CHAPTER 40 Glaucoma and corneal surgery
Introduction
Eduard Zirm is credited with performing the first successful human corneal transplant in 19051. Since then, most corneal transplants have been penetrating keratoplasties (PKPs), replacing all layers of a recipient’s cornea even if the pathology was limited to a single layer. With the advent of endothelial keratoplasty, the ‘big bubble’ technique, and femtosecond laser assisted keratoplasty, the selective replacement of layers of the cornea has become increasingly popular2.
From 2005 to 2008, the Eye Bank Association of America reported an increase in the number of endothelial transplants from 1429 to 17 4683. During this same period, the number of PKPs declined from 45 821 to 32 524. While the number of corneas distributed for lamellar transplantation increased almost 20% from 2005 to 2008, only 1072 lamellar surgeries were performed in 20083. The significance of the shift away from PKP to endothelial and lamellar keratoplasties, and its effect on post-keratoplasty glaucoma, has not been well studied; but, may result in lower rates of post-keratoplasty glaucoma.
Keratoprosthesis have also gained greater acceptance. Of these devices, the Boston Keratoprosthesis is the most popular with nearly 5000 having been implanted during the past decade4. Outside the United States, the osteo-odonto-keratoprosthesis has gained popularity as a surgical option for those with severe ocular surface diseases. Even in patients who have not had a history of glaucoma, implantation of these devices can induce glaucoma. Management difficulties are made worse because the implanted devices prevent accurate IOP measurements.
Irvine and Kaufman reported increased intraocular pressure (IOP) after PKP in 19695. Angle closure, a common cause of post-PKP glaucoma, is difficult to control medically and frequently requires surgical intervention. Trabeculectomy and glaucoma drainage devices (GDDs) have become the mainstays of post-PKP glaucoma management.
Just as corneal transplantation is a risk factor for the development of glaucoma, glaucoma is associated with an increased risk of corneal failure and the subsequent need for corneal transplantation6,7. The mechanisms for corneal failure secondary to glaucoma are not clear. Glaucoma surgery, particularly the placement of GDDs, may contribute to corneal failure through peripheral corneal–tube touch, the close proximity of tube tips to the corneal endothelium, and induced anterior chamber turbulence. In patients for whom vision is already compromised by glaucoma, worsening vision may indicate sub-clinical corneal decompensation.
Epidemiology
The incidence of glaucoma after penetrating keratoplasty is linked to the clinical indication for the transplant. Glaucoma occurs in approximately 1–6% of eyes that undergo surgery for keratoconus8–10, in 3–16% of patients with corneal dystrophies and Fuchs’ endothelial dystrophy8,10,11, and in 17–44% of eyes with pseudophakic or aphakic bullous keratopathy8,9,11–13. Eyes with repeated corneal grafts have higher rates of both graft failure and glaucoma.
Pre-existing glaucoma is often more difficult to control after PKP14. Other factors that contribute to the development of glaucoma after PKP are a history of ocular trauma, pre-existing peripheral anterior synechiae, and combined PKP and cataract surgery9,10,13,15.
Since endothelial keratoplasty (EK) is a relatively new procedure, the incidence of glaucoma after EK has not yet been well established. Early reports suggest that glaucoma is diagnosed or glaucoma medications are started in 0–18% of EK patients16–18. In the first year after DSEK, Vajaranant et al. reported starting glaucoma medications in 18% of patients without pre-existing glaucoma and increasing glaucoma therapy in 33% of patients with a history of glaucoma18. While only 0.3% patients without pre-existing glaucoma went on to require glaucoma surgery, 7 of 85 (8%) patients with a history of glaucoma required additional glaucoma surgery after EK. In this study, median IOP peaked 3 months after EK, which led the authors to speculate that many of the patients were steroid responders. Although no long-term data were presented, Lee et al. reported elevations of IOP to greater than 30 mmHg in 13% of patients in the first 6 months after EK16. Most IOP elevations occurred during the first week after surgery.
Thirty-six to 76% of patients undergoing keratoprosthesis surgery were treated preoperatively for glaucoma19,20. The onset or progression of glaucoma as determined by finger tension, visual field progression, or optic nerve head cupping has been reported in 6–28% of cases after keratoprosthesis surgery19,21.
Diagnosis
The diagnosis of glaucoma after PKP can be made challenging immediately postoperatively, by the presence of large epithelial defects, stromal edema, or irregular astigmatism, all of which can complicate applanation tonometry. Early postoperatively, the Tonopen (Reichert, Depew, New York) or the pneumotonometer may more accurately measure IOP. With high regular astigmatism, IOP is best obtained by averaging two Goldmann applanation measurements taken with the prisms 90° apart22. In some cases, digital palpation may be the best way to obtain an estimate of the IOP.
Etiology
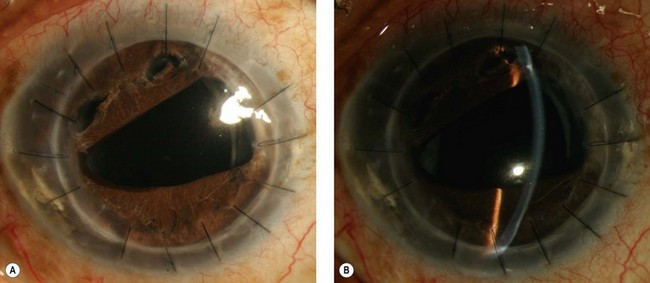
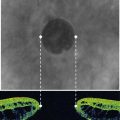
The development of glaucoma in eyes with a history of corneal surgery is usually attributed to changes in angle anatomy. Peripheral anterior synechiae (PAS) are present in up to 87% of patients after PKP10,15. In our experience, PAS caused by corneal transplantation leads to progressive angle closure which is often difficult to manage without surgical intervention. The slowly progressive and recurrent nature of the angle closure prevents goniosynechialysis from being effective: synechiae tend to re-form. Figure 40.1 demonstrates a patient with areas of synechial angle closure.
Distorted angle anatomy, anterior and posterior to the trabecular meshwork, has been implicated in the development of open angle glaucoma after PKP23. Anterior to the angle, tight and long sutures may compress the trabecular meshwork24. Posterior to the angle, loss of the support normally provided by the ciliary body–lens system allows for collapse of the trabecular meshwork.
Donor tissue diameter may also contribute to the development of glaucoma post-keratoplasty. Using an oversized graft may decrease the incidence of glaucoma after PKP in the early postoperative period. This effect may persist in the long term, but there is limited supporting data23–26. In contrast, an undersized graft may decrease angle width and increase the risk for development of PAS with subsequent angle closure.
Immediately post-PKP, elevated IOP may be caused by iritis, hemorrhage, ciliary block glaucoma, and retained viscoelastic. In eyes inflamed preoperatively, the risk of pupillary block due to iritis or hemorrhage may be reduced by an iridotomy. Postoperative steroid response (in 20–32% of patients depending on the definition of steroid induced glaucoma) may elevate IOP in susceptible patients27,28.
EK involves injecting air into the anterior chamber and keeping the patient supine to assist in graft attachment. Typically this involves an anterior chamber air fill of 10–60 minutes followed by an exchange of approximately half of the air for balanced salt solution. This can lead to complications: immediately postoperatively, patients in whom air bubbles extend beyond the inferior pupillary border in the supine position may experience pupillary block. In Tillett’s original description of posterior lamellar keratoplasty, the cornea was clear on the first postoperative day, but the iris was pushed forward against the graft creating anterior synechiae and angle closure with elevated IOP29. A second mechanism for IOP elevations occurring within one week of EK has been postulated by Lee and co-authors: air displaced behind the iris leads to iridocorneal adhesions and elevated IOP16.
Postoperatively, steroid response appears to be a common cause of post-EK IOP elevation18. Fuchs’ endothelial dystrophy, which typically carries a lower risk of glaucoma after PKP, was the most common indication for surgery in the study presented by Vajaranant et al. The median IOP peaked at 3 months postoperatively. Topical steroids were commonly used four times daily for the first 4 months then slowly tapered18. Using a more rapid steroids taper, Lee et al. reported only one patient with IOP elevation after 6 months follow-up16.
Medical treatment of glaucoma after PKP
While prostaglandin analogs are effective in lowering IOP, increased risks of intraocular inflammation and cystoid macular edema have been associated with their use. Increased intraocular inflammation can compromise long-term graft survival. As latanoprost has been associated with recurrent herpetic keratitis, prostaglandin agonists should be used with caution in patients with a history of herpetic eye disease30. We use prostaglandin agonists in patients with post-keratoplasty glaucoma, if the alternative is surgery for IOP control. Many of the reported side effects of prostaglandin analoge have been based upon case reports and no controlled or prospective studies have demonstrated any deleterious effects of prostaglandin analoge on graft survival.
Surgical decision making
When deciding between combined or staged glaucoma and corneal transplant surgery, retrospective and non-randomized reports have not demonstrated clear differences in graft outcomes31,32. In light of common postoperative complications of glaucoma surgery (such as anterior chamber shallowing or flattening and the development of choroidals), we prefer a staged approach. We aim to control IOP prior to visual rehabilitation with corneal surgery. Should other factors necessitate combined surgery, the pitfalls associated with the type of glaucoma procedure planned need to be considered within the context of graft survival.
What type of glaucoma surgery should be performed? Cyclodestruction was among the first surgeries performed for post-PKP glaucoma33. Drawbacks include resultant inflammation which may lead to a greater incidence of graft failure or rejection and the frequent need for repeat treatment to control IOP adequately. Due to its tendency to produce less inflammation, diode cyclophotocoagulation (CPC) is preferred over older techniques such as cryotherapy or use of the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser. A recent study on the use of trans-scleral diode laser to control post-PKP glaucoma showed a single treatment success rate of 72% after 12 months without ensuing graft failure or phthisis bulbi34.
Trabeculectomy with mitomycin C has been studied for the control of post-PKP glaucoma. Two small studies with 22 and 24 months of follow-up demonstrated IOP control rates of 62% and 50% respectively35,36. Approximately 60% of the grafts remained clear during the study period.
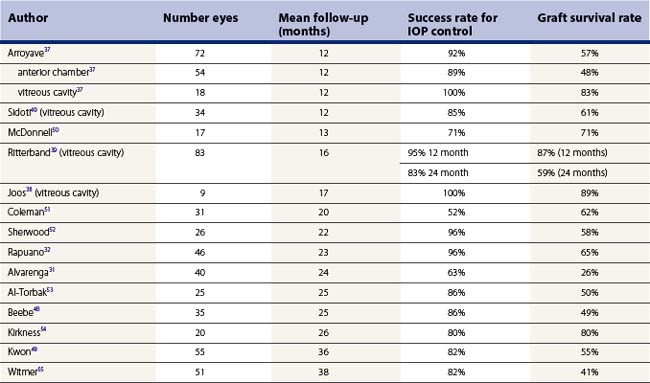
In our opinion, GDDs appear to offer superior IOP control post-PKP. However, the rate of graft survival, while roughly similar to that seen after trabeculectomy, is lower than desired. The outcomes of studies in which GDDs have been performed for post-PKP glaucoma are summarized in Table 40.1. In a few published studies, patients with pars plana tubes demonstrated slightly improved graft survival rates (and similar success rates for IOP control) when compared with those with anterior chamber tubes37–40.
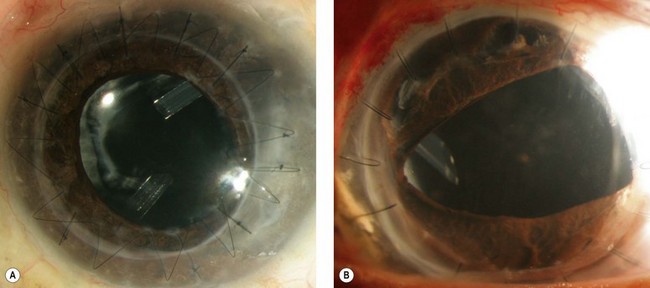
In a phakic eye not requiring cataract extraction, we prefer anterior chamber tubes. In an eye with a stable sulcus or in-the-bag posterior chamber intraocular lens (IOL), the tube can be placed into the ciliary sulcus (Fig. 40.2). If pars plana vitrectomy is planned or anterior chamber or sulcus tube insertion are not desirable, pars plana tube insertion is possible. Insertion of the tube into the pars plana requires shaving of the vitreous base to minimize the risk of vitreous incarceration in the tube and tube failure.
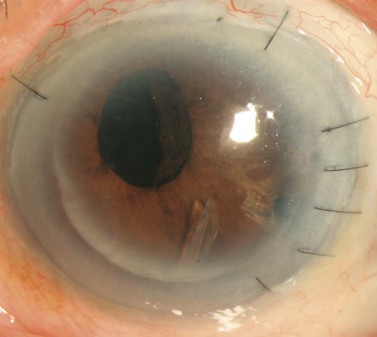
In patients with endothelial dysfunction, EK may be preferable over PKP. Concurrent EK and GDD surgery can be complicated by the escape of air around a tube sclerostomy site that has not yet fibrosed or through a tube that has not been ligated. In our experience, if concurrent EK and GDD surgery is performed, the tube should be completely ligated without fenestrations. EK combined with trabeculectomy can be complicated by air leakage through the sclerostomy and into the bleb. As with PKP, postoperative courses complicated by hypotony and hypertensive phases may have consequences for newly transplanted endothelial cells. For these reasons, we prefer glaucoma intervention and maximal IOP control prior to EK. Although it may be difficult to maintain a full air fill after penetrating glaucoma surgery, successful subsequent EK has been reported in eyes with valved and non-valved GDDs (Fig. 40.3)41,42. Case reports have described successful EK in eyes with a functioning trabeculectomy as well as in an eye with two tube shunts41,43.
Trabeculectomy
A bleb that is low, diffuse, and amenable to rigid gas permeable (RGP) contact lens wear is ideal in an eye with a corneal transplant. We use an anti-metabolite (mitomycin C or 5-fluorouracil) to create a broad, diffuse fornix-based bleb. Mitomycin C allows for statistically improved, long-term IOP control. However, the focal and/or ischemic blebs that Mytomycin C’s use tends to produce may be more susceptible to the development of leaks and infection, especially with contact lens use. In reports of trabeculectomy following PKP with greater than 18-month follow-up, success rates for IOP control ranged from 50% to 77%35,36,44,45.
Glaucoma drainage devices
Insertion of a tube into the ciliary sulcus may offer the best compromise for pseudophakic eyes, not undergoing vitrectomy, and without vitreous prolapse into the anterior chamber. Sulcus tubes should be posteriorly beveled and long enough so that the tip of the tube can just be visualized through an undilated pupil. The tract should allow the tube to rest parallel to the anterior surface of the IOL (see Fig. 40.2).
Endothelial keratoplasty (EK)
EK can be successfully performed after penetrating glaucoma surgery. If the GDD tube is too anterior or its position will limit the size of the EK graft, tube shortening during the EK or tube repositioning prior to EK is preferred. Figure 40.3 demonstrates an inferonasal tube encroaching on the visual axis. Since the tube was well positioned close to the iris surface, it did not interfere with graft placement. Alternatively, to accommodate an AC tube, a small notch can be placed in the transplanted tissue allowing for a larger size graft (and more endothelial cells) to fit within the anterior chamber46. A well positioned sulcus or pars plana tube is unlikely to affect EK.
Postoperative care
Postoperative inflammation should be managed aggressively to minimize both harm to transplanted tissue and the risk of graft rejection. Topical steroids should be used liberally particularly in pseudophakic eyes. While there is not strong evidence for long-term topical steroids, in eyes with glaucoma and corneal transplants we feel they are indicated. Early reports of rejection rates for EK appear to be lower than for PKP. This may be attributable to increased topical steroid use post-EK (as it does not have to be balanced against wound healing as in PKP)47. Newer steroid formulations with lower incidences of steroid-induced ocular hypertension may also benefit those with a history of or who subsequently develop steroid response glaucoma.
Surgical outcomes assessment
In eyes requiring both glaucoma and corneal procedures, visual rehabilitation is dependent on the success of both surgeries. The reported success in eyes requiring both interventions has been fair with moderate follow-up times31,32,37,48,49.
1 Zirm E. Eine erfolgreiche totale Keratoplastik. Graefes Arch Ophthalmol. 1906;64:580-593.
2 Anwar M, Teichmann KD. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28(3):398-403.
3 Eye Banking Statistical Report. Washington, DC: Eye Bank Association of America, 2008.
4 Personal communication with Dr. Claes Dohlman MEEI.
5 Irvine AR, Kaufman HE. Intraocular pressure following penetrating keratoplasty. Am J Ophthalmol. 1969;68(5):835-844.
6 Rahman I, Carley F, Hillarby C, et al. Penetrating keratoplasty: indications, outcomes, and complications. Eye. 2009;23(6):1288-1294.
7 Sugar A, Tanner JP, Dontchev M, et al. Recipient risk factors for graft failure in the cornea donor study. Ophthalmology. 2009;116(6):1023-1028.
8 Sekhar GC, Vyas P, Nagarajan R, et al. Post-penetrating keratoplasty glaucoma. Indian J Ophthalmol. 1993;41(4):181-184.
9 Ing JJ, Ing HH, Nelson LR, et al. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105(10):1855-1865.
10 Kirkness CM, Ficker LA. Risk factors for the development of postkeratoplasty glaucoma. Cornea. 1992;11(5):427-432.
11 Goldberg DB, Schanzlin DJ, Brown SI. Incidence of increased intraocular pressure after keratoplasty. Am J Ophthalmol. 1981;92(3):372-377.
12 Sihota R, Sharma N, Panda A, et al. Post-penetrating keratoplasty glaucoma: risk factors, management and visual outcome. Aust NZ J Ophthalmol. 1998;26(4):305-309.
13 Foulks GN. Glaucoma associated with penetrating keratoplasty. Ophthalmology. 1987;94(7):871-874.
14 Simmons RB, Stern RA, Teekhasaenee C, et al. Elevated intraocular pressure following penetrating keratoplasty. Trans Am Ophthalmol Soc. 1989;87:79-91. discussion
15 Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994;101(9):1536-1547.
16 Lee JS, Desai NR, Schmidt GW, et al. Secondary angle closure caused by air migrating behind the pupil in Descemet stripping endothelial keratoplasty. Cornea. 2009;28(6):652-656.
17 Lee WB, Jacobs DS, Musch DC, et al. Descemet’s stripping endothelial keratoplasty: safety and outcomes. A Report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(9):1818-1830.
18 Vajaranant TS, Price MO, Price FW, et al. Visual acuity and intraocular pressure after Descemet’s stripping endothelial keratoplasty in eyes with and without preexisting glaucoma. Ophthalmology. 2009;116(9):1644-1650.
19 Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology. 1998;105(4):751-757.
20 Aldave AJ, Kamal KM, Vo RC, et al. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009;116(4):640-651.
21 Khan BF, Harissi-Dagher M, Pavan-Langston D, et al. The Boston keratoprosthesis in herpetic keratitis. Arch Ophthalmol. 2007;125(6):745-749.
22 Holladay JT, Allison ME, Prager TC. Goldmann applanation tonometry in patients with regular corneal astigmatism. Am J Ophthalmol. 1983;96(1):90-93.
23 Olson RJ, Kaufman HE. A mathematical description of causative factors and prevention of elevated intraocular pressure after keratoplasty. Invest Ophthalmol Vis Sci. 1977;16(12):1085-1092.
24 Zimmerman T, Olson R, Waltman S, et al. Transplant size and elevated intraocular pressure. Postkeratoplasty. Arch Ophthalmol. 1978;96(12):2231-2233.
25 Bourne WM, Davison JA, O’Fallon WM. The effects of oversize donor buttons on postoperative intraocular pressure and corneal curvature in aphakic penetrating keratoplasty. Ophthalmology. 1982;89(3):242-246.
26 Heidemann DG, Sugar A, Meyer RF, et al. Oversized donor grafts in penetrating keratoplasty. A randomized trial. Arch Ophthalmol. 1985;103(12):1807-1811.
27 Erdurmus M, Cohen EJ, Yildiz EH, et al. Steroid-induced intraocular pressure elevation or glaucoma after penetrating keratoplasty in patients with keratoconus or Fuchs dystrophy. Cornea. 2009;28(7):759-764.
28 Fan JC, Chow K, Patel DV, et al. Corticosteroid-induced intraocular pressure elevation in keratoconus is common following uncomplicated penetrating keratoplasty. Eye (Lond). 2009;23(11):2056-2062.
29 Tillett CW. Posterior lamellar keratoplasty. Am J Ophthalmol. 1956;41(3):530-533.
30 Wand M, Gilbert CM, Liesegang TJ. Latanoprost and herpes simplex keratitis. Am J Ophthalmol. 1999;127(5):602-604.
31 Alvarenga LS, Mannis MJ, Brandt JD, et al. The long-term results of keratoplasty in eyes with a glaucoma drainage device. Am J Ophthalmol. 2004;138(2):200-205.
32 Rapuano CJ, Schmidt CM, Cohen EJ, et al. Results of alloplastic tube shunt procedures before, during, or after penetrating keratoplasty. Cornea. 1995;14(1):26-32.
33 Binder PS, Abel RJr, Kaufman HE. Cyclocryotherapy for glaucoma after penetrating keratoplasty. Am J Ophthalmol. 1975;79(3):489-492.
34 Ocakoglu O, Arslan OS, Kayiran A. Diode laser transscleral cyclophotocoagulation for the treatment of refractory glaucoma after penetrating keratoplasty. Curr Eye Res. 2005;30(7):569-574.
35 Ishioka M, Shimazaki J, Yamagami J, et al. Trabeculectomy with mitomycin C for post-keratoplasty glaucoma. Br J Ophthalmol. 2000;84(7):714-717.
36 WuDunn D, Alfonso E, Palmberg PF. Combined penetrating keratoplasty and trabeculectomy with mitomycin C. Ophthalmology. 1999;106(2):396-400.
37 Arroyave CP, Scott IU, Fantes FE, et al. Corneal graft survival and intraocular pressure control after penetrating keratoplasty and glaucoma drainage device implantation. Ophthalmology. 2001;108(11):1978-1985.
38 Joos KM, Lavina AM, Tawansy KA, et al. Posterior repositioning of glaucoma implants for anterior segment complications. Ophthalmology. 2001;108(2):279-284.
39 Ritterband DC, Shapiro D, Trubnik V, et al. Penetrating keratoplasty with pars plana glaucoma drainage devices. Cornea. 2007;26(9):1060-1066.
40 Sidoti PA, Mosny AY, Ritterband DC, et al. Pars plana tube insertion of glaucoma drainage implants and penetrating keratoplasty in patients with coexisting glaucoma and corneal disease. Ophthalmology. 2001;108(6):1050-1058.
41 Esquenazi S, Rand W. Safety of DSAEK in patients with previous glaucoma filtering surgery. J Glaucoma. 2010;19(3):219-220.
42 Riaz KM, Sugar J, Tu EY, et al. Early results of Descemet-stripping and automated endothelial keratoplasty (DSAEK) in patients with glaucoma drainage devices. Cornea. 2009;28(9):959-962.
43 Duarte MC, Herndon LW, Gupta PK, et al. DSEK in eyes with double glaucoma tubes. Ophthalmology. 2008;115(8):1435. e1
44 Ayyala RS, Pieroth L, Vinals AF, et al. Comparison of mitomycin C trabeculectomy, glaucoma drainage device implantation, and laser neodymium:YAG cyclophotocoagulation in the management of intractable glaucoma after penetrating keratoplasty. Ophthalmology. 1998;105(8):1550-1556.
45 Gilvarry AM, Kirkness CM, Steele AD, et al. The management of post-keratoplasty glaucoma by trabeculectomy. Eye. 1989;3(Pt 6):713-718.
46 Sansanayudh W, Bahar I, Rootman D. Novel technique in preparing a donor DSAEK lenticule in a patient with a glaucoma drainage device. Br J Ophthalmol. 2009;93(9):1267-1269.
47 Allan BD, Terry MA, Price FWJr, et al. Corneal transplant rejection rate and severity after endothelial keratoplasty. Cornea. 2007;26(9):1039-1042.
48 Beebe WE, Starita RJ, Fellman RL, et al. The use of Molteno implant and anterior chamber tube shunt to encircling band for the treatment of glaucoma in keratoplasty patients. Ophthalmology. 1990;97(11):1414-1422.
49 Kwon YH, Taylor JM, Hong S, et al. Long-term results of eyes with penetrating keratoplasty and glaucoma drainage tube implant. Ophthalmology. 2001;108(2):272-278.
50 McDonnell PJ, Robin JB, Schanzlin DJ, et al. Molteno implant for control of glaucoma in eyes after penetrating keratoplasty. Ophthalmology. 1988;95(3):364-369.
51 Coleman AL, Mondino BJ, Wilson MR, et al. Clinical experience with the Ahmed Glaucoma Valve implant in eyes with prior or concurrent penetrating keratoplasties. Am J Ophthalmol. 1997;123(1):54-61.
52 Sherwood MB, Smith MF, Driebe WTJr, et al. Drainage tube implants in the treatment of glaucoma following penetrating keratoplasty. Ophthalmic Surg. 1993;24(3):185-189.
53 Al-Torbak AA. Outcome of combined Ahmed glaucoma valve implant and penetrating keratoplasty in refractory congenital glaucoma with corneal opacity. Cornea. 2004;23(6):554-559.
54 Kirkness CM, Ling Y, Rice NS. The use of silicone drainage tubing to control post-keratoplasty glaucoma. Eye. 1988;2(Pt 5):583-590.
55 Witmer MT, Tiedeman JS, Olsakovsky LA, et al. Long-term intraocular pressure control and corneal graft survival in eyes with a pars plana Baerveldt implant and corneal transplant. J Glaucoma. 2010 Feb;19(2):124-131.