Chapter 91 Haemostatic failure
Failure of haemostasis is common in critically ill patients and may be complex and multifactorial in pathogenesis. As haemostatic failure may complicate a wide range of medical, surgical and obstetric disorders, definitive diagnosis and specific therapy can significantly impact on outcome. Frequently, complex tests are required for definitive diagnosis, but the urgency of the situation cannot always wait for the results, and therapy may be initiated on clinical evidence with minimal laboratory information. Consultation with a clinical haematologist is strongly recommended.
NORMAL HAEMOSTASIS
The haemostatic system is a delicately controlled component of the host defence system, the role of which is to initiate haemostasis where and when required and in adequate, but not in excessive, amounts. The system closely interacts with other components of the host defence system, including the acute stress responses, inflammation, healing and immune functions. There has been a paradigm shift in our understanding of the haemostatic system from one of interacting humoral factors to an integrated cell-based system.1,2 In view of the increasing range of focused therapies available that act on the haemostatic system to modulate its activity, it is important that core aspects of the structure and function of the system are summarised.
The conversion of blood from its fluid to solid state is an increasingly understood physiological process. The triad of vascular constriction, platelet plugging and fibrin clot formation forms haemostatic plugs and provides the framework on which haemostasis operates (Figure 91.1) and sets the scene for healing to occur. Thrombin is the potent proteolytic enzyme of the coagulation sequence converting fibrinogen to fibrin-soluble monomers, which subsequently polymerise to form the fibrin clot. Fibrinogen is the bulk protein of the coagulation system and fibrin is the end-product of this cascade of proteolytic activity, in which precursor coagulation proteins are activated to potent proteolytic enzymes, with the aid of cofactors, to produce active precursors further down the coagulation ‘amplifier’. The polymerised fibrin is further acted on by factor XIII to form a stable fibrin clot. The process of thrombin generation in small amounts initially occurs in relationship to tissue factor-bearing cells and the process is subsequently transferred to the activated platelet surface where amplification occurs.
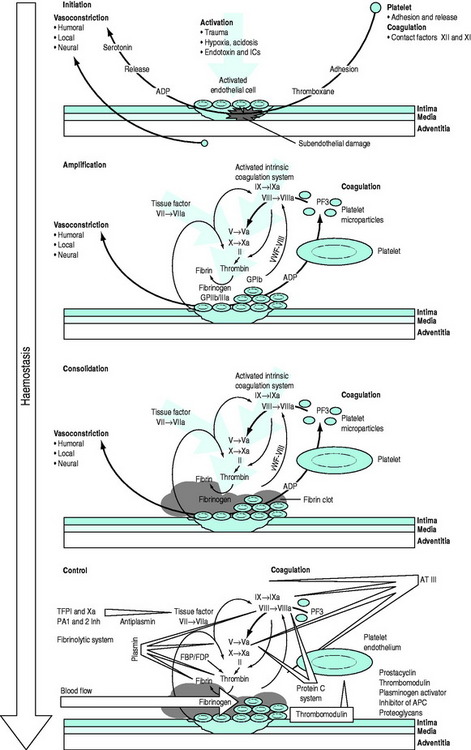
Figure 91.1 Overview of the haemostatic system. APC, activated protein C; AT III, antithrombin III; FBP, fibrinogen breakdown product; FDP, fibrinogen degradation product; ICs, immune complexes; TFPI, tissue factor pathway inhibitor; vWF, von Willebrand factor.
Following injury, vascular constriction reduces bleeding, and allows time to initiate haemostasis. With large volume haemorrhage, resulting systemic hypotension is an important physiological mechanism to minimise blood loss and facilitate stabilisation of the haemostatic plug. Controlled or ‘tolerated’ hypotension is now accepted as an important aspect of managing critical haemorrhage.3,4 This vascular constriction is further accentuated by vasoconstrictors released in association with platelet plug formation. Vascular endothelial cells play an active part by synthesising substances which act at the membrane surface and/or interact with platelets and the coagulation system (e.g. prostacyclin, antithrombin III, plasminogen activator, von Willebrand factor, thrombomodulin, heparin cofactor II and nitric oxide).5 Following the initial vascular reactions, successful haemostasis depends on adequate numbers of functioning platelets, coagulation cascade function, and poorly understood contributions from red cells and leukocytes.
Von Willebrand factor (vWF) is a multimeric glycoprotein that plays a central role in haemostasis by mediating adhesion of platelets to the exposed subendothelium and linking the primary vascular/platelet phase with coagulation by being the carrier protein for coagulant factor VIII which dissociates from vWF to form a complex on the activated platelet surface with IXa (tenase complex) to activate factor X to Xa. Further vWF is released from Weibel–Palade bodies in nearby endothelial cells and platelet α-granules. Platelets interact with vWF via their surface glycoprotein Ib-IX-V complexes. This slows their motion along the subendothelium, allowing receptor interaction leading to platelet binding to collagen. Adhesion to collagen is facilitated by the GPIa-IIa receptor. GPIIb-IIIa can then bind vWF, fibrinogen, fibronectin, vitronectin and thrombospondin, providing further important sites of anchorage for the spreading platelet. The initiation phase of platelet plug formation leads to the development of a platelet monolayer over the injured subendothelium. With the concomitant activation of the coagulation cascade there is local generation of thrombin. Thrombin is a potent platelet agonist via the protease-activated receptor (PAR) to produce a haemostatic plug. Further extension occurs by platelet recruitment by platelet agonists, such as thrombin and mediators, adenosine 5-diphosphate (ADP) and thromboxane A2 released directly from platelets. P-selectin is expressed during activation and also plays a role in platelet-to-platelet cohesion.
Parallel to and within the coagulation system are complex feedback mechanisms to ensure fine-tuning and protection against inappropriate and excessive activation.6–8 There are several inhibitory proteins, including antithrombin III, thrombomodulin, proteins C and S, tissue factor pathway inhibitor as well as the fibrinolytic system, which are all important in controlling the degree and site of fibrin formation. Indeed, thrombin itself acts as either a procoagulant or an anticoagulant depending on context. Massive activation, as may be seen in trauma and severe infections, can precipitate systemic activation, but in general, the system is well controlled and activity is localised to the area of the stimulus. Perturbations in this complex defence system can produce a wide range of clinical disorders from excessive arterial or venous thrombosis, microvascular obstruction and atheroma to haemostatic failure.
SYSTEMIC HAEMOSTATIC ASSESSMENT
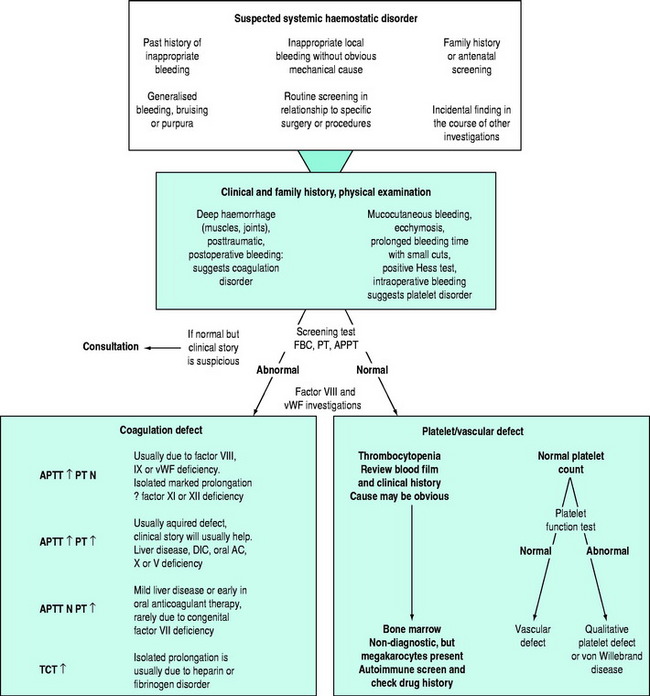
There may be clinical features suggesting local or generalised failure of the haemostatic system (Figure 91.2). Clinical history is important, especially with respect to previous bleeding problems, family history, comorbid medical conditions and medications. The nature of surgery or an invasive intervention may have haemostatic issues that need specific consideration.
TESTS OF THE HAEMOSTATIC SYSTEM
Tests of whole blood clotting time and clot observation do not generally have a role in assessing haemostasis. However, in emergency settings, while waiting for laboratory results, observation of blood collected into a glass tube and maintained at 37°C for clot formation, size, retraction and possible lysis may provide crude information of a possible coagulopathy. The thromboelastogram is a more accurate and controlled procedure for ‘holistic’ assessment of the haemostatic system at the bedside, but requires close attention to technique and quality control. In most clinical settings, laboratory haemostatic screening tests are readily available, with near-patient testing techniques continuing to improve. A full blood count, prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen level, D-Dimer ± thrombin clotting time (TCT) provide a broad screen for most clinically significant haemostatic disorders9–11 (Figure 91.3). Based on these results, and following consultation with a haematologist, further specific tests of haemostasis may be performed (e.g. mixing studies, factor assays, platelet function tests and test of fibrinolytic function). In broad terms:
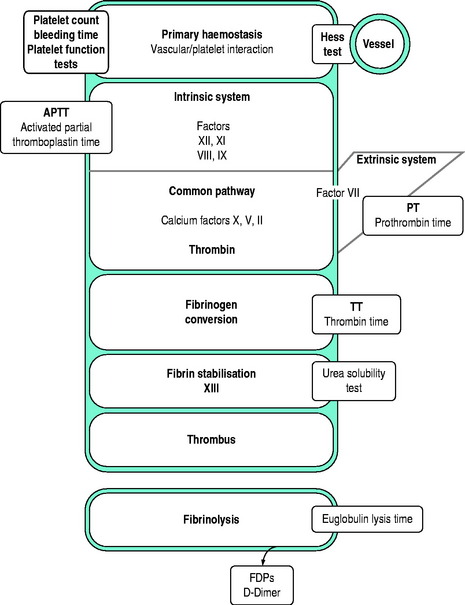
Figure 91.3 Investigations for a suspected haemostatic defect. FDPs, fibrinogen degradation products.
There are several important principles in the collection of blood samples for haemostatic investigations. Most samples are collected into citrated tubes and the amount of anticoagulant in the tube is related to the intended amount of blood to be collected into the tube. It is important that the correct amount of blood is added to the tube and gently mixed. Attention to venepuncture technique is crucial and rapid transfer of the sample to the laboratory is important. Contamination of the sample with tissue factor due to traumatic venepuncture will activate the sample and invalidate results. Collection from vascular access lines may result in diluted or heparin-contaminated samples. Incorrect laboratory results may be more dangerous to a patient than no results at all.
BLEEDING TIME (BT) (NORMAL 3–9 MINUTES)
The bleeding time is progressively prolonged when the platelet count falls below 75–100 × 109/l assuming platelet function is normal. It is also prolonged when platelet function is defective, in capillary/vascular disease and in some coagulation defects. The bleeding time is an invasive and operator-dependent test. It is not good for screening unselected patients and has generally been replaced by the platelet function analyser, PFA-100(R).
D-DIMER (NORMAL < 0.25 ng/l)
The D-Dimer assay measures cleavage fragments resulting from the proteolytic action of plasmin of fibrin. Elevation of D-Dimer may be seen in the postoperative state, trauma, renal impairment, sepsis and venous thrombosis. High levels of D-Dimer suggest excessive fibrinolysis, as seen in disseminated intravascular coagulation. The test is specific for fibrinolysis and not primary fibrinogenolysis.
PLATELET FUNCTION TESTS
Platelet function tests are used for the diagnosis of platelet disorders.12,13 In recent years there has been increasing interest in the use of platelet function tests to monitor antiplatelet therapy. Unfortunately, platelet function testing is poorly standardised and good quality control is commonly lacking. The diagnostic utility of some of the newer simpler assays of platelet function have been evaluated in a range of clinical settings, but their value remains controversial.
A popular technology is the platelet function analyser (PFA-100) in which citrated whole blood is placed in a disposable cartridge containing a membrane coated with collagen/adrenaline or collagen/ADP into which a microscopic aperture has been cut. With high shear rates contact of blood with the membrane causes platelets to aggregate and occlude the aperture. The time to occlude the aperture is recorded as the end-point and referred to as the closure time.14 The technique is simple and rapid, but subject to a number of variables such as the platelet count, haematocrit, ABO blood group and plasma vWF levels. A normal result has a high negative predictive value and is increasingly becoming popular as a screening test for platelet dysfunction; however, false positive and negative results may occur. The test is more sensitive than the bleeding time which is now generally regarded as an obsolete test.
THROMBOELASTOGRAM (TEG)
Thrombelastography was originally described 50 years ago, but failed to establish a role in routine laboratory haemostasis testing. Improvements in the technology, especially standardisation and computerisation, have now made the TEG a valuable tool for monitoring haemostasis as a holistic dynamic process supplementing the long-standing conventional coagulation screens. The TEG measures the viscoelastic properties of blood as it clots under low shear conditions. The patterns of changes in shear elasticity provide information about the kinetics of clot formation, growth, strength and stability of the formed clot. The TEG is increasing being used as a point of care test, providing ‘real time’ information about a patient’s haemostatic function.15
CONGENITAL HAEMOSTATIC DEFECTS
THE HAEMOPHILIAS
HAEMOPHILIA A (CLASSIC HAEMOPHILIA)
Haemophilia A is an X-linked disorder due to a deficiency of factor VIII. The mainstay of haemophilia treatment is coagulation factor replacement.16,17 Coagulation factor concentrates or recombinant factor VIII are given to prevent bleeding and to control existing haemorrhage. The dose of clotting factor is calculated on the basis that one unit of coagulation factor is that amount present in 1 ml of pooled normal plasma. An individual with a plasma volume of 3000 ml would have 3000 U of the clotting factor in the circulation. If such person was a haemophiliac with <1% factor VIII level in the plasma, a dose of 3000 U of factor VIII would be required to raise the plasma level to 100% of normal.
VON WILLEBRAND’S DISEASE
Classification of vWD is complex and controversial. Therapy may be determined by the specific subtype and expert haematological consultation is important.18,19 Clinical heterogeneity is reflected in the classification of subtypes corresponding to specific pathophysiological mechanisms.
CONGENITAL PLATELET DISORDERS
Congenital platelet disorders are rare, the commonest severe disorder being Glanzmann’s thrombaesthenia.20 Platelet transfusions may be required for acute bleeds or in relation to elective surgery; DDAVP ± antifibrinolytic therapy and recombinant factor VIIa may all have a role.
ACQUIRED HAEMOSTATIC DISORDERS
CRITICAL HAEMORRHAGE AND MASSIVE BLOOD TRANSFUSION
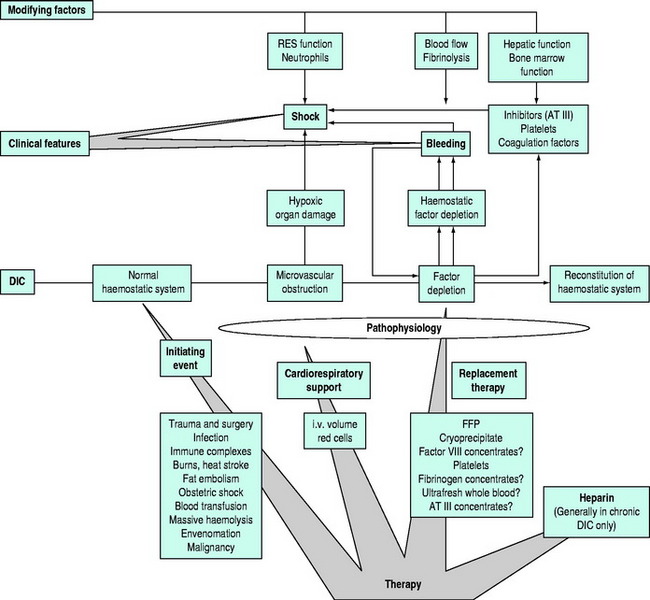
The nature and management of haemostatic defects secondary to acute and/or massive blood loss and transfusion remains poorly understood.21 The coagulopathy of trauma is characterised by non-surgical bleeding from mucosal lesions, serosal surfaces, wounds and vascular access sites. Hypothermia, acidosis and haemodilution are commonly present, occasionally with features of disseminated intravascular coagulation (DIC). DIC typically occurs acutely after trauma when brain, fat, amniotic fluid or other potent thromboplastins enter the circulation. It may occur less acutely when endothelial inflammation or failure reduces clearing of activated coagulation factors. The coagulopathy of trauma should be anticipated in massive transfusion situations and there is increasing support for early treatment with plasma, which may prevent or delay its onset.
Further aggravation of the complications of massive blood transfusion can be avoided or minimised if correctable defects in the haemostatic system are identified (Table 91.1). The relative importance of the different potential mechanisms responsible for haemostatic failure is difficult to determine, but the identification of correctable defects will avoid or minimise complications. Haemostatic failure correlates poorly with the volume of transfusion or components administered, but better with the nature of the insult, degree of hypovolaemia, and time to resuscitation. Trauma patients with major coagulopathy and microvascular haemorrhage usually have abnormal laboratory parameters prior to massive blood transfusion. Except for severe abnormalities, haemostatic laboratory parameters correlate poorly with clinical evidence of haemostatic failure. Thrombocytopenia and impaired platelet function are probably the most consistent significant haematological abnormalities, correction of which may be associated with control of microvascular bleeding.
Table 91.1 Possible factors contributing to haemostatic failure following massive blood transfusion
Coagulation deficiency from massive blood loss is initially confined to factors V and VIII. APTT, PT and fibrinogen assay should be performed, but the urgency of the situation does not usually allow for other specific factor assays, and fresh frozen plasma (FFP), cryoprecipitate or factor concentrates should be infused if the test results are abnormal. Correction of hypofibrinogenaemia is probably more important than thought previously.22A case can be made for prophylactic FFP in infusions in patients with massive blood loss which has been replaced with red cell concentrates and plasma substitutes. Hypothermia must be avoided, recognised and treated rapidly.23 Potential problems associated with large volume stored blood replacement are summarised in Table 91.2.
Table 91.2 Potential problems with stored blood components
With ongoing bleeding with associated microvascular oozing, various approaches may be taken. Having ensured that all identifiable haemostatic defects have been corrected, questions arise as to the roles of fresh blood and, more recently, recombinant activated factor VII (rFVIIa).24 The use of freshly collected whole blood remains controversial and is an ‘emotive’ subject needing some comment.25,26 The provision of fresh whole blood can present logistic, ethical and safety problems needing consideration. Many transfusion medicine specialists categorically state that there is never an indication for fresh whole blood. Such dogma can be difficult to defend in the clinical setting of the massive haemorrhage and transfusion syndrome where pathophysiology remains poorly understood and specific blood component therapy may be ineffective. The current author, from his own experience and on the basis of current evidence, feels that the following statements reasonably summarise the current status of fresh blood transfusion:
HAEMOSTATIC FAILURE ASSOCIATED WITH LIVER DISEASE
The liver is the production site of nearly all the factors involved in the formation and control of coagulation (Table 91.3). Bleeding in association with liver disease can be difficult to manage. There may be a combination of excessive consumption of coagulation factors and impaired synthesis of both clotting factors and coagulation inhibitor proteins. Excessive activation of fibrinolysis may occur and defective clearing of activated clotting factors compounds the problem. Moreover, the effects of massive blood loss, shock and transfusion must also complicate the problem.
Haemostatic defects due to deficient vitamin K-dependent coagulation factors (in patients with predominantly cholestatic liver disease) may be rapidly reversed with vitamin K therapy, and blood transfusion may not be required. If the abnormality is not reversed by vitamin K, hepatocellular damage is likely to be present and FFP is appropriate replacement therapy.27 When an elective procedure is planned, prophylactic FFP is appropriate. Low fibrinogen levels in liver disease usually indicate advanced disease and are associated with a poor prognosis, or the presence of DIC. Cryoprecipitate is generally the most readily available form of fibrinogen if concentrates are not available.
DISSEMINATED INTRAVASCULAR COAGULATION28
Disseminated intravascular coagulation (DIC) is a pathophysiological process and not a disease in itself. Clinical manifestations of DIC relate to occlusion of the microvessels during the obstructive phase of the syndrome and haemorrhage secondary to consumption of plasma and cellular components of the haemostatic system.29
PATHOPHYSIOLOGY
DIC is characterised by the consumption of clotting factors and platelets within the circulation, resulting in varying degrees of microvascular obstruction due to fibrin deposition (Figure 91.4). When significant platelet and coagulation factor consumption occurs, bleeding may become a major feature.
Mechanisms which may inappropriately activate the haemostatic system include:
CLINICAL FEATURES
The clinical presentation of DIC varies, with patients showing thrombotic, haemorrhagic or mixed manifestations in various organ systems. The major clinical problem and presenting feature of acute DIC is bleeding. This may manifest as generalised bruising or bleeding at sites of therapeutic or traumatic invasion (venepuncture sites and surgical wounds). DIC may occur in association with a wide range of clinical disorders (see Table 91.5). When DIC occurs in acutely ill patients with multisystem organ failure the prognosis is poor. DIC may, in some patients, be an agonal event and should not be treated.
LABORATORY FINDINGS
Results of tests may be variable and difficult to interpret. Significant DIC can be present despite normal standard coagulation tests (i.e. PT, APTT and TCT). The key tests in diagnosis are those that provide evidence for excessive conversion of fibrinogen to fibrin within the circulation and its subsequent lysis. Platelet–fibrin clots create a mesh in the microcirculation in which passing red cells may be traumatised, resulting in red cell fragmentation and haemolysis. The blood film may demonstrate fragmentation of the red cells (microangiopathic haemolytic anaemia), but this is more commonly seen in chronic DIC, especially in association with malignancy.
The diagnosis is usually based on a combination of the appropriate clinical picture with a supportive pattern of laboratory tests of the haemostatic system27 (Table 91.4). Thrombocytopenia and hypofibrinogenaemia with prolongation of the APTT, PT and TCT in conjunction with an elevation of fibrin degradation products (D-Dimer test) are supportive evidence for the diagnosis. The D-Dimer test is specific for fibrin breakdown versus primary fibrinogenolysis. More specialised test such as elevation of fibrinopeptide A and reduced levels of antithrombin III add further weight to the diagnosis.
Table 91.4 Laboratory test for the diagnosis of disseminated intravascular coagulation
| Analysis | Early | Late |
|---|---|---|
| Platelet count | ↓ | ↓↓ |
| Activated partial thromboplastin time (APTT) | ↑ | ↑↑ |
| Prothrombin time (PT) | ↑ | ↑ |
| Thrombin clotting time (TCT) | ↑ | ↑ |
| Fibrin degradation products (D-Dimer assay) | ↑ | ↑↑ |
| Hypofibrinogen | ↓ | ↓↓ |
| Other coagulation factors II, VII, VIII, X | ↓ | ↓↓ |
| Coagulation inhibitors: antithrombin III, protein C | ↓ | ↓↓↓ |
| Blood film | Usually normal in early stages | Fragmented red cells + in subacute or chronic cases |
| Supplementary and research tests: prothrombin fragment 1+2, thrombin–antithrombin complex (TAT complex), procalcitonin (PCT), plasmin–antiplasmin complexes (PAP complex) | ↑ | ↑ |
Table 91.5 Conditions associated with disseminated intravascular coagulation
DISORDERS ASSOCIATED WITH DIC
DIC may occur in association with a wide range of clinical disorders (Table 91.5).
THERAPY
ANTITHROMBOTIC THERAPY
The availability of an increasing variety of antithrombotic agents, and wider clinical use of antithrombotic therapy generally, has inevitably resulted in an increase in iatrogenic bleeding in patients presenting with other medical conditions (e.g. trauma, surgery and invasive procedures) and intensivists must be aware of the methods for rapid reversal of therapy.32,33 Bleeding problems in patients on well-controlled anticoagulation are usually due to surgery, trauma or a local lesion (e.g. peptic ulceration). Otherwise, bleeding is due to over-anticoagulation, sometimes as a result of drug interactions. Elective surgery in this group of patients needs careful planning if haemorrhagic and thrombotic complications are to be avoided. Patients on antithrombotic therapy involved in traumatic events are a challenge in whom there is the risk of progression of the original insult, increased risk of posttraumatic complications, and mortality.
HEPARINS
With the wide clinical use of fractionated low molecular weight (LMW) heparins, this subject has become more complex. Heparins act by potentiating the action of antithrombin III, a natural coagulation inhibitor. Unfractionated heparin acts in neutralising both thrombin and factor Xa, in contrast to the LMW heparins which predominantly neutralise factor Xa. The anticoagulant effect of unfractionated heparin is usually measured by prolongation of the APTT. LMW heparins do not prolong the APTT, are generally administered on weight basis and are not routinely monitored by a laboratory test. When monitoring is required, the anti-Xa assay is used.
REVERSAL OF ANTITHROMBOTIC AGENTS
Life-threatening bleeding in patients on warfarin requires urgent reversal and this is best achieved with prothrombin concentrate (PCC) administration in conjunction with FFP depending on the factor VII level of the PCC.34–36 The advantages of PCC over FFP include more timely correction, absence of volume overload and potentially better restoration of the vitamin K-dependent coagulation factors. Vitamin K1 is essential for sustained reversal, but the dose should be determined on the basis of whether temporary reversal is indicated. When there is an indication to continue warfarin therapy, vitamin K1 should be administered in a dose that will lower or maintain the INR to a safe, but not subtherapeutic, range and will not cause resistance once warfarin is reinstated.
Antiplatelet agents, particularly acetylsalicylic acid, clopidogrel and GPIIb/IIIa inhibitors, now have a central role in preventing arterial thrombosis. Clinicians are now regularly confronted by balancing the haemorrhagic risks associated with these agents, especially in the perisurgical setting, against the thrombotic risks associated with their discontinuation. It is generally agreed that continuing acetylsalicylic acid during vascular surgery procedures and in most other settings in which the bleeding risk is likely to be low is acceptable. It is generally recommended that clopidogrel be ceased 5 days before surgery to allow regeneration of half the platelet pool. Although withdrawal of the competitive GPIIb/IIIa inhibitors at the beginning of surgery will decrease the risk of bleeding, it should be emphasised that thrombotic events in patients waiting for urgent myocardial revascularisation are a significant risk and acceptance of perioperative bleeding risk may take priority. Patient blood management techniques to minimise blood loss and transfusion should be used.37 Platelet concentrate transfusions may be indicated in the acute setting.
NEWER ANTITHROMBOTIC AGENTS
Limitations of the currently available antithrombotic agents continue to stimulate research into new anticoagulants with improved pharmacological activity and safety.38
ACQUIRED INHIBITORS OF COAGULATION
Major haemostatic failure may be seen with rare inhibitors of coagulation factors. Autoantibodies against factors VIII (commonest), IX, X, V and vWF have been reported. A coordinated approach to haemotherapy is essential if bleeding is to be arrested. The patients are relatively resistant to factor replacement therapy and removal of the offending autoantibody by plasma exchange and replacement with FFP ± factor concentrates is indicated. Recombinant activated factor VII may have a role yet to be defined.
EXTRACORPOREAL CIRCULATION CARDIAC SURGERY
A range of haemostatic disturbances may be associated with extracorporeal circulation, most commonly cardiopulmonary bypass, and the clinician must constantly balance the risks of bleeding versus thrombosis.39 The abnormalities are generally directly related to the time on bypass and are more likely to be seen in multiple valve replacements or re-operations. Fresh blood components have generally been advocated in cardiac bypass surgery to ensure optimal oxygen delivery and function of the haemostatic components of blood. Haemostatic failure usually becomes apparent in the operating room or soon after and appropriate laboratory investigations should be performed to allow logical therapy. It is pointless infusing large volumes of plasma products or platelets if heparin has not been adequately reversed. Increased fibrinolytic activity may in some cases also contribute to bleeding.
A predictable thrombocytopenia occurs after extracorporeal blood circulation. The postoperative platelet count in the majority of cardiac bypass patients is seldom below 100 000/μl, but platelet functional defects may contribute to bleeding and occasionally platelet concentrates are indicated. Increasingly, rFVIIa is being used to control bleeding associated with cardiac surgery. More evidence is required before definitive recommendations can be made.40
THROMBOCYTOPENIA AND QUALITATIVE PLATELET DEFECTS
IDIOPATHIC THROMBOCYTOPENIC PURPURA (ITP)
Therapy of ITP remains controversial.41 Corticosteroids should generally be regarded as short-term therapy. In adults, the majority of patients respond with a rise in the platelet count. After an initial high dose (50–75 mg) of prednisolone a day until response occurs, the dose is gradually reduced. There is little evidence that corticosteroids will raise the platelet count in acute disease. However, their short-term role in reducing the incidence of bleeding, which is highest in the first weeks, may be important. High dose i.v. immunoglobulin is effective in most patients and is specifically indicated in fulminant acute disease, preoperatively and in pregnancy.
DRUG-INDUCED THROMBOCYTOPENIA
Heparin-induced thrombocytopenia and thrombosis syndrome (HITTS) is an important and potentially life-threatening complication of heparin therapy.42–44 An immune reaction occurs to heparin after 7–10 days of therapy, at which time the patient’s platelets aggregate and thrombocytopenia develops; this reactivity can be demonstrated by a variety of laboratory tests. In contrast to other causes of drug-induced thrombocytopenia, there may be arterial, microvascular or venous thrombosis associated with the platelet aggregation. The best way to avoid this potentially devastating complication is to keep heparin therapy as brief as possible, and be always alert to the diagnostic possibility. The incidence is less with the use of LMW heparins, and if cross-reactivity is not demonstrated in the heparin antibody test, LMW heparins or heparinoids may have a part to play in therapy.
Quinine-induced immune thrombocytopenia with haemolytic uraemic syndrome (HUS) can be a severe drug reaction, characterised by the onset of chills, sweating, nausea and vomiting, abdominal pain, oliguria and petechial rash following quinine or quinidine exposure. Quinine-dependent platelet-reactive antibodies can usually be identified. Plasma exchange in conjunction with renal dialysis has been found to be a beneficial adjunct to therapy.
1 Hoffman M, Monroe DM. Coagulation 2006: a modern view of hemostasis. Hematol Oncol Clin North Am. 2007;21:1-11.
2 Roberts HR, Hoffman M, Monroe DM. A cell-based model of thrombin generation. Semin Thromb Hemost. 2006;32(Suppl 1):32-38.
3 Duggan JM. Review article: transfusion in gastrointestinal haemorrhage – if, when and how much? Aliment Pharmacol Ther. 2001;15:1109-1113.
4 Bickell WH, Wall MJJr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105-1109.
5 Verhamme P, Hoylaerts MF. The pivotal role of the endothelium in haemostasis and thrombosis. Acta Clin Belg. 2006;61:213-219.
6 Tanaka KA, Levy JH. Regulation of thrombin activity – pharmacologic and structural aspects. Hematol Oncol Clin North Am. 2007;21:33-50.
7 Dahlback B. Blood coagulation and its regulation by anticoagulant pathways: genetic pathogenesis of bleeding and thrombotic diseases. J Intern Med. 2005;257:209-223.
8 Macias WL, Yan SB, Williams MD, et al. New insights into the protein C pathway: potential implications for the biological activities of drotrecogin alfa (activated). Crit Care. 2005;9(Suppl 4):S38-45.
9 Adams M, Ward C, Thom J, et al. Emerging technologies in hemostasis diagnostics: a report from the Australasian Society of Thrombosis and Haemostasis Emerging Technologies Group. Semin Thromb Hemost. 2007;33:226-234.
10 Rochon AG, Shore-Lesserson L. Coagulation monitoring. Anesthesiol Clin. 2006;24:839-856.
11 Soliman DE, Broadman LM. Coagulation defects. Anesthesiol Clin. 2006;24:549-578. vii
12 Hayward CP, Eikelboom J. Platelet function testing: quality assurance. Semin Thromb Hemost. 2007;33:273-282.
13 Cardigan R, Turner C, Harrison P. Current methods of assessing platelet function: relevance to transfusion medicine. Vox Sang. 2005;88:153-163.
14 Karger R, Donner-Banzhoff N, Muller HH, et al. Diagnostic performance of the platelet function analyzer (PFA-100(R)) for the detection of disorders of primary haemostasis in patients with a bleeding history: a systematic review and meta-analysis. Platelets. 2007;18:249-260.
15 Traverso CI, Caprini JA, Arcelus JI. The normal thromboelastogram and its interpretation. Semin Thromb Hemost. 1995;21(Suppl 4):7-13.
16 Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361:1801-1809.
17 Bolton-Maggs PH, Stobart K, Smyth RL. Evidence-based treatment of haemophilia. Haemophilia. 2004;10(Suppl 4):20-24.
18 Mannucci PM. Treatment of von Willebrand’s disease. N Engl J Med. 2004;351:683-694.
19 Laffan M, Brown SA, Collins P, et al. The diagnosis of von Willebrand disease: a guideline from the UK Haemophilia Centre Doctors’ Organization. Haemophilia. 2004;10:199-217.
20 Hayward CP, Rao AK, Cattaneo M. Congenital platelet disorders: overview of their mechanisms, diagnostic evaluation and treatment. Haemophilia. 2006;12(Suppl 3):128-136.
21 Hardy JF, de Moerloose P, Samama CM. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth. 2006;53(6 Suppl):S4-58.
22 Fries D, Innerhofer P, Reif C, et al. The effect of fibrinogen substitution on reversal of dilutional coagulopathy: an in vitro model. Anesth Analg. 2006;102:347-351.
23 Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 60(6 Suppl), 2006. S–8
24 Levy JH, Fingerhut A, Brott T, et al. Recombinant factor VIIa in patients with coagulopathy secondary to anticoagulant therapy, cirrhosis, or severe traumatic injury: review of safety profile. Transfusion. 2006;46:919-933.
25 Kauvar DS, Holcomb JB, Norris GC, et al. Fresh whole blood transfusion: a controversial military practice. J Trauma. 2006;61:181-184.
26 Repine TB, Perkins JG, Kauvar DS, et al. The use of fresh whole blood in massive transfusion. J Trauma. 2006;60(6 Suppl):S5-69.
27 Ramsey G. Treating coagulopathy in liver disease with plasma transfusions or recombinant factor VIIa: an evidence-based review. Best Pract Res Clin Haematol. 2006;19:113-126.
28 Toh CH, Hoots WK. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost. 2007;5:604-606.
29 Levi M. Current understanding of disseminated intravascular coagulation. Br J Haematol. 2004;124:567-576.
30 Wiedermann CJ, Kaneider NC. A systematic review of antithrombin concentrate use in patients with disseminated intravascular coagulation of severe sepsis. Blood Coagul Fibrinolysis. 2006;17:521-526.
31 Levi M, de Jonge E, van der Poll T. Plasma and plasma components in the management of disseminated intravascular coagulation. Best Pract Res Clin Haematol. 2006;19:127-142.
32 Pengo V, Pegoraro C, Iliceto S. New trends in anticoagulant therapy. Isr Med Assoc J. 2004;6:479-481.
33 Kessler CM. Current and future challenges of antithrombotic agents and anticoagulants: strategies for reversal of hemorrhagic complications. Semin Hematol. 2004;41(1 Suppl 1):44-50.
34 Lankiewicz MW, Hays J, Friedman KD, et al. Urgent reversal of warfarin with prothrombin complex concentrate. J Thromb Haemost. 2006;4:967-970.
35 Baker RI, Coughlin PB, Gallus AS, et al. Warfarin reversal: consensus guidelines, on behalf of the Australasian Society of Thrombosis and Haemostasis. Med J Aust. 2004;181:492-497.
36 Schulman S, Bijsterveld NR. Anticoagulants and their reversal. Transfus Med Rev. 2007;21:37-48.
37 Ferraris VA, Ferraris SP, Saha SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(5 Suppl):S2-86.
38 Von Heymann C, Schoenfeld H, Sander M, et al. New antithrombotics – pharmacologic profile and implications for anesthesia and surgery. Trans Altern Trans Med. 2006;8:164-174.
39 Vincentelli A, Jude B, Belisle S. Antithrombotic therapy in cardiac surgery. Can J Anaesth. 2006;53(6 Suppl):S89-102.
40 Walsham J, Fraser JF, Mullany D, et al. The use of recombinant activated factor VII for refractory bleeding post complex cardiothoracic surgery. Anaesth Intensive Care. 2006;34:13-20.
41 Cines DB, McMillan R. Management of adult idiopathic thrombocytopenic purpura. Annu Rev Med. 2005;56:425-442.
42 Cines DB, Rauova L, Arepally G, et al. Heparin-induced thrombocytopenia: an autoimmune disorder regulated through dynamic autoantigen assembly/disassembly. J Clin Apher. 2007;22:31-36.
43 Levy JH, Hursting MJ. Heparin-induced thrombocytopenia, a prothrombotic disease. Hematol Oncol Clin North Am. 2007;21:65-88.
44 Selleng K, Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia in intensive care patients. Crit Care Med. 2007;35:1165-1176.