tips of the other fingers is perhaps the ultimate expression of functional health of this digit—and arguably of the entire hand. This complex motion is a composite of the other primary motions already described for the CMC joint.57
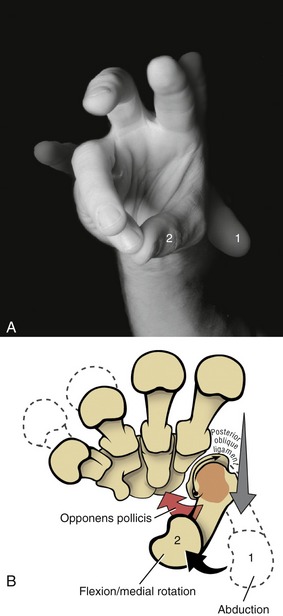
For ease of discussion, Figure 8-18, A shows the full arc of opposition divided into two phases. In phase one, the thumb metacarpal abducts. In phase two, the abducted metacarpal flexes and medially rotates across the palm toward the small finger. Figure 8-18, B shows the detail of the kinematics of this complex movement. During abduction, the base of the thumb metacarpal takes a path in a palmar direction across the surface of the trapezium. During flexion–medial rotation, the base of this metacarpal turns slightly medially, led by the groove on the surface of the trapezium.115 Muscle force, especially from the opponens pollicis, helps guide and rotate the metacarpal to the medial side of the articular surface of the trapezium. The partially abducted CMC joint increases passive tension in most connective tissues associated with the CMC joint. Increased tension in the stretched posterior oblique ligament, for instance, promotes the medial rotation (spin) of the thumb metacarpal.115
As evidenced by the change in orientation of the thumbnail, full opposition incorporates 45 to 60 degrees of medial rotation of the thumb.13 The CMC joint of the thumb accounts for most but not all of this rotation. Lesser amounts of axial rotation occur in the form of accessory motions at the MCP and IP joints. The trapezium also medially rotates slightly against the scaphoid and the trapezoid, thereby amplifying the final magnitude of the metacarpal rotation.75 The small finger contributes indirectly to opposition through a cupping motion at the fifth CMC joint. This motion allows the tip of the thumb to more easily contact the tip of the small finger.
Full opposition is often considered the CMC joint’s close-packed position.63,101 This position is stabilized not only by a twisting of several ligaments, but by activation of muscle. Although maximum in full opposition, only about half of the surface area within the joint makes articular contact. Considering the large and frequent forces that cross this joint, the relatively small contact area may naturally predispose the joint to large and potentially damaging pressures.
Metacarpophalangeal Joints
General Features and Ligaments: The metacarpophalangeal (MCP) joints of the fingers are relatively large, ovoid articulations formed between the convex heads of the metacarpals and the shallow concave proximal surfaces of the proximal phalanges (Figure 8-19). Motion at the MCP joint occurs predominantly in two planes: flexion and extension in the sagittal plane, and abduction and adduction in the frontal plane.
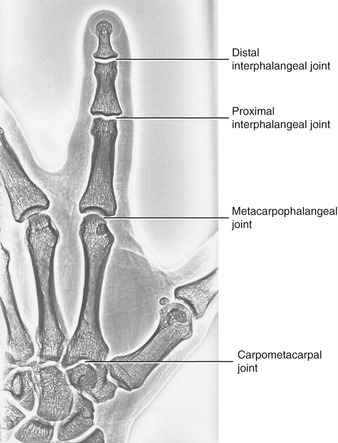
FIGURE 8-19. The joints of the index finger.
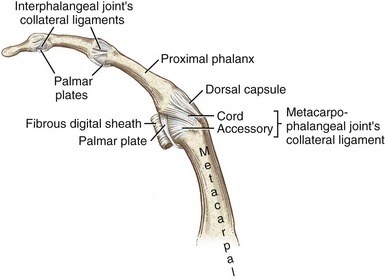
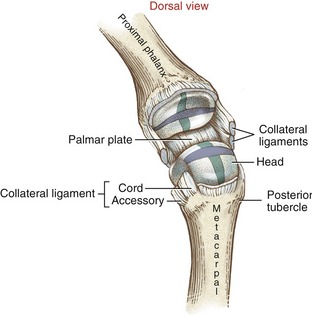
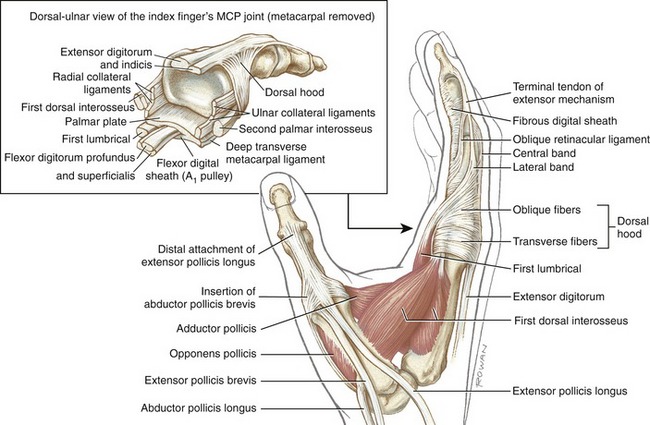
Mechanical stability at the MCP joint is critical to the overall biomechanics of the hand. As discussed earlier, the MCP joints serve as keystones that support the mobile arches of the hand. In the healthy hand, stability at the MCP joints is achieved by an elaborate set of interconnecting connective tissues. Embedded within the capsule of each MCP joint are a pair of radial and ulnar collateral ligaments and one palmar plate (Figure 8-20). Each collateral ligament has its proximal attachment on the posterior tubercle of the metacarpal head. Crossing the MCP joint in an oblique palmar direction, the ligament forms two distinct parts. The more dorsal cord part of the ligament is thick and strong, attaching distally to the palmar aspect of the proximal end of the phalanx. The accessory part consists of fan-shaped fibers, which attach distally along the edge of the palmar plate.
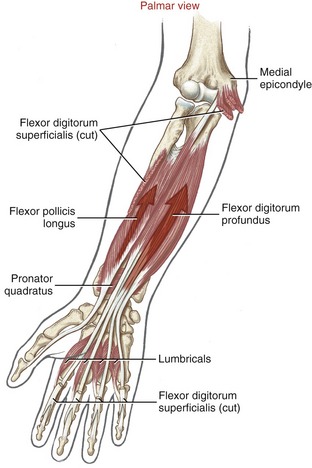
Located palmar to each MCP joint are ligamentous-like structures called palmar (or volar) plates (see Figure 8-20). The term plate describes a composition of dense, thick fibrocartilage. The distal end of each plate attaches to the base of each proximal phalanx. At this region the plates are relatively thick and stiff. The thinner and more elastic proximal end attaches to the metacarpal bone, just proximal to the head. Fibrous digital sheaths, which form tunnels or pulleys for the extrinsic finger flexors, are anchored on the palmar (anterior) surface of the palmar plates. The primary function of the palmar plates is to strengthen the structure of the MCP joints, and limit the extremes of extension.
Figure 8-21 illustrates several anatomic aspects of the MCP joints. The concave component of an MCP joint is formed by the articular surface of the proximal phalanx, the collateral ligaments, and the dorsal surface of the palmar plate. These tissues form a three-sided receptacle aptly suited to accept the large metacarpal head. This structure adds to the stability of the joint while also increasing the area of articular contact. Attaching between the palmar plates of each MCP joint are three deep transverse metacarpal ligaments. The three ligaments merge into a wide, flat structure that interconnects and loosely binds the second through the fifth metacarpals.
Osteokinematics: In addition to the volitional motions of flexion-extension and abduction-adduction at the MCP joints, substantial accessory motions are possible. With the MCP joint relaxed and nearly extended, the ample passive mobility of the proximal phalanx relative to the head of the metacarpal can be appreciated. The joint can be distracted-compressed, translated in anterior-to-posterior and side-to-side directions, and axially rotated. The extent of passive axial rotation is particularly remarkable. These ample accessory motions at the MCP joints permit the fingers to better conform to the shapes of held objects, thereby increasing control of grasp (Figure 8-22). The range of this passive axial rotation at the MCP joints is greatest at the ring and small fingers, with average rotations of about 30 to 40 degrees.50
The overall range of flexion and extension at the MCP joints increases gradually from the second to the fifth digit: the second (index) flexes to about 90 degrees, and the fifth to about 110 to 115 degrees.4 The greater mobility allowed at the more ulnar MCP joints is similar to that expressed at the CMC joints. The MCP joints can be passively extended beyond the neutral (0-degree) position for a considerable range of 30 to 45 degrees. Abduction and adduction at the MCP joints occur to about 20 degrees on both sides of the midline reference formed by the third metacarpal.
Arthrokinematics: The head of each metacarpal has a slightly different shape but in general is rounded at the apex and nearly flat on the palmar surface (see Figure 8-6). Articular cartilage covers the entire head and most of the palmar surface. The convex-concave relationship of the joint surfaces is readily apparent (Figure 8-23). The longitudinal diameter of the joint follows the sagittal plane; the shorter transverse diameter follows the frontal plane.
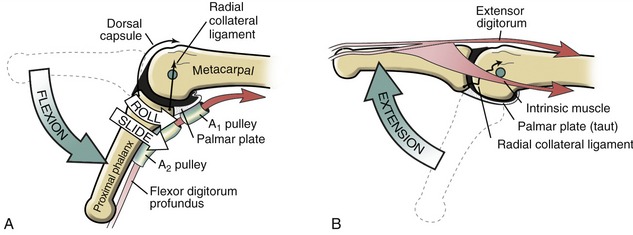
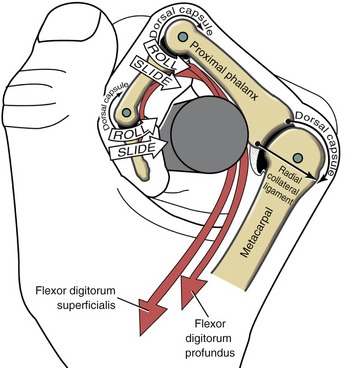
The arthrokinematics at the MCP joint are based on the concave articular surface of the phalanx moving against the convex metacarpal head. Figure 8-24, A shows the arthrokinematics of active flexion, driven by one of the extrinsic flexor muscles: the flexor digitorum profundus. Flexion stretches and therefore increases the passive tension in both the dorsal capsule and the collateral ligaments. In the healthy state this passive tension helps guide the joint’s natural arthrokinematics.7 For example, as depicted in Figure 8-24, A, the increased tension in the stretched dorsal capsule (depicted by the thin elongated arrow) prevents the joint from unnaturally “hinging” outward on its dorsal side. The tension helps maintain firm contact between the articular surfaces as the proximal phalanx slides and rolls in a palmar direction. The increased tension in the dorsal capsule and collateral ligaments stabilizes the joint in flexion, which is useful during grasp.
Figure 8-24, B illustrates active extension of the MCP joint, driven through a coordinated coactivation of the extensor digitorum and one of the intrinsic muscles (to be further described later in this chapter). The arthrokinematics of extension are similar to those illustrated for flexion except that the roll and slide of the proximal phalanx occur in a dorsal direction. By 0 degrees of extension, the collateral ligaments have slackened while the palmar plate has elongated and unfolded to support the head of the metacarpal. The relative slackness created in the collateral ligaments accounts, in part, for the increased passive mobility (“play”) within the joint in the extended position. Extension beyond the 0-degree position is normally blocked by contraction of an intrinsic muscle, such as a lumbrical.
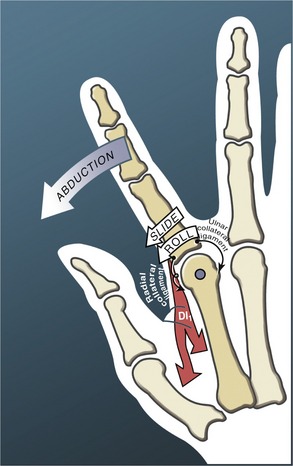
The arthrokinematics of abduction and adduction of the MCP joints are similar to those described for flexion and extension. During abduction of the index MCP joint, for instance, the proximal phalanx rolls and slides in a radial direction (Figure 8-25). The first dorsal interosseus muscle not only directs the arthrokinematics of abduction, but stabilizes the joint radially as the radial collateral ligament progressively slackens.7
The extent of active abduction and adduction at the MCP joints is significantly less when the motions are performed in full flexion compared with full extension. (This can be readily verified on your own hand.) Two factors can account for this difference. First, the collateral ligaments are taut near full flexion. Stored passive tension in these ligaments theoretically increases the compression force between the joint surfaces, thereby reducing available motion. Second, in the position of about 70 degrees of flexion, the articular surface of the proximal phalanges contacts the flattened palmar part of the metacarpal heads (see Figure 8-24, A). This relatively flat surface blocks the natural arthrokinematics required for maximal abduction and adduction range of motion.
THUMB
General Features and Ligaments: The MCP joint of the thumb consists of the articulation between the convex head of the first metacarpal and the concave proximal surface of the proximal phalanx of the thumb (Figure 8-27). The basic structure and arthrokinematics of the MCP joint of the thumb are similar to those of the fingers. Marked differences exist, however, in osteokinematics. Active and passive motions at the MCP joint of the thumb are significantly less than those at the MCP joints of the fingers. For all practical purposes, the MCP joint of the thumb allows only one degree of freedom: flexion and extension within the frontal plane.94 Unlike the MCP joints of the fingers, extension of the thumb MCP joint is usually limited to just a few degrees. The arthrokinematics of active flexion at the metacarpophalangeal joint of the thumb is illustrated in Figure 8-28. From full extension, the proximal phalanx of the thumb can actively flex about 60 degrees across the palm toward the middle digit.41
Although the limited abduction and adduction at the MCP joint provide some natural stability to thumb, the normally taut collateral ligaments at the joint are particularly vulnerable to injury from excessively large external torques. This is well exemplified by the relatively common “skier’s injury” in which the handle and strap of the ski pole of a falling skier create a large abduction torque against the MCP joint, damaging the joint’s ulnar collateral ligament. The rupture point of this ligament occurs at about 45 degrees of abduction.25 Furthermore, the ligament is most vulnerable to rupture when the abduction torque is applied with the MCP joint flexed to about 30 degrees, a scenario likely present at the time of the skiing accident.
Interphalangeal Joints
Distal to the MCP joints are the proximal and distal interphalangeal joints of the fingers (see Figure 8-27). Each joint allows only one degree of freedom: flexion and extension. From both structural and functional perspectives, these joints are simpler than the MCP joints.
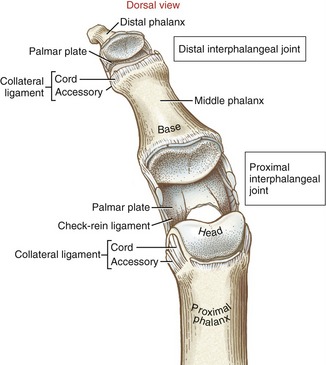
General Features and Ligaments: The proximal interphalangeal (PIP) joints are formed by the articulation between the heads of the proximal phalanges and the bases of the middle phalanges. The articular surface of the joint appears as a tongue-in-groove articulation similar to that used in carpentry to join planks of wood (Figure 8-29). The head of the proximal phalanx has two rounded condyles separated by a shallow central groove. The opposing surface of the middle phalanx has two shallow concave facets separated by a central ridge. The tongue-in-groove articulation helps guide the motion of flexion and extension as it restricts axial rotation.
Each PIP joint is surrounded by a capsule that is reinforced by radial and ulnar collateral ligaments.101 The cord portion of the collateral ligament at the PIP joint significantly limits abduction and adduction motion. As with the MCP joint, the accessory portion of the collateral ligament blends with and reinforces the palmar plate (see Figure 8-29). The anatomic connections between the palmar plate and collateral ligaments form a secure seat for the head of the proximal phalanx.53 The palmar plate is the primary structure that limits hyperextension of the PIP joint.113 In addition, the palmar surface of the plate serves as the attachment for the base of the fibrous digital sheath—the structure that houses the tendons of the extrinsic finger flexor muscles (see index and small fingers, Figure 8-21).
The proximal lateral regions of each palmar plate at the PIP joints thicken longitudinally, forming a fibrous tissue referred to as check-rein ligaments (see Figure 8-29).101,113 These tissues reinforce the proximal attachments of the palmar plate, as well as assist in limiting hyperextension of the joint. When enlarged, check-rein ligaments are often considered a pathologic tissue and as such are often excised during surgical release of a flexion contracture at the PIP joint.
The distal interphalangeal (DIP) joints are formed through the articulation between the heads of the middle phalanges and the bases of the distal phalanges (see Figure 8-29). The structure of the DIP joint and the surrounding connective tissue are similar to those of the PIP joint, except for the absence of the check-rein ligaments.
Kinematics: The PIP joints flex to about 100 to 120 degrees. The DIP joints allow less flexion, to about 70 to 90 degrees. As with the MCP joints, flexion at the PIP and DIP joints is greater in the more ulnar digits. Minimal hyperextension is usually allowed at the PIP joints. The DIP joints, however, normally allow up to about 30 degrees of extension beyond the neutral (0 degree) position.
Similarities in joint structure cause similar arthrokinematics at the PIP and DIP joints. During active flexion at the PIP joint, for instance, the concave base of the middle phalanx rolls and slides in a palmar direction by the pull of the extrinsic finger flexors (Figure 8-30). During flexion, the passive tension created in the dorsal capsule helps guide and stabilize the roll-and-slide arthrokinematics.
In contrast to the MCP joints, passive tension in the collateral ligaments at the IP joints remains relatively constant throughout the range of motion.62 Perhaps the more spheric shape of the heads of the phalanges prevents a significant change in length in these collateral ligaments (see Figure 8-26).18 The close-packed position of the PIP and DIP joints is considered to be full extension,101 most likely because of the stretch placed on the palmar plates. During periods of immobilization of the hand, the PIP and DIP joints are often splinted in near extension. This position places a stretch on the palmar plates, reducing the likelihood of a flexion contracture developing in these joints.
THUMB
The structure and function of the interphalangeal (IP) joint of the thumb are similar to those of the IP joints of the fingers. Motion is limited primarily to one degree of freedom, allowing active flexion to about 70 degrees (see Figure 8-28).41 The IP joint of the thumb can be passively extended beyond neutral to about 20 degrees. This motion is often employed to apply a force between the pad of the thumb and an object, such as pushing a thumbtack into a board. The amount of passive hyperextension often increases throughout life owing to years of stretch placed on palmar structures, including the palmar plate.
MUSCLE AND JOINT INTERACTION
Innervation of Muscles, Skin, and Joints of the Hand
The highly complex and coordinated functions of the hand require a rich source of motor and sensory innervation of the region’s muscles, skin, and joints. Consider, for instance, the very precise and delicate movements of the digits performed by a concert violinist. One fact allowing such precision is that a single axon traveling to an intrinsic muscle of the hand, such as the thumb, may innervate as few as 100 muscle fibers.74 In this case, one axon would simultaneously activate all 100 muscle fibers. By contrast, a single axon traveling to the medial head of the gastrocnemius muscle—a muscle not involved with fine movements—may innervate about 2000 muscle fibers.24 The smaller fiber-per-axon ratio typical of most intrinsic muscles of the hand allows for a more precise gradation between levels of force, ultimately permitting finer control of movement.
MUSCLE AND SKIN INNERVATION
Innervation to the muscles and the skin of the hand is illustrated in Figure 6-32. The radial nerve innervates the extrinsic extensor muscles of the digits. These muscles, located on the dorsal aspect of the forearm, are the extensor digitorum, extensor digiti minimi, extensor indicis, extensor pollicis longus, extensor pollicis brevis, and abductor pollicis longus. The radial nerve is responsible for the sensation on the dorsal aspect of the wrist and hand, especially around the dorsal region of the thenar web space.
As a reference, the primary nerve roots that supply the muscles of the upper extremity are listed in Appendix II, Part A. In addition, Appendix II, Parts B to D include additional reference items to help guide the clinical assessment of the functional status of the C5-T1 nerve roots and several major peripheral nerves of the upper limb.
SENSORY INNERVATION TO THE JOINTS
For the most part, the joints of the hand receive sensation from sensory nerve fibers that supply the overlying dermatomes. (See dermatome chart in Appendix II, Part D.) These afferent nerve fibers merge with the following dorsal nerve roots at the spinal cord: C6, carrying sensation from the thumb and index finger; C7, carrying sensation from the middle finger; and C8, carrying sensation from the ring and small fingers.30,39,101
Muscular Function of the Hand
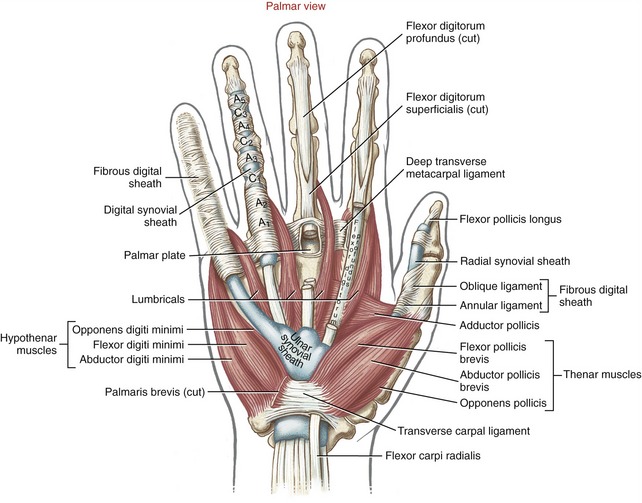
Muscles that operate the digits are classified as either extrinsic or intrinsic to the hand (Table 8-3). Extrinsic muscles have their proximal attachments in the forearm or, in some cases, as far proximal as the epicondyles of the humerus. Intrinsic muscles, in contrast, have both their proximal and distal attachments within the hand. As a summary and reference, the detailed anatomy and nerve supply of the muscles of the hand are included in Appendix II, Part E.
EXTRINSIC FLEXORS OF THE DIGITS
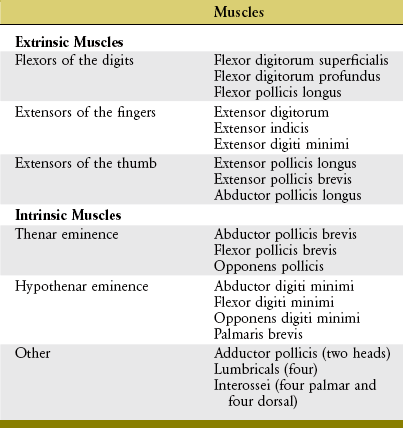
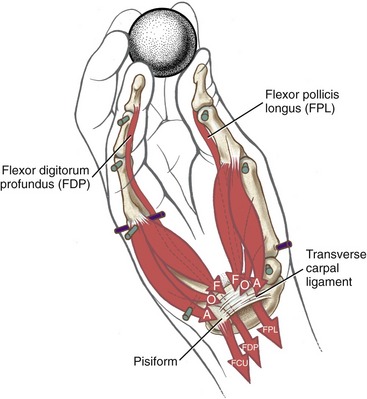
Anatomy and Joint Action of the Extrinsic Flexors of the Digits: The extrinsic flexor muscles of the digits are the flexor digitorum superficialis, flexor digitorum profundus, and flexor pollicis longus (Figures 8-32 and 8-33). These muscles have extensive proximal attachments from the medial epicondyle of the humerus and regions of the forearm.
The muscle belly of the flexor digitorum superficialis is located in the anterior forearm, just deep to the three wrist flexors and the pronator teres muscle (see Figure 8-32). Its four tendons cross the wrist and enter the palmar aspect of the hand. At the level of the proximal phalanx, each tendon splits to allow passage of the tendon of the flexor digitorum profundus (Figure 8-34, middle and index finger). The two split parts of each tendon partially reunite, cross the PIP joint, and attach on the sides of the palmar aspect of the middle phalanx.
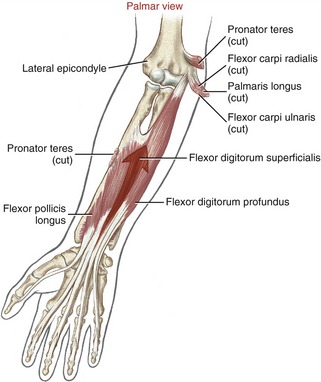
The muscle belly of the flexor digitorum profundus is located in the deepest muscular plane of the forearm, deep to the flexor digitorum superficialis muscle (see Figure 8-33). Once in the digit, each tendon passes through the split tendon of the flexor digitorum superficialis. Each profundus tendon then continues distally to attach to the palmar side of the base of the distal phalanx. The flexor digitorum profundus is the sole flexor of the DIP joint, but, like the superficialis, can assist in flexing every joint it crosses.
The flexor pollicis longus resides in the deepest muscular plane of the forearm, just lateral to the flexor digitorum profundus (see Figure 8-33). This muscle crosses the wrist and attaches distally to the palmar side of the base of the distal phalanx of the thumb. The flexor pollicis longus is the sole flexor at the IP joint of the thumb, and it also exerts a substantial flexion torque at the MCP and CMC joints of the thumb. If not opposed, the flexor pollicis longus also flexes the wrist.
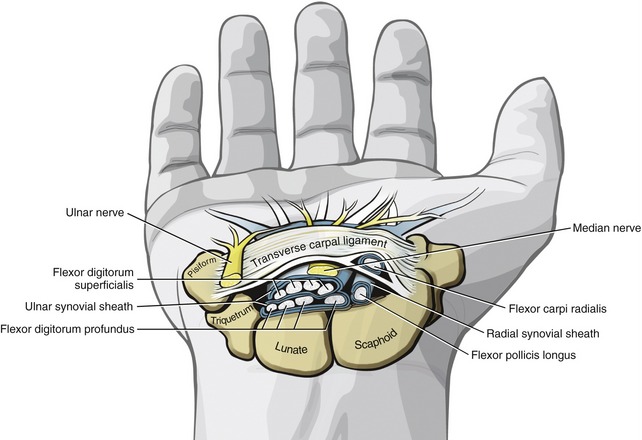
Distal to the carpal tunnel, the ulnar synovial sheath surrounds the flexor digitorum superficialis and profundus tendons. This sheath ends in the proximal palm, except for a distal continuation around the tendons of the small finger (see Figure 8-34). The radial synovial sheath remains in contact with the tendon of the flexor pollicis longus to its distal insertion on the thumb.
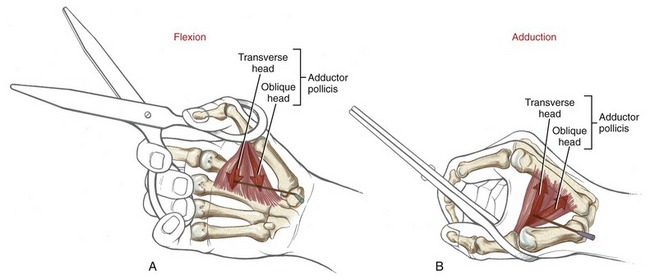
The extrinsic flexor tendons of the digits travel to their distal attachment in protective fibro-osseous tunnels known as fibrous digital sheaths (see Figure 8-34, small finger). Sheaths start proximally as a continuation of the thick aponeurosis just under the skin of the palm. Throughout the length of each digit, the sheaths are anchored to the phalanges and the palmar plates (see Figure 8-21, index finger). Embedded within each digital sheath are discrete bands of tissue called flexor pulleys (see Figure 8-34, A1 to A5, C 1 to C3 in ring finger). Deep to these pulleys is a digital synovial sheath, surrounding the flexor tendons from the distal palmar crease to the DIP joint. This sheath serves as a nutritional and lubrication source for the enclosed tendons. The synovial fluid secreted from the sheath reduces the friction between the flexor digitorum superficialis and profundus tendons. After injury or laceration, adhesions may develop between the tendon and adjacent digital sheath or between tendons. After surgical repair of a lacerated tendon, the therapist usually initiates a closely monitored exercise program to facilitate gliding of the tendon. This therapy is performed according to a strict time schedule as determined by the surgeon and the therapist, depending on the type of repair and other parameters.
Anatomy and Function of the Flexor Pulleys: Figure 8-34 shows the flexor pulleys that are embedded within the fibrous digital sheath. Five annular pulleys have been described for each finger, designated as A1 to A5.17 The major pulleys (A2 and A4) attach to the shafts of the proximal and middle phalanges. The minor pulleys (A1, A3, and A5) attach directly to the palmar plate at each of the three joints within a finger. Three less distinct cruciate pulleys (C1 to C3) have also been described.17 The cruciate pulleys are made of thin, flexible fibers that crisscross over the tendons at regions where the digital sheaths bend during flexion.
The annular and oblique ligaments of the thumb function as pulleys for the passage of the tendon of the flexor pollicis longus (see Figure 8-34).
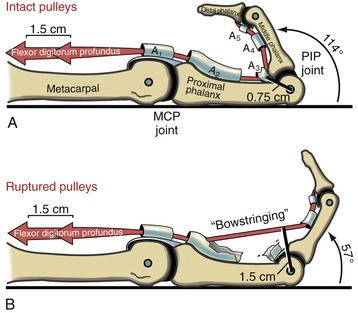
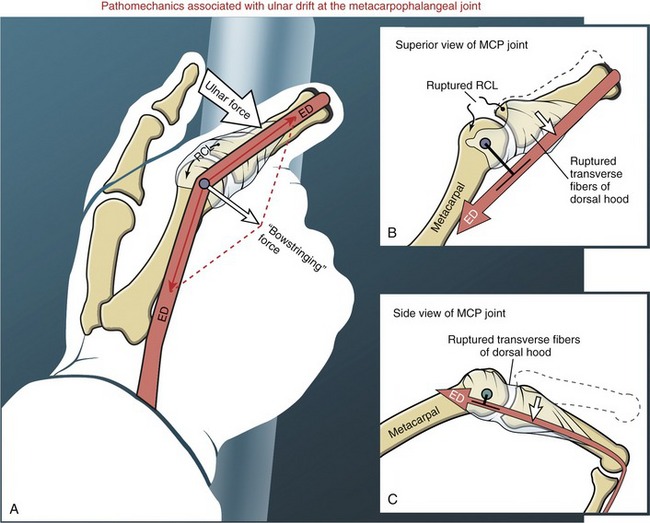
Flexor pulleys, palmar aponeurosis, and skin share a similar function of holding the underlying tendons at a relatively close distance to the joints.29 Without the restraint provided by these tissues, the force of a strong contraction of the extrinsic finger flexors causes the tendon to pull away from the joint’s axis of rotation, a phenomenon referred to as “bowstringing” of the tendon.
The flexor pulleys of the digits have a particularly important role in stabilizing the position of the tendons relative to the underlying joints.90 The pulleys may be overstretched or torn secondary to trauma, overuse, or disease. (Of interest, overstretching and subsequent bowstringing of the flexor tendons have been observed in 26% of elite rock climbers, most often in the ring and middle fingers.89,109) A severing or overstretching of the major A2 or A4 pulley significantly alters the moment arms of the flexor tendons and subsequently alters the biomechanics at the MCP and PIP joints.29 Preservation of these two major pulleys is therefore a major goal of hand surgeons.
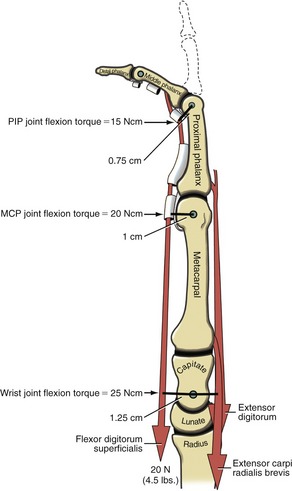
Role of Proximal Stabilizer Muscles during Active Finger Flexion: The extrinsic digital flexors are mechanically capable of flexing multiple joints, from the DIP joint to, at least theoretically for the flexor digitorum superficialis, the elbow. In order for these muscles to isolate their flexion potential across a single joint, other muscles contract synergistically with the extrinsic digital flexors. Consider the flexor digitorum superficialis performing isolated PIP joint flexion (Figure 8-36). At the onset of contraction, the extensor digitorum must act as a proximal stabilizer to prevent the flexor digitorum superficialis from flexing the MCP joint and the wrist. Because the flexor moment arm length of the flexor digitorum superficialis progressively increases at the more proximal joints, a relatively small force applied to a distal joint is amplified to a greater torque at the more proximal joints. Figure 8-36 shows that a 20-N (4.5-lb) force within the superficialis tendon produces a 15-Ncm torque at the PIP joint, a 20-Ncm torque at the MCP joint, and a 25-Ncm torque at the midcarpal joint of the wrist. The greater the force produced by the flexor digitorum superficialis, the greater the force demands placed on the proximal stabilizers. The proximal stabilizers include the extensor digitorum and, if needed, the wrist extensors. The amount of muscle force and muscular coordination required for a simple action of PIP joint flexion is actually more than what it first appears. Paralysis or weakness of proximal stabilizers can significantly disrupt the effectiveness of more distal muscle function.
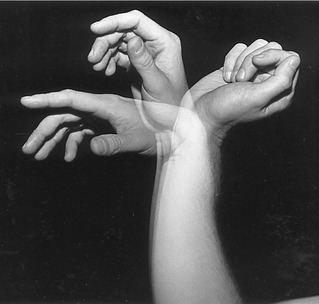
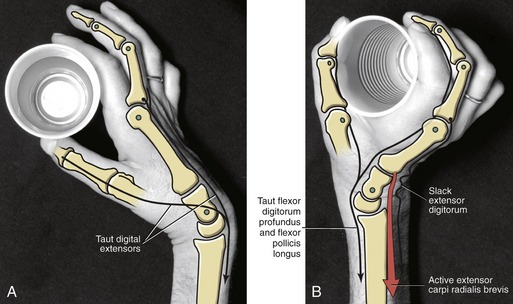
Passive Finger Flexion via “Tenodesis Action” of the Extrinsic Digital Flexors: The extrinsic flexors of the digits—namely, the flexor digitorum profundus and superficialis and the flexor pollicis longus—cross anterior to the wrist. The position of the wrist therefore significantly alters the length and subsequent passive tension in these muscles. One implication of this arrangement can be appreciated by actively extending the wrist and observing the passive flexion of the fingers and thumb (Figure 8-37). The digits automatically flex because of the increased passive tension in the stretched flexor muscles. The stretching of a polyarticular muscle across one joint, which generates a passive movement at other joints, is referred to as a tenodesis action of a muscle.
The amount of passive flexion of the fingers caused by the aforementioned tenodesis action is surprisingly large; in healthy subjects, on average, completely extending the wrist from full flexion automatically flexes the DIP joint about 20 degrees, the PIP joint about 50 degrees, and the MCP joint about 35 degrees.104 Figure 8-37 also demonstrates that in the position of full wrist flexion, the fingers, most notably the index, passively extend owing to a similar tenodesis action of the stretched extrinsic digital extensor muscles. Essentially all polyarticular muscles in the body demonstrate some degree of tenodesis action.
EXTRINSIC EXTENSORS OF THE FINGERS
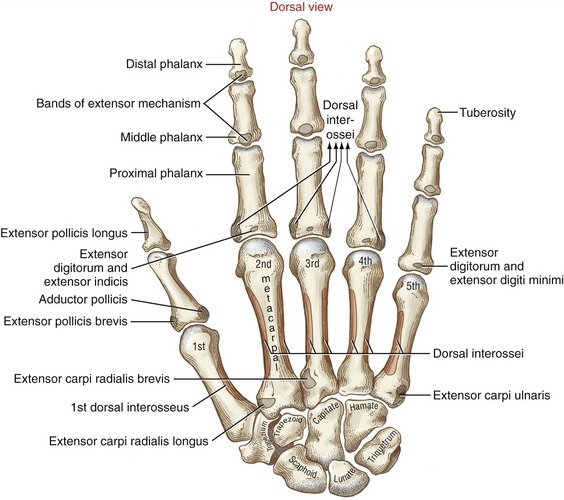
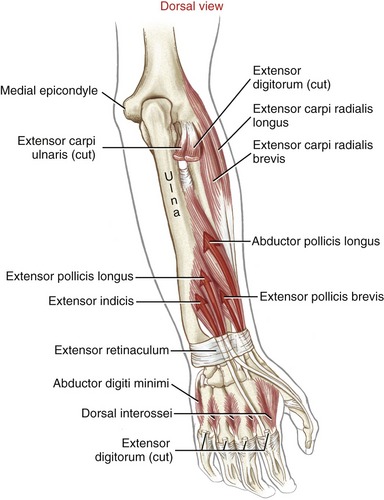
Muscular Anatomy: The extrinsic extensors of the fingers are the extensor digitorum, the extensor indicis, and the extensor digiti minimi (see Figure 7-22). The extensor digitorum and the extensor digiti minimi originate from a common tendon off the lateral epicondyle of the humerus. The extensor indicis has its proximal attachment on the dorsal region of the forearm. The extensor digitorum, in terms of cross-sectional area, is by far the predominant finger extensor. In addition to functioning as a finger extensor, the extensor digitorum has an excellent moment arm as a wrist extensor (see Figure 7-24).
Dissecting away the extensor digitorum and extensor minimi exposes the deeper extensor indicis and the extrinsic extensor muscles of the thumb (Figure 8-39). The extensor indicis muscle has only one tendon, which serves the index finger. The extensor digiti minimi is a small fusiform muscle often interconnected with the extensor digitorum. As depicted in Figure 8-40, the extensor digiti minimi often has two tendons.
Tendons of the extensor digitorum, extensor indicis, and extensor digiti minimi cross the wrist in synovial-lined compartments located within the extensor retinaculum (see Figure 7-23). Distal to the extensor retinaculum, the tendons course toward the fingers, dorsal to the metacarpals (see Figure 8-40). The tendons of the extensor digitorum are interconnected by several juncturae tendinae. These thin strips of connective tissue stabilize the angle of approach of the tendons to the base of the MCP joints and may limit independent movement of the individual tendons.
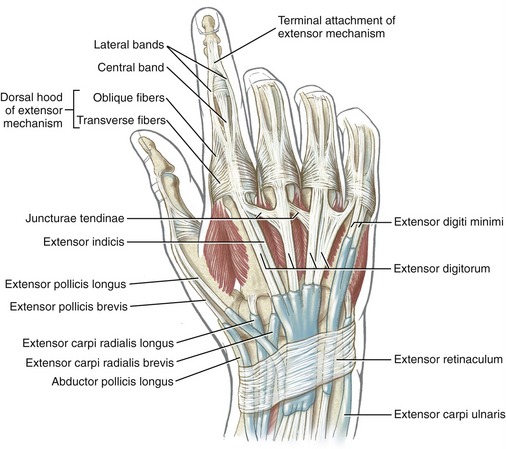
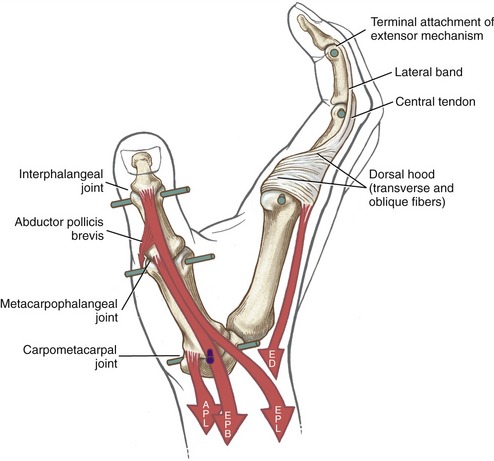
The anatomic organization of the extensor tendons of the fingers is very different from that of the finger flexors. The flexor tendons travel in well-defined digital sheaths toward single bony attachments. In contrast, distal to the wrist, the extensor tendons lack a defined digital sheath or pulley system. The extensor tendons eventually become integrated into a fibrous expansion of connective tissues, located along the length of the dorsum of each finger (see Figure 8-40). The complex set of connective tissue is called the extensor mechanism, although other terms have been used over the years, including the extensor expansion, extensor apparatus, and extensor assembly.14,100 The extensor mechanism serves as a primary distal attachment for the extensor digitorum, indicis, and digiti minimi and for most of the intrinsic muscles acting on the fingers. The following section describes the anatomy of the extensor mechanism. A similar but less organized extensor mechanism exists for the thumb.
Extensor Mechanism of the Fingers: A small slip of the tendon of the extensor digitorum attaches to the base of the dorsal side of the proximal phalanx. The remaining tendon flattens into a central band, forming the “backbone” of the extensor mechanism to each finger (see Figures 8-40 and 8-41). The central band courses distally to attach to the dorsal base of the middle phalanx. Before crossing the PIP joint, two lateral bands diverge from the central band. More distally, the lateral bands fuse into a single terminal tendon that attaches to the dorsal base of the distal phalanx. The multiple attachments of the extensor mechanism into the phalanges allow the extensor digitorum to transfer extensor force distally throughout the entire finger.
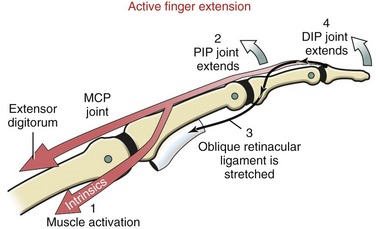
The position of lateral bands relative to the PIP joint is stabilized bilaterally on each finger by a set of thin connective tissues, generally known as retinacular ligaments.93,110 The more substantial of these connective tissues is a pair of oblique retinacular ligaments. Figure 8-41 shows the radial oblique retinacular ligament of the index finger. The slender fibers arise proximally from the fibrous digital sheath, just proximal to the PIP joint, and course obliquely and distally to insert into the lateral bands. The ligaments help coordinate movement between the PIP and DIP joints of the fingers, a point to be discussed later in this chapter.
The most prominent feature of the proximal end of the extensor mechanism is the dorsal hood (see Figures 8-40 and 8-41). This specialized tissue consists of a nearly triangular sheet of thin aponeurosis that contains both transverse and oblique fibers. The transverse fibers (also called “sagittal” bands) run nearly perpendicular to the long axis of the tendon of the extensor digitorum. The transverse fibers from both sides of the extensor tendon attach into the palmar plate, forming a “sling” around the base of the proximal phalanx (Figure 8-42). This sling is used by the extensor digitorum muscle to extend the MCP joint. In addition, the transverse fibers stabilize the tendon of the extensor digitorum over the dorsum of the MCP joint. The oblique fibers of the dorsal hood course distally and dorsally to fuse primarily with the lateral bands (see Figure 8-41).
As a general rule, the intrinsic muscles of the hand (specifically the lumbricals and interossei) attach into the extensor mechanism via the oblique fibers and, to a lesser extent, the transverse fibers of the dorsal hood. Figure 8-41 shows this arrangement for the first dorsal interosseus and lumbrical of the index finger. Via these important connections, the intrinsic muscles assist the extensor digitorum with extension of the PIP and DIP joints.
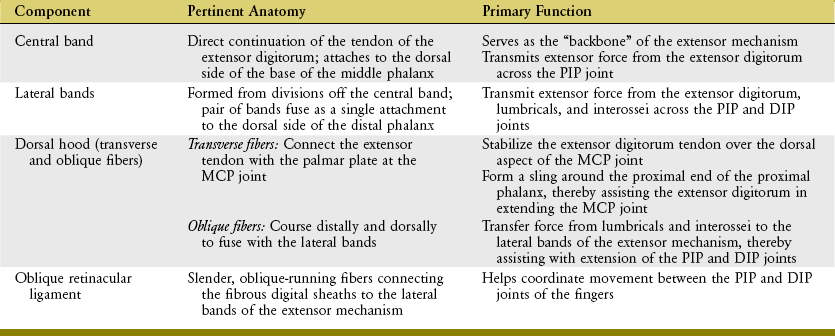
The anatomic and functional components of the extensor mechanism are summarized in Table 8-4.
Action of the Extrinsic Finger Extensors: Isolated contraction of the extensor digitorum produces hyperextension of the MCP joints. Only in the presence of activated intrinsic muscles of the fingers can the extensor digitorum fully extend the PIP and DIP joints. This important point will be reinforced later in this chapter.
EXTRINSIC EXTENSORS OF THE THUMB
Anatomic Considerations: The extrinsic extensors of the thumb are the extensor pollicis longus, extensor pollicis brevis, and abductor pollicis longus (see Figures 8-39 and 8-41). These radial innervated muscles have their proximal attachments on the dorsal region of the forearm. The tendons of these muscles compose the “anatomic snuffbox” located on the radial side of the wrist. The tendons of the abductor pollicis longus and the extensor pollicis brevis together pass through first dorsal compartment within the extensor retinaculum of the wrist (see Figure 7-23). Distal to the extensor retinaculum, the tendon of the abductor pollicis longus inserts primarily into the radial-dorsal surface of the base of the thumb metacarpal. Additional distal attachments of this muscle have been observed attaching into the trapezium and blending with fibers of the intrinsic thenar muscles.91 The extensor pollicis brevis attaches distally to the dorsal base of the proximal phalanx of the thumb. The tendon of the extensor pollicis longus crosses the wrist in the third compartment in a groove just medial to the dorsal tubercle of the radius (see Figure 7-23). The extensor pollicis longus attaches distally to the dorsal base of the distal phalanx of the thumb. Fibers from both extrinsic extensor tendons contribute to the central tendon of the extensor mechanism of the thumb.
Functional Considerations: The multiple actions of the extensor pollicis longus, extensor pollicis brevis, and abductor pollicis longus can be understood by noting their line of force relative to the axes of rotation at the joints they cross (see Figure 8-42). The extensor pollicis longus extends the IP, MCP, and CMC joints of the thumb. The muscle passes to the dorsal side of the medial-lateral axis of the CMC joint and is therefore also capable of adducting this joint. The extensor pollicis longus is unique in its ability to perform all three actions that compose the repositioning of the thumb to the anatomic position: extension (with slight lateral rotation) and adduction of the first metacarpal.
Figure 8-42 also illustrates that the extensor pollicis brevis is an extensor of the MCP and CMC joints of the thumb; the abductor pollicis longus extends only at the CMC joint. The long abductor muscle is also a prime abductor of the CMC joint, based on its line of force passing anterior (palmar) to the joint’s medial-lateral axis of rotation. The combined extension-abduction action of the abductor pollicis longus reflects its attachment on the radial-dorsal corner of the base of the thumb metacarpal. The actions of all the muscles that cross the joints of the thumb are summarized in Box 8-1.
The extensor pollicis longus and the abductor pollicis longus are potent radial deviators at the wrist (see Figure 7-24). During extension of the thumb, therefore, an ulnar deviator muscle must be activated to stabilize the wrist against unwanted radial deviation. This activation is apparent by palpating the raised tendon of the flexor carpi ulnaris, located just proximal to the pisiform, during rapid and full extension of the thumb.
INTRINSIC MUSCLES OF THE HAND
Muscles of the Thenar Eminence:
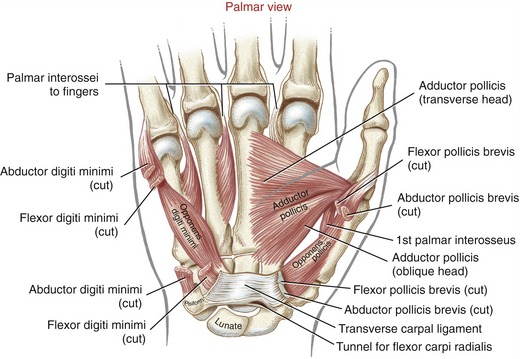
Anatomic Considerations: The abductor pollicis brevis, flexor pollicis brevis, and opponens pollicis make up the bulk of the thenar eminence (see Figure 8-34). The flexor pollicis brevis has two parts: a superficial head, which comprises most of the muscle, and a deep head, which consists of a small set of poorly defined fibers, often described as part of the oblique fibers of the adductor pollicis.58,101 This chapter considers only the superficial head when discussing the flexor pollicis brevis. Deep to the abductor pollicis brevis is the opponens pollicis (Figure 8-43). All three thenar muscles have their proximal attachments on the transverse carpal ligament and adjacent carpal bones. Both the short abductor and flexor muscles have their distal attachments on the radial side of the base of the proximal phalanx. In addition, the abductor pollicis brevis attaches to the radial side of the extensor mechanism of the thumb; the flexor pollicis brevis frequently attaches to a sesamoid bone. The deeper opponens pollicis attaches distally to the entire radial border of the thumb metacarpal.
Functional Considerations: A primary responsibility of the muscles of the thenar eminence is to position the thumb in varying amounts of opposition, usually to facilitate grasping. As discussed earlier, opposition combines elements of CMC joint abduction, flexion, and medial rotation. Each muscle within the thenar eminence is a prime mover for at least one component of opposition, and an assistant for several others (see Box 8-1).45,97
The actions of the thenar muscles across the CMC joint become apparent when each muscle’s line of force relative to a particular axis of rotation is viewed (Figure 8-44).97 Note that the opponens pollicis has a line of force to medially rotate the thumb toward the fingers. Because the opponens pollicis has its distal attachment on the metacarpal (and proximal to the MCP joint), its entire contractile force is dedicated to controlling the CMC joint.
Implications of Median Nerve Injury: A severance of the median nerve paralyzes all three muscles of the thenar eminence: namely the opponens pollicis, flexor pollicis brevis, and abductor pollicis brevis. Consequently, opposition of the thumb is essentially disabled. The thenar eminence region of the hand also becomes flat because of muscle atrophy. The functional loss of opposition, in conjunction with the anesthesia of the tips of the thumb and radial fingers, greatly reduces precision grip and other manipulative functions of the hand.
In addition to the important medial rotation function of the opponens pollicis, all three muscles of the thenar eminence independently perform the combined actions of flexion and abduction of the CMC joint. These kinematics are essential to lifting the thumb up and over the palm during opposition. Figure 8-45 compares these and other combined actions of the muscles that cross the CMC joint of the thumb. As noted by the location of the black dots, almost all the muscles have a combined action as a flexor-abductor, a flexor-adductor, an extensor-adductor, or an extensor-abductor. As indicated, the median nerve is the only source of innervation of the flexion-abduction quadrant of muscles. Although abduction of the thumb is still possible primarily because of the radial nerve–innervated abductor pollicis longus,8 this action is usually overruled by the stronger remaining adduction torque potential of the ulnar nerve–innervated adductor pollicis muscle (see ADPo and ADPt). For this reason, persons with a median nerve injury are susceptible to an adduction contracture of the CMC joint of the thumb. As described earlier, an adduction bias to the thumb is certainly counterproductive to the natural kinematics of opposition.
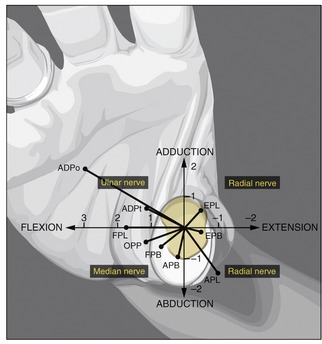
FIGURE 8-45. An illustration that associates the potential torque (strength) and combined actions of the muscles that cross the carpometacarpal (CMC) joint of the right thumb. The trapezium is outlined in light yellow at the base of the thumb. The black dots represent the location of each muscle relative to the two (primary) degrees of freedom of movement at the CMC joint: flexion-extension and adduction-abduction. With the exception of the flexor pollicis longus (FPL), each muscle is classified as a flexor-abductor, a flexor-adductor, an extensor-adductor, or an extensor-abductor. Furthermore, the length of each line associated with each muscle is proportional to the maximum torque potential of the muscle, which considers both the muscle’s moment arm and the cross-sectional area. The units used on the axes indicate torque in Nm. Observe that the muscles that fall within each of the four quadrants share the same source of innervation. ADPo, adductor pollicis, oblique head; ADPt, adductor pollicis, transverse head; APB, abductor pollicis brevis; APL, abductor pollicis longus; EPB, extensor pollicis brevis; EPL, extensor pollicis longus; FPB, flexor pollicis brevis; FPL, flexor pollicis longus; OPP, opponens pollicis. (The diagram is based on data originally plotted by Smutz WP, et al.97 From Neumann DA, Bielefeld TB: The carpometacarpal joint of the thumb: stability, deformity, and therapeutic intervention, J Orthop Sports Phys Ther 33:386, 2003.)
Muscles of the Hypothenar Eminence:
Anatomic Considerations: The muscles of the hypothenar eminence consist of the flexor digiti minimi, abductor digiti minimi, opponens digiti minimi, and palmaris brevis (see Figures 8-34 and 8-43). The abductor digiti minimi is the most superficial and medial of these muscles, occupying the extreme ulnar border of the hand. The relatively small flexor digiti minimi is located just lateral to, and often blended with, the abductor. Deep to these muscles is the opponens digiti minimi, the largest of the hypothenar muscles. The palmaris brevis is a thin and relatively insignificant muscle about the thickness of a postage stamp. It attaches between the transverse carpal ligament and an area of skin just distal to the pisiform bone (see Figure 8-34). The palmaris brevis raises the height of the hypothenar eminence, typically to assist with a deepening of the concavity of the palm.
Functional Considerations: A common function of the hypothenar muscles is to raise and “cup” the ulnar border of the hand. This action deepens the distal transverse arch, and enhances digital contact with held objects (see Figure 8-44). When necessary, the abductor digiti minimi can spread the small finger for greater control of grasp. The opponens digiti minimi rotates, or opposes, the fifth metacarpal toward the middle digit. Contraction of the long finger flexors of the small finger, such as the flexor digitorum profundus, also contributes to raising the ulnar border of the hand. The actions of all the muscles that cross the joints of the small finger are listed in Box 8-2.
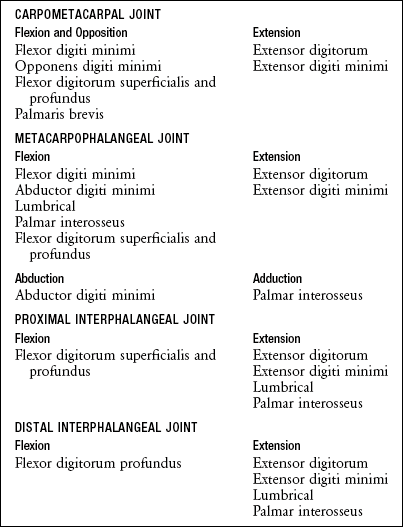
Adductor Pollicis Muscle: The adductor pollicis is a two-headed muscle lying deep in the web space of the thumb, palmar to the second and third metacarpals (see Figure 8-43). The muscle has its proximal attachments on the most stable skeletal region of the hand. The thicker oblique head arises from the capitate bone, bases of the second and third metacarpals, and other adjacent connective tissues.58 The thinner, triangular transverse head attaches on the palmar surface of the third metacarpal bone. Both heads join for a common distal attachment on the ulnar side of the base of the proximal phalanx of the thumb; additional attachments include a sesamoid bone located near the MCP joint.
The adductor pollicis is a dominant muscle at the CMC joint, producing the greatest combination of flexion and adduction torque.9 This important source of torque is applied to many activities, such as pinching an object between the thumb and index finger or closing a pair of scissors (Figure 8-46). The transverse head of the adductor pollicis uses a very long moment arm to generate both flexion (Figure 8-46, A) and adduction (Figure 8-46, B) torque at the base of the thumb. Although the transverse fibers have the greater leverage at the CMC joint, the thicker oblique head generates the greater flexion and adduction torque (compare ADPo and ADPt in Figure 8-45).58,97
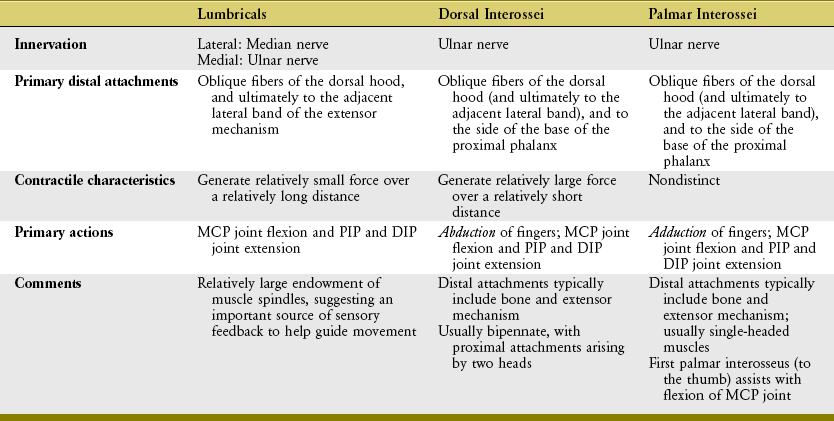
Lumbricals and Interosseus Muscles: The lumbricals (from the Latin root lumbricus, earthworm) are four very slender muscles originating from the tendons of the flexor digitorum profundus (see Figures 8-33 and 8-34). Like the flexor digitorum profundus, the lumbricals have a dual source of innervation: the two lateral lumbricals by the median nerve, and the two medial lumbricals by the ulnar nerve.
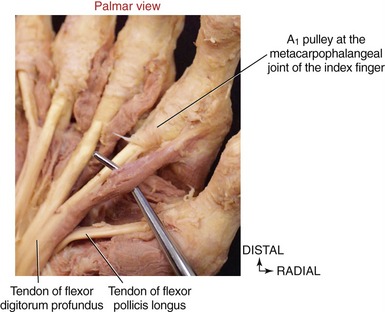
All four lumbricals show marked variation in both size and attachments.23,101 From their tendinous proximal attachments, the lumbricals course palmar to the deep transverse metacarpal ligament and then radial to the MCP joints (see Figure 8-41, first lumbrical). Distally, a typical lumbrical attaches to the adjacent lateral band of the extensor mechanism, most often via the oblique fibers of the dorsal hood (see close-up view of first lumbrical in Figure 8-47). This distal attachment enables the lumbricals to exert a proximal pull throughout the extensor mechanism.
The function of the lumbricals has been studied and debated for many years.14,54,60,86,107 What is universally agreed on is that their contraction produces flexion at the MCP joints and extension at the PIP and DIP joints.112 This seemingly paradoxic action is possible because the lumbricals pass palmar to the MCP joints but dorsal to the PIP and DIP joints (Figure 8-48).
Of all the intrinsic muscles of the hand, the lumbricals have the longest fiber length but the smallest cross-sectional area.11,40,58 This anatomic design suggests that these muscles are capable of generating small amounts of force over a relatively long distance. Although a low force potential in a muscle generally suggests a limited role in controlling movement, this is not always the case. Muscles have other important kinesiologic functions besides producing force. The first lumbrical, for instance, possesses a very rich source of muscle spindles—sensory organs that closely monitor changes in the length of the muscle. The average spindle density of the first lumbrical is approximately three times greater than that of the interosseus muscles within the hand and eight times greater than that of the biceps brachii muscle.81 This large density of muscle spindles in the lumbricals suggests an important role in providing sensory feedback during complex movements.86,99 By also attaching to the tendons of the flexor digitorum profundus, perhaps the lumbricals are in position to help coordinate the interactions between the intrinsic and extrinsic muscles.
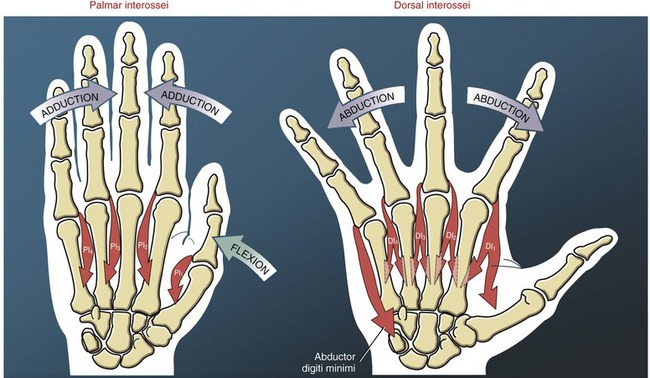
The interosseus muscles are named according to their general location between the metacarpal bones (see Figures 8-4 and 8-5). As with the lumbricals, variations in attachments and morphology are more the rule than the exception.22,101 In general, the interossei act at the MCP joints to spread the digits apart (abduction) or bring them together (adduction).
The four palmar interossei of the hand are slender, typically single-headed muscles that occupy the palmar region of the interosseous spaces. The three palmar interossei to the fingers have their proximal attachments on the palmar surfaces and sides of the second, fourth, and fifth metacarpals (see Figure 8-43). The muscles’ distal attachments vary but typically include the oblique fibers of the dorsal hood, and the sides of the bases of the proximal phalanges.22 These muscles adduct the second, fourth, and fifth MCP joints toward the midline of the hand (Figure 8-49).12 The palmar interosseus muscle to the thumb occupies the first palmar interosseous space. This deep muscle has its distal attachment on the ulnar side of the proximal phalanx of the thumb, and often attaches to a sesamoid bone at the MCP joint.101 The first palmar interosseus is often small or partially formed and therefore is ignored in most biomechanical analysis. In theory, this muscle is positioned to help flex the MCP joint of the thumb, bringing the first metacarpal toward the midline of the hand.
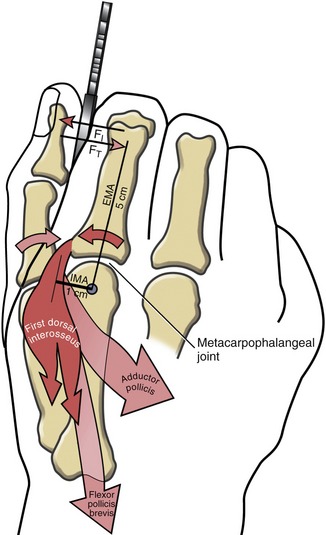
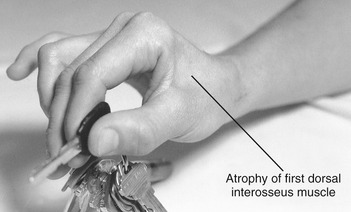
The four dorsal interossei fill the dorsal sides of the interosseous spaces (see Figure 8-39). In contrast to the palmar interossei, the dorsal muscles typically have a bipennate shape. As a general rule, the dorsal interossei have distal attachments to the oblique fibers of the dorsal hood, as well as to the sides of the bases of the proximal phalanges. Some distal attachments may blend with more palmar aspects of the transverse fibers of the dorsal hood and the palmar plate.22 The first dorsal interosseus (formerly abductor indicis) is the largest and most accessible for clinical inspection. With the index finger well stabilized, the first dorsal interosseus can assist the adductor pollicis in adducting the thumb at the CMC joint. (This can be visualized by reversing the direction of the arrow of the first dorsal interosseus to the thumb in Figure 8-48.)
As a set, the dorsal interossei abduct the MCP joints of the index, middle, and ring fingers away from an imaginary reference line through the middle digit (see Figure 8-49). Abduction of the fifth MCP joint is performed by the abductor digiti minimi of the hypothenar group.
In addition to abducting and adducting the fingers, the interossei and abductor digiti minimi provide an important source of dynamic stability to the MCP joints. When the two hands shown in Figure 8-49 are visually superimposed, it is apparent that each MCP joint of the fingers is equipped with a pair of abducting and adducting muscles. The pairs act as dynamic collateral ligaments, providing strength to the MCP joints. Acting in pairs, this interosseus musculature also controls the extent of axial rotation permitted at the MCP joints.
To varying degrees, both palmar and dorsal interossei have a line of force that passes palmar to the MCP joints, especially when the MCP joints are flexed. The interossei, via their attachments into the extensor mechanism, pass dorsal to the IP joints of the fingers (see index finger in Figure 8-48). Like the lumbricals, therefore, contraction of the interossei flexes the MCP joints and extends the IP joints. The interossei produce greater flexion torques at the MCP joints than the lumbricals. Even though the lumbricals have the larger moment arm for this action, the overwhelmingly larger cross-section area of the interossei empowers them with the greater flexion torque potential. In contrast to the lumbricals, the interossei produce relatively larger forces but over a shorter contraction distance (Table 8-5).40
Interaction of the Extrinsic and Intrinsic Muscles of the Fingers
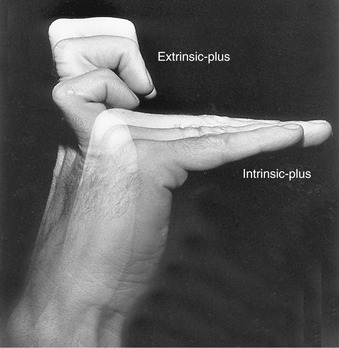
As described in Figure 8-48, simultaneous contraction of the intrinsic muscles of the fingers (lumbricals and interossei) produces a combined MCP joint flexion and IP joint extension. This position of the hand is referred to as the intrinsic-plus position. In contrast, simultaneous contraction of the extrinsic muscles of the fingers (extensor digitorum, flexor digitorum superficialis, and flexor digitorum profundus) produces MCP joint hyperextension and IP joint flexion: the extrinsic-plus position. The two opposite positions of the fingers are presented in Figure 8-50. A very important kinesiologic principle of the hand is that most functional or complex digital movements require a synergistic blending of these two opposite actions. This point is reinforced in the next sections.
The interaction between the extrinsic and intrinsic muscles of the hand can produce many combinations of movements used to perform a seemingly infinite number of functions. The following analysis, however, addresses the muscular interaction within a typical finger during two fundamental functions: opening and closing of the hand. The precise muscular interactions used to perform these actions is controversial and not completely understood, despite years of research and study based on anatomy, biomechanics, electromyography, and computer-simulated modeling.* Part of the obstacle in understanding the muscular interactions is that similar movements can be performed by different combinations of muscles, both within and between persons.42 Precise muscular interaction also depends on the speed or power of an activity, the skill of the performer, weight and shape of the manipulated object, and natural human variability. Of interest, much of what is known for certain has been learned by carefully observing the pathomechanical impairments of the hand that resulted from a disruption of the neuromusculoskeletal system.9,49
OPENING THE HAND: FINGER EXTENSION
Primary Muscular Activity: Opening the hand is often performed in preparation for grasp. The greatest resistance to complete extension of the fingers across the MCP and IP joints is usually not from gravity but from the viscoelastic resistance generated by the stretching of the extrinsic finger flexors, in particular the flexor digitorum profundus. The passive “recoil” force generated within this muscle is largely responsible for the partially flexed posture of a relaxed hand.
The primary extensors of the fingers are the extensor digitorum and the intrinsic muscles, specifically the lumbricals and interossei. In general, the lumbricals show a greater and more consistent level of electromyographic (EMG) activity than the interossei during finger extension.60
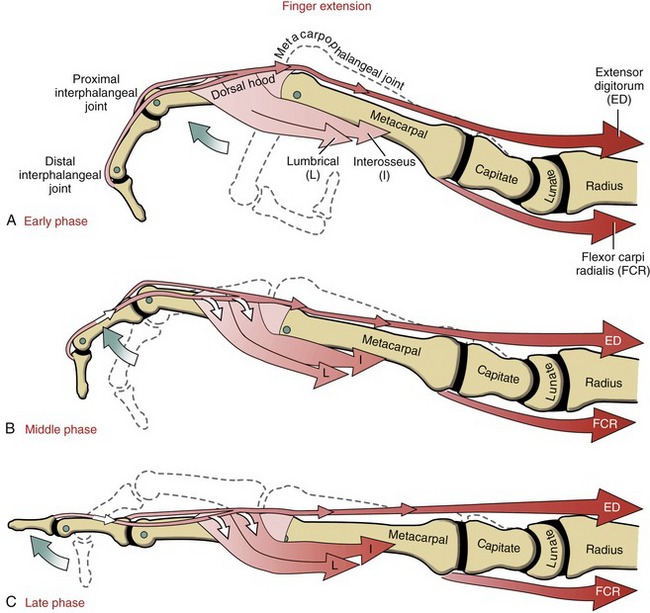
Figure 8-51, A shows the extensor digitorum exerting a force on the extensor mechanism, pulling the MCP joint toward extension. The intrinsic muscles of the fingers furnish both direct and indirect effects on the mechanics of extension of the IP joints (see Figure 8-51, B and C). The direct effect is provided by the proximal pull placed on the extensor mechanism; the indirect effect is provided by the production of a flexion torque at the MCP joint.47 The flexion torque prevents the extensor digitorum from hyperextending the MCP joint—an action that prematurely dissipates most of its contractile force. Only with the MCP joint blocked from being hyperextended can the extensor digitorum effectively tense the extensor mechanism sufficiently to completely extend the IP joints.
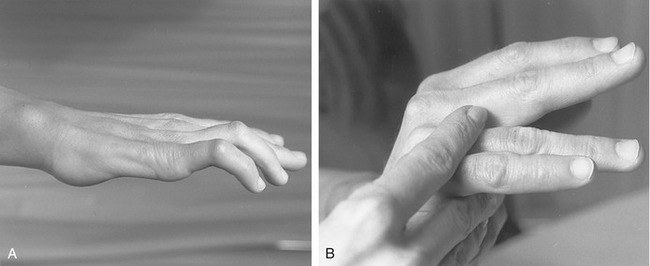
The extensor digitorum and intrinsic muscles of the fingers cooperate synergistically to extend the finger. Paradoxically, it is the opposing actions of the extensor digitorum and the intrinsic muscles across the MCP joint that allow them to synergistically extend the IP joints. This relationship is apparent on observation of a person with an injury of the ulnar nerve (Figure 8-52, A). Without active resistance from either of the intrinsic muscles of the medial two fingers, activation of the extensor digitorum causes a characteristic “clawing” of the digits: the MCP joints hyperextend and the IP joints remain partially flexed. This is often called the “intrinsic-minus” posture because of the lack of intrinsic-innervated muscles. (This posture is functionally similar to the “extrinsic-plus” posture depicted in Figure 8-50.) Without the MCP joint flexion torque normally provided by the intrinsic muscles, the extensor digitorum functions only to hyperextend the MCP joints. This posture stretches the flexor digitorum profundus, thereby adding further resistance against extension of the IP joints. As shown in Figure 8-52, B, with manual application of a flexion torque across the MCP joint (i.e., a force normally furnished by the intrinsic muscles), contraction of the extensor digitorum is able to fully extend the IP joints. Blocking the MCP joint from hyperextending also slackens the profundus tendon, thereby minimizing the muscle’s passive resistance to extension of the IP joints. Preventing the MCP joints from hyperextending is one form of therapeutic intervention after paralysis of the intrinsic muscles of the fingers. Therapists may fabricate a splint that limits extension of the MCP joints; surgeons may devise a muscular block against hyperextension by rerouting a tendon from a stronger, innervated muscle to the flexor side of the involved MCP joints.33
Function of Wrist Flexors during Finger Extension: Activation of the wrist flexor muscles normally accompanies active finger extension, especially when performed rapidly. Although this activity is depicted only in the flexor carpi radialis in Figure 8-51, other wrist flexors are also active. The wrist flexors offset the large extension potential of the extensor digitorum at the wrist. The wrist actually flexes slightly during rapid and complete finger extension. (Compare Figure 8-51, A with Figure 8-51, C.) Wrist flexion helps maintain optimal length of the extensor digitorum during active finger extension.
CLOSING THE HAND: FINGER FLEXION
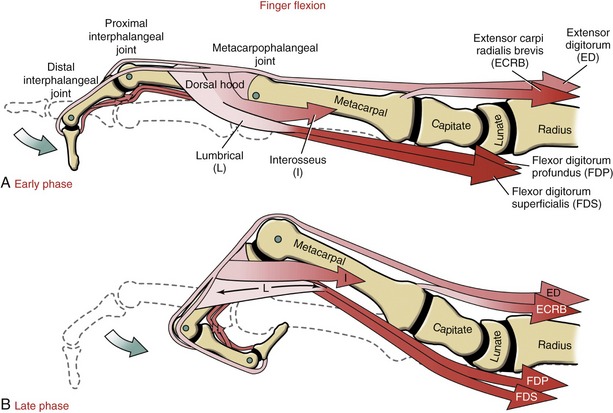
Primary Muscle Action: The muscles used to close the hand depend in part on the specific joints that need to be flexed and on the force requirements of the action. Flexing the fingers against resistance or at relatively high speed requires activation of the flexor digitorum profundus, flexor digitorum superficialis, and, to a lesser extent, the interosseus muscles (Figure 8-54, A). Forces produced by the flexor digitorum profundus and superficialis flex all three joints of the fingers; the flexing finger pulls the extensor mechanism distally by several millimeters.
Although typically inactive while the hand is closing, the lumbricals may still passively assist with this action. Recall that the lumbricals attach between the flexor digitorum profundus and the extensor mechanism. During active finger flexion, the lumbricals are stretched in a proximal direction owing to the contracting flexor digitorum profundus and at the same time are stretched in a distal direction owing to the distal migration of the extensor mechanism (see Figure 8-54, B, bidirectional arrow in lumbrical). Between full extension and full active flexion, a lumbrical must stretch an extraordinary distance.86 The stretch generates a passive flexion torque across the MCP joint. Although small, this passive torque may supplement the active flexion torque produced by the interossei and, primarily, the extrinsic flexor musculature.55
The extensor digitorum shows consistent EMG activity while the hand is closing.59 This activity reflects the muscle’s role as an extension brake at the MCP joint. This important stabilization function allows the long finger flexors to shift their action distally to the PIP and DIP joints. Without coactivation of the extensor digitorum, the long finger flexors exhaust most of their flexion potential over the MCP joints, reducing their potential for more refined actions at the more distal joints.
Function of Wrist Extensors during Finger Flexion: Making a strong fist requires strong synergistic activation from the wrist extensor muscles (see Figure 8-54, extensor carpi radialis brevis). Wrist extensor activity can be verified by palpating the dorsum of the forearm while a fist is made. As explained in Chapter 7, the primary function of the wrist extensors, including the extensor digitorum, is to neutralize the strong wrist flexion tendency of the activated extrinsic finger flexor muscles (review Figure 7-25). While the hand is closing, wrist extension also helps maintain more optimal length of the extrinsic finger flexors. If the wrist extensors are paralyzed, attempts at making a fist result in a posture of wrist flexion and finger flexion. When combined with the increased passive tension in the overstretched extensor digitorum, the overshortened, activated finger flexors are incapable of producing an effective grip (see Figure 7-27).
HAND AS AN EFFECTOR ORGAN
Prehension describes the ability of the fingers and thumb to grasp or to seize, often for holding, securing, and picking up objects. Several terms have evolved over the years to describe the many forms of prehension.52,73 Most forms of prehension can be described as a grip (or grasp), in which all digits are used, or as a pinch, in which primarily the thumb and index finger are used. Each form can be further classified based on the need for power (loosely defined as high force without regard to the exactness of the task) or precision (i.e., high level of exactness with low force). The specific classifications of prehension subsequently described are not intended to include all possible ways that the hand can be used. These definitions are nevertheless useful to establish a common reference for clinical communication.
Basically, most types of prehension activities fall into one of the following five types:
1. The power grip is used when stability and large forces are needed, without the need for precision. The shape of held objects tends to be spheric or cylindric. Using a hammer is a good example of a power grip (Figure 8-55, A). This activity requires strong forces from the finger flexors, especially from the fourth and fifth digits; intrinsic muscles of the fingers, especially the interossei; and the thumb adductor and flexor musculature. Wrist extensors are needed to stabilize the partially extended wrist.
2. The precision grip is used when control and/or delicate action is needed during prehension (see Figure 8-55, B and C). The thumb is usually held partially abducted, and the fingers are partially flexed. The precision grip uses the thumb and one or more of the digits to improve grip security or, if needed, to add variable amounts of force. The precision grip is modified to fit objects of varied sizes by altering the contour of the distal transverse arch of the hand (see Figure 8-55, D to F).
3. The power (key) pinch is used when large forces are needed to stabilize an object between the thumb and the lateral border of the index finger (see Figure 8-55, G). The power pinch is an extremely useful form of prehension, combining the force of the adductor pollicis and first dorsal interosseus with the dexterity and sensory acuity of the thumb and index finger.
4. The precision pinch is used to provide fine control to objects held between the thumb and index finger, without the need for high power. This type of pinch has many forms, such as the tip-to-tip or pulp-to-pulp method of holding an object (see Figure 8-55, H and I, respectively). The tip-to-tip pinch is used especially for tiny objects, when skill and precision are required. The pulp-to-pulp pinch provides greater surface area for contact with larger objects, thereby increasing prehensile security.
5. The hook grip is a form of prehension that does not involve the thumb. A hook grip is formed by the partially flexed PIP and DIP joints of the fingers. This grip is often used in a static manner for prolonged periods of time, such as holding a luggage strap (see Figure 8-55, J). The force of the hook grip is usually provided primarily by the flexor digitorum profundus.
JOINT DEFORMITIES TYPICALLY CAUSED BY RHEUMATOID ARTHRITIS
Zigzag Deformity of the Thumb
Advanced rheumatoid arthritis often results in a zigzag deformity of the thumb. As defined in Chapter 7, a zigzag deformity results from the collapse of multiple interconnected joints in alternating directions. Although several combinations of deformity have been described,3,71,102 one relatively common deformity involves CMC joint flexion and adduction, MCP joint hyperextension, and IP joint flexion (Figure 8-56).5 In this example the collapse of the thumb starts with instability at the CMC joint. Ligaments that normally reinforce the medial (ulnar) side of the joint, such as the anterior oblique and ulnar collateral ligaments, can become weak and rupture because of the disease process. Subsequently the base of the thumb metacarpal dislocates off the radial or dorsal-radial edge of the trapezium (see arrow at base of first metacarpal in Figure 8-56). Altered moment arms of some of the muscles that cross the CMC joint may further contribute to this dislocation.79 Once this dislocation occurs, the adductor and short flexor muscles, which are often in spasm, hold the thumb metacarpal rigidly against the palm. In time, rheumatoid disease may cause the muscles to become fibrotic and permanently shortened, maintaining the deformity at the CMC joint. In efforts to extend the rigid thumb out of the palm, a compensatory hyperextension deformity at the MCP joint often occurs.3 A weakened and overstretched palmar plate at this joint offers little resistance to the extension forces produced by the extensor pollicis longus and brevis or contact forces produced during pinch. Eventual bowstringing of these tendons across the MCP joint increases their leverage as extensors, thereby further contributing to the hyperextension deformity. The IP joint tends to remain flexed as a result of the passive tension in the stretched flexor pollicis longus.
Clinical interventions for a zigzag deformity of the thumb depend on the specific mechanics of the collapse and the severity of the underlying rheumatoid disease. Nonsurgical intervention includes splinting to encourage more normal joint alignment, medicine to reduce chronic inflammation, and teaching patients ways to minimize stress on the joint.76 Surgery may be considered if more conservative intervention fails to slow the progression of the deformity.
Destruction of the Metacarpophalangeal Joints of the Finger
Advanced rheumatoid arthritis is often associated with deformities at the MCP joint of the fingers. The two most common deformities are palmar dislocation and ulnar drift (Figure 8-57). Although these two deformities typically occur together, they are discussed separately in the following sections.
PALMAR DISLOCATION OF THE METACARPOPHALANGEAL JOINT
When the fingers flex during grip, the tendons of the flexor digitorum superficialis and profundus are deflected palmarly across the MCP joint (Figure 8-58, A). This natural bend generates a bowstringing force in the palmar direction. As indicated in Figure 8-58, A, the bowstringing force is transferred through much of the MCP joint’s periarticular connective tissue: from flexor A1 pulley, palmar plate, collateral ligaments, and, finally, to posterior tubercle of the metacarpal head. The greater the degree of flexion at the MCP joint, the greater the magnitude of the bowstringing force. In the healthy hand, this force is safely dissipated throughout the natural elasticity and strength of the tissues.
In the hand with severe rheumatoid arthritis, the collateral ligaments may rupture because of the constant bowstringing force. In time, the proximal phalanx may translate excessively in a palmar direction, resulting in a completely dislocated MCP joint (see Figure 8-58, B). Palmar dislocation may collapse both the longitudinal and transverse arches of the hand, causing it to appear flat.
Patient education on ways to “protect” the MCP joint from further palmar dislocation is an important part of treatment.7 Patients are instructed on how to perform functional activities that place limited demands on their finger flexor muscles.
ULNAR DRIFT
Ulnar “drift” deformity at the MCP joint consists of an excessive ulnar deviation and ulnar translation (slide) of the proximal phalanx. This deformity is common in advanced stages of rheumatoid arthritis and often occurs in conjunction with a palmar dislocation of the MCP joint (as indicated in Figure 8-57).
To fully understand the pathomechanics of ulnar drift, it is important to realize that all hands—healthy or otherwise—are constantly subjected to factors that favor an ulnar-deviated posture of the fingers.7,27,105 These factors include gravity, an asymmetric slope of the metacarpal heads, and the prevailing ulnar (medial) line of pull of the extrinsic flexor tendons as they pass the MCP joints. But perhaps the most influential factor stems from the relentless, ulnar-directed forces applied against the proximal phalanges of the radial fingers. These forces are produced by contact from hand-held objects and large “pinching” forces generated by the flexor muscles of the thumb. Figure 8-59, A shows these ulnar-directed forces pushing the index finger in an ulnar direction. The subsequent ulnar deviation of the MCP joint increases the ulnar deflection—or bend—in the extensor digitorum (ED) tendon as its crosses the dorsal side of the joint. The deflection creates a potentially destabilizing bowstringing force on the tendon. In the healthy hand, however, the transverse fibers of the dorsal hood and radial collateral ligament maintain the extensor tendon over the axis of rotation, thereby protecting the joint from drifting further into ulnar deviation.
The previous description reinforces the important role that healthy connective tissue plays in maintaining the stability of a joint. Often, in severe cases of rheumatoid arthritis, the radial transverse fibers of the dorsal hood rupture or overstretch, allowing the tendon of the extensor digitorum to slip toward the ulnar side of the joint’s axis of rotation (see Figure 8-59, B). In this position, the force produced by the extensor digitorum acts with a moment arm that amplifies the ulnar deviated posture. This situation initiates a self-perpetuating process: the greater the ulnar deviation, the greater the associated moment arm, and the greater the deforming ulnar deviation torque. In time, a weakened and overstretched radial collateral ligament may rupture, allowing the proximal phalanx to rotate and slide ulnarly, leading to complete joint dislocation. Persons with severe ulnar drift that affects multiple fingers are typically most concerned about appearance and the reduced function—especially related to pinch and power grip.
The pathomechanics of ulnar drift often involve a secondary destabilizing process at the MCP joints. In addition to ulnar migration, the tendon of the extensor digitorum may also slip palmarly into the natural “gullies” between the prominent metacarpal heads. This abnormal palmar position reduces the moment arm of the extensor digitorum for extending the MCP joint. As depicted in a side view in Figure 8-59, C, the extensor tendon may actually displace palmar to the medial-lateral axis of rotation. In this case the displaced tendon creates a flexion torque at the MCP joint. These abnormal mechanics favor the palmar dislocation deformity previously described.
Treatment for ulnar drift typically includes normalizing the alignment of the joint and, when possible, minimizing the underlying mechanics that are responsible for the instability or deformity.7 Common nonsurgical treatment includes using splints and specialized adaptive equipment and advising patients on how to minimize the deforming forces across the MCP joint.66,77 Consider the strong ulnar deviation torque placed on the MCP joints of the right hand when the lid of a jar is tightened or a pitcher of water is held. This torque may, over time, predispose or accentuate ulnar drift. In general, patients are advised to avoid most heavy gripping and forceful key pinch activities, especially during the acute inflammation or painful stage of the rheumatoid arthritis.
Surgical intervention for excessive ulnar drift may include transferring the extensor digitorum tendon to the radial side of the MCP joint’s anterior-posterior axis of rotation.33 In more severe cases the damaged MCP joint may be replaced with a total joint arthoplasty. This usually provides relief of pain and restores some function, although the patient typically will not regain full range of motion. This surgery is often performed in conjunction with reconstruction of the joint’s periarticular connective tissues. A fusion or arthroplasty of the wrist may also be indicated because the mechanics associated with a misaligned wrist can create potentially deforming forces on the MCP joint. Regardless of the specific surgery, proper postsurgical treatment is critical for successful rehabilitation.7 This treatment is typically provided by certified hand therapists who have specialized in hand rehabilitation. A close working relationship is essential between the surgeon and the therapist.
Zigzag Deformities of the Fingers
Two classic zigzag deformities of the finger can occur, typically associated with advanced rheumatoid arthritis: swan-neck deformity and boutonniere deformity (see Figure 8-57). As noted in the previous photograph, both deformities often occur in conjunction with ulnar drift and palmar dislocation of the MCP joints.
SWAN-NECK DEFORMITY
Swan-neck deformity is characterized by hyperextension of the PIP joint with flexion at the DIP joint (see Figure 8-57, middle finger). The position of the MCP joint is variable. The intrinsic muscles in the hand affected by rheumatoid arthritis often become fibrotic and contracted. With weakened palmar plates at the PIP joint, the tension within the intrinsic muscles may eventually collapse the PIP joints into hyperextension (Figure 8-60, A). The hyperextended position of the PIP joint causes the lateral bands of the extensor mechanism to bowstring dorsally, away from the joint’s axis of rotation. Bowstringing increases the moment arm for the intrinsic muscles to extend the PIP joint, thereby accentuating the hyperextension deformity. The DIP joint tends to remain flexed because of the stretch placed on the tendon of the flexor digitorum profundus across the PIP joint.
BOUTONNIERE DEFORMITY
The boutonniere deformity is described as flexion of the PIP joint and hyperextension of the DIP joint (see Figure 8-57, index finger). (The term boutonniere—a French word meaning buttonhole—describes the appearance of the head of the proximal phalanx as it slips through the “buttonhole” created by the displaced lateral bands.) The interphalangeal joints collapse essentially in a reciprocal pattern to that described for the swan-neck deformity. The primary cause of the boutonniere deformity is abnormal displacement of the bands of the extensor mechanism at the PIP joint and rupture of the central band, usually the result of chronic synovitis. The lateral bands slip toward the palmar side of the axis of rotation at the PIP joint (see Figure 8-60, B). Consequently, forces transferred across the slipped lateral bands (either from active or passive sources) cause flexion at the PIP joint instead of normal extension. Essentially, the PIP joint loses all sources of extension.
As depicted in Figure 8-60, the DIP joint in the boutonniere deformity remains hyperextended owing to the increased tension in the stretched lateral bands. The inability to flex the DIP joint interferes with the ease of picking up small objects, such as a coin from a table.
REFERENCES
1. An, KN, Ueba, Y, Chao, EY, et al. Tendon excursion and moment arm of index finger muscles. J Biomech. 1983;16:419–425.
2. Arimitsu, S, Murase, T, Hashimoto, J, et al. A three-dimensional quantitative analysis of carpal deformity in rheumatoid wrists. J Bone Joint Surg Br. 2007;89:490–494.
3. Armbruster, EJ, Tan, V. Carpometacarpal joint disease: Addressing the metacarpophalangeal joint deformity. Hand Clin. 2008;24:295–299.
4. Batmanabane, M, Malathi, S. Movements at the carpometacarpal and metacarpophalangeal joints of the hand and their effect on the dimensions of the articular ends of the metacarpal bones. Anat Rec. 1985;213:102–110.
5. Belt, E, Kaarela, K, Lehtinen, J, et al. When does subluxation of the first carpometacarpal joint cause swan-neck deformity of the thumb in rheumatoid arthritis: A 20-year follow-up study. Clin Rheumatol. 1998;17:135–138.
6. Bettinger, PC, Linscheid, RL, Berger, RA, et al. An anatomic study of the stabilizing ligaments of the trapezium and trapeziometacarpal joint. J Hand Surg [Am]. 1999;24:786–798.
7. Bielefeld, T, Neumann, DA. The unstable metacarpophalangeal joint in rheumatoid arthritis: Anatomy, pathomechanics, and physical rehabilitation considerations. J Orthop Sports Phys Ther. 2005;35:502–520.
8. Boatright, JR, Kiebzak, GM. The effects of low median nerve block on thumb abduction strength. J Hand Surg [Am]. 1997;22:849–852.
9. Brand, PW. Clinical biomechanics of the hand. St. Louis: Mosby; 1985.
10. Brand, PW. The reconstruction of the hand in leprosy. 1952. Clin Orthop Relat Res. 2002;396:4–11.
11. Brand, PW, Beach, RB, Thompson, DE. Relative tension and potential excursion of muscles in the forearm and hand. J Hand Surg [Am]. 1981;6:209–219.
12. Buford WL, Andersen CR: Predicting moment arms in diarthrodial joints—3D computer simulation capability and muscle-tendon model validation. Conference Proceedings. 2006; Annual International Conference of the IEEE Engineering in Medicine & Biology Society. 1:3407-3410, 2006.
13. Cheema, TA, Cheema, NI, Tayyab, R, Firoozbakhsh, K. Measurement of rotation of the first metacarpal during opposition using computed tomography. J Hand Surg [Am]. 2006;31:76–79.
14. Close, JR, Kidd, CC. The functions of the muscles of the thumb, the index, and long fingers. Synchronous recording of motions and action potentials of muscles. J Bone Joint Surg Am. 1969;51:1601–1620.
15. Cooney, WP, 3rd., Lucca, MJ, Chao, EY, Linscheid, RL. The kinesiology of the thumb trapeziometacarpal joint. J Bone Joint Surg Am. 1981;63:1371–1381.
16. Cope, JM, Berryman, AC, Martin, DL, Potts, DD. Robusticity and osteoarthritis at the trapeziometacarpal joint in a Bronze Age population from Tell Abraq, United Arab Emirates. Am J Phys Anthropol. 2005;126:391–400.
17. Doyle, JR, Blythe, W. The finger flexor tendon sheath and pulleys: Anatomy and reconstruction. In: American academy of orthopaedic surgeons symposium on tendon surgery in the hand. St. Louis: Mosby; 1975.
18. Dubousset, JF. The Digital Joints. In: Tubiana R, ed. The hand. Philadelphia: Saunders, 1981.
19. Dvir, Z. Biomechanics of muscle. In: Dvir Z, ed. Clinical biomechanics. Philadelphia: Churchill Livingstone, 2000.
20. Eaton, RG, Littler, JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55:1655–1666.
21. El Shennawy, M, Nakamura, K, Patterson, RM, Viegas, SF. Three-dimensional kinematic analysis of the second through fifth carpometacarpal joints. J Hand Surg [Am]. 2001;26:1030–1035.
22. Eladoumikdachi, F, Valkov, PL, Thomas, J, Netscher, DT. Anatomy of the intrinsic hand muscles revisited: part I. Interossei. Plast Reconstr Surg. 2002;110:1211–1224.
23. Eladoumikdachi, F, Valkov, PL, Thomas, J, Netscher, DT. Anatomy of the intrinsic hand muscles revisited: part II. Lumbricals. Plast Reconstr Surg. 2002;110:1225–1231.
24. Enoka, RM. Neuromechanics of human movement, ed 3. Champaign, Ill: Human Kinetics; 2002.
25. Firoozbakhsh, K, Yi, IS, Moneim, MS, Umada, Y. A study of ulnar collateral ligament of the thumb metacarpophalangeal joint. Clin Orthop Relat Res. 2002;403:240–247.
26. Flatt, AE. The care of the rheumatoid hand, ed 3. St. Louis: Mosby; 1974.
27. Flatt, AE. Ulnar drift. J Hand Ther. 1996;9:282–292.
28. Fontana, L, Neel, S, Claise, JM, et al. Osteoarthritis of the thumb carpometacarpal joint in women and occupational risk factors: A case-control study. J Hand Surg [Am]. 2007;32:459–465.
29. Goodman, HJ, Choueka, J. Biomechanics of the flexor tendons. Hand Clin. 2005;21:129–149.
30. Gray, DJ, Gardner, E. The innervation of the joints of the wrist and hand. Anat Rec. 1965;151:261–266.
31. Greenwald, D, Shumway, S, Allen, C, Mass, D. Dynamic analysis of profundus tendon function. J Hand Surg [Am]. 1994;19:626–635.
32. Hahn, P, Krimmer, H, Hradetzky, A, Lanz, U. Quantitative analysis of the linkage between the interphalangeal joints of the index finger. An in vivo study. J Hand Surg [Br]. 1995;20:696–699.
33. Hentz, VR, Chase, RA. Hand surgery: a clinical atlas. Philadelphia: Saunders; 2001.
34. Holzbaur, KR, Delp, SL, Gold, GE, Murray, WM. Moment-generating capacity of upper limb muscles in healthy adults. J Biomech. 2007;40:2442–2449.
35. Holzbaur, KR, Murray, WM, Gold, GE, Delp, SL. Upper limb muscle volumes in adult subjects. J Biomech. 2007;40:742–749.
36. Hume, MC, Gellman, H, McKellop, H, Brumfield, RH, Jr. Functional range of motion of the joints of the hand. J Hand Surg [Am]. 1990;15:240–243.
37. Imaeda, T, An, KN, Cooney, WP, 3rd., Linscheid, R. Anatomy of trapeziometacarpal ligaments. J Hand Surg [Am]. 1993;18:226–231.
38. Imaeda, T, Niebur, G, Cooney, WP, 3rd., et al. Kinematics of the normal trapeziometacarpal joint. J Orthop Res. 1994;12:197–204.
39. Inman, VT, Saunders, JB. Referred pain from skeletal structures. J Nerv Ment Dis. 1944;99:660–667.
40. Jacobson, MD, Raab, R, Fazeli, BM, et al. Architectural design of the human intrinsic hand muscles. J Hand Surg [Am]. 1992;17:804–809.
41. Jenkins, M, Bamberger, HB, Black, L, Nowinski, R. Thumb joint flexion. What is normal? J Hand Surg [Br]. 1998;23:796–797.
42. Johanson, ME, Skinner, SR, Lamoreux, LW. Phasic relationships of the intrinsic and extrinsic thumb musculature. Clin Orthop Relat Res. 1996;322:120–130.
43. Kamper, DG, George, HT, Rymer, WZ. Extrinsic flexor muscles generate concurrent flexion of all three finger joints. J Biomech. 2002;35:1581–1589.
44. Katarincic, JA. Thumb kinematics and their relevance to function. Hand Clin. 2001;17:169–174.
45. Kaufman, KR, An, KN, Litchy, WJ, et al. In-vivo function of the thumb muscles. Clin Biomech (Bristol, Avon). 1999;14:141–150.
46. Keir, PJ, Bach, JM, Hudes, M, Rempel, DM. Guidelines for wrist posture based on carpal tunnel pressure thresholds. Hum Factors. 2007;49:88–99.
47. Koh, S, Buford, WL, Jr., Andersen, CR, Viegas, SF. Intrinsic muscle contribution to the metacarpophalangeal joint flexion moment of the middle, ring, and small fingers. J Hand Surg [Am]. 2006;31:1111–1117.
48. Kovler, M, Lundon, K, McKee, N, Agur, A. The human first carpometacarpal joint: Osteoarthritic degeneration and 3-dimensional modeling. J Hand Ther. 2004;17:393–400.
49. Kozin, SH, Porter, S, Clark, P, Thoder, JJ. The contribution of the intrinsic muscles to grip and pinch strength. J Hand Surg [Am]. 1999;24:64–72.
50. Krishnan, J, Chipchase, L. Passive axial rotation of the metacarpophalangeal joint. J Hand Surg [Br]. 1997;22:270–273.
51. Kuczynski, K. Carpometacarpal joint of the human thumb. J Anat. 1974;118:119–126.
52. Landsmeer, JF. Power grip and precision handling. Ann Rheum Dis. 1962;21:164–170.
53. Leibovic, SJ, Bowers, WH. Anatomy of the proximal interphalangeal joint. Hand Clin. 1994;10:169–178.
54. Leijnse, JN. Why the lumbrical muscle should not be bigger—a force model of the lumbrical in the unloaded human finger. J Biomech. 1997;30:1107–1114.
55. Leijnse, JN, Kalker, JJ. A two-dimensional kinematic model of the lumbrical in the human finger. J Biomech. 1995;28:237–249.
56. Leijnse, JN, Spoor, CW, Shatford, R. The minimum number of muscles to control a chain of joints with and without tenodeses, arthrodeses, or braces—application to the human finger. J Biomech. 2005;38:2028–2036.
57. Li, ZM, Tang, J. Coordination of thumb joints during opposition. J Biomech. 2007;40:502–510.
58. Linscheid, RL, An, KN, Gross, RM. Quantitative analysis of the instrinsic muscles of the hand. Clin Anat. 1991;4:265–284.
59. Long, C. Intrinsic-extrinsic muscle control of the fingers. Electromyographic studies. J Bone Joint Surg Am. 1968;50:973–984.
60. Long, C, Brown, TD. Electromyographic kinesiology of the hand: Muscles moving the long finger. J Bone Joint Surg Am. 1964;46:1683–1706.
61. Loos, B, Puschkin, V, Horch, RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskel Disorders. 2007;8:60.
62. Loubert, PV, Masterson, TJ, Schroeder, MS, Mazza, AM. Proximity of collateral ligament origin to the axis of rotation of the proximal interphalangeal joint of the finger. J Orthop Sports Phys Ther. 2007;37:179–185.
63. MacConaill, MA, Basmajian, JV. Muscles and movements: a basis for human kinesiology. New York: Robert E. Krieger; 1977.
64. MacMoran, JW, Brand, PW. Bone loss in limbs with decreased or absent sensation: Ten year follow-up of the hands in leprosy. Skeletal Radiol. 1987;16:452–459.
65. Marklin, RW, Simoneau, GG, Monroe, JF. Wrist and forearm posture from typing on split and vertically inclined computer keyboards. Hum Factors. 1999;41:559–569.
66. Masiero, S, Boniolo, A, Wassermann, L, et al. Effects of an educational-behavioral joint protection program on people with moderate to severe rheumatoid arthritis: A randomized controlled trial. Clin Rheumatol. 2007;26:2043–2050.
67. Melvin, JL. Therapist’s management of osteoarthritis in the hand. In Mackin EJ, Callahan AD, Skriven TM, et al, eds.: Rehabilitation of the hand and upper extremity, ed 5, St. Louis: Mosby, 2005.
68. Mo, JH, Gelberman, RH. Ligament reconstruction with trapezium retention arthroplasty for carpometacarpal arthritis. J Hand Surg [Am]. 2004;29:240–246.
69. Momose, T, Nakatsuchi, Y, Saitoh, S. Contact area of the trapeziometacarpal joint. J Hand Surg [Am]. 1999;24:491–495.
70. Nakamura, K, Patterson, RM, Viegas, SF. The ligament and skeletal anatomy of the second through fifth carpometacarpal joints and adjacent structures. J Hand Surg [Am]. 2001;26:1016–1029.
71. Nalebuff, EA. Diagnosis, classification and management of rheumatoid thumb deformities. Bull Hosp Jt Dis. 1968;29:119–137.
72. Nanno, M, Buford, WL, Jr., Patterson, RM, et al. Three-dimensional analysis of the ligamentous attachments of the first carpometacarpal joint. J Hand Surg [Am]. 2006;31:1160–1170.
73. Napier, JR. The prehensile movements of the human hand. J Bone Joint Surg Br. 1956;38:902–913.
74. Neto, HS, Filho, JM, Passini, R, Jr., Marques, MJ. Number and size of motor units in thenar muscles. Clin Anat. 2004;17:308–311.
75. Neumann, DA, Observations from cineradiography analysis, 2000.
76. Neumann, DA, Bielefeld, T. The carpometacarpal joint of the thumb: Stability, deformity, and therapeutic intervention. J Orthop Sports Phys Ther. 2003;33:386–399.
77. Niedermann, K, Forster, A, Hammond, A, et al. Development and validation of a German version of the joint protection behavior assessment in patients with rheumatoid arthritis. Arthritis Rheum. 2007;57:249–255.
78. Nilsson, A, Liljensten, E, Bergström, C, Sollerman, C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg [Am]. 2005;30:380–389.
79. Omokawa, S, Ryu, J, Tang, JB, et al. Trapeziometacarpal joint instability affects the moment arms of thumb motor tendons. Clin Orthop Relat Res. 2000;372:262–271.
80. Pagalidis, T, Kuczynski, K, Lamb, DW. Ligamentous stability of the base of the thumb. Hand. 1981;13:29–36.
81. Peck, D, Buxton, DF, Nitz, A. A comparison of spindle concentrations in large and small muscles acting in parallel combinations. J Morphol. 1984;180:243–252.
82. Pellegrini, VD, Jr. Osteoarthritis at the base of the thumb. Orthop Clin North Am. 1992;23:83–102.
83. Pellegrini, VD, Jr. Osteoarthritis of the trapeziometacarpal joint: The pathophysiology of articular cartilage degeneration. I. Anatomy and pathology of the aging joint. J Hand Surg [Am]. 1991;16:967–974.
84. Pelligrini, VD, Jr. Osteoarthritis of the trapeziometacarpal joint: The pathophysiology of articular cartilage degeneration. II. Articular wear patterns in the osteoarthritic joint. J Hand Surg [Am]. 1991;16:975–982.
85. Pellegrini, VD, Jr. Pathomechanics of the thumb trapeziometacarpal joint. Hand Clin. 2001;17:175–184.
86. Ranney, D, Wells, R. Lumbrical muscle function as revealed by a new and physiological approach. Anat Rec. 1988;222:110–114.
87. Rempel, DM, Keir, PJ, Bach, JM. Effect of wrist posture on carpal tunnel pressure while typing. J Orthop Res. 2008;26:1269–1273.
88. Rispler, D, Greenwald, D, Shumway, S, et al. Efficiency of the flexor tendon pulley system in human cadaver hands. J Hand Surg [Am]. 1996;21:444–450.
89. Rohrbough, JT, Mudge, MK, Schilling, RC. Overuse injuries in the elite rock climber. Med Sci Sports Exer. 2000;32:1369–1372.
90. Roloff, I, Schöffl, VR, Vigouroux, L, Quaine, F. Biomechanical model for the determination of the forces acting on the finger pulley system. J Biomech. 2006;39:915–923.
91. Schulz, CU, Anetzberger, H, Pfahler, M, et al. The relation between primary osteoarthritis of the trapeziometacarpal joint and supernumerary slips of the abductor pollicis longus tendon. J Hand Surg [Br]. 2002;27:238–241.
92. Schneider, LW. Tendon transfers: An overview. In: Mackin EJ, Callahan AD, Skirven TM, et al, eds. Rehabilitation of the hand and upper extremity. St Louis: Mosby, 2002.
93. Schweitzer, TP, Rayan, GM. The terminal tendon of the digital extensor mechanism: Part I, anatomic study. J Hand Surg [Am]. 2004;29:898–902.
94. Shaw, SJ, Morris, MA. The range of motion of the metacarpo-phalangeal joint of the thumb and its relationship to injury. J Hand Surg [Br]. 1992;17:164–166.
95. Simoneau, GG, Marklin, RW, Monroe, JF. Wrist and forearm postures of users of conventional computer keyboards. Hum Factors. 1999;41:413–424.
96. Smith, KL. Nerve response to injury and repair. In: Mackin EJ, Callahan AD, Skirven TM, et al, eds. Rehabilitation of the hand and upper extremity. St Louis: Mosby, 2002.
97. Smutz, WP, Kongsayreepong, A, Hughes, RE, et al. Mechanical advantage of the thumb muscles. J Biomech. 1998;31:565–570.
98. Sonne-Holm, S, Jacobsen, S. Osteoarthritis of the first carpometacarpal joint: A study of radiology and clinical epidemiology. Results from the Copenhagen Osteoarthritis Study. Osteoarthritis Cartilage. 2006;14:496–500.
99. Soukup, T, Pedrosa-Domellöf, F, Thornell, LE. Intrafusal fiber type composition of muscle spindles in the first human lumbrical muscle. Acta Neuropathol. 2003;105:18–24.
100. Stack, HG. Muscle function in the fingers. J Bone Joint Surg Br. 1962;44:899–902.
101. Standring, S. Gray’s anatomy: the anatomical basis of clinical practice, ed 40. St Louis: Elsevier; 2009.
102. Stein, AB, Terrono, AL. The rheumatoid thumb. Hand Clin. 1996;12:541–550.
103. Strong, CL, Perry, J. Function of the extensor pollicis longus and intrinsic muscle of the thumb. J Am Phys Ther Assoc. 1966;46:939–945.
104. Su, FC, Chou, YL, Yang, CS, et al. Movement of finger joints induced by synergistic wrist motion. Clin Biomech (Bristol, Avon). 2005;20:491–497.
105. Taguchi, M, Zhao, C, Zobitz, ME, et al. Effect of finger ulnar deviation on gliding resistance of the flexor digitorum profundus tendon within the A1 and A2 pulley complex. J Hand Surg [Am]. 2006;31:113–117.
106. Taylor, EJ, Desari, K, D’Arcy, JC, Bonnici, AV. A comparison of fusion, trapeziectomy and silastic replacement for the treatment of osteoarthritis of the trapeziometacarpal joint. J Hand Surg [Br]. 2005;30:45–49.
107. Thomas, DH, Long, C. Biomechanical considerations of lumbricalis behavior in the human finger. J Biomech. 1968;1:107–115.
108. Ugbolue, UC, Hsu, WH, Goitz, RJ, Li, ZM. Tendon and nerve displacement at the wrist during finger movements. Clin Biomech (Bristol, Avon). 2005;20:50–56.
109. Vigouroux, L, Quaine, F, Paclet, F, et al. Middle and ring fingers are more exposed to pulley rupture than index and little during sport-climbing: A biomechanical explanation. Clin Biomech (Bristol, Avon). 2008;23:562–570.
110. von Schroeder, HP, Botte, MJ. The dorsal aponeurosis, intrinsic, hypothenar, and thenar musculature of the hand. Clin Orthop Relat Res. 2001;383:97–107.
111. Wariyar, B. Hansen’s disease (leprosy). Nebr Med J. 1996;81:147–148.
112. Wells, RP, Ranney, DA. Lumbrical length changes in finger movement: A new method of study in fresh cadaver hands. J Hand Surg [Am]. 1986;11:574–577.
113. Williams, EH, McCarthy, E, Bickel, KD. The histologic anatomy of the volar plate. J Hand Surg [Am]. 1998;23:805–810.
114. Xu, L, Strauch, RJ, Ateshian, GA, et al. Topography of the osteoarthritic thumb carpometacarpal joint and its variations with regard to gender, age, site, and osteoarthritic stage. J Hand Surg [Am]. 1998;23:454–464.
115. Zancolli, EA, Ziadenberg, C, Zancolli, E, Jr. Biomechanics of the trapeziometacarpal joint. Clin Orthop Relat Res. 1987;220:14–26.
STUDY QUESTIONS
1. Compare the relative mobility permitted at the proximal and distal transverse arches of the hand.
2. List regions within the hand where you would most expect muscle atrophy after a longstanding (a) ulnar neuropathy and (b) median neuropathy.
3. The adductor pollicis is a forceful muscle requiring stable proximal bony attachments. After reviewing the muscle’s proximal attachments, state whether this requirement has been met.
4. Which movements at the carpometacarpal joint of the thumb constitute opposition? Which muscles are most responsible for performing these individual movements?
5. Describe the path of the lumbrical muscle of the index finger, from its proximal to its distal attachment. Explain how this muscle can flex the metacarpophalangeal joint and simultaneously extend the interphalangeal joints.
6. Figure 8-42 shows the line of force of the extensor pollicis longus, extensor pollicis brevis, and abductor pollicis longus at the carpometacarpal joint. Of the three muscles, which (a) is capable of adduction, (b) is capable of abduction, and (c) has neither potential? Finally, which of these muscles can extend the carpometacarpal joint?
7. What is the role of the lumbricals and interossei in opening the hand (i.e., extending the fingers)?
8. Contrast the underlying pathomechanics in the swan-neck and boutonniere deformities.
9. Which of three intrinsic muscles illustrated in Figure 8-48 has the greatest moment arm for flexion of the metacarpophalangeal joint of the index finger?
10. Clinicians frequently splint the hand of a person with a fractured metacarpal bone in a position of the flexion of the metacarpophalangeal joint and near extension of the interphalangeal joint. What is the reason for doing this? Which muscle could eventually become tight (contracted) from this prolonged position?
11. A person with a damaged ulnar nerve at the level of the pisiform bone typically shows marked weakness of adduction of the carpometacarpal joint of the thumb. Why would this be? Which muscle could substitute for some of the loss of adduction at this joint?
12. How does the saddle-shaped joint structure of the carpometacarpal joint of the thumb influence the arthrokinematics of flexion and extension and abduction and adduction?
13. Rank the passive mobility of the carpometacarpal joints of the hand from least to most. What is the functional significance of this mobility pattern?
14. A patient shows marked weakness in the active movements of abduction and adduction of the fingers and in making a “key pinch.” In addition, the patient shows atrophy of the muscles of the hypothenar eminence and decreased sensation over the ulnar border of the hand and distal forearm. Based on information displayed in Appendix II, Parts A through D, which spinal nerve roots are most likely associated with these impairments?
15. Assume a person has a completely lacerated flexor digitorum profundus (FDP) tendon of the ring finger at the level of the A4 pulley. Furthermore, the person reports that attempts at making a fist result in extension rather than flexion of the distal interphalangeal joint of the ring finger. (This observation is often referred to by clinicians as “paradoxic extension.”) Please offer a possible kinesiologic explanation for this phenomenon.
 Answers to the study questions can be found on the Evolve website.
Answers to the study questions can be found on the Evolve website.