Growth Hormone Deficiency In Children
Postnatal growth is best described by the ICP (infancy-childhood-pubertal) model of growth.1 These three phases are regulated by different components of the endocrine system. During the infancy phase, growth is rapid but at a sharply decelerating rate. Growth at this stage is principally dependent on nutrition, although endocrine factors in the form of the growth hormone (GH)-IGF axis play an increasingly important role during the first year of life. Over the first 2 years, a period of “catch-up” or “catch down” growth commonly occurs while the infant establishes his/her own growth trajectory, with a marked increase in the correlation between current height and final height (r = 0.8) by 3 years of age. As a result, growth along a predictable channel is a hallmark of the healthy child. Poor growth may be a manifestation of any underlying illness reflecting a wide variety of genetic, constitutional, and pathologic conditions, of which GH deficiency (GHD) is but one cause. Stature itself is merely an indication of a potential abnormal physical state and not a diagnosis. That comes from the answer to the question, what is the explanation for this abnormality?
By 4 years of age, average height velocity has declined to 7 cm/year, with a further decline to a rate of 5 to 5.5 cm/year at 8 years of age (Fig. 19-1). The onset of the childhood phase of growth is apparent from the age of 6 months, when there is overlap between the childhood and infancy phases of growth. This childhood growth is dependent mainly on endocrine factors such as GH and thyroxine.2 The third phase of postnatal growth, the pubertal phase, is dependent upon the normal secretion of GH and sex steroids. It is extremely variable in terms of timing, with marked sexual dimorphism that gives rise to the average difference of 12.5 cm in adult height between the sexes.
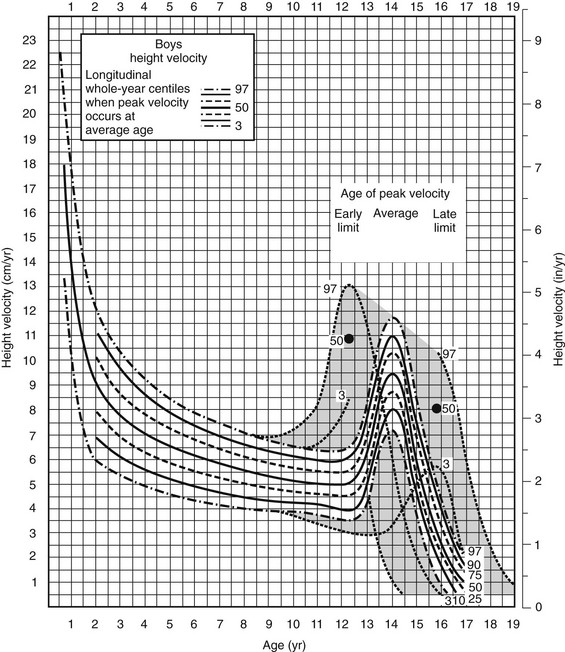
FIGURE 19-1 Height velocity chart for boys aged 0 to 19 years. Centiles 3 to 97 illustrated with 50th centile in bold. Shaded zone, Variation in timing of pubertal growth spurt. Visually, the chart depicts the rapid but rapidly decelerating growth during the first 4 years of life followed by a much slower declination until the onset of the pubertal growth spurt. (Copyright Castlemead Publications.)
Growth hormone is the main mediator of postnatal growth,2 and virtually any chronic childhood illness will modify secretion. As such, care needs to be exercised in the evaluation of GH secretion in these situations. Although GH deficiency (GHD) may be considered a form of IGF deficiency,3 this approach may be limited. Since GH receptor and post-receptor issues and GHD in adults are considered elsewhere, this chapter will focus primarily on GHD as related to disorders of the hypothalamo-pituitary axis in children.
History
The history of GHD starts with the pursuit of therapeutic interventions which antedate attempts to measure serum concentrations of GH. In 1932, a treatment to promote growth with a crude anterior pituitary extract was reported,4 but it was not until Raben’s observations of 19585 and the general availability of methods of GH extraction that large studies could be conducted. These larger studies showed a beneficial effect of human GH in promoting growth in children with clear physical signs suggestive of GH deficiency.6–9
Growth hormone immunoassays postdated the initial therapeutic studies of GH.10 The advent of radioimmunoassay allowed the measurement of the concentration of GH in blood in response to a variety of pharmacologic stimuli, thereby paving the way to a better understanding of which children might benefit from the then limited supplies of GH. This limitation in supply largely dictated clinical research until the advent of unlimited supplies of biosynthetic human GH (r-hGH) resulting from bioengineering technology in the late 1970s and early 1980s.
Our understanding of the physiology of GH secretion stemmed from the pioneering work of Geoffrey Harris and his group in Oxford, who suggested that release of GH from the pituitary was under the control of a releasing factor secreted from the hypothalamus. The demonstration by Brazeau et al.11 of a GH release-inhibiting factor (somatostatin) led to a radical change in the thinking around the control of GH secretion. The final demonstration of a GH stimulating factor came in 1982 when GH releasing hormone was isolated and characterized from two pancreatic tumors.12,13 During the search for the releasing factor, little was made of a further stimulating factor described by Bowers et al., which although synthetic in nature formed the basis from which GH releasing substances and their receptors were identified. Finally the natural ligand, ghrelin, was isolated from the stomach.14
For a considerable period of time, GH was believed to act via the generation of a further endocrine factor from the liver, somatomedin-C or IGF-I.15 Further work led to the realization that liver was not the only source of IGF-I, and in a series of classic experiments, Green16 and Isaksson17 demonstrated in adipose tissue and cartilage, respectively, that IGF-I was generated locally and acted in a paracrine manner to promote clonal expansion of the cell population.
Epidemiology
The reported incidence of GHD is to a large extent dependent on the criteria employed to establish the diagnosis and reflects the wide variation in the stringency of diagnostic testing. In one U.K. study, an incidence of 1 in 60,000 live births was reported,18 although a survey of Scottish schoolchildren led to a calculated prevalence of 1 per 4000 live births,19 a value similar to that of the Utah Growth Study (1 in 3480 live births).20
Several large surveys have indicated that approximately 25% of children diagnosed with GHD have an underlying “organic” cause for their condition, such as trauma, CNS tumors, inflammation, irradiation, or anatomic abnormalities of the hypothalamus or pituitary.21,22 The remainder are labeled as “idiopathic” GHD. Such surveys are likely to overestimate the number of true cases of idiopathic GHD because of variation in the diagnosis of GHD. Recent advances in developmental endocrinology suggest that many patients labeled previously as idiopathic GHD have genetic abnormalities or subtle anatomic abnormalities affecting the hypothalamus, pituitary, or both.
Pathogenesis
A list of causes of GHD is provided in Table 19-1. As mentioned already, “idiopathic” GHD constitutes by far the largest group of patients, although advances in developmental biology are forcing a rethink in this area.
Table 19-1
Congenital
Genetic:
See Table 19-2
Associated With Structural Defects of the Brain:
Associated With Midline Facial Defects:
Idiopathic
Acquired
Trauma:
Infection:
CNS tumors:
Following Cranial Irradiation
Following Chemotherapy
Pituitary Infarction
Neurosecretory Dysfunction
Transient:
Genetic and Structural Abnormalities
The pituitary gland, which consists of anterior, intermediate, and posterior lobes, is a central regulator of growth, metabolism, and development. Its complex functions are mediated via hormone-signaling pathways that act to regulate the finely balanced homeostatic control in vertebrates by coordinating signals from the hypothalamus to peripheral endocrine organs (thyroid, adrenals, and gonads). The mature anterior pituitary gland is populated by five neuroendocrine cell types defined by the hormone produced: corticotropes (corticotropin [formerly adrenocorticotropic hormone, ACTH]), thyrotropes (thyroid-stimulating hormone [TSH]), gonadotropes (luteinizing hormone [LH], follicle-stimulating hormone [FSH]), somatotropes (GH) and lactotropes (prolactin [PrL]).23 The posterior gland secretes vasopressin and oxytocin. The origins of the anterior and posterior lobes of the pituitary gland are embryologically distinct. Rathke’s pouch, the primordium of the anterior pituitary, arises from the oral ectoderm, whereas the posterior pituitary derives from neural ectoderm. Development of the anterior gland follows a similar pattern in a number of different species but has been best studied in rodents.
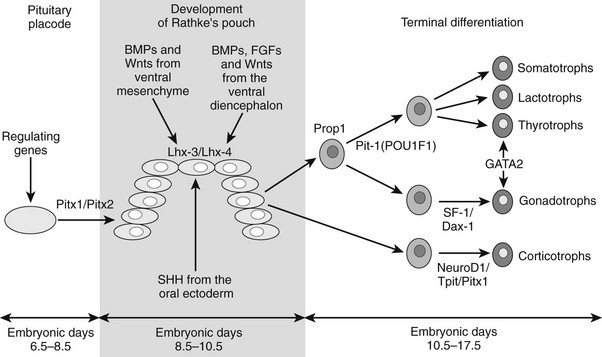
In the mouse, anterior pituitary development occurs in four distinct stages: pituitary placode formation; the development of a rudimentary Rathke’s pouch; the formation of a definitive pouch; and finally the terminal differentiation of the various cell types in a temporally and spatially regulated manner (Fig. 19-2). The apposition of Rathke’s pouch and the diencephalon, which later develops into the hypothalamus, is maintained throughout the early stages of pituitary organogenesis24 and appears to be critical for normal anterior pituitary development. A number of signaling molecules—fibroblast growth factor-8 (Fgf8),24–26 bone morphogenetic protein 4 (Bmp4),24,25 and Nkx2.126—that are expressed in the neural ectoderm and not in Rathke’s pouch are thought to play a significant role in normal anterior pituitary development, as illustrated by the phenotype of mouse mutants that are either null or hypomorphic for these alleles. These signaling molecules activate or repress key regulatory genes encoding transcription factors such as Hesx1, LIM homeobox 3 (Lhx3), and LIM homeobox 4 (Lhx4) within the developing Rathke’s pouch that are essential for subsequent development of the pituitary.23,24
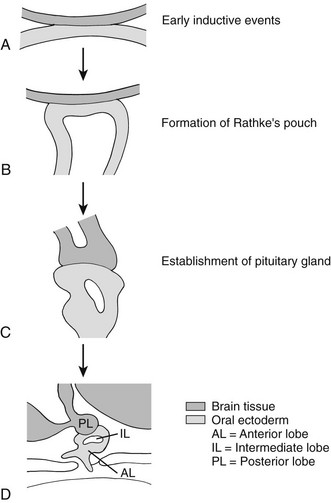
FIGURE 19-2 Formation of the pituitary gland. Four-stage process commencing with early inductive events as the infundibulum of the diencephalon abuts the roof of the oral cavity. Pituitary established with signaling gradients generating spatial defined patterns of gene expression and specific cell lineages in the definitive gland. (Reproduced from Valette-Kasic and Enjalbert, Topical Endocrinology, February 2003.)
The final stage of pituitary gland development entails the terminal differentiation of the progenitor cells into the distinct cell types found within the mature pituitary gland. This process is tightly regulated by extrinsic factors (Fgf8, Bmp2, Bmp4, and Bmp7) that emanate from the surrounding infundibulum and the juxtapituitary mesenchyme. These then establish gradients of transcription factors (Lhx3, Six3, prophet of Pit1 [Prop1], Pit1, Nkx3.1, Islet-1 [Isl1], Lhx4, Six1, Brain-4 [Brn4], and pituitary forkhead [Pfrk]).25,26 These genetic gradients lead to a wave of cell differentiation. Each of the five anterior pituitary cell types differentiates in a temporally and spatially regulated manner (Fig. 19-3),29–32 and this process is dependent upon a number of transcription factors such as Pit1, T-pit, and steroidogenic factor 1 (Sf1).33,34
Disorders of Pituitary and Extra-Pituitary Development in Humans
A number of genetic abnormalities have been identified in children who were previously thought to have idiopathic GHD or combined pituitary hormone deficiency (CPHD)35–37 (Table 19-2). In some cases, extra-pituitary manifestations may be associated. Mutations within the paired-like homeobox gene HESX1 are associated with the phenotypes of GHD, CPHD, and septo-optic dysplasia, a condition characterized by forebrain, pituitary, and eye abnormalities such as optic nerve hypoplasia.38,39 The inheritance and phenotypes are variable, with both dominant and recessive modes of inheritance described. Intriguingly, HESX1 mutations are classically associated with anterior pituitary hypoplasia with an undescended posterior pituitary and an absent or thin infundibulum.40
Table 19-2
Genes Implicated in Isolated Growth Hormone Deficiency and Combined Pituitary Hormone Deficiencies
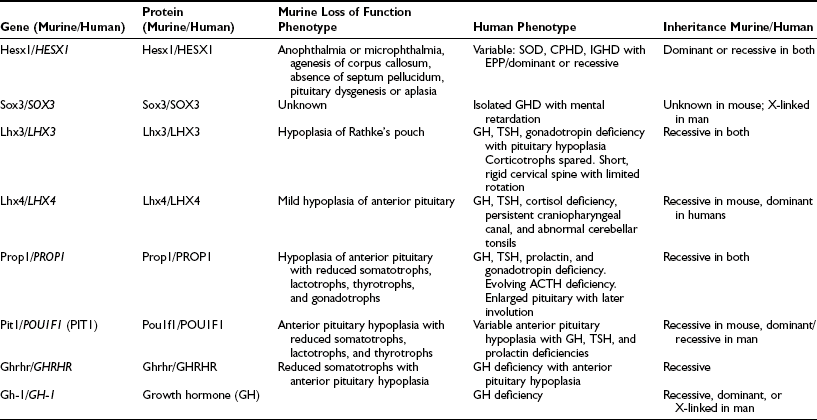
Mutations within the LIM-domain genes LHX3 and LHX4 are associated with CPHD with extrapituitary manifestations such as a short neck and steep cervical spine in the case of LHX341 and an abnormal cerebellum in the case of LHX4.42 The inheritance of LHX3 mutations is recessive, whereas that associated with LHX4 mutations is dominant, unlike the murine phenotype.
Mutations within the gene encoding the transcription factor Sox2 is associated with hypopituitarism in the mouse and humans. SOX2 is one of the earliest known genes to be expressed in embryonic stem cells and neural progenitors. Although mutations in the mouse are associated with a generalized reduction in all pituitary cell types, in the human, the most frequent pituitary defect is hypogonadotropic hypogonadism, with GHD less frequent. Other features include severe eye defects, esophageal atresia, hypothalamic hamartomata, learning difficulties, and sensorineural hearing loss.43,44
Recent studies with SOX3 suggest a possible explanation for the predominance of males in many series of GHD. SOX3 is sited on the X chromosome (Xq26-27) and appears not only to be important in pituitary development but is also associated with mental retardation.45 These observations of GHD and brain developmental abnormalities are particularly important because neurodevelopmental handicap has often been ascribed to untreated neonatal hypoglycemia, whereas structural developmental problems may be a more pertinent explanation.
Growth Hormone–Releasing Hormone and Its Receptor
Because growth hormone–releasing hormone (GHRH) and its receptor (GHRHR; GHD Type 1B) are critical to somatotroph population expansion, abnormalities in either are likely to be associated with severe GHD. No mutations of the human GHRH gene have been identified, but mutations in GHRHR have been identified in a number of pedigrees.46 Two large pedigrees have been identified in Pakistan (Glu72Stop mutation)47 and in northeastern Brazil (donor splice mutation in position 1 of intron 1; IVS1, G-A, +1).48 All patients reported to date have been either homozygous or compound heterozygous for mutations of the GHRHR gene. Serum GH concentrations fail to rise following standard provocative testing, as well as after GHRH administration. The patients resemble the little mouse (lit/lit), which has a mutation of the GHRH receptor gene affecting the ligand-binding domain (Asp60Gly; D60G).49
Somatotroph Development
A number of mouse models exist in which somatotrope development has been impaired. These include the Ames, the Jackson, and the Snell dwarf mice. A missense point mutation within the prophet of Pit1, or PROP1 gene (S83P), has been shown to be responsible for the Ames dwarf mouse.50 The phenotype results from a failure of initial determination of the Pit1 lineage required for production of GH, PrL, and TSH. The Ames pituitary gland contains less than 1% of the normal complement of somatotrophs and decreased numbers of lactotrophs and thyrotrophs. In humans, mutations within the transcription factor Prop1 are associated with CPHD in the form of GH, prolactin, TSH, and gonadotropin deficiency.51,52 A proportion of individuals with PROP1 mutations will develop cortisol deficiency.53 Additionally, a number of individuals with mutations within PROP1 develop transient pituitary masses with subsequent involution (Fig. 19-4).54 The exact mechanism underlying this phenomenon remains unclear, but it is clearly important to exclude mutations within PROP1 in patients with pituitary “tumors” especially the nonfunctional variety. There is considerable variability in the timing of the endocrinopathy, and a number of patients will actually commence puberty but then arrest halfway through. Recently, a mutation within PROP1 was identified in a patient who actually achieved a normal final height without receiving any GH treatment. PROP1 mutations are thought to be the commonest cause of familial CPHD and are usually recessive.
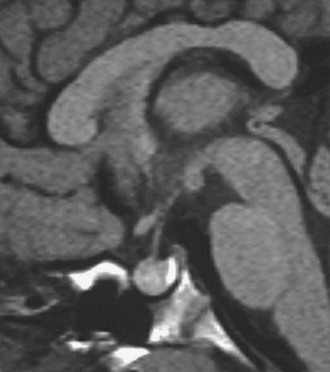
FIGURE 19-4 Pituitary “tumor” in patient with PROP-1 mutation. Saggital MRI scan of the pituitary revealing a large, globular anterior pituitary with normal posterior pituitary enhancement. Subsequent scans revealed involution of the mass and resultant empty sella.
Pit1 (now known as Pou1f1)55,56 is a member of the POU family of homeodomain proteins and contains a highly conserved bipartite DNA-binding domain consisting of the POU homeodomain, required for low-affinity DNA binding, and a POU-specific domain, responsible for the specificity of DNA binding and potential interactions with other proteins. The Snell dwarf mouse, characterized by pituitary hypoplasia and GH, PrL, and TSH deficiencies, has a point mutation (W261C) within the Pit1 gene, affecting the third helix of the POU homeodomain. This abrogates binding of Pit1 to its target promoter sequences. Several mutations and deletions of the POU1F1 gene have been identified in humans with CPHD, characterized by the combination of GH, PrL, and TSH deficiency.57,58 Mutations have been described which separately affect the DNA-binding capacity of POU1F1 or its transactivation properties. Autosomal dominant transmission, resulting from a dominant negative effect, has been observed in mutations affecting dimerization of POU1F1, transactivation (P24L), or in the relatively common R271W mutation, which results in increased binding to promoter elements and disruption of transcriptional activation.59 Autosomal recessive transmission is found with other mutations, such as A172stop, E250stop, R143G, A158P, and P239S.60 Variability in phenotype has been reported, although most patients exhibit growth retardation during the first year of life. GH and PrL deficiency is complete; TSH secretion may be observed during infancy but declines progressively during the early months of life. Magnetic resonance imaging (MRI) scanning revealed a marked variability in the size of the anterior pituitary, with some patients demonstrating a normal pituitary and others having a hypoplastic pituitary. After appropriate GH and thyroxine replacement, patients appear to enter puberty normally and have normal fertility. Lactation may be impaired. In some patients, TSH secretion may be normal.61
GH1 Gene
The GH1 gene, located at chromosome 17q22-24, is part of a cluster of five structurally related genes: GH1, CSHP (chorionic somatomammotropin pseudogene), CSH (chorionic somatomammotropin), GH2 (or placental variant), and CSH2. Mutations within the GH1 gene are associated with isolated GH deficiency (Table 19-3). Large, recessive, inherited deletions are associated with absence of GH protein (Type 1A GHD). Complete loss of pituitary GH secretion occurs secondary to deletions resulting from nonhomologous crossing over at different sites in the GH and chorionic somatomammotropin (CS) gene cluster. The most common deletion is 6.7 kb, but deletions of 7.0, 7.6, and greater than 45 kb have also been observed.62 Wagner et al.63 have also described the GHD IA phenotype in a patient with a point mutation in the GH signal peptide, E23X, resulting in premature termination of translation. Patients typically have an excellent initial response to GH therapy, but because of the absence of a normal GH molecule in fetal life, an attenuation of the growth response to exogenous GH may result from the development of anti-GH antibodies,64 although this event has been described less frequently with newer GH preparations.
Type II GHD is autosomal dominant and associated with splice-site mutations.65 These mutations lead to the production of two alternatively spliced GH molecules, 20 and 17.5 kD hGH. Mutations in an exon splice enhancer within exon 3 of the GH1 gene have also been associated with autosomal dominant GHD.66 The generation of the 17.5 kD form of hGH has a dominant negative effect and prevents the secretion of the normal wild-type 22kD hGH, with a consequent deleterious effect on pituitary somatotropes. In a murine model of this dominant negative mutation, there is loss of somatotroph number67 and progressive damage to adjacent pituitary cells (with later failure of PrL, TSH, and gonadotropin secretion).68
Seven different splice-site mutations have been reported to date. In addition, three missense mutations (R183H, P89L, and V110F) were also recently implicated in IGHD Type II. These patients have a normal GH1 allele but are unable to secrete the normal form of GH in appropriate concentrations. The mutant protein therefore exerts a dominant negative effect. As in the mouse evolution of other hormonal deficiencies, including ACTH, TSH, and gonadotropin, deficiencies have been described in patients with some dominant GH1 mutations.69
GHD Type III, an X-linked form of isolated GH deficiency (IGHD), has been reported in patients with hypogammaglobulinemia. To date, no alteration in the GH1 gene has been identified in this condition, and the genetic mechanisms remain unknown. Recently a polyalanine expansion within the transcription factor Sox3, which lies at Xq26-27, has been associated with X-linked GHD and mental retardation.45 Intriguingly, duplications of this region of the X chromosome have been associated with X-linked panhypopituitarism, and SOX3 has been implicated as the gene associated with this phenotype.70,71
Bioinactive Growth Hormone Molecule
Since the GH molecule exists in multiple molecular forms resulting from alternative splicing or posttranslational processing, some cases of short stature have been hypothesized to be the consequence of abnormal ratios of the various GH forms.72 The first report of two individuals heterozygous for point mutations in the GH gene was described by Takahashi et al. and detailed the biochemistry and molecular genetics.73 The mutant GH molecules (R77C and D112G) were capable of binding to the GH receptor, perhaps even with increased affinity, but were unable to stimulate tyrosine phosphorylation of GH-activated intracellular signaling intermediates in a normal manner. The ability of the R77C mutant to behave in a dominant negative manner was demonstrated by its ability to inhibit the in vitro actions of wild-type GH.
Structural Abnormalities
As methods of radiologic evaluation of the CNS have improved, an increasing percentage of patients with idiopathic GHD have been identified to have structural abnormalities.74–80 Many of these are associated with some of the genetic abnormalities described above, but the findings are worthy of separate consideration. In particular, the finding on MRI of an undescended (erroneously called “ectopic”) posterior pituitary (PPE) was commoner in males than females (3:1 when PPE present versus 1:1 if normal anatomy) in patients with CPHD as compared with IGHD (49% versus 12%), breech delivery (32% versus 7%), and associated congenital brain anomalies (12% versus 7%).
These findings appear to be best explained by a defect in induction of the mediobasal structure of the brain in the early embryo rather than the product of birth trauma, as previously suggested.77 Whether pituitary insufficiency is the result of hypothalamic or pituitary dysgenesis or the product of hypoplasia or sectioning of the pituitary stalk is not always clear. Perinatal problems, however, including breech presentation, may prove to be the consequence rather than the cause of underlying CNS abnormality. The concept that PPE, stalk section or hypoplasia, and pituitary hypoplasia may represent abnormal embryonic development rather than the consequences of birth trauma is supported by the finding of similar anatomic abnormalities in patients with septo-optic dysplasia, type I Arnold-Chiari syndrome, holoprosencephaly, and increasingly in patients with mutations in the genes controlling pituitary development.
In the empty sella syndrome, abnormalities of the sellar diaphragm allow herniation of the suprasellar subarachnoid space into the region of the sella turcica.81 This may result in damage to the sella, including the pituitary. Empty sella syndrome may be the consequence of surgery or irradiation, or may be idiopathic. It is often found in patients with mutations in PROP-1, when it may have been preceded by a pituitary mass.
Acquired Defects
Destructive Lesions of the Hypothalamus and Pituitary
A wide range of destructive lesions involving the hypothalamus or pituitary may present with isolated GHD or CPHD. Birth trauma, associated with abrupt delivery, prolonged labor, or extensive use of forceps, has been associated frequently with subsequent hypothalamic or pituitary dysfunction.82,83 An increased incidence of GHD has been reported in breech deliveries, although it is still unclear whether such deliveries lead to acquisition of pituitary dysfunction or, on the other hand, whether preexisting CNS abnormalities result in higher rates of abnormal birth presentations.
Tumors: Central nervous system tumors are an important cause of isolated GHD and CPHD and must be excluded in every child with GHD who does not have an obvious alternative explanation for growth failure. Midline brain tumors include germinomas, meningiomas, gliomas, colloid cysts of the third ventricle, ependymomas, and optic nerve gliomas. GHD or CPHD may also occur from local extension of tumors affecting the head or neck, such as craniopharyngeal carcinomas and lymphomas.
The major pediatric tumor involving the pituitary is craniopharyngioma, which is probably an evolving congenital malformation which develops from remnants of Rathke’s pouch.84 It accounts for 5% to 15% of intracranial tumors in childhood and 80% of tumors in the hypothalamo-pituitary region. Arising from rests of squamous cells at the embryonic junction of the adenohypophysis and neurohypophysis, it forms an enlarging cyst filled with degenerating cells, leading to cyst fluid or calcification but never to malignant degeneration (Fig. 19-5). These calcifications may be seen at times on skull films and constitute an important diagnostic sign. Although craniopharyngiomas represent the consequences of a congenital malformation, they may present clinically at any age. Significant growth failure may be observed at the time of diagnosis, but patients most commonly present with complaints of increased intracranial pressure, such as headaches, vomiting, and oculomotor disturbances; visual field defects are frequently noted at the time of diagnosis.84 Deficiency of at least one pituitary hormone, most commonly GH or gonadotropin, is present in 50% to 80% of patients. Diabetes insipidus is reported in 25% to 50% of patients at diagnosis.84,85
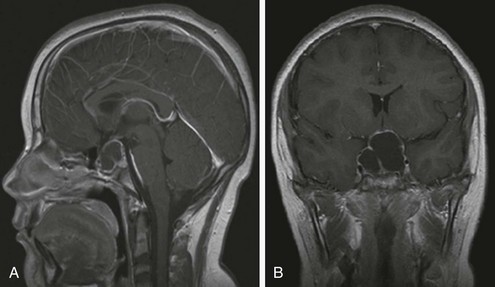
FIGURE 19-5 MRI of cystic craniopharyngioma. Saggital MRI scan (A) revealing large multicystic craniopharyngioma arising from the pituitary fossa and extending up to hypothalamus. Coronal section (B) of same lesion delineating upward and lateral spread. Both images are T-1 weighted, gadolinium-enhanced scans.
Langerhans cell histiocytosis may also present at any age. Langerhans cell histiocytosis (LCH) is characterized by clonal proliferation and accumulation of abnormal dendritic cells that can affect either a single site or many systems, causing multi-organ dysfunction. In children, the median age of diagnosis ranges between 1.8 and 3.4 years.86 LCH infiltrates the hypothalamo-pituitary area in 15% to 35% of patients, with subsequent development of at least one pituitary hormone deficiency.87 In a multicenter French national study of 589 pediatric patients with LCH, 145 patients (25%) had pituitary dysfunction. In 60 patients, pituitary involvement was already present at the time of diagnosis, and in 20 of them, it was the first manifestation of the disease. Patients at high risk of pituitary involvement seem to be those with multi-system disease involving skull and facial bones, mastoid, sinuses, and mucous membranes (i.e., gums, ear, nose, and throat region). Furthermore, compared to patients without pituitary involvement, patients with pituitary involvement have a higher rate of relapse (10% at 5 years versus 4.8% at 5 years) and a higher incidence of neurodegenerative LCH.88
Diabetes insipidus is the most frequently reported permanent consequence of LCH and the commonest endocrinopathy; almost all patients with pituitary involvement have DI. The second commonest endocrinopathy is GHD, which occurs in 14% of all patients with LCH and in more than 40% of patients who have pituitary involvement.87 In the vast majority of patients, GHD is associated with DI, with a median interval of 2.9 to 3.5 years between the diagnosis of DI and development of GHD. Isolated GHD, or the association of GHD with other anterior pituitary hormone deficiencies, occurs less commonly.
Pituitary MRI findings in patients with LCH include thickening of the pituitary stalk, suggestive of the infiltrative process enhancing changes in the pituitary gland and hypothalamus, and absence of the bright signal of the posterior pituitary in T1-weighted images, caused by the loss of the phospholipid-rich ADH secretory granules. The latter is an invariable feature of patients who develop DI.89 Although at the time of diagnosis of DI, 75% show a thickened pituitary stalk, only 24% have persistent stalk thickening after 5 years. These changes are variable and do not correlate with treatment or with clinical recovery; DI persists in all cases.
Long-term follow-up of patients with LCH has shown that the already established hormone deficiencies cannot be reversed by treatment.89 Recently, however, isolated case reports have suggested that treatment with the purine analogue 2-chlorodeoxyadenosine (2-CDA) may reverse established DI.90 Subsequent studies of this form of therapy, used in refractory cases of LCH involving the CNS, showed that 2-CDA may result in partial or complete radiologic improvement of the mass lesion, but the endocrine consequences of the disease, including DI and panhypopituitarism, do not reverse.91 Patients treated with the JLSG-96 protocol who have been followed up for 5 years developed DI with an incidence of 3.1% to 8.9%, depending on the extension of the disease (single system multisite versus multisystem).92
Irradiation of the Central Nervous System: Cranial irradiation used for the therapy of solid brain tumors and as prophylaxis for leukemia can lead to abnormal hypothalamo-pituitary function. The sensitivity of the HP axis to radiation depends upon the dose, fractionation, tissue location, and the age of the patient.93 Such damage is typically difficult to assess precisely, since the hypothalamus and pituitary may differ in the extent of involvement, and the loss of function may evolve with time. Sensitivity to CNS radiation may differ among patients, although the majority of children will experience some degree of hypothalamic or pituitary dysfunction within 5 years of receiving 30 Gy.94 GHD also occurs with doses of 18-24 Gy,95 and subtle dysfunction may be observed at even lower doses. GH secretion generally appears to be the most sensitive to irradiation, followed by TSH, gonadotropins, and finally ACTH. This may relate to the unique position of the GHRH neurons on the surface of the hypothalamus and not deep within the structure as previously thought.96
Pituitary dysfunction evolves over several years following irradiation, so such children should be monitored for growth deceleration. Provocative GH testing may be within normal limits, but measures of spontaneous GH secretion frequently demonstrate abnormalities.97 Serum concentrations of IGF-1 or insulin-like growth factor–binding protein-3 (IGFBP-3) may not be reduced in the early years following cranial irradiation.98
Cranial irradiation may also result in precocious puberty, leading to an early pubertal growth spurt, advanced skeletal maturation, and ultimately reduced stature. This may be superimposed upon any growth restriction that results from the spinal irradiation for the primary problem.99 Low-dose irradiation is frequently associated with a precocious onset of puberty; higher doses may result in gonadotropin deficiency and pubertal delay. In the irradiated child with early puberty, therapy with gonadotropin-releasing hormone (GnRH) analogues should be considered, with or without GH treatment, to delay epiphyseal fusion.
Traumatic Brain Injury: Traumatic brain injury (TBI) has been recognized as a cause of acquired hypopituitarism in a number of adult studies. Data on pediatric patients are sporadic, but TBI is probably underdiagnosed.100 These effects may be significant, considering the scale of the problem. In the United Kingdom, 180 children per 100,000 population per year sustain a head injury, with 5.6 per 100,000 requiring intensive care and almost one third of those admitted to ICU undergoing neurosurgery.
Although the pituitary gland is protected within the bony cavity of the sella turcica, the rich vascular network of the hypothalamus and pituitary and the structure of the pituitary stalk make it vulnerable to the effects of traumatic brain injury. The hypothalamus and pituitary have a complex vascular supply consisting of an arterial supply via the superior and inferior hypophyseal arteries from the internal carotid artery, as well as long hypophyseal vessels and a rich network of portal capillaries that surround the pituitary and infundibulum. The pathophysiology of hypopituitarism related to TBI is not clearly defined, but it is thought that it is the result of direct trauma or of vascular injury resulting in ischemia and infarction,101 an observation supported by the anatomical findings of autopsies following head trauma which include anterior lobe necrosis, pituitary fibrosis, hemorrhage, infarction, or necrosis of the pituitary stalk.102
In the acute phase, alterations in the endocrine function may reflect an adaptive response to acute illness. The clinically significant alterations involve mainly the regulation of fluid and electrolyte balance (diabetes insipidus, SIADH, cerebral salt wasting) and the hypothalamo-pituitary adrenal axis. Most of the pituitary hormone changes observed in the acute phase are transient, and their development cannot predict the development of permanent hypopituitarism.103
Until recently, there were only sporadic reports of hypopituitarism following TBI in children, but prospective studies designed to address the problem in the pediatric and adolescent population are in progress. The incidence of hypopituitarism is reported to range from 10% to 60%, and although this is lower in children as compared with adults, it is not uncommon.104 In general, the long-term outcome of TBI seems to be more favorable in children, although quality-of-life issues and minor disability may persist. The extent to which endocrine dysfunction contributes to these outcomes has yet to be defined.
Growth hormone deficiency appears to be the main endocrine manifestation, followed by gonadotropin deficiency. GHD can present as growth failure, whereas delayed or arrested puberty and secondary amenorrhea may present in adolescents and in patients in the transition phase. In a number of case reports, central precocious puberty has also been described in association with head injury, presenting 0.4 to 1.6 years after the event.105
Patients with hypopituitarism after head injury may have no clinical signs and symptoms suggestive of this disorder; its correct identification requires a high degree of suspicion. A consensus guideline on the screening of patients post TBI suggests that all patients who had TBI, regardless of its severity, should undergo baseline endocrine evaluation 3 and 12 months after the event or discharge from ITU.106
Infiltrative and Inflammatory Disorders: Infiltrative diseases are uncommon causes of GHD in the pediatric population, but pituitary insufficiency may be observed secondary to CNS involvement in tuberculosis,107 sarcoidosis,108 or toxoplasmosis. Inflammation associated with bacterial, viral, fungal, or parasitic disease may also result in hypothalamic-pituitary dysfunction. Lymphadenoid hypophysitis has also been reported.
Thalassemia is a hereditary disorder characterized by quantitative defects in synthesis of globin chains that result in ineffective erythropoiesis and, in its more severe forms, transfusion dependence. The majority of complications are the consequence of the toxic effects of iron which is deposited in organs of the reticuloendothelial system, the heart, and all target organs of the endocrine system, including the pituitary.109 The anterior pituitary is very sensitive to iron overload, resulting in defective GH secretion, reduced responsiveness of GH to GHRH, and hypogonadotropic hypogonadism. The gonadotroph cells seem to be particularly vulnerable to the toxic effects of iron deposition, which may be related to the way iron is transported in cells. Failure of pubertal development and growth impairment are the most prominent endocrine complications and may occur despite early initiation of chelation therapy. It is estimated that 56% of thalassemic patients have at least one endocrinopathy; almost half have hypogonadism (40% to 59%), and 33% to 36% manifest growth failure.110 The growth impairment is the result of a number of factors that include chronic anemia and tissue hypoxia, overchelation due to the toxic effects of desferrioxamine on spinal cartilage, GH insufficiency, and possible GH insensitivity.
Psychosocial Dwarfism
Psychosocial dwarfism is a form of poor growth associated with bizarre eating and drinking behavior, social withdrawal, delayed speech, and on occasion other evidence of developmental delay.111 Periodic hyperphagia is associated with decreased GH responsiveness to standard provocative stimuli but also with subnormal responses to exogenous GH therapy. Removal from the stressful environment, which usually involves removal from the home, is accompanied by a restoration of normal GH secretion, typically within weeks, and a period of catch-up growth.112,113 The mechanisms for this reversible form of GHD are unclear, but it is of note that a variety of psychiatric conditions in adults may be associated with decreased spontaneous and provocative GH secretion. Establishing the diagnosis of psychosocial dwarfism requires documentation of catch-up growth and restoration of normal GH secretion following correction of the environmental situation.
Clinical Features
The Hypothalamo-Pituitary-Somatotroph Axis
Growth hormone is secreted by somatotropes in the anterior pituitary gland. The secretory pattern is pulsatile, with discrete pulses of GH every 3 to 4 hours and virtually undetectable GH concentrations in between. Secretion of GH varies considerably with age114 and shows a sexually dimorphic pattern,115 with a greater average daily GH output in women. This pattern is the result of an interaction between the hypothalamic peptides GHRH and somatostatin (SS). The amplitude of the GH peak is determined by GHRH that stimulates the pituitary somatotrophs to increase both the secretion of stored GH and GH gene transcription. SS determines trough levels of GH by inhibition of GHRH release from the hypothalamus and GH release from the pituitary. Withdrawal of SS, on the other hand, determines the timing of a GH pulse.
More recently, the use of synthetic GH-releasing peptides (GHRP) has led to the identification of a GH secretagogue (GHS) receptor (GHS-R type 1a). The receptor is strongly expressed in the hypothalamus, but specific binding sites for GHRP have also been identified in other regions of the CNS and peripheral endocrine and nonendocrine tissues in both humans and other organisms.116,117 The endogenous ligand for the GHS receptor, ghrelin, was isolated from the stomach and is an octynylated peptide consisting of 28 amino acids.118 It is expressed predominantly in the stomach, but lower amounts are present within the bowel, pancreas, kidney, immune system, placenta, pituitary, testis, ovary, and hypothalamus.119 Ghrelin not only leads to the secretion of GH but also stimulates prolactin and ACTH secretion. Additionally, it influences endocrine pancreatic function and glucose metabolism, gonadal function, appetite, and behavior. It can also control gastric motility and acid secretion and has cardiovascular and antiproliferative effects. The role of endogenous ghrelin in normal growth during childhood remains unclear. Both ghrelin and GHRPs release GH synergistically with GHRH.
The expression of the human GH gene is regulated not only by a proximal promoter but also by a locus control region (LCR) 15 to 32 kb upstream of the GH-1 gene. The LCR confers pituitary-specific, high-level expression of GH.120,121 The full-length transcript from the GH1 gene encodes a 191-amino-acid, 22-kD protein that accounts for 85% to 90% of circulating GH. Alternative splicing of the mRNA transcript generates a 20-kD form of GH that accounts for the remaining 10% to 15%. Within both the proximal promoter and the LCR are located binding sites for the pituitary-specific transcription factor Pit1. Additional binding sites for the transcription factor Zn15 are also located within the proximal promoter.
In the circulation, GH binds to two binding proteins, high-affinity GHBP and low-affinity GHBP.122 Little is known about the low-affinity GHBP, which accounts for approximately 10% to 15% of GH binding, with a preference for binding to 20-kD hGH. The high-affinity GHBP is a 61-kD, glycosylated protein that represents a soluble form of the extracellular domain of the GH receptor that can bind to both 20 and 22 kD hGH and thereby prolong the half-life of GH. In-vivo studies that have co-administered GH and GHBP to hypophysectomized and GH-deficient rats have demonstrated a potentiation of weight gain and bone growth, although similar studies have not as yet been performed in man.123
The GH receptor (GHR) is present in a number of tissues. The hormone sequentially dimerizes its receptor, activating a receptor-associated tyrosine kinase JAK2 that in turn is auto-phosphorylated and also phosphorylates the GHR. This then leads to signal transduction using the MAPK, STAT, and PI3 kinase pathways. The end result is activation of a number of genes that mediate the effects of GH. These include early-response genes encoding transcription factors such as c-jun, c-fos, and c-myc—implicated in cell growth, proliferation, and differentiation—and IGF-I, which mediates the growth-promoting effects of GH.124,125
Neonatal Presentation
Recent studies in humans and in animal models have demonstrated marked similarities but also critical differences between the clinical features of GHD and various forms of IGF deficiency.126–128 In GHD, prenatal growth is near normal, although mild reductions in birth length and weight have been observed. GHD does not cause severe IUGR, whereas loss of placental GH does.129 However, loss of IGF-I in utero results in severe intrauterine growth restriction in both humans and mice,130,131 suggesting that IGF-I and the IGF-I receptor are critically involved in intrauterine growth. IGF-I synthesis and secretion in utero are not regulated primarily by pituitary GH.
Infant and Childhood Years
After the perinatal period, the defining feature of GHD is growth failure. Reduced skeletal growth may be observed during the first 6 months of life in congenital GHD, but by 6 to 12 months of age, early growth failure is almost inevitable.132–134 Height velocity is usually between −2 and −5 standard deviations (SD) from the mean, leading to progressive height centile crossing. In patients with acquired GHD, the critical feature is a change in growth rate. Between the age of 2 years and the onset of puberty, children maintain their height percentile with remarkable integrity. Deviation from this channel (either acceleration or deceleration) needs investigation. Thus a child who has been growing along the 75th percentile but moves across to the 25th percentile warrants evaluation, even though his/her height may still be within the normal range.
Bone age is often delayed in patients with GHD, but this may not be so in acquired GHD. The close proximity of time to the growth failure or acquired GHD accompanied by accelerated puberty is occasionally seen in patients with intracranial tumors, when bone age may be accelerated.93,94 Delayed dentition may be observed, but in the absence of midline craniofacial abnormalities is otherwise normal. Other skeletal appearances include hypoplasia of facial bones, hypoplastic nasal bridge, frontal bossing, and delayed closure of sutures. Head circumference is usually at the lower limits of normal, indicating normal brain growth.
Limited data are available on the adult height of untreated GHD patients. These results are often difficult to interpret because of (1) heterogeneity in the timing of GHD, (2) heterogeneity in the severity of GHD, (3) the presence or absence of other pituitary deficiencies, and (4) delay in puberty, resulting in late epiphyseal fusion. Wit et al.135 summarized the results from studies of 22 untreated men and 14 untreated women with severe isolated GHD and reported a mean adult height of −4.7 SD. In patients with untreated autosomal recessive GHD, Rimoin and colleagues reported mean adult heights of −7.4 SD.136 In patients with CPHD, adult height is often not as severely affected as in IGHD, presumably reflecting pubertal delay and late epiphyseal fusion.137
Diagnosis of Growth Hormone Deficiency in Childhood
The diagnostic evaluation of children with growth failure is complex because there are multiple causes for short stature (Table 19-4). In the pursuit of the diagnosis of GHD, other causes for short stature need to be considered and excluded. This is because the diagnosis of GHD is one of exclusion.138 GH is the final common pathway for postnatal growth, and many causes of poor growth may secondarily affect GH secretion.139 There are a number of tests available for assessing GH status. Considerable attention has been paid to the underlying mechanisms assessed by the tests, how the samples should be collected, and what type of measurement should be performed. Less attention has been paid to the statistical assumptions underlying the performance of diagnostic tests. The statistical theory behind many tests is complex because the results do not follow an all-or-none law. Rather than being left with a clear-cut answer to the initial diagnostic question, the clinician is more likely to be left with a series of probabilities as to whether or not the patient is likely to have GHD.
Table 19-4
Nonpathogenic
Low Birth Weight
Systemic Disorders
Cardiovascular disease (e.g., congenital heart disease)
Renal (e.g., chronic renal failure, renal tubular disease)
Respiratory (e.g., cystic fibrosis, asthma)
Gastrointestinal disease (e.g., Crohn’s disease)
Endocrine Causes
Growth hormone (GH) deficiency: isolated or combined with other hormone deficiencies
Genetic Causes
Guidance Derived From Clinical Assessment
Several pointers to the diagnosis of GHD have already been considered in this discussion, but in the neonatal period, GHD may be isolated or associated with other pituitary hormone deficiencies. Small genitalia may point to associated gonadotropin deficiency. Hypoglycemia in the newborn period is often a feature of ACTH deficiency, although on an arbitrary basis, a serum GH of less than 10 ng/mL is considered consistent with a diagnosis of GHD under these circumstances.140 This is not universal, however, and caution needs to be exercised in interpreting the GH response to hypoglycemia under different circumstances.141 Prolonged neonatal jaundice raises the question of thyroxine (unconjugated) or cortisol (conjugated hyperbilirubinemia) deficiency. Given these features, it might be possible on the basis of pattern recognition to ascribe the diagnosis of GHD to a patient with a high degree of certainty. MRI of the brain should be obtained to look for an undescended posterior pituitary, anterior pituitary hypoplasia, hypoplasia or absence of the pituitary stalk, hypoplasia of the optic chiasm, and absence or hypoplasia of the corpus callosum and septum pellucidum.74–80
Infancy and Childhood
Diagnostic evaluation in children must be based upon auxology. Although there are a number of clinical features of GHD which are said to be classic, none is specific. For example, obesity is listed as a clinical feature of GHD, but if we simply restricted biochemical evaluation to patients with obesity as the main feature, testing the GH axis would yield a large number of individuals with a poor GH response, because obesity per se is associated with blunted GH responses to various stimuli.142 Individuals who are GHD are often obese, but the converse is clearly not the case.
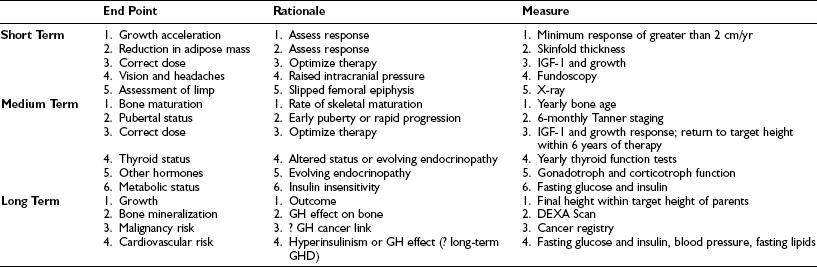
The manifestation of GHD as a result of a GH gene deletion is early, and poor growth can be detected as early as the sixth month of postnatal life. With advancing age, more GH has to be secreted to maintain concentrations of GH sufficient for growth, so idiopathic isolated pituitary GHD may present at any time. It is the degree of deficiency that dictates when the individual comes to medical attention. Table 19-5 provides general clinical rules that are a useful aid when selecting patients for further study of the GH axis.
Table 19-5
Clinical Indicators for Further Evaluation of the GH Axis
1. Height, at any age, below the 0.4th centile on the U.K. Reference Charts.143 The 0.4th level is chosen to improve diagnostic return from evaluation. The previous cutoff (3rd or 5th centile) lacks sensitivity and specificity.
2. Crossing of one or more height centiles on the U.K. Reference Charts over a period of 1 or more years. Centiles are equispaced (0.7 SD), allowing general rules to be applied at all ages.
3. A height that is inappropriately low for the heights of the parents.
4. Predisposing condition (tumor, radiation, etc.) or features suggestive of an underlying syndrome.
5. Neonatal signs consistent with pituitary hormone deficiencies.
Principles of Testing
The aim of any diagnostic test is to progress the clinical history and examination to the point where the care of the patient is altered. No test will ever benefit a patient. It is only when subsequent treatment has to differ depending on the test result that patients will be better off. There is a vast and bewildering literature on GH testing, but the clinician can be guided by asking the questions detailed in Table 19-6. It is important to remember that a diagnostic test is not just about whether a disease is present or not. It might also be important in determining severity and prognosis, responsiveness to and monitoring of therapy, and as a screening tool. As such, how the test performs under one circumstance may not be the same in another. Measuring serum IGF-1 concentrations may be unhelpful in screening for GHD but may be excellent as a marker of response to therapy.
Table 19-6
Underlying Principles of Assessing Tests
1. Has there been an independent blind comparison with the diagnostic “gold standard”?
2. Was the test conducted in a wide range of patients with and without the condition?
4. What was the definition of “normal” in the test situation?
5. How might the test interact with others in a diagnostic sequence?
6. Does the test entail risk or reduce risk for the patient?
Two points deserve special mention. First, it is unusual in endocrinology for there to be a diagnostic gold standard. The anterior pituitary is not accessible, and molecular biology is not sufficiently advanced to give definitive answers. Second, care needs to be taken in ascribing the role of a gold standard. It may change with time, and the test must be well validated by application to large numbers of individuals with and without the condition. The temptation is to use the extremes, but this may lead to a considerable overestimate of sensitivity and specificity144 which may not be borne out in field studies.145,146
Two principles operate when using diagnostic tests.147 First, probability is a useful marker of diagnostic uncertainty. This is when the sensitivity (ability to detect a target disorder when present or true positive rate) and specificity (ability to identify correctly the absence of the disorder or true negative rate) become important. If both were 85%, 15% of patients with disease would have a negative result (false negative) and 15% without disease would have a positive result (false positive). Abnormal results would occur in patients with and without disease. Whatever the result, new information has been generated that may or may not influence decision making. Second, diagnostic tests should be obtained only when they can alter the management of the case—that is, if the test result alters the probability of the disease.
Pre- and Post-test Probability
The relationship between the probability of disease after the results of diagnostic tests are known (the post-test probability) and pretest probability of disease depends on the sensitivity and specificity of the test as shown in Figure 19-6. There are two important points to note: the first is that the more certain the clinician is of the diagnosis before the test is performed, the less effect the confirmatory test has on the probability of disease. The obverse is also true. The second point is that tests will have major effects on probability of disease in the intermediate zone. Testing is not likely to be beneficial if the pretest probability is very high or low. This is one reason why screening for GH problems in short children on the basis of biochemical tests is unhelpful; the pretest probability is 1 in 3000 or 0.003%.
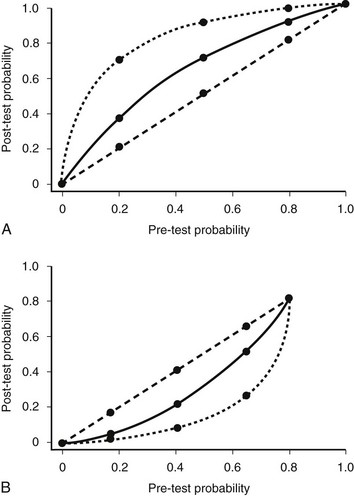
FIGURE 19-6 The relation between pre-test and post-test probability of disease. The data were constructed by using Bayes’ theorem with a test sensitivity and specificity of either 70% (solid line) or 90% (dashed line). A, The post-test probability if the test were positive; B, the post-test probability if the test were negative. If the post-test probability were the same as the pre-test probability, then the relation would be given by the line of identity. (Reproduced from Brook CGD, Hindmarsh PC, Jacobs HS [eds]: Clinical Pediatric Endocrinology, 4th ed. London: Blackwell Publishers, 2001.)
Clinicians are often faced with the situation where they feel really sure the patient has the condition, but the test does not confirm this. Table 19-7 analyzes this concept. Here, specificity and sensitivity have been fixed, and the effects on post-test probability are considered. In the situation where there is a 90% pretest probability that the patient is GH-deficient, then even if the test is negative in the individual, there is still a 67% probability (reduced by 23%) that they have the condition, so treatment would still be justified. When the pretest probability was 5% (very certain that the patient does not have GHD) and the test is positive, all the result says is that the patient has a 1 in 4 chance of having the condition, so we would probably not treat. In the middle ground, certainty in either direction is dramatically improved.
Table 19-7
Effect on Post-test Probability of Differing Pre-test Certainty, Assuming Constant Sensitivity and Specificity

Multiple Tests: Table 19-7 could have been made much larger by introducing any number of pretreatment probabilities. There comes a point, however, when post-test probability changes to a level where a decision has to be made to stop and either accept or reject the proposal that the condition is present. The decision to stop investigation and to treat or not depends on how convinced the clinician is of the diagnosis, the benefits and risks of the therapy, and the potential yield and risks of further tests. There are two ways to assist this situation: conduct another test or use a more sophisticated analysis rather than a simple positive or negative.
This is problematic in the GH field because the methodology assumes that the results of the two tests are independent. In normal individuals undergoing repeat GHRH tests, dependence cannot be assumed.148 Where repeat tests have been performed in children, concordance was observed 50% of the time, a value close to that calculated for independent events using a test with 70% to 85% efficiency. If all tests are treated as independent, there is a risk of over- or underestimating the presence of the condition. Another important issue is whether the test might change in individuals as they age. There is evidence that the clonidine test is less effective in releasing GH in young adults compared with children.149 Whether the magnitude of the response to other stimuli can be assumed to remain unchanged is unknown.
One special area of two-tests is the question of re-testing after completion of GH therapy. Several recent publications have suggested that individuals who were originally diagnosed with GHD do not appear to have the biochemical abnormality when the test is repeated later.150 This has led to statements being made that these individuals are no longer GHD. However, such statements reflect a subtle mind shift that has taken place. The clinicians have shifted from wishing to make a diagnosis to one of excluding a diagnosis, without taking into account the laws of probability. Two issues are worth considering: The first is that the population studied during the second test is not the same as that during the first. Those thought unlikely to have the condition have been excluded. The second point also relates to some extent to the original diagnosis. It is worth rehearsing the scenario that has led to the second test. The child was initially evaluated because of concerns over short stature and poor growth. At that point, a test was conducted because the clinician required an answer with which to rule in or rule out the diagnosis, and the result was sufficient in terms of post-test probability for therapy to be offered. If the post-test probability of the child having the condition was 87%, then this value now forms the pretest probability for the second test, not the 50:50 situation the clinician faced prior to the original investigation.
Diagnosis of Growth Hormone Deficiency
Assessment of GH secretion is problematic, in part because of the pulsatile nature of GH secretion.151 The most consistent GH surges accompany slow-wave electroencephalographic rhythms during phases 3 and 4 of sleep. Although this rhythmicity is characteristic of GH secretion at all ages, the size of the amplitudes and the total integrated GH secretion varies with sex, age, pubertal status, and nutrition.152,153 Between pulses, serum GH concentrations are extremely low, often less than 0.1 ng/mL. Consequently, measurement of random serum GH concentrations is of no value in the diagnosis of GHD. Measurement of spontaneous GH secretion requires multiple sampling, typically every 15 minutes over a 12- to 24-hour period.151–155 Such methodologies are inconvenient and expensive, and while they allow identification of the patient with severe GHD, it is not clear that they can discriminate between partial GHD and normal secretory variation.156 However, even the reproducibility of GH secretory patterns in children from day to day is uncertain. Rose and colleagues156 reported that measurement of spontaneous GH secretion identified only 57% of children diagnosed as GHD by provocative testing. Lanes157 reported that approximately 25% of normally growing children have low overnight GH concentrations. A longitudinal study of GH secretion in normal boys during puberty indicated wide intersubject variation,152,153 and day-to-day variation has been noted among normal subjects.158
An alternative approach has been the measurement of urinary GH concentrations.159–163 This methodology requires a timed urinary collection and a GH assay of high sensitivity because urinary GH concentrations are low. The theoretic advantages of this approach include its relative ease of performance and noninvasive nature, as well as the requirement for only a single GH measurement. This must be balanced, however, by the need to assess the effects of renal function, the wide interindividual variation, and the lack of adequate age- and sex-related reference ranges.
As a result of these difficulties, the standard for the diagnosis of GHD has been provocative testing of GH “secretory reserve”140 (Table 19-8). Physiologic stimuli for such tests have included sleep164 and exercise,165,166 and pharmacologic stimuli have included a wide variety of agents.167–175 None of these tests truly mimics normal GH secretory physiology, and none has been evaluated adequately in normal children and normal short children. The limitations of provocative GH testing in the diagnosis of GHD are described below and need to be considered in the light of statistical theory (see above):
Table 19-8
Growth Hormone Stimulation Tests
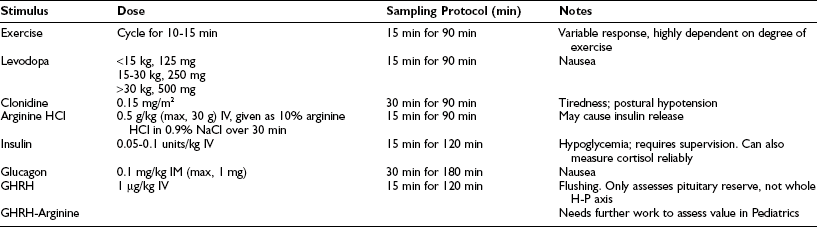
Tests should be performed after an overnight fast.
Patients should be documented to be euthyroid.
Prepubertal children should be primed with gonadal steroids.
GHRH, Growth hormone–releasing hormone; IM, intramuscular; IV, intravenous.
1. Provocative testing by its nature is nonphysiologic. None of the commonly used stimuli truly mimics normal regulation of GH secretion.
2. The definition of a “normal” response to stimulation is arbitrary. Normal values are difficult to obtain in pediatric practice, and reference ranges would be needed for tall, normal, and short children, because their GH secretion differs.176 In addition, both age and pubertal stage influence GH secretion,152,153,176 as does body composition.142 Values for these would also have to be included.
The question of cutoff points for use in tests becomes more important as individuals with differing severities of the disorder are considered. In constructing normal ranges, it is clearly best if large sample sizes are chosen. Choosing populations of disease positive and disease negative is unwise, either for this task or assessing test performance, since it is unlikely that in the less severe cases the test will perform as well.177 In the assessment of studies, some useful points are outlined in Table 19-9. Referral bias remains a major issue in many studies. In studies emanating from referral centers, the strength of a factor such as short stature may appear to be less important in that patients are already selected for this in the referral. Changes in prevalence of a condition do not change test properties, whereas changes in the spectrum of the disorder do.178
Table 19-9
3. The dependence of GH secretion on other factors needs to be taken into account. Marin and colleagues179 demonstrated that when exercise and arginine stimulation tests were performed on normal-stature children without sex steroid priming, the lower-limits-of-normal (−2 SD) peak serum GH concentration for prepubertal children was as low as 1.9 ng/mL and rose to 7.2 ng/mL on estrogen priming. Thyroxine and cortisol, which directly alter gene transcription, influence the results obtained, and these need to be controlled before undertaking a diagnostic study. Similarly, the presence of high levels of glucose or free fatty acids may influence the response obtained.180
4. GH assays may measure a variety of immunoreactive molecular forms of GH.181 These GH variants do not necessarily possess equivalent growth-promoting actions. Furthermore, considerable interassay variability exists in the measurement of these GH molecular variants.182,183 Individual standards need to be established for each laboratory.184 Assay precision, accuracy, and sensitivity play an important role in determining the success or failure of the diagnostic test. For example, assays for IGF-1—despite having good standardization from the technical aspect (elimination of interference of IGF-I-binding proteins [IGFBP] and the use of high affinity, high specificity antisera)—may have different performance characteristics when concentrations are either above or below the normal range.185
5. Most endocrine tests are conducted over short periods of time, and results are extrapolated to longer time frames. GH provocation tests take 2 hours to perform, and the results are then compared with height velocity measurements obtained over a longer period of time, often 1 year. That there is a relationship is perhaps surprising; that there are high false-positive and false-negative rates probably not.
6. Hormone pulsatility may also influence diagnostic tests if the test itself is influenced by oscillations (e.g., the stimulus applied) within the system under study. The GH response at any point in time is going to be heavily dependent on the interplay between the hypothalamic regulatory peptides involved in GH release, namely GHRH and somatostatin.186 Somatostatin, in particular, is a key determinant of the amount of GH released as a result of GHRH stimulation. Attempts have been made to take control of this variable187 by pretreatment with somatostatin. GHRH combined with arginine is an alternative approach.188 It was hoped that the ghrelin-like agents would be an advance in this area because of their potent GH-releasing qualities, but they appear to suffer the same problems of reproducibility.189
7. Endocrine systems are also subject to feedback from target tissues, and this is an issue not only in the interpretation of single provocation tests but also where second tests are performed in rapid succession to the first. A diminished response to GHRH can be observed if the second stimulus is applied 1, 2, or 3 hours after the first.147 The implication of doing two tests on the same day, often following each other, are immense; the cutoff that might be implied to determine normality or not may not be the same for the second test as for the first, especially if the second stimulus is different from the first.
8. Provocative testing fails to give any consideration to the effect of negative feedback by serum IGF-1.190–193 It probably makes more sense to interpret serum GH concentrations in the light of serum IGF concentrations, much as TSH concentrations are best assessed with a knowledge of circulating thyroxine concentrations.
9. In assessing the results of endocrine evaluations, it is generally assumed that the single or multiple samples measured are relatively stable, at least over short periods. When important changes are postulated to be taking place, for example in a disease process, some knowledge of the inherent variability within the measurement system is required. In the short term, a number of studies have demonstrated variability within and between individuals in terms of GH tests.158,194 Group data are usually reproducible, but problems can arise if it is assumed that individual oscillatory profiles are consistent from day to day.
10. In considering provocative tests, the situation may arise where no response is observed. A possible explanation is that the strength of the stimulus was insufficient to provoke hormone release. In such a situation, it is valuable to have an independent marker of stimulus application. In the insulin induced hypoglycemia test, this marker is glucose and the attainment of adequate hypoglycemia. In the glucagon test, it may be the release of glucose. In other tests, there may be no independent markers, so doubt may be cast on the reliability of the nonresponse.
11. Careful consideration needs to be given to the age of the child under study. Not only might the cutoff point criteria differ at different ages, but the likelihood of disease presence will change with age. It is highly unlikely that GHD will manifest itself during puberty. It is possible, but it is more likely that any growth or GH secretory problems at this age relate more to delayed puberty rather than an abnormality in the GH axis. Even in childhood, the return in terms of diagnosis of GHD is not high if height screening is undertaken at school entry so that with increasing age there is a diminishing diagnostic return.
12. Provocative GH testing is expensive, uncomfortable, and has risk. Insulin-induced hypoglycemia should only be performed in a supervised setting. Deaths have been documented in patients rendered hypoglycemic and corrected in an overly vigorous manner.
Of the provocative tests listed in Table 19-8, it should be noted that stimulation with GHRH is not designed to document whether a patient has GHD but rather whether GHD, established by other methodologies, is the result of pituitary or hypothalamic dysfunction.195 Failure to respond to GHRH suggests that the abnormality is at the pituitary level. This test may be enhanced by the addition of arginine or pyridostigmine.196
Treatment
The first successful treatment of human GHD with human pituitary–derived GH (hGH) was in the 1950s, and this was followed by a series of publications that documented the efficacy of GH in patients with GHD.6–9 Treatment utilized human cadaver pituitary–derived hGH, which brought with it a series of problems surrounding supply. Limited supplies mandated the use of suboptimal dosages, interrupted treatment periods, and frequent cessation of therapy before maximal height had been attained. The use of human pituitary–derived hGH was halted in 1985 following the discovery of several cases of Creutzfeldt-Jakob disease associated with its use. Pituitary-derived hGH was replaced with recombinant DNA–derived rhGH, which allowed for potentially unlimited supplies, obviating the need for low-dose usage and interrupted therapeutic regimens. The initial rhGH preparation was an N-terminal methionine, met-rhGH, which was fully active biologically but was ultimately replaced by the mature 191–amino acid protein. The biopotency of current preparations of rhGH, expressed as International Units per milligram of the new World Health Organization (WHO) rhGH reference reagent for somatropin (88/624), is 3 IU/mg.
Dose Studies
Investigations of optimal dosing of rhGH have been complicated by the use of heterogeneous study populations, so studies frequently include patients with unequivocal and complete GHD together with patients with partial GHD. Several studies have demonstrated a dose-response relationship for hGH, but the slope of the response is relatively shallow.197 MacGillivray and colleagues198 compared the growth responses of 99 children treated with pituitary hGH at a mean dosage of 0.1 mg/kg/week with those of 77 children treated with rhGH at a dosage of 0.3 mg/kg/week. The mean time required to reach normal height (greater than −2 SD) was 48 months for the low-dose group and 27 months for the high-dose group. Fifty-one percent of the low-dose group never reached this point compared with 23% in the high-dose group. Cohen et al.199 compared the growth responses of prepubertal, naive patients randomized to rhGH at a dosage of 0.175, 0.35, or 0.7 mg/kg/week for the first 2 years of treatment. Significantly greater height velocities and gains in height SD resulted from the 0.35 mg/kg/week versus 0.175 mg/kg/week, but no further significant improvement was observed with the 0.7 mg/kg/week dosage. Ultimately, the issues that should determine dosage in the child with GHD are (1) how best to return the GHD child to the normal growth curve, (2) how best to ensure that the child attains his or her genetic height potential, (3) risks, and (4) cost. For the child with severe GHD, weekly dosages of 0.175 mg/kg, administered in seven daily doses either by a subcutaneous or intramuscular route, are usually sufficient to increase growth rates from 3 to 4 cm/year to more than 10 cm/year.
Response to GH therapy varies even when the diagnosis is homogeneous. This probably reflects differences in tissue responsivity which may relate in part to the function of the GH receptor. A polymorphism in the GH receptor (GHR) gene leading to retention (full length [fl]) or deletion of exon 3 (d3), which encodes a 22–amino acid (aa) residue sequence in the extracellular domain,200,201 has been associated with the degree of height increase in response to GH replacement in children born short for gestational age (SGA), those with idiopathic short stature (ISS),202 and in a GHD population.203 Patients with at least one d3 allele had a significantly better first-year response leading to an improved adult height on GH treatment than patients with homozygosity for GHR-fl. However, reported studies are not all consistent, which may reflect differing populations and conditions.204–208 False-positive findings are more likely with small sample sizes, and for quantitative trait loci, phenotypic variations tend to be overestimated with small sample sizes.209,210
Weight- or body-size-based dosing is common practice in pediatrics; whether it is better to dose on the basis of growth response and serum IGF-1 concentration is worth consideration. Current data suggest that upwards of 2.5 times the standard dose of r-hGH is required to attain serum IGF-1 concentrations in the upper zone of the normal distribution, although the benefit in terms of height improvement was minimal.211
Frequency of Administration
Several studies have compared the short-term effects of administering hGH either daily or thrice weekly.212 Generally, daily injections are more effective, but increasing the frequency more than this makes little difference.213 Sustained-release rhGH preparations, which may be administered as infrequently as every 2 to 4 weeks,214 are unlikely to be effective and have potentially greater side effects due to the pulsatile nature of GH physiology.
Prediction Models
A series of models have been derived8,215 that describe factors that might influence response, but none have gone on to test these in formal randomized control trials. Further problems arise when large multicontributor databases are used because of the accuracy of the data entered; when prognosis is considered, factors that may be important may not have been recorded. It has been reported that in children with GHD, auxologic parameters, such as chronologic age (the younger the patient, the better the response) and the difference between target height and actual height (the smaller the patient, the better the response), are better predictors of growth response than the cumulative weekly GH dosage. There are several problems with these types of models:
1. Although prediction models are useful to give an average effect, they are not individualizable.
2. They often only focus on one outcome, usually short-term growth, whereas interest may be more centered on final height. The two need not necessarily be related, and the factors that influence response in the first year of treatment may differ totally from those that lead to prediction of the individual’s final height.
3. Very few prediction models have been constructed from an a-priori hypothesis, and care needs to be taken that there has been no interference from other factors accompanying the disease that might affect prognosis. The problem is that importance can be ascribed to factors that are merely markers for other factors of real importance. Examples of this can be seen in models which demonstrate that individuals who are extremely short, growing very poorly, and whose heights are subsequently further away from their genetic height respond best to treatment. All these factors are simply a marker of “how bad the disease is” and could perhaps be more easily summarized by a similar single factor that actually describes the severity of the condition.
4. Rules derived from one data set may reflect associations that have occurred by chance and often result from overfitting of the data.
5. There is always the possibility that the predictors are idiosyncratic to the population, the setting, to the clinicians, or to other aspects of the original study. Specific issues associated with growth-response models are summarized in Table 19-10.
Table 19-10
Specific Issues With Prediction Models of Response to Growth Hormone Treatment Development
a. The first problem relates to the method by which response to GH is defined. In several studies, acceleration in growth rate or the difference between the pretreatment growth rate and that observed during the year of treatment is used. Using either, however, leads potentially to the generation of artifact. This is because if difference (e.g., change in height velocity) is plotted against pretreatment states (e.g., pretreatment height velocity), then a good relationship will always be demonstrated purely and simply because pretreatment height velocity is contributing to both variables.216,217 Examination of the association between change in a variable and its initial value is complicated. It is possible, for example, that if high values are recorded at one stage and the same measure is performed at a later date, low values may be recorded even in the absence of treatment. This effect, “regression to the mean,” will be most marked in those with highest or lowest initial recorded values and will induce a spurious association between change and initial value. Several methods are available to overcome this problem.217
b. The prediction gives the average effect, and the confidence interval of the response is smallest at the average value of the independent variable. The difficulty comes in applying this information to the individual. Simply stating the average expected improvement is not much help. To convey this information, consideration needs to be given to the patient’s perception of what they think the treatment can do. If the patient wishes a growth response that will put them into the upper half of the normal height distribution, then disappointment might ensue. It is far better to provide the individual with an understanding of the chance of a successful outcome, and the concept “number needed to treat” is useful.218
c. If the treatment effect varies among individuals with the same initial height value, then spurious trends may emerge in the overall model prediction. Even though it is clear that most treatments will not affect all individuals equally, this is not well understood. Whether a child with GHD with a tall or a short parent will have a better or worse response than a child with average-height parents is difficult to determine, because at present there are no easy markers of GH sensitivity.
d. There are inherent problems with making predictions from previous data (e.g., how does year 1 influence year 2) or from the original data set. If we are looking at serial effects on height, it is important to recognize that the data going into such analyses are highly correlated. A person who is taller than average after 1 year will also tend to be taller than average after 2 years of follow up. It is important to realize that any two quantities changing over time will show a statistical association. Methods are available to assist in this area, but of necessity the time component needs to be removed.219
Height Outcomes and the Influence of Puberty
Early initiation of therapy, combined with careful attention to dosage adjustments and compliance, is the best predictor of cumulative growth response in patients with GHD. Final height correlates with height at the initiation of puberty, so it is important to maximize growth during the prepubertal period, within the limits of safety and economy.220–225 Analysis of data on final heights of rhGH-treated GHD is complicated by the heterogeneity of patient groups and dosage, but the most common observation is a general failure of children to reach their full genetic height potential, especially in the case of IGHD and particularly in females. Price and Ranke226 have reported final heights of −1.26 SD and −1.45 SD from the mean in males and females, respectively, with IGHD, and −0.22 SD and −0.52 SD from the mean in males and females, respectively, with CPHD. In patients with longer durations of treatment and higher dosages of rhGH, adult heights tended to be greater, although still failing to achieve full genetic height potential.
It is clear that the timing of puberty has a significant impact on adult height of rhGH-treated GHD.223–225 The duration of rhGH treatment and the height gained prepubertally are typically greater when puberty is induced rather than spontaneous. In the study by Ranke and colleagues,227 final height was attained at 17.8 and 19.2 years in boys and at 16.0 and 17.0 years in girls following spontaneous and induced puberty, respectively. Final heights were greater after induced puberty compared with spontaneous puberty in boys (171.3 versus 166.0 cm) and in girls (157.0 versus 155.0 cm). Therapy designed at delaying the onset of puberty (both normal and precocious) may augment the cumulative growth response to rhGH.
Burns et al.224 reported that final height in GHD patients who enter puberty spontaneously is less than in patients in whom puberty needs to be induced because of gonadotropin deficiency. Final height gain can be particularly variable in children who have had treatment for malignancies. The GHD is often complicated by skeletal damage following TBI or craniospinal irradiation, early puberty, hypothyroidism, gonadotropin deficiency, malnutrition, and concomitant chemotherapy. Gonadotropin releasing hormone analogue (GnRHa) therapy to arrest early puberty has been used in conjunction with GH treatment in this group of patients, with encouraging results. The use of GnRHa reduces the concentration of sex steroid, with a consequent delay in epiphyseal fusion. However, the use of GH and GnRHa combination therapy in children with GHD228 is not widely used at present. It may be beneficial under certain circumstances—for example, where the diagnosis of GHD has been delayed. The effects of GnRHa in the long term are unknown; additionally, the cost of this combination therapy would need to be weighed against the benefits.
Although considerable attention has been paid to growth, it is important to realize that GH treatment in childhood can also normalize body composition, with a reduction in body fat, although effects on lean body mass are less evident. It is also associated with reversible insulin insensitivity and an increase in the ratio of high-density lipoprotein (HDL) to total cholesterol. Glomerular filtration rate (GFR) is increased and bone remodeling accelerated, with a marked increase in bone mineral mass.229
Adverse Effects of Human Growth Hormone
Treatment with rhGH has had an excellent safety record.230,231 Reports of anti-GH antibodies in patients receiving rhGH have been few, with no untoward effect on the growth response. Fluid retention and carpal tunnel syndrome are observed in adults but are seldom significant in children. Whereas the incidence of type 1 diabetes mellitus is not higher in patients with idiopathic GHD than in the general population, the incidence of type 2 diabetes mellitus is greater in those patients treated with GH, although this tends to be in those predisposed to developing the condition.232 Idiopathic intracranial hypertension (pseudotumor cerebri) has been observed occasionally but resolves with cessation of treatment and then a gradual reintroduction of rhGH but commencing at a lower dose.231 Slipped capital femoral epiphysis has been reported, but it is not clear that the incidence is greater than that observed in normal children during rapid periods of growth. Other possible rare and still unproven complications include acute pancreatitis, increased growth of pigmented nevi, and prepubertal gynecomastia.
The most important theoretical risk that has been raised with rhGH therapy has been that of malignancy.230,231,233–239 Epidemiologic assessment has been complicated by the fact that many rhGH recipients are at increased risk of malignancy because of chromosomal abnormalities, prior malignancies, or prior histories of chemotherapy or irradiation. Additionally, it has been suggested that in some cases, GH deficiency itself may predispose to development of malignancy. Although a number of early reports suggested a link between hGH treatment and leukemia, Blethen and colleagues230 reported that an analysis of 47,000 patient-years of treatment in greater than 19,000 children indicated that in children without known risk factors, rhGH therapy was not associated with an increased occurrence of tumors or recurrence rate of leukemia or CNS tumors. Despite these assurances, it is customary to delay rhGH treatment in children with treated brain tumors until they have been shown to be tumor free for at least 1 to 2 years.
More recently, the long-term follow up of these patients has revealed a higher than expected incidence and mortality of colonic cancer and Hodgkin’s disease.239 However, these data need to be put in context. The affected patients had been treated with high GH doses given two to three times a week. Hence one could speculate that the IGF-I concentrations generated by this mode of GH therapy may be excessive. IGF-I concentrations at the upper end of the normal range (top quartile for colon and prostate and top tertile for breast) have been associated with colon, breast, and prostatic cancer.240–243 Given that lower doses of GH are given on a daily basis, it would be incorrect—at this stage at least—to extrapolate the data from the earlier studies to the present treatment regimens.
Growth Hormone–Releasing Hormone
In children whose GHD is due to a hypothalamic abnormality, treatment with GHRH would appear to be an appropriate therapeutic option.244,245 Both GHRH1-44 and GHRH1-29 are biologically active in humans. Unfortunately, although GHRH can be absorbed nasally, this route of administration has not proved to be effective,246 and treatment must be via subcutaneous or intramuscular injection, as is the case with rhGH. Direct comparisons of rhGH and GHRH have not been performed, but a number of studies indicate that GHRH administered once or twice daily can increase the growth rates of children with GHD. No specific therapeutic advantage of GHRH over rhGH has been demonstrated to date, although further studies are warranted to investigate optimization of dosage and frequency of administration.
Growth Hormone–Releasing Peptides and Nonpeptidyl Growth Hormone Secretagogues
Since the discovery of growth hormone–releasing peptides (GHRPs) by Bowers et al. in the 1980s,14 a variety of small GHRPs and nonpeptidyl small-molecule GH secretagogues have been manufactured.247 These molecules are potent stimulators of GH release, especially when administered together with GHRH, and may be active when administered by intravenous, intramuscular, subcutaneous, nasal, and oral routes. These potential advantages must be balanced by the likelihood that normal GHRH production is required to see maximal benefit from such agents.248,249 Clinical trials have proven disappointing however.
Transition To Adult Care
It has been suggested that at the end of statural growth, GH secretion should be reassessed in all patients after a wash-out period of at least 1 to 3 months.250–252 The investigation of choice is an insulin tolerance test (ITT),253 although the arginine + GHRH test has recently been proposed as a safer alternative, particularly in patients who have a contraindication to an ITT.254 For between 25% to 75% of patients, the GH response to provocation is in the normal range, as would be expected from the previous discussion on testing, and probability theory needs to be employed to determine whether therapy should be continued or not.255 In the remainder, continuation of GH therapy should be considered in those with a peak GH less than 3 µg/L, who can be described as having severe GHD. Patients with a peak GH between 3 and 7 µg/L have moderate GHD and should be followed up by an adult endocrinologist. In these individuals, adverse changes in body composition, quality of life, and bone mineral density may be an indication to recommence GH treatment,256 although it is less likely that these individuals will develop adult GHD syndrome.255 In those patients with multiple pituitary hormone deficiency, GHD due to a congenital lesion, and GHD secondary to radiotherapy, surgery, or a mass lesion, the GHD is highly unlikely to reverse.257
References
1. Karlberg, J. On the modelling of human growth. Stat Med. 1987;6:185–192.
2. Hindmarsh, PC, Smith, PJ, Brook, CGD, et al. The relationship between growth velocity and growth hormone secretion in short prepubertal children. Clin Endocrinol. 1987;27:581–591.
3. Rosenfeld, RG. Disorders of growth hormone/IGF secretion and action. In: Sperling M, ed. Pediatric Endocrinology. Philadelphia: WB Saunders; 1996:117–169.
4. White, P. Diabetes in Childhood and Adolescence. Philadelphia: Lea and Febiger; 1932.
5. Raben, MS. Treatment of a pituitary dwarf with human growth hormone. J Clin Endocrinol Metab. 1958;18:901–903.
6. Soyka, ZF, Ziskind, A, Crawford, JD. Treatment of short stature in children and adolescents with human pituitary growth hormone (Raben). Experience with 35 cases. N Engl J Med. 1964;271:754–764.
7. Prader, A, Zachmann, M, Poley, JR, et al. Long term treatment with human growth hormone (Raben) in small doses. Evaluation of 18 hypopituitary patients. Helv Paediatr Acta. 1967;22:423–439.
8. Tanner, JM, Whitehouse, RH, Hughes, PCR, et al. Effect of human growth hormone treatment for 1 to 7 years on growth of 100 children with growth hormone deficiency, low birth weight, inherited smallness, Turner Syndrome and other complaints. Arch Dis Child. 1971;45:745–779.
9. Aceto, T, Frasier, SD, Hayles, AB, et al. Collaborative study of the effects of human growth hormone in growth hormone deficiency. 1. First year of therapy. J Clin Endocrinol Metab. 1972;35:483–496.
10. Hunter, WM, Greenwood, FC. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962;194:495–496.
11. Brazeau, P, Vale, W, Burgus, R, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;178:77–79.
12. Guillemin, R, Brazeau, P, Bohien, P, et al. Growth hormone releasing factor from a human pancreatic tumour that caused acromegaly. Science. 1982;218:585–587.
13. Rivier, J, Spiess, J, Thorner, MO, et al. Characterisation of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature. 1982;300:276–278.
14. Bowers, CY, Momany, F, Reynolds, GA, et al. Structure-activity relationships of a synthetic pentapeptide that specifically releases growth hormone in vitro. Endocrinology. 1980;106:663–667.
15. Salmon, WD, Daughaday, WH. A hormonally controlled serum factor which stimulates sulphate incorporation by cartilage in vivo. J Lab Clin Med. 1957;49:825–836.
16. Zezulak, KM, Green, H. The generation of insulin-like growth factor 1 sensitive cells by growth hormone action. Science. 1986;233:551–553.
17. Isgaard, J, Nilsson, A, Lindahl, A, et al. Effects of local administration of GH and IGF-1 on longitudinal bone growth in rats. Am J Physiol. 1986;250:E367–E372.
18. Parkin, JM. Incidence of growth hormone deficiency. Arch Dis Child. 1974;49:904–905.
19. Vimpani, GV, Vimpani, AF, Lidgard, GP, et al. Prevalence of severe growth hormone deficiency. BMJ. 1977;2:427–430.
20. Lindsay, R, Feldkamp, M, Harris, D, et al. Utah Growth Study. Growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125:29–35.
21. Wilton, P. Progress in Growth Hormone Therapy—5 Years of KIGS. Mannheim, Germany: JJ Verlag; 1994.
22. Genentech National Cooperative Growth Study Summary Report 18. San Francisco, 1994, Genentech, pp 6–13.
23. Dasen, JS, Rosenfeld, MG. Signaling and transcriptional mechanisms in pituitary development. Annu Rev Neurosci. 2001;24:327–355.
24. Takuma, N, Sheng, HZ, Furuta, Y, et al. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840.
25. Ericson, J, Norlin, S, Jessell, TM, et al. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015.
26. Lazzaro, D, Price, M, De Felice, M, et al. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104.
27. Sheng, HZ, Moriyama, K, Yamashita, T, et al. Multistep control of pituitary organogenesis. Science. 1997;278:1809–1812.
28. Treier, M, Gleiberman, AS, O’Connell, SM, et al. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704.
29. Rosenfeld, MG, Briata, P, Dasen, J, et al. Multistep signaling and transcriptional requirements for pituitary organogenesis in vivo. Recent Prog Horm Res. 2000;55:1–13.
30. Sheng, HZ, Westphal, H. Early steps in pituitary organogenesis. Trends Genet. 1999;15:236–240.
31. Simmons, DM, Voss, JW, Ingraham, HA, et al. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990;4:695–711.
32. Japon, MA, Rubinstein, M, Low, MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem. 1994;42:1117–1125.
33. Li, S, Crenshaw, EB, III., Rawson, EJ, et al. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533.
34. Lamolet, B, Pulichino, AM, Lamonerie, T, et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104(6):849–859.
35. Dattani, MT, Robinson, ICAF. The molecular basis for developmental disorders of the pituitary gland in man. Clin Genet. 2000;57:337–346.
36. Cohen, LE, Wondisford, FE, Salvatoni, A, et al. A “hot spot” in the PIT1 gene responsible for combined pituitary hormone deficiency: clinical and molecular correlates. J Clin Endocrinol Metab. 1995;80:679–684.
37. Cohen, LE, Radovick, S. Molecular basis of combined pituitary hormone deficiencies. Endocr Rev. 2002;23:431–442.
38. Dattani, MT, Martinez-Barbera, JP, Thomas, PQ, et al. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet. 1998;19:125–133.
39. Thomas, PQ, Dattani, MT, Brickman, JM, et al. Heterozygous HESX1 mutations associated with isolated congenital pituitary hypoplasia and septo-optic dysplasia. Hum Mol Genet. 2001;10:39–45.
40. Carvalho, LR, Woods, KS, Mendonca, BB, et al. A homozygous mutation in HESX1 is associated with evolving hypopituitarism due to impaired repressor-corepressor interaction. J Clin Invest. 2003;112(8):1192–1201.
41. Netchine, I, Sobrier, ML, Krude, H, et al. Mutations in LHX3 result in a new syndrome revealed by combined pituitary hormone deficiency. Nat Genet. 2000;25:182–186.
42. Machinis, K, Pantel, J, Netchine, I, et al. Syndromic short stature in patients with a germline mutation in the LIM homeobox LHX4. Am J Hum Genet. 2001;69:961–968.
43. Kelberman, D, Rizzoti, K, Avilion, A, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116(9):2442–2455.
44. Kelberman, D, de Castro, SC, Huang, S, et al. SOX2 plays a critical role in the pituitary, forebrain and eye during human embryonic development. J Clin Endocrinol Metab. 2008;93(5):1865–1873.
45. Laumonnier, F, Ronce, N, Hamel, BC, et al. Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am J Hum Genet. 2002;71:1450–1455.
46. Wajnrajch, MP, Gertner, JM, Harbison, MD, et al. Nonsense mutations of the human growth hormone releasing hormone receptor (GHRHR) causes growth failure analogous to that of the little (lit) mouse. Nat Genet. 1996;12:88–90.
47. Baumann, G, Maheshwari, H. Severe growth hormone (GH) deficiency caused by a mutation in the GH-releasing hormone receptor gene. Acta Paediatr Suppl. 1997;423:33–38.
48. Salvatori, R, Hagashida, CY, Aguiar-Olivera, MH, et al. Familial dwarfism due to a novel mutation of the growth hormone–releasing hormone receptor gene. J Clin Endocrinol Metab. 1999;84:917–923.
49. Godfrey, P, Rahal, JO, Beamer, WG, et al. GHRH receptor of little mouse contains missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993;4:227–432.
50. Sornson, MW, Wu, W, Daser, JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333.
51. Wu, W, Cogan, JD, Pfaffle, RW, et al. Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat Genet. 1998;18:147–149.
52. Deladoey, J, Fluck, C, Buyukgebiz, A, et al. “Hot spot” in the PROP1 gene responsible for combined pituitary hormone deficiency. J Clin Endocrinol Metab. 1999;84:1645–1650.
53. Pernasetti, F, Toledo, SP, Vasilyev, VV, et al. Impaired adrenocorticotropin-adrenal axis in combined pituitary hormone deficiency caused by a two-base pair deletion (301–302delAG) in the prophet of PIT1 gene. J Clin Endocrinol Metab. 2000;85:390–397.
54. Mendonca, BB, Osorio, MG, Latronico, AC, et al. Longitudinal hormonal and pituitary imaging changes in two females with combined pituitary hormone deficiency due to deletion of A301,G302 in the PROP1 gene. J Clin Endocrinol Metab. 1999;84:942–945.
55. Bodner, M, Castrillo, J-L, Theill, LE, et al. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988;55:505–518.
56. Mangalam, HJ, Albert, VR, Ingraham, HA, et al. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989;3:946–958.
57. Li, S, Crenshaw, EB, Rawson, EJ, et al. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene. Nature. 1990;347:528–533.
58. Radovick, S, Nations, M, Du, Y, et al. A mutation in the POV-homeodomain of Pit-1 responsible for combined pituitary hormone deficiency. Science. 1992;257:1115–1118.
59. Pfaffle, R, Kim, C, Otten, B, et al. Pit-1: Clinical aspects. Horm Res. 1996;45(suppl 1):25–28.
60. Pernasetti, F, Milner, RDG, Al Ashwal, AAL, et al. Pro239Ser: A novel recessive mutation of the PIT1 gene in seven Middle Eastern children with growth hormone, prolactin, and thyrotropin deficiency. J Clin Endocrinol Metab. 1998;83:2079–2083.
61. Turton, JP, Reynaud, R, Mehta, A, et al. Novel mutations within the POU1F1 gene associated with variable Combined Pituitary Hormone Deficiency (CPHD). J Clin Endocrinol Metab. 2005;90(8):4762–4770.
62. Procter, A, Phillips, JA, III., Cogan, J. The molecular genetics of growth hormone deficiency. Hum Genet. 1998;103:255–272.
63. Wagner, JK, Eble, A, Hindmarsh, PC, et al. Prevalence of human GH1 gene alterations in patients with isolated growth hormone deficiency. Pediatr Res. 1998;43:105–110.
64. Illig, R, Prader, A, Ferrandez, A, et al. Hereditary prenatal growth hormone deficiency with increased tendency to growth hormone antibody formation (“A-type” of isolated growth hormone deficiency). Acta Paediatr Scand. 1971;60:607.
65. Phillips, JA, III., Cogan, J. Genetic basis of endocrine disease. 6. Molecular basis of familial human growth hormone deficiency. J Clin Endocrinol Metab. 1994;78:11–16.
66. Moseley, C, Mullis, P, Prince, M, et al. An exon splice enhancer mutation causes autosomal dominant GH deficiency. J Clin Endocrinol Metab. 2002;87:847–852.
67. McGuiness, L, Magoulas, C, Mathers, K, et al. Autosomal dominant growth hormone deficiency disrupts secretory vesicles: in vitro and in vivo studies in transgenic mice. Endocrinology. 2003;144:720–731.
68. Ryther, RCC, McGuiness, LM, Phillips, JA, III., et al. Disruption of exon definition produces a dominant-negative growth hormone isoform that causes somatotroph death and IGHD II. Hum Genet. 2003;113:140–148.
69. Mullis, PE, Robinson, IC, Salemi, S, et al. Isolated autosomal dominant growth hormone deficiency: an evolving pituitary deficit? A multicenter follow-up study. J Clin Endocrinol Metab. 2005;90(Apr (4)):2089–2096.
70. Solomon, NM, Nouri, S, Warne, GL, et al. Increased gene dosage at Xq26-q27 is associated with X-linked hypopituitarism. Genomics. 2002;79(Apr (4)):553–559.
71. Woods, KS, Cundall, M, Turton, J, et al. Over- and underdosage of SOX3 is associated with infundibular hypoplasia and hypopituitarism. American Journal of Human Genetics. 2005;76(May (5)):833–849.
72. Kowarski, AA, Schneider, J, Ben-Galim, E, et al. Growth failure with normal serum RIA-GH and low somatomedin activity: Somatomedin restoration and growth acceleration after exogenous GH. J Clin Endocrinol Metab. 1978;47:461–464.
73. Takahashi, Y, Shirono, H, Arisaka, O, et al. Biologically inactive growth hormone caused by an amino acid substitution. J Clin Invest. 1997;100:1159–1165.
74. Fujisawa, I, Kikuchi, K, Nishimura, K, et al. Transection of the pituitary stalk: Development of an ectopic posterior lobe assessed with MR imaging. Radiology. 1987;165:487–489.
75. Abrahams, JJ, Trefelner, E, Boulware, SD. Idiopathic growth hormone deficiency MR findings in 35 patients. Am J Neuroradiol. 1991;12:155–160.
76. Cacciari, E, Zucchini, S, Carla, G, et al. Endocrine function and morphological findings in patients with disorders of the hypothalamo-pituitary area: A study with magnetic resonance. Arch Dis Child. 1990;65:1199–1202.
77. Maghnie, M, Larizza, D, Triulzi, F, et al. Hypopituitarism and stalk agenesis: A congenital syndrome worsened by breech delivery? Horm Res. 1991;35:104–108.
78. Root, AW, Martinez, CR. Magnetic resonance imaging in patients with hypopituitarism. Trends Endocrinol Metab. 1992;3:283–287.
79. Argyopoulou, M, Perignon, F, Brauner, R, et al. Magnetic resonance imaging in the diagnosis of growth hormone deficiency. J Pediatr. 1992;120:886–891.
80. Triulzi, F, Scotti, G, diNatale, B, et al. Evidence of a congenital midline brain anomaly in pituitary dwarfs: A magnetic resonance imaging study in 101 patients. Pediatrics. 1994;93:409–416.
81. Wilkinson, IA, Duck, SC, Gager, WE, et al. Empty sella syndrome. Occurrence in childhood. Am J Dis Child. 1982;136:245–248.
82. Albertsson-Wikland, K, Niklasson, A, Karlberg, P. Birth data for patients who later develop growth hormone deficiency: Preliminary analysis of a national register. Acta Paediatr Suppl. 1990;370:115–120.
83. Craft, WH, Underwood, LE, Van Wyk, JJ. High incidence of perinatal insult in children with idiopathic hypopituitarism. J Pediatr. 1980;96:397–402.
84. DeVile, CJ, Grant, DB, Hayward, RD, et al. Growth and endocrine sequelae of craniopharyngioma. Arch Dis Child. 1996;75:108–114.
85. Tiulpakov, AN, Mazerkina, NA, Brook, CG, et al. Growth in children with craniopharyngioma following surgery. Clin Endocrinol (Oxf). 1998;49:733–738.
86. Howarth, DM, Gilchrist, GS, Mullan, BP, et al. Langerhans cell histiocytosis: diagnosis, natural history, management, and outcome. Cancer. 1999;85(10):2278–2290.
87. Nanduri, VR, Bareille, P, Pritchard, J, et al. Growth and endocrine disorders in multisystem Langerhans’ cell histiocytosis. Clin Endocrinol (Oxf). 2000;53:509–515.
88. Donadieu, J, Rolon, MA, Thomas, C, et al. Endocrine involvement in pediatric-onset Langerhans’ cell histiocytosis: a population-based study. J Pediatr. 2004;144(3):344–350.
89. Kaltsas, GA, Powles, TB, Evanson, J, et al. Hypothalamo-pituitary abnormalities in adult patients with Langerhans cell histiocytosis: clinical, endocrinological, and radiological features and response to treatment. J Clin Endocrinol Metab. 2000;85(4):1370–1376.
90. Ottaviano, F, Finlay, JL. Diabetes insipidus and Langerhans cell histiocytosis: a case report of reversibility with 2-chlorodeoxyadenosine. J Pediatr Hematol Oncol. 2003;25(7):575–577.
91. Dhall, G, Finlay, JL, Dunkel, IJ, et al. Analysis of outcome for patients with mass lesions of the central nervous system due to Langerhans cell histiocytosis treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2008;50(1):72–79.
92. Morimoto, A, Ikushima, S, Kinugawa, N, et al. Improved outcome in the treatment of pediatric multifocal Langerhans cell histiocytosis: Results from the Japan Langerhans Cell Histiocytosis Study Group-96 protocol study. Cancer. 2006;107(3):613–619.
93. Ogilvy-Stuart, AL, Clark, DJ, Wallace, WH, et al. Endocrine deficit after fractionated total body irradiation. Arch Dis Child. 1992;67:1107–1110.
94. Rappaport, R, Brauner, R. Growth and endocrine disorders secondary to cranial irradiation. Pediatr Res. 1989;25:561–567.
95. Sklar, C, Mertens, A, Walter, A, et al. Final height after treatment for childhood acute lymphoblastic leukemia: Comparison of no cranial irradiation with 1800 and 2400 centigrays of cranial irradiation. J Pediatr. 1993;123:56–64.
96. Balthasar, N, Mery, PF, Magoulas, CB, et al. Growth hormone-releasing hormone (GHRH) neurons in GHRH-enhanced green fluorescent protein transgenic mice: a ventral hypothalamic network. Endocrinology. 2003;144:2728–2740.
97. Blatt, J, Bercu, BB, Gillin, JC, et al. Reduced pulsatile growth hormone secretion in children after therapy for acute lymphoblastic leukemia. J Pediatr. 1984;104:182–186.
98. Sklar, CA, Sarafoglou, K, Whittam, E. Effects of insulin-like growth factor binding protein 3 in predicting the growth hormone response to provocative testing in children treated with cranial irradiation. Acta Endocrinol. 1993;129:511–515.
99. Quigley, C, Cowell, C, Jimenez, M, et al. Normal or early development of puberty despite gonadal damage in children treated for acute lymphoblastic leukemia. N Engl J Med. 1989;321:143–151.
100. Acerini, CL, Tasker, RC, Bellone, S, et al. Hypopituitarism in childhood and adolescence following traumatic brain injury: the case for prospective endocrine investigation. Eur J Endocrinol. 2006;155(5):663–669.
101. Oertel, M, Boscardin, WJ, Obrist, WD, et al. Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. J Neurosurg. 2005;103(5):812–824.
102. Agha, A, Thompson, CJ. Anterior pituitary dysfunction following traumatic brain injury (TBI). Clin Endocrinol (Oxf). 2006;64(5):481–488.
103. Tanriverdi, F, Senyurek, H, Unluhizarci, K, et al. High risk of hypopituitarism after traumatic brain injury: a prospective investigation of anterior pituitary function in the acute phase and 12 months after trauma. J Clin Endocrinol Metab. 2006;91(6):2105–2111.
104. Niederland, T, Makovi, H, Gal, V, et al. Abnormalities of pituitary function after traumatic brain injury in children. J Neurotrauma. 2007;24(1):119–127.
105. Einaudi, S, Bondone, C. The effects of head trauma on hypothalamic-pituitary function in children and adolescents. Curr Opin Pediatr. 2007;19(4):465–470.
106. Ghigo, E, Masel, B, Aimaretti, G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19(9):711–724.
107. Bartsocas, CS, Pantelakis, SN. Human growth hormone therapy in hypopituitarism due to tuberculous meningitis. Acta Paediatr Scand. 1973;62:304–306.
108. Stuart, CA, Neelon, FA, Lebovitz, HE. Hypothalamic insufficiency: The cause of hypopituitarism in sarcoidosis. Ann Intern Med. 1978;88:589–594.
109. Rund, D, Rachmilewitz, E. Beta-thalassemia. N Engl J Med. 2005;353(11):1135–1146.
110. Fung, EB, Harmatz, PR, Lee, PD, et al. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006;135(4):574–582.
111. Powell, GF, Brasel, JA, Blizzard, RM. Emotional deprivation and growth retardation simulating idiopathic hypopituitarism. N Engl J Med. 1967;276:1271–1278.
112. Skuse, D, Albanese, A, Stanhope, R. A new stress-related syndrome of growth failure and hyperphagia in children, associated with reversibility of growth-hormone insufficiency. Lancet. 1996;348:353–358.
113. Albanese, A, Hamill, G, Jones, J, et al. Reversibility of physiological growth hormone secretion in children with psychosocial dwarfism. Clin Endocrinol. 1994;40:687–692.
114. Hindmarsh, PC, Matthews, DR, Brook, CGD. Growth hormone secretion in children determined by time series analysis. Clin Endocrinol (Oxf). 1988;29:35–44.
115. Jaffe, CA, Ocampo-Lim, B, Guo, W, et al. Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest. 1998;102:153–164.
116. Papotti, M, Ghe, C, Cassoni, P, et al. Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab. 2000;85:3803–3807.
117. Gnanapavan, S, Kola, B, Bustin, SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988.
118. Kojima, M, Hosoda, H, Date, Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660.
119. Smith, RG, Palyha, OC, Feighner, SD, et al. Growth hormone releasing substances: types and their receptors. Horm Res. 1999;51(Suppl 3)):1–8.
120. Bennani-Baiti, IM, Asa, SL, Song, D, et al. DNase-I hypersensitive sites I and II of the human growth hormone locus control region are a major developmental activator of somatotrope gene expression. Proc Natl Acad Sci U S A. 1998;95:10655–10660.
121. Shewchuk, BM, Asa, SL, Cooke, NE, et al. Pit-1 binding sites at the somatotrope-specific DNase-I hypersensitive sites I, II of the human growth hormone locus control region are essential for in-vivo hGH-N gene activation. J Biol Chem. 1999;274:35725–35733.
122. Baumann, G. Growth hormone heterogeneity: genes, isohormones, variants, and binding proteins. Endocr Rev. 1991;12:424–449.
123. Clark, RG, Mortensen, DL, Carlsson, LM, et al. Recombinant human growth hormone (GH)-binding protein enhances the growth-promoting activity of human GH in the rat. Endocrinology. 1996;137:4308–4315.
124. Carter-Su, C, Schwartz, J, Smit, LS. Molecular mechanism of growth hormone action. Annu Rev Physiol. 1996;58:187–207.
125. Smit, LS, Meyer, DJ, Billestrup, N, et al. The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol Endocrinol. 1996;10:519–533.
126. Rosenfeld, RG, Rosenbloom, AL, Guevara-Aguirre, J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocr Rev. 1994;15:369–390.
127. Rosenfeld, RG, Rosenbloom, AL, Guevara-Aguirre, J. Abnormalities of growth hormone action. In: Kelnar CJH, Savage MO, Stirling HF, et al, eds. Growth Disorders. Pathophysiology and Treatment. London: Chapman & Hall; 1998:549–564.
128. Woods, KA, Dastot, F, Preece, MA, et al. Phenotype-genotype relationships in growth hormone insensitivity syndrome. J Clin Endocrinol Metab. 1997;82:3529–3535.
129. Rygaard, K, Revol, A, Esquivel-Escobedo, D, et al. Absence of human placental lactogen and placental growth hormone (hGH-V) during pregnancy: PCR analysis of the deletion. Hum Genet. 1998;102:87–92.
130. DeChiara, TM, Efstratiadis, A, Robertson, EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80.
131. Liu, JP, Baker, J, Perkins, AS, et al. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75:73–82.
132. Goossens, M, Brauner, R, Czernichow, P, et al. Isolated growth hormone deficiency Type 1A associated with a double deletion in the human growth hormone gene cluster. J Clin Endocrinol Metab. 1986;62:712–716.
133. Wit, JM, Van Unen, H. Growth of infants with neonatal growth hormone deficiency. Arch Dis Child. 1982;67:920–924.
134. Gluckman, PD, Gunn, A-J, Wray, A. Congenital idiopathic growth hormone deficiency associated with early postnatal growth failure. J Pediatr. 1992;121:920–923.
135. Wit, JM, Kamp, G, Rikken, B. Spontaneous growth and response to growth hormone treatment in children with growth hormone deficiency and idiopathic short stature. Pediatr Res. 1996;39:295–302.
136. Rimoin, DL, Merimee, TJ, Rabinowitz, D, et al. Genetic aspects of clinical endocrinology. Recent Prog Horm Res. 1968;24:365–437.
137. van der Werff ten Bosch, JJ, Bot, A. Growth of males with idiopathic hypopituitarism without growth hormone treatment. Clin Endocrinol (Oxf). 1990;32:707–717.
138. Milner, RDG, Russell-Fraser, T, Brook, CGD, et al. Experience with human growth hormone in Great Britain: the report of the MRC working Party. Clin Endocrinol. 1979;11:15–38.
139. Vanderschuren-Lodeweyckx, M, Wolter, R, Mulla, A, et al. Plasma growth hormone in coeliac disease. Acta Pediatr (Helv). 1973;28:349–357.
140. Rosenfeld, RG, Albertsson-Wikland, K, Cassorla, F, et al. The diagnosis of childhood growth hormone deficiency revisited. J Clin Endocrinol Metab. 1995;80:1532–1540.
141. Hussain, K, Hindmarsh, P, Aynsley_Green, A. Spontaneous hypoglycemia in childhood is accompanied by paradoxically low serum growth hormone and appropriate cortisol counterregulatory hormonal responses. J Clin Endocrinol Metab. 2003;88:3715–3723.
142. Iranmanesh, A, Lizaralde, G, Veldhuis, JD. Age and relative adiposity are specific negative determinants of the pregnancy and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73:1081–1088.
143. Freeman, JV, Cole, TJ, Chinn, S, et al. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73:17–24.
144. Blum, WF, Ranke, MB, Kietzmann, K, et al. A specific radioimmunoassay for the growth hormone (GH)-dependent somatomedin-binding protein: its use for diagnosis of GH deficiency. J Clin Endocrinol Metab. 1990;70:1292–1298.
145. Tillman, V, Buckler, JM, Kibirge, MS, et al. Biochemical tests in the diagnosis of childhood growth hormone deficiency. J Clin Endocrinol Metab. 1997;82:531–535.
146. Mitchell, H, Dattani, MT, Nanduri, V, et al. Failure of IGF-1 and IGFBP-3 to diagnose growth hormone insufficiency. Arch Dis Child. 1999;80:443–447.
147. Sox, HC, Jr. Probability theory in the use of diagnostic tests. Ann Int Med. 1986;104:60–66.
148. Suri, D, Hindmarsh, PC, Matthews, DR, et al. The pituitary gland is capable of responding to two successive doses of growth hormone–releasing hormone (GHRH). Clin Endocrinol. 1991;34:13–17.
149. Rahim, A, Toogood, A, Shalet, SM. The assessment of growth hormone status in normal young adult males using a variety of provocative tests. Clin Endocrinol. 1996;45:557–562.
150. Tauber, M, Houlin, P, Pienkowski, C, et al. Growth Hormone (GH) retesting and auxological data in 131 GH-deficient patients after completion of treatment. J Clin Endocrinol Metab. 1997;82:352–356.
151. Thomas, GB, Robinson, ICAF. Central regulation of growth hormone secretion. In: Kelnar CJH, Savage MO, Stirling HF, et al, eds. Growth Disorders. Pathophysiology and Treatment. London: Chapman & Hall; 1998:99–125.
152. Martha, PM, Jr., Rogol, AD, Veldhuis, JD, et al. Alterations in the pulsatile properties of circulating growth hormone concentrations during puberty in boys. J Clin Endocrinol Metab. 1989;69:563–570.
153. Martha, PM, Gorman, KM, Blizzard, RM, et al. Endogenous growth hormone secretion and clearance rates in normal boys as determined by deconvolution analysis: Relationship to age, pubertal status and body mass. J Clin Endocrinol Metab. 1992;74:336–344.
154. Spiliotis, BE, August, GP, Hung, W, et al. Growth hormone neurosecretory dysfunction: A treatable cause of short stature. JAMA. 1984;252:2223–2230.
155. Bercu, BB, Shulman, D, Root, AW, et al. Growth hormone (GH) provocative testing frequently does not reflect endogenous GH secretion. J Clin Endocrinol Metab. 1986;86:709–716.
156. Rose, SR, Ross, JL, Uriarte, M, et al. The advantage of measuring stimulated as compared with spontaneous growth hormone levels in the diagnosis of growth hormone deficiency. N Engl J Med. 1988;319:201–207.
157. Lanes, R. Diagnostic limitations of spontaneous growth hormone measurements in normally growing prepubertal children. Am J Dis Child. 1989;143:1284–1286.
158. Donaldson, DL, Hollowell, JG, Pan, F, et al. Growth hormone secretory profiles: Variation on consecutive nights. J Pediatr. 1989;115:51–56.
159. Hourd, P, Edwards, R. Current methods for the measurement of growth hormone in urine. Clin Endocrinol (Oxf). 1994;40:155–170.
160. Albini, CH, Quattrin, T, Vandlen, RL, et al. Quantitation of urinary growth hormone in children with normal and subnormal growth. Pediatr Res. 1988;23:89–92.
161. Granada, ML, Sanmarti, ALA, et al. Clinical usefulness of urinary growth hormone measurements in normal and short children according to different expressions of urinary growth hormone data. Pediatr Res. 1992;32:73–76.
162. Phillip, M, Chalew, SA, Stene, MA, et al. The value of urinary growth hormone determination for assessment of growth hormone deficiency and compliance with growth hormone therapy. Am J Dis Child. 1993;147:553–557.
163. Skinner, AM, Clayton, PE, Price, DA, et al. Urinary growth hormone excretion in the assessment of children with disorders of growth. Clin Endocrinol (Oxf). 1993;39:201–206.
164. Underwood, LE, Azumi, K, Voina, SJ, et al. Growth hormone levels during sleep in normal and growth hormone deficient children. Pediatrics. 1971;48:946–954.
165. Buckler, JMH. Plasma growth hormone response to exercise as a diagnostic aid. Arch Dis Child. 1973;48:565–567.
166. Lacey, KA, Hewison, A, Parkin, JM. Exercise as a screening test for growth hormone deficiency in children. Arch Dis Child. 1973;48:508–512.
167. Coller, R, Leboeuf, G, Letarte, J. Stimulation of growth hormone secretion by levodopa propranolol in children and adolescents. Pediatrics. 1975;56:262–266.
168. Youlton, R, Kaplan, SL, Grumbach, MM. Growth and growth hormone. IV limitations of the growth hormone response to insulin and arginine in the assessment of growth hormone deficiency in children. Pediatrics. 1969;43:989–1004.
169. Lanes, R, Hurtado, E. Oral clonidine: an effective growth hormone–releasing agent in prepubertal subjects. J Pediatr. 1982;100:710–714.
170. Mitchell, ML, Bryne, MJ, Sanchez, Y, et al. Detection of growth deficiency. The glucagon simulation test. N Engl J Med. 1970;282:539–541.
171. Merimee, TJ, Rabinowitz, D, Fineberg, SE. Arginine-initiated release of human growth hormone. N Engl J Med. 1969;280:1434–1438.
172. Fass, B, Lippe, BM, Kaplan, SA. Relative usefulness of three growth hormone stimulation screening tests. Am J Dis Child. 1979;133:931–933.
173. Weldon, VV, Gupta, SK, Klingensmith, G. Evaluation of growth hormone release in children using arginine and L-dopa in combination. J Pediatr. 1975;87:540–544.
174. Reiter, EO, Martha, PM, Jr. Pharmacological testing of growth hormone secretion. Horm Res. 1990;33:121–127.
175. Raiti, S, Davis, WT, Blizzard, RM. A comparison of the effects of insulin hypoglycemia and arginine infusion on release of human growth hormone. Lancet. 1967;2:1182–1183.
176. Albertsson-Wikland, K, Rosberg, S. Analysis of 24-hour growth hormone profiles in children: relation to growth. J Clin Endocrinol Metab. 1988;67:493–500.
177. Lijmer, JG, Mol, BW, Heisterkamp, S, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. 1999;282:1061–1066.
178. Ware, J. The limitations of risk factors as prognostic tools. N Engl J Med. 2006;355:2615–2617.
179. Marin, G, Domene, HM, Barnes, KM, et al. The effects of estrogen priming and puberty on the growth hormone response to standardized treadmill exercise and arginine-insulin in normal girls and boys. J Clin Endocrinol Metab. 1994;79:537–541.
180. Cordido, F, Fernandez, T, Martinez, T, et al. Effect of acute pharmacological reduction of plasma free fatty acids on growth hormone (GH) releasing hormone—induced GH secretion in obese adults with and without hypopituitarism. J Clin Endocrinol Metab. 1998;83:4350–4354.
181. Lewis, UJ, Singh, RNP, Tutwiler, GH, et al. Human growth hormone: A complex of proteins. Recent Prog Horm Res. 1980;36:477–508.
182. Celniker, AC, Chem, AB, Wert, RM, Jr., et al. Variability in the quantitation of circulating growth hormone using commercial immunoassays. J Clin Endocrinol Metab. 1989;68:469–476.
183. Barth, JH, Smith, JH, Clarkson, P. Wide diversity in measurements of growth hormone after stimulation tests in short children are due to assay variability. Ann Clin Biochem. 1995;32:369–372.
184. Dattani, MT, Pringle, PJ, Hindmarsh, PC, et al. What is a normal stimulated growth hormone concentration? J Endocr. 1992;133:447–450.
185. Clemmons, DR. IGF-1 assays: Current assay methodologies and their limitations. Pituitary. 2007;10:121–128.
186. Devesa, J, Lima, L, Lois, N, et al. Reasons for the variability in growth hormone (GH) responses to GHRH challenge: the endogenous hypothalamic—somatotroph rhythm (HSR). Clin Endocrinol. 1989;30:367–377.
187. Tzanela, M, Guyda, H, Van Vliet, G, et al. Somatostatin pretreatment enhances growth hormone responsiveness to GH-releasing hormone: a potential new diagnostic approach to GH deficiency. J Clin Endocrinol Metab. 1996;81:2487–2494.
188. Bernasconi, S, Volta, C, Cozzini, A, et al. GH response to GHRH, insulin, clonidine and arginine after GHRH pretreatment in children. Acta Endocrinol. 1992;126:105–108.
189. Massoud, AF, Hindmarsh, PC, Matthews, DR, et al. The effect of repeated administration of hexarelin, a growth hormone releasing peptide, and growth hormone releasing hormone (GHRH) on growth hormone (GH) responsivity. Clin Endocrinol. 1996;44:555–562.
190. Berelowitz, M, Szabo, M, Frohman, LA, et al. Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and pituitary. Science. 1981;212:1279–1281.
191. Yamashita, S, Melmed, S. Insulin-like growth factor I action on rat anterior pituitary cells: Suppression of growth hormone secretion and messenger ribonucleic acid levels. Endocrinology. 1986;118:176–182.
192. Abe, H, Molitch, M, Van Wyk, JJ, et al. Human growth hormone and somatomedin-C suppress the spontaneous release of growth hormone in unanesthetized rats. Endocrinology. 1983;113:1319–1324.
193. Ceda, GP, Davis, WT, Rosenfeld, RG, et al. The growth hormone (GH) releasing hormone (GHRH)-GH-somatomedin axis: Evidence for rapid inhibition of GHRH-elicited GH release by insulin-like growth factors I and II. Endocrinology. 1987;120:1658–1662.
194. Saini, S, Hindmarsh, PC, Matthews, DR, et al. Reproducibility of 24hour serum growth hormone profiles in man. Clin Endocrinol. 1991;34:455–462.
195. Shriock, EA, Hulse, JA, Harris, DA, et al. Evaluation of hypothalamic dysfunction in growth hormone (GH)–deficient patients using single versus multiple doses of growth hormone–releasing hormone (GHRH-44) and evidence for diurnal variation in somatotroph responsiveness to GHRH in GH deficient patients. J Clin Endocrinol Metab. 1987;65:1177–1182.
196. Ghigo, E, Bellone, J, Aimasetti, G, et al. Reliability of provocative tests to assess growth hormone secretory status. Study in 472 normally growing children. J Clin Endocrinol Metab. 1996;81:3323–3327.
197. Frasier, SD. Human pituitary growth hormone (hGH) therapy in growth hormone deficiency. Endocr Rev. 1983;4:155–170.
198. MacGillivray, MH, Baptista, J, Johanson, A, et al. Outcome of a four year randomized study of daily versus three times weekly somatropin treatment in prepubertal naive growth hormone deficient children. J Clin Endocrinol Metab. 1996;81:1806–1809.
199. Cohen, P, Bright, GM, Rogol, AD, et al. Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J Clin Endocrinol Metab. 2002;87:90–98.
200. Pantel, J, Machinis, K, Sobrier, ML, et al. Species-specific alternative splice mimicry at the growth hormone receptor locus revealed by the lineage of retroelements during primate evolution. J Biol Chem. 2000;275:18664–18669.
201. Pantel, J, Grulich-Henn, J, Bettendorf, M, et al. Heterozygous nonsense mutation in exon 3 of the growth hormone receptor (GHR) in severe GH insensitivity (Laron syndrome) and the issue of the origin and function of the GHRd3 isoform. J Clin Endocrinol Metab. 2003;88:1705–1710.
202. Dos Santos, C, Essioux, L, Teinturier, C, et al. A common polymorphism of the growth hormone receptor is associated with increased responsiveness to growth hormone. Nat Genet. 2004;36:720–724.
203. Jorge, AA, Marchisotti, FG, Montenegro, LR, et al. Growth hormone (GH) pharmacogenetics: influence of GH receptor exon 3 retention or deletion on first-year growth response and final height in patients with severe GH deficiency. J Clin Endocrinol Metab. 2006;91:1076–1080.
204. Pilotta, A, Mella, P, Filisetti, M, et al. Common polymorphisms of the growth hormone (GH) receptor do not correlate with the growth response to exogenous recombinant human GH in GH-deficient children. J Clin Endocrinol Metab. 2006;91:1178–1180.
205. Carrascosa, A, Esteban, C, Espadero, R, et al. The d3/fl-growth hormone (GH) receptor polymorphism does not influence the effect of GH treatment (66 microg/kg per day) or the spontaneous growth in short non-GH-deficient small-for-gestational-age children: results from a two-year controlled prospective study in 170 Spanish patients. J Clin Endocrinol Metab. 2006;91:3281–3286.
206. Audi, L, Esteban, C, Carrascosa, A, et al. Exon 3-deleted/full-length growth hormone receptor polymorphism genotype frequencies in Spanish short small-for-gestational-age (SGA) children and adolescents (n = 247) and in an adult control population (n = 289) show increased fl/fl in short SGA. J Clin Endocrinol Metab. 2006;91:5038–5043.
207. Blum, WF, Machinis, K, Shavrikova, EP, et al. The growth response to growth hormone (GH) treatment in children with isolated GH deficiency is independent of the presence of the exon 3-minus isoform of the GH receptor. J Clin Endocrinol Metab. 2006;91:4171–4174.
208. Räz, B, Janner, M, Petkovic, V, et al. Influence of growth hormone (GH) receptor deletion of exon 3 and full-length isoforms on GH response and final height in patients with severe GH deficiency. J Clin Endocrinol Metab. 2008;93:974–980.
209. Beavis, WD. QTL analyses: power, precision and accuracy. In: Paterson AH, ed. Molecular Analysis of Complex Traits. New York: CRC Press; 1998:145–161.
210. Xu, S. Theoretical basis of the Beavis effect. Genetics. 2003;165:2259–2268.
211. Cohen, P, Rogol, AD, Howard, CP, et al. Insulin growth factor–based dosing of growth hormone therapy in children: a randomized controlled study. J Clin Endocrinol Metab. 2007;92:2480–2486.
212. Albertsson-Wikland, K. The effect of human growth hormone injection frequency on linear growth rate. Acta Paediatr Scand Suppl. 1987;337:110–116.
213. Hakeem, V, Hindmarsh, PC, Brook, CGD. Intermittent versus continuous administration of growth hormone treatment. Arch Dis Child. 1993;68:783–784.
214. Johnson, OL, Cleleand, TL, Lee, HJ, et al. A month-long effect from a single injection of microencapsulated human growth hormone. Nat Med. 1996;2:795–799.
215. Ranke, MB, Lindberg, A, Guilbaud, O. Prediction of growth in response to treatment with growth hormone. In: Ranke MB, Gunnarsson R, eds. Progress in Growth Hormone Therapy: 5 Years of KIGS. Mannheim, Germany: JJ Verlag; 1994:97–111.
216. Blomqvist, N. On the bias caused by regression to the mean in studying the relation between change and initial value. J Clin Periodontol. 1986;13:34–37.
217. Hayes, RJ. Methods for assessing whether change depends on initial value. Stat Med. 1988;7:915–927.
218. Taback, SP, Van Vliet, G. Managing the short stature of Turner syndrome: an evidence-based approval to the suggestion of growth hormone supplementation. In: Hindmarsh PC, ed. Current Indications for Growth Hormone Therapy. Basel: Karger; 1999:102–117.
219. Matthews, JNS, Altman, DG, Campbell, MJ, et al. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235.
220. Bramswig, JH, Schlosser, H, Kiese, K. Final height in children with growth hormone deficiency. Horm Res. 1995;43:126–128.
221. Frisch, H, Birnbacher, R. Final height and pubertal development in children with growth hormone deficiency after long-term treatment. Horm Res. 1995;43:132–134.
222. Severi, F. Final height in children with growth hormone deficiency. Horm Res. 1995;43:138–140.
223. Blethen, SL, Compton, P, Lippe, BM, et al. Factors predicting the response to growth hormone (GH) therapy in prepubertal children with GH deficiency. J Clin Endocrinol Metab. 1993;74:574–579.
224. Burns, EC, Tanner, JM, Preece, MA, et al. Final height and pubertal development in 55 children with idiopathic growth hormone deficiency, treated for between 2 and 15 years with human growth hormone. Eur J Pediatr. 1981;137:155–164.
225. Bourguignon, JP, Vandeweghe, M, Vanderschuren-Lodeweyckx, M, et al. Pubertal growth and final height in hypopituitary boys: A minor role of bone age at onset of puberty. J Clin Endocrinol Metab. 1986;63:376–382.
226. Price, DA, Ranke, MB. Final height following growth hormone treatment. In: Ranke MB, Gunnarsson R, eds. Progress in Growth Hormone Therapy: 5 Years of KIGS. Mannheim, Germany: JJ Verlag; 1994:574–579.
227. Ranke, MB, Price, DA, Albertsson-Wikland, K, et al. Factors determining pubertal growth and final height in growth hormone treatment of idiopathic growth hormone deficiency. Horm Res. 1997;48:62–71.
228. Tanaka, T, Satoh, M, Yasunaga, T, et al. GH and GnRH analog treatment in children who enter puberty at short stature. J Pediatr Endocrinol Metab. 1997;10:623–628.
229. Saggese, G, Baroncelli, GI, Bertelloni, S, et al. The effect of long-term growth hormone (GH) treatment on bone mineral density in children with GH deficiency. Role of GH in the attainment of peak bone mass. J Clin Endocrinol Metab. 1996;81:3077–3083.
230. Blethen, SL, Alster, DK, Graves, D, et al. Safety of recombinant DNA–derived growth hormone (rhGH): The National Cooperative Growth Study experience. J Clin Endocrinol Metab. 1996;81:1704–1710.
231. Wilton, P. Adverse events during growth hormone treatment: 5 years’ experience. In: Ranke B, Gunnarsson R, eds. Progress in Growth Hormone Therapy: 5 Years of KIGS. Mannheim, Germany: JJ Verlag; 1994:291–307.
232. Cutfield, WS, Wilton, P, Bennmarker, H, et al. Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatment. Lancet. 2000;355:610–613.
233. Watanabe, S, Yamagucki, N, Tsunematsu, Y, et al. Risk factors for leukemia occurrence among growth hormone users. Jpn J Cancer. 1989;80:822–825.
234. Fisher, DA, Job, J, Preece, M, et al. Leukemia in patients treated with growth hormone. Lancet. 1988;1:1159–1160.
235. Fradkin, JE, Mills, JL, Schonberger, LB, et al. Risk of leukemia after treatment with pituitary growth hormone. JAMA. 1993;270:2829–2832.
236. Oglivy-Stuart, AL, Ryder, WD, Gattamaneni, HR, et al. Growth hormone and tumor recurrence. BMJ. 1992;304:1601–1605.
237. Moshang, T, Rundle, AC, Graves, DA, et al. Brain tumor recurrence in children treated with growth hormone: The National Cooperative Growth Study experience. J Pediatr. 1996;128:S4–S7.
238. Swerdlow, AJ, Reddingius, RE, Higgins, CD, et al. Growth hormone treatment of children with brain tumors and risk of tumor recurrence. J Clin Endocrinol Metab. 2000;85(12):4444–4449.
239. Swerdlow, AJ, Higgins, CD, Adlard, P, et al. Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959–1985: a cohort study. Lancet. 2002;360:273–277.
240. Ma, J, Pollak, MN, Giovannucci, E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–625.
241. Hankinson, SE, Willett, WC, Colditz, GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396.
242. Chan, JM, Stampfer, MJ, Giovannucci, E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566.
243. Palmqvist, R, Hallmans, G, Rinaldi, S, et al. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002;50:642–646.
244. Thorner, MO, Rogol, AD, Blizzard, RM, et al. Acceleration of growth rate in growth hormone–deficient children treated with human growth hormone–releasing hormone. Pediatr Res. 1988;24:145–151.
245. Thorner, MO, Rochiccioli, P, Colle, M, et al. Geraf International Study Group. Once-daily subcutaneous growth hormone–releasing hormone therapy accelerates growth in growth hormone–deficient children during the first year of therapy. J Clin Endocrinol Metab. 1996;81:1189–1196.
246. Hummelink, R, Sippell, WG, Benoit, KG. Intranasal administration of growth hormone–releasing hormone (1–29)-NH2 in children with growth hormone deficiency: effects on growth hormone secretion and growth. Acta Paediatr Suppl. 1993;388:23–26.
247. Bowers, CY, Alster, DK, Frentz, JM. The growth hormone–releasing activity of a synthetic hexapeptide in normal men and short stature children after oral administration. J Clin Endocrinol Metab. 1992;74:292–298.
248. Bowers, CY. On a peptidomimetic growth hormone–releasing peptide. J Clin Endocrinol Metab. 1994;79:940–942.
249. Smith, RG, van der Ploey, LHT, Howard, AD, et al. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18:621–645.
250. Allen, DB. Issues in the transition from childhood to adult growth hormone therapy. Pediatrics. 1999;104:1004–1010.
251. Monson, JP, Hindmarsh, P. The assessment of growth hormone deficiency in children and adults with particular reference to the transitional period. Clin Endocrinol (Oxf). 2000;53:545–547.
252. Rosenfeld, RG. Transitioning patients with childhood-onset growth hormone deficiency to treatment in adulthood. J Pediatr Endocrinol Metab. 2002;15:1361–1365.
253. GH Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab. 2000;85:3990–3993.
254. Donaubauer, J, Kiess, W, Kratzsch, J, et al. Re-assessment of growth hormone secretion in young adult patients with childhood-onset growth hormone deficiency. Clin Endocrinol (Oxf). 2003;58:456–463.
255. de Boer, H, van der Veen, EA. Why retest young adults with childhood-onset growth hormone deficiency? J Clin Endocrinol Metab. 1997;82:2032–2036.
256. Saggese, G, Ranke, MB, Saenger, P, et al. Diagnosis and treatment of growth hormone deficiency in children and adolescents: towards a consensus. Ten years after the Availability of Recombinant Human Growth Hormone Workshop held in Pisa, Italy, 27–28 March 1998. Horm Res. 1998;50:320–340.
257. Shalet, SM, Toogood, A, Rahim, A, et al. The diagnosis of growth hormone deficiency in children and adults. Endocr Rev. 1998;19:203–223.